Abstract
Background and purpose:
Obestatin, encoded by the ghrelin gene may inhibit gastrointestinal (GI) motility. This activity was re-investigated.
Experimental approach:
Rat GI motility was studied in vitro (jejunum contractility and cholinergically-mediated contractions of forestomach evoked by electrical field stimulation; EFS) and in vivo (gastric emptying and intestinal myoelectrical activity). Ghrelin receptor function was studied using a GTPγS assay and transfected cells.
Key results:
Contractions of the jejunum or forestomach were unaffected by obestatin 100 nM or 0.01–1000 nM, respectively (P>0.05 each; n=4-18). Obestatin (0.1-1 nM) reduced the ability of ghrelin 1 μM to facilitate EFS-evoked contractions of the stomach (increases were 42.7±7.8% and 21.2±5.0 % in the absence and presence of obestatin 1 nM; P<0.05; n=12); higher concentrations (10–1000 nM) tended to reduce the response to ghrelin but changes were not statistically significant. Similar concentrations of obestatin did not significantly reduce a facilitation of contractions caused by the 5-HT4 receptor agonist prucalopride, although an inhibitory trend occurred at the higher concentrations (increases were 69.3±14.0% and 42.6±8.7% in the absence and presence of 1000 nM obestatin; n=10). Obestatin (up to 10 μM) did not modulate recombinant ghrelin receptor function. Ghrelin increased gastric emptying and reduced MMC cycle time; obestatin (1000 and 30,000 pmol kg−1 min−1) had no effects. Obestatin (2500 pmol kg−1 min−1, starting 10 min before ghrelin) did not prevent the ability of ghrelin (500 pmol kg−1 min−1) to shorten MMC cycle time.
Conclusions and implications:
Obestatin has little ability to modulate rat GI motility.
Keywords: obestatin, GPR39, ghrelin, rat, gastrointestinal
Introduction
Recently, a novel peptide, thought to be the endogenous ligand for the orphan G-protein-coupled receptor GPR39, was identified through sequence analysis of the ghrelin-precursor gene, proghrelin. This peptide was named obestatin to reflect its ability to suppress feeding and weight gain in mice (Zhang et al., 2005). In addition, the authors hypothesized that obestatin may play a role in the regulation of gastrointestinal motility, as it provoked a sustained reduction in rat gastric emptying and reduced spontaneous contractile activity in the jejunum, isolated from the same species. Subsequently, Moechars et al. (2006) suggested that the rate of gastric emptying is increased in GPR39 knockout mice, an observation which may be consistent with a gastric regulatory function for obestatin. Interestingly, this ability of obestatin to reduce gastric emptying is the opposite to the prokinetic stimulator function of ghrelin, widely shown to increase gastric emptying rate in a number of mammalian species, including humans (Murray et al., 2005; Tack et al., 2005; Binn et al., 2006; Levin et al., 2006), dogs (Trudel et al., 2003) and rodents (Asakawa et al., 2001; Trudel et al., 2002; Levin et al., 2005). This opposing activity of two proteins apparently derived from the same precursor gene, was further demonstrated by the ability of obestatin to inhibit the increased spontaneous contractility caused by ghrelin in the mouse isolated jejunum, in the absence of any direct interaction with the ghrelin binding site (Zhang et al., 2005). However, these exciting observations have now been at least partially questioned by Holst et al. (2006) and Wigglesworth et al. (2006) who were unable to replicate the interaction between obestatin and the GPR39 receptor. Consequently, we have re-examined the effects of obestatin on different in vitro and in vivo models of rat gastrointestinal motility, in part replicating but also extending the studies of Zhang et al. (2005) and looked for evidence of any selective interaction between the effects of obestatin and ghrelin in these models. Our findings suggest a subtle but insignificant ability of obestatin to modulate gastrointestinal (GI) motility via an action that is not selectively dependent on the activity of ghrelin.
Methods
Ghrelin GTPγS binding assay
Activity of obestatin at the human ghrelin receptor was assessed using high-affinity [35S]-GTPγS binding in a 96-well scintillantion proximity assay (SPA). In brief, HEK293 membranes co-expressing the human ghrelin receptor and the rat G protein subunit Gαi were resuspended in assay buffer (50 mM HEPES, 10 mM MgCl2, 100 mM NaCl, pH 7.4 at 25°C) and preincubated with 4 μM GDP. Five micrograms of membrane/well were incubated with obestatin (30 pM–10 μM) or human ghrelin (3 pM–1 μM) in the presence of 0.38 nM guanosine 5′ [35-S]-thiotriphosphate triethylamine for 30 min. Wheat germ agglutinin (WGA) SPA beads (0.5 mg/well) were then added and the mixture shaken for 30 min at room temperature. Bound [35S]-GTPγS was determined by scintillation counting. In experiments designed to examine antagonist activity, the assay protocol had the following changes: different concentrations of obestatin were preincubated with membranes for 30 min before the addition of an EC80 concentration of human ghrelin and [35S]-GTPγS incubation.
Rat isolated tissues
Adult male Sprague–Dawley rats (Charles River, Thanet, UK, 250–350 g), were culled by CO2 asphyxiation followed by cervical dislocation. All efforts were made to minimize the number of animals used and culling was performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by an animal care committee. Following a midline incision, the stomach and small intestine were blunt-dissected and placed immediately in Krebs solution (NaCl 121.5, CaCl2 2.5, KH2PO4 1.2, KCl 4.7, MgSO4 1.2, NaHCO3 25.0, glucose 5.6 mM) previously equilibrated with 5% CO2 in O2 at room temperature. Sections of the jejunum or forestomach (∼4 × 8 mm) were cut approximately parallel to the longitudinal muscle fibres and the mucosa was either fully (jejunum) or partially (stomach) removed. Tissues were suspended under 10 mN for isometric recording between two platinum ring electrodes 1 cm apart, in 5 ml tissue baths containing Krebs solution bubbled with 5% CO2/95% O2 and maintained at 37°C. Force was measured using the Pioden dynamometer UF1 force-displacement transducers (Pioden Control Ltd, Cantebury, UK). Data acquisition and analysis were performed using MP100 hardware and AcqKnowledge software (Biopac Systems, Inc., Santa Barbara, CA, USA). For experiments investigating the effects of obestatin on jejunal contractility, the methods published by Zhang et al. (2005) were followed as closely as possible. Briefly, tissues were allowed to equilibrate for 90 min during which time bath solutions were changed every 20 min. Tissue viability was initially assessed by measuring the contraction induced by 10 μM acetylcholine chloride. Following a wash and a 20 min recovery period to allow basal contractility activity to stabilize, vehicle followed by 100 nM obestatin (5 min contact each) was applied. Baseline muscle contractility following vehicle or obestatin challenges was measured and expressed as a percentage of the 10 μM acetylcholine chloride-induced contraction.
For experiments investigating the effects of obestatin on isolated forestomach preparations, tissues were allowed to equilibrate for 45 min during which time bath solutions were changed every 15 min. Electrical field stimulation (EFS) was achieved using biphasic square-wave pulses of 0.5 ms pulse width, at a submaximally effective voltage (±25 V; Digitimer, Welwyn Garden City, UK). For consistency, the frequency was adjusted between 2.5 and 5 Hz to give contractile responses between 5 and 40 mN. EFS was applied for 10 s every 1 min for 30-min -periods; each 30-min period was separated by a 5 min interval in which the bath solutions were changed. Using this protocol, the contractions evoked by EFS are cholinergically mediated (abolished by scopolamine 10 μM, unpublished observations). After obtaining consistent EFS-evoked contractions, a single concentration of obestatin or vehicle was applied to each tissue strip (15 min contact). The mean amplitude of three maximum responses after obestatin or vehicle application was calculated and the change expressed as a percentage of the mean amplitude of three pre-drug responses. In separate experiments, ghrelin (0.1 μM) or the 5HT4-receptor agonist, prucalopride (3 μM) was added 1 min after the application of obestatin or vehicle and responses were measured as described above.
Rat GI motility in vivo
Surgery
Experiments were performed on male Sprague–Dawley (300–350 g) rats kept under standardized conditions on a commercial diet (Beekay Feeding AB, Sollentuna, Sweden). The studies were approved by the local Ethics committee for animal experimentation in northern Stockholm, Sweden. After the rats had been deprived of food overnight, surgery was performed under anaesthesia with pentobarbitone (50 mg kg−1 intraperitoneally; Apoteksbolaget, Umeå, Sweden). The experimental protocol was approved by the Ethics Committee in northern Stockholm for the humane use of experimental animals in research.
For small bowel motility studies, three bipolar stainless-steel electrodes (SS-5T, Clark Electromedical Instruments, Reading, UK) were implanted into the muscular wall of the small intestine, 5(D), 15(J1) and 25(J2) cm distal to the pylorus through a midline incision. For gastric emptying studies and to measure the return of fasting motility after feeding and a period of fed motility, an indwelling polyethylene catheter (PE 50, Clay Adams, Becton Dickinson, Parsippany, NJ, USA) was implanted into the gastric forestomach through a midline incision for administration of the radioactive marker solution or nutrient solution. In all studies, silastic catheters (Dow Corning Co., Midland, MI, USA) were inserted into each jugular vein. All catheters and electrodes were tunnelled subcutaneously to exit at the back of the animal's neck. In all studies, the animals were housed singly after surgery and allowed to recover for at least 7 days before experiments were undertaken. During the recovery periods, rats were trained to comply with experimental conditions. Experiments were carried out in conscious animals after an 18 h fasting period in wire-bottomed cages, with free access to water.
Gastric emptying
Gastric emptying was studied both in a non-nutrient and nutrient setting with a liquid solution. Obestatin (1000 and 30 000 pmol kg−1 min−1) or saline was infused at a rate of 15 μl min−1 for 10 min. After this loading period, 0.5 ml of a radioactive marker solution consisting of polyethylene glycol (PEG) 4000 containing 1.48 MBq of [51Cr]-Na2CrO4 ml−1 of solution (pH 7.2, 300 mOsm kg−1) or 50% radioactive PEG 4000 and 50% enteral feeding solution (Isosource, Novartis, Täby, Sweden) was administered slowly over a period of 30 s via the forestomach catheter, whereas the infusion of obestatin or saline was continued for 20 min. The time of 20 min was chosen as this has been shown previously to correspond to 50% emptying using the current model (Levin et al., 2005). The rats were then killed with an overdose of pentobarbitone, the abdomen opened, the pylorus and lower esophagus were ligated and the stomach removed en bloc. The amount of radioactivity remaining in the stomach was calculated in a gamma counter (Wallac, Turku, Finland) for 60 s. Gastric emptying was determined from the percentage of radioactive marker retained in the stomach after 20 min compared with a standard of an equal volume of the radioactive marker.
Small bowel motility
Rats were placed in Bollman cages and the electrodes were connected to an EEG preamplifier (7P5B) operating a Grass Polygraph 7B (Grass Instruments, Quincy, MA, USA). The time constant was set at 0.015 s and the low and high cutoff frequencies were set at 10 and 35 Hz, respectively. The main characteristic feature of myoelectrical activity of the small intestine in the fasted state, the activity front (phase III) of the migrating motor complex (MMC), was identified as a period of clearly differentiated intense spiking activity with an amplitude at least twice that of the preceding baseline, propagating aborally through the whole recording segment and followed by a period of quiescence. The MMC cycle length, duration and propagation velocity of the activity fronts were calculated as a mean of the study period. The MMC cycle length, reflecting the interval between the activity fronts that were propagated throughout the study segment, was always calculated at the J2 recording site. All experiments started with a control recording of basal myoelectrical activity, during which four activity fronts of the MMC were observed, propagating over all three recording sites during a period of 60 min. All intravenous (i.v.) infusions were given using a microinjection pump (CMA 100, Carnegie Medicine, Stockholm, Sweden).
In an initial set of experiments with ghrelin, the time for return of fasting motility after fed motility was induced was studied. A bolus of 2 ml of Isosource (Novartis, Täby, Sweden; 1.0 kcal ml−1, protein 16 energy percent (E%), carbohydrate 54 E%, fat 30 E%; 1 kcal ml−1) was administered intragastrically over 3 min. Ten minutes later, either saline or ghrelin (300, 1000 or 3000 pmol kg−1 min−1) was administered i.v. at a rate of 5–45 μl min−1 and continued until the fasting motility pattern resumed (one propagated activity front). In a second set of experiments, to assess the effect of long-term infusion of ghrelin on fasting small bowel motility two experimental procedures were used. First, after a 60 min control period, ghrelin (1000 pmol kg−1 min−1) or saline was administered at a rate of 15 μl min−1 continuously over 4 h and the effects on the fasting motility pattern studied. Saline and ghrelin (1000 pmol kg−1 min−1) were administered at 15 μl min−1 for alternating 60 min periods over 6 h (three periods of saline and ghrelin, respectively in the same rat) and the effects on the fasting motility were again studied.
To study the effect of obestatin on fasting motility, this peptide was infused for 60 min at 1000 pmol kg−1 min−1 with 15 μl min−1. In a second set of experiments with fasted rats, ghrelin was infused for 60 min (500 pmol kg−1 min−1; 7 μl min−1). Two days later, in each of the same rats as studied earlier with ghrelin alone, an infusion of obestatin was begun (2500 pmol kg−1 min−1; 40 μl min−1) and 10 min later an infusion of ghrelin (500 pmol kg−1 min−1; 7 μl min−1) was started. Both infusions then continued for 60 min.
Data analysis and statistical procedures
Data are expressed as means±s.e.m. The statistical significance of any differences between unpaired data was determined using Student's two-tailed t-test. For the experiments with obestatin in vivo, the results were compared using the Mann–Whitney U-test or Wilcoxon signed rank-test as appropriate using GraphPad Prism, version 4 (San Diego, CA, USA); n values are the number of animals used. P<0.05 was considered statistically significant.
Chemical reagents used
All drugs were freshly prepared before use. Obestatin (Cambridge Research Biochemicals, Cambridge, UK or NeoMPS, Strasbourg, France) and rat ghrelin (Bachem Ltd, St Helens, UK or NeoMPS) were dissolved in 0.9% NaCl containing 0.01% bovine serum albumin (Sigma, Gillingham, UK). The obestatin obtained from Cambridge Research Biochemicals was made using standard Fmoc peptide synthesis methodology. Purification by reverse-phase high-pressure liquid chromatography (HPLC) and purity was >95% as determined by analytical HPLC (Vydac 218TP54, eluent A 0.1% TFA/water, eluent B 0.1% TFA/acetonitrile, gradient 0–50% B over 30 min, wavelength 230 nm, flow 1.5 ml min−1). Theoretical monoisotopic mass: 2515.3, found [M+H]+ 2516.4. Prucalopride (in house) and acetylcholine chloride (Sigma, Gillingham, UK) were both dissolved in water.
In all experiments conducted in vitro, obestatin was obtained from Cambridge Research Biochemicals. In the studies conducted in vivo, obestatin was obtained from Cambridge Research Biochemicals and NeoMPS. Similar results were obtained when using obestatin from either source, so the results were combined.
Results
Ghrelin GTPγS-binding assay
Obestatin was found to be devoid of any agonist or antagonist activity at the human ghrelin receptor. Ghrelin produced a concentration-dependent increase in GTPγS binding yielding a pEC50 of 8.86±0.03. Obestatin failed to stimulate GTPγS binding up to concentrations of 10 μM. In antagonist assays, a pre-determined EC80 concentration of ghrelin (10 nM) was used. This concentration gave a 3.1±0.4-fold stimulation of GTPγS binding over basal, which obestatin (0.1 nM–10 μM) failed to change (2.9±0.3-fold stimulation; P>0.05). n=4 in all the experiments.
Rat isolated jejunum
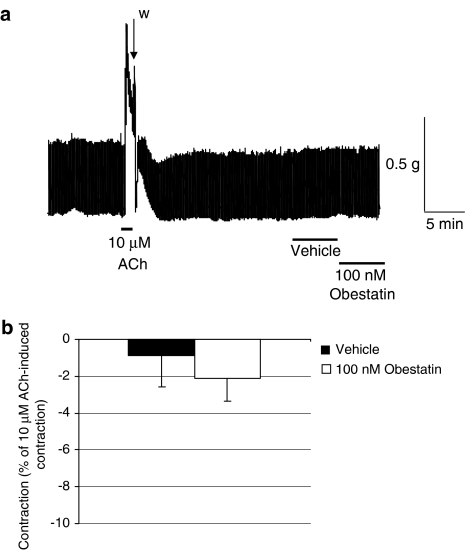
Compared with vehicle, obestatin 100 nM had no effects on jejunal baseline contractility (n=4, P>0.05; Figure 1).
Figure 1.
Effects of obestatin on rat isolated jejunum basal muscle contractility. (a) Representative trace of acetylcholine chloride 10 μM, vehicle and obestatin 100 nM; w indicates a wash period. (b) Obestatin 100 nM had no effect on rat isolated jejunum basal muscle contractility compared to vehicle controls; 5 min contact time; n=4, P>0.05.
Rat isolated forestomach
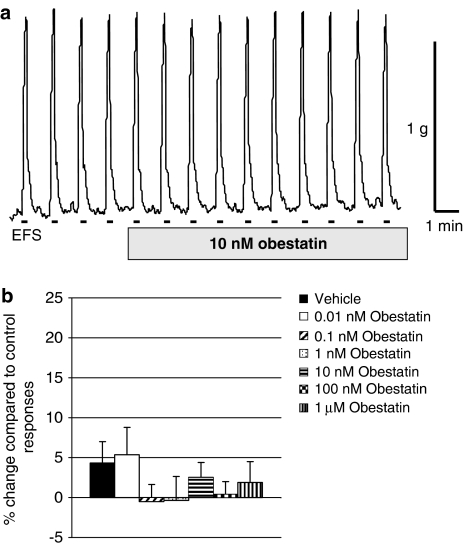
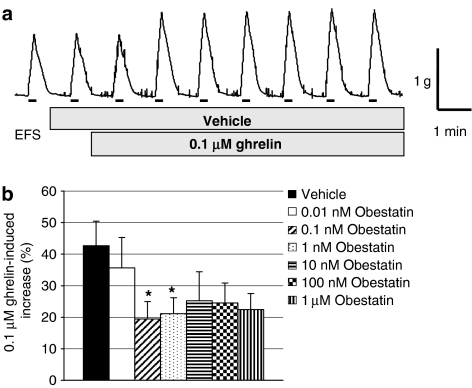
Compared with vehicle-treated tissues, obestatin 0.01 nM–1 μM had no effects on EFS-evoked contractions (n=7–18, P>0.05; Figure 2). However, compared with vehicle-treated tissues, in which ghrelin 0.1 μM increased the EFS-evoked contractions (by 42.7±7.8%; n=15, see Figure 3a for example trace), obestatin 0.1 nM–1 μM significantly inhibited or tended to reduce the excitatory response of ghrelin (Figure 3b). In similar experiments in which prucalopride was administered instead of ghrelin, a larger increase in EFS-evoked contractions was observed (increased by 69.3±14.0%; n=12). This action of prucalopride was not significantly reduced by the presence of obestatin 1–1000 nM, although at the higher concentrations of obestatin there was a tendency for the effects of prucalopride to be smaller (e.g., increases caused by prucalopride in the presence of obestatin 1, 10, 100 or 1000 nM were, respectively, 82.1±18.4, 59.3±14.8, 40.8±10.0 and 42.6±8.7%, n=10, P>0.1, each).
Figure 2.
Effects of obestatin on EFS-evoked, nerve-mediated contractions in rat isolated forestomach. (a) Representative trace of obestatin 10 nM. The bars indicate the periods of EFS, as described in Methods. (b) Obestatin 0.01 nM–1 μM had no effect on EFS-evoked, nerve-mediated contractions in rat isolated forestomach compared with vehicle controls; 15 min contact time; n=7–18, P>0.05.
Figure 3.
Interactions between obestatin and ghrelin on EFS-evoked, nerve-mediated contractions in rat isolated forestomach. (a) Representative trace of ghrelin 0.1 μM-induced increase of EFS-evoked contractions. The bars indicate the periods of EFS, as described in Methods. (b) Effects of obestatin, 0.01 nM–1 μM, on ghrelin 0.1 μM-induced potentiation of EFS-evoked, nerve-mediated contractions in rat isolated forestomach compared to vehicle controls; obestatin administered 1 min before 15 min application of ghrelin; n=8–18, *P<0.05.
Gastric emptying
During saline infusion, the remaining radioactive non-nutrient PEG 4000 meal or nutrient liquid meal was, respectively, 32.2±2.2 and 35.4±2.3% of the meal originally administered (n=4 each). Obestatin 1000 or 30 000 pmol kg−1 min−1 (infused for a 10 min loading period followed by 20 min after the radioactive solution was administered into the stomach) had no significant effect on the gastric retention of either the non-nutrient (31.3±2.1 and 34.8±3.0%) or nutrient (34.1±2.2 and 33.5±3.8%) meal, respectively; n=4 each.
Small bowel motility in vivo
Effect of ghrelin
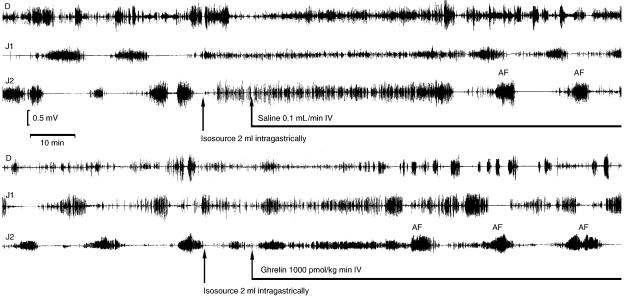
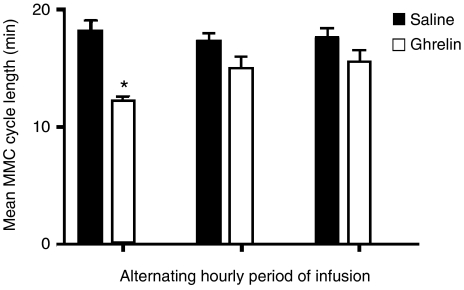
During fasting, the MMC cycle length was 15.6±0.8 min (n=7). Following dosing with Isosource, the first propagated activity fronts, measured at J2, were seen after 39.4±1.9, 37.8±13.2, 33.4±1.9 and 29.5±0.5 min in rats infused with, saline or ghrelin at 300, 1000 and 3000 pmol kg−1 min−1, respectively (P<0.05 for 1000 and 3000 pmol kg−1 min−1 vs saline; n=6 each) (Figure 4). In fasted rats and during 4 h infusion of ghrelin 1000 pmol kg−1 min−1, the MMC cycle length was decreased compared with saline during the first hour (11.4±1.3 and 14.2±0.8, respectively; P<0.05; n=6 each). However, this effect was transient and after the first hour there was no statistically significant difference (data not shown). Similar results were seen when infusion of saline and ghrelin 1000 pmol kg−1 min−1 was alternated over 6 h at hourly intervals. During the first hour of ghrelin infusion, the interval between the activity fronts was significantly shorter compared with saline, but this effect was also transient (Figure 5). There was no effect of ghrelin on the propagation velocity of the activity fronts or the duration of the activity fronts (data not shown).
Figure 4.
Representative recordings of small bowel electrical activity in the rat. Records are before and after oral administration of the Isosource meal and subsequent i.v. infusion with saline or ghrelin (1000 pmol kg−1 min−1). Electrodes had been previously implanted into the muscular wall of the small intestine 5 (D), 15 (J1) and 25 (J2) cm distal to the pylorus.
Figure 5.
The effect of alternating infusions of ghrelin on the interval between the activity fronts of fasting small bowel motility. Infusions of saline or ghrelin (1000 pmol kg−1 min−1) were administered at 15 μ min−1 for alternating 60 min periods over 6 h. Results are given as means and s.e.m. (vertical lines); comparisons were made between the changes measured during saline or ghrelin infusions during each of the 3 h periods of infusion (*P<0.05; n=6 each).
Effect of obestatin alone or in combination with ghrelin
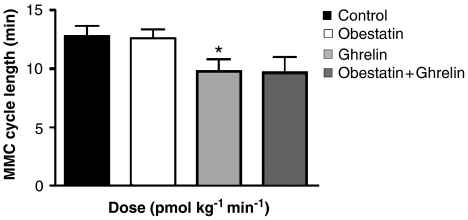
In the fasting state, the interval between the propagated activity fronts of the MMC was 13.5±1.5 min during the control period with saline infusion. The interval between the activity fronts was not different between infusion of saline or obestatin 1000 pmol kg−1 min−1 (Figure 6). Obestatin had no effect on the duration of the activity fronts or the rate at which they were propagated (data not shown). Ghrelin 500 pmol kg−1 min−1 decreased the MMC cycle length (Figure 6). When combined with obestatin 2500 pmol kg−1min−1, ghrelin tended to reduce the MMC cycle length by a similar amount, but an increased scatter of data meant that this apparent effect of ghrelin was not statistically significant (P>0.08; Figure 6).
Figure 6.
Interactions between obestatin and ghrelin on the interval between the activity fronts of the migrating myoelectric complex. Obestatin (1000 pmol kg−1 min−1) and ghrelin (500 pmol kg−1 min−1) were infused for 60 min at, respectively, 15 and 7 μl min−1. When combined, obestatin infusion (2500 pmol kg−1 min−1; 40 μl min−1) was started 10 min before the infusion of ghrelin (500 pmol kg−1 min−1), and both then continued for 60 min. Data are given as mean and s.e.m. (vertical lines); n=7 each; *P<0.05 compared to control.
Discussion and conclusions
The effects of obestatin were studied in models of rat GI motility that were sensitive to the prokinetic activity of ghrelin. The ability of ghrelin to induce a small facilitation of cholinergically mediated contractions in rat isolated forestomach has been observed previously (Dass et al., 2003; Bassil et al., 2006). Similarly, our experiments confirm this ability of ghrelin to increase rat gastric emptying (Trudel et al., 2002; De Winter et al., 2004; Levin et al., 2005; Poitras et al., 2005) and shorten the rat small intestinal MMC cycle time (Edholm et al., 2004).
Our studies on the effects of ghrelin on small bowel motility extend those previously obtained. Thus, the ability of ghrelin to shorten the intervals between the MMCs seems to be transient, as after 1 h of ghrelin treatment this activity could no longer be observed. The reasons for this are not clear. Circulating ghrelin is present as acylated or des-acyl ghrelin, with the majority being des-acyl ghrelin. It is possible that some of the infused acylated ghrelin may have been converted to des-acyl ghrelin over time. Des-acyl ghrelin has been shown to inhibit gastric emptying in rats, at least when given intracisternally (Chen et al., 2005) and some of the metabolic effects of acylated ghrelin are counteracted by des-acyl ghrelin (Broglio et al., 2004). Accordingly, it becomes a possibility that in our experiments, the transient ability of ghrelin to shorten the MMC cycle length may be related to a build-up of the levels of des-acyl ghrelin, somehow leading to an inhibition of the effects of the acylated form of ghrelin.
In the present study, obestatin, in contrast to ghrelin, had no ability to inhibit or excite GI motility, either in vitro or in vivo, the latter studies being carried out using obestatin obtained from two different suppliers and during both fasting small bowel motility and gastric emptying after administration of a nutrient or non-nutrient meal. These data contrast with the observations of Zhang et al. (2005), who found that obestatin could provoke a sustained inhibition of rat gastric emptying, inhibit spontaneous contractile activity in the isolated jejunum and functionally antagonize the excitatory effects of ghrelin in the same preparation. Further, the validity of these negative data is supported by the demonstration of biological activity with obestatin in rat isolated forestomach. Thus, we were able to confirm that obestatin has the ability to inhibit a prokinetic-like activity of ghrelin. In these experiments, low concentrations of obestatin (0.1 and 1 nM, but not 0.01 nM) prevented the ability of ghrelin to increase EFS-evoked, cholinergically mediated contractions, without directly affecting the EFS-evoked contractions. These were difficult experiments to conduct, as the response to ghrelin is highly variable (Bassil et al., 2006) and a clear concentration-dependent activity of obestatin could not be obtained; the statistical significance of the effect observed with low concentrations of obestatin was lost at the higher concentrations (10–1000 nM). Nevertheless, these data with obestatin, obtained in rat isolated forestomach, confirm that the obestatin peptide used in our experiments was capable of biological activity.
Obestatin had no statistically significant ability to reduce the facilitation of EFS-evoked contractions caused by the 5-HT4 receptor agonist (Briejer et al., 2001) prucalopride, although there was an inhibitory trend for both the 0.1 and 1 μM concentrations. These observations suggest that the ability of obestatin to interact with the functions of ghrelin may be a nonspecific activity, independent of direct protein–protein interactions and consistent with the inability of obestatin to interact with the ghrelin binding site, also reported by Zhang et al. (2005).
The inability of obestatin to affect gastrointestinal motility in vivo, contrasts with the findings of Zhang et al. (2005). In the present experiments, obestatin was obtained from two sources (Cambridge Research Biochemicals and NeoMPS) and neither compound had any effect on gastrointestinal motility in vivo. Further, to avoid a possible rapid degradation of obestatin following i.v. injection (Pan et al., 2006), obestatin was infused at a constant rate, even at doses similar to those used by Zhang et al. (2005) where infusion of 30 000 pmol kg−1 min−1 (i.v.) should correspond to the 1 mmol kg−1 i.p. bolus dose. Nevertheless, despite these attempts to demonstrate activity with obestatin, the possibility remains that the inactivity of obestatin in the present experiments may reflect a failure of sufficient amounts of the peptide to reach its site of action. Finally, the inability of obestatin to reduce the prokinetic activity of ghrelin in vivo contrasts with the present experiments in vitro and suggests that any ability of obestatin to interfere with the actions of ghrelin may be dependent on the model used to detect the different actions of ghrelin. For example, the in vitro experiments used stomach preparations, whereas the experiments in vivo focused on the small intestine. In addition, studies in vivo suggest that ghrelin may affect gastric emptying and perhaps intestinal motility via an action within the gastric–vagal–brainstem pathway (e.g., Asakawa et al., 2001), whereas in vitro the prokinetic-like activities of ghrelin (Dass et al., 2003) and also prucalopride (Briejer et al., 2001) may be mediated predominantly via the enteric nervous system (ENS). Accordingly, it is possible that these differences in the models used and/or in the sites of action of ghrelin may explain the different effects of obestatin in vitro and in vivo.
In conclusion, our experiments have demonstrated a weak ability of obestatin to inhibit the prokinetic-like activity of ghrelin in vitro, without directly modulating normal GI motility itself. These observations demonstrate some biological activity of obestatin, but suggest that obestatin does not have a role to play in the modulation of GI gastric motility in healthy individuals. The recent demonstration of an ability of centrally administered obestatin to modulate patterns of sleep in conscious rats (Szentirmai and Krueger, 2006) suggests the need to continue investigating the functions of this exciting new peptide, possibly outside the GI tract.
Acknowledgments
We thank Steven Ratcliffe (DR, GSK, UK) for liaison with Cambridge Research Biochemicals.
Abbreviations
- EFS
electrical field stimulation
Conflict of Interest
The authors state no conflict of interest.
References
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Bassil A, Dass NM, Sanger GJ. The prokinetic-like activity of ghrelin in rat isolated stomach is mediated via cholinergic and tachykininergic motor neurones. Eur J Pharmacol. 2006;544:146–152. doi: 10.1016/j.ejphar.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Binn M, Albert C, Gougeon A, Maerki H, Coulie B, Lemoyne M, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Briejer MR, Bosmans JP, Van Daele P, Jurzak M, Heylen L, Leysen JE, et al. The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound. Eur J Pharmacol. 2001;423:71–83. doi: 10.1016/s0014-2999(01)01087-1. [DOI] [PubMed] [Google Scholar]
- Broglio F, Cottero C, Prodam F, Gauna C, Muccioli G, Papotti M, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89:3062–3069. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- Chen CY, Chao Y, Chang FY, Chien EJ, Lee SD, Doong ML. Intracisternal des-acyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. Int J Mol Med. 2005;16:695–699. [PubMed] [Google Scholar]
- Dass NB, Monunyara M, Bassil AK, Hervieu GJ, Osbourne S, Corcoran S, et al. Growth hormone secretagogue receptors in the rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- De Winter BY, De Man JG, Seerden TC, Depoortere I, Herman AG, Peeters TL, et al. Effect of ghrelin and growth hormone-releasing peptide 6 on septic ileus in mice. Neurogastroenterol Motil. 2004;16:439–446. doi: 10.1111/j.1365-2982.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- Edholm T, Levin F, Hellström PM, Schmidt PT. Ghrelin stimulates motility in the small instestine in rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25–30. doi: 10.1016/j.regpep.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach L-O, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin Endocrinol 2006. doi:10.1210/en.2006-0933 [DOI] [PubMed]
- Levin F, Edholm T, Ehrstrom M, Wallin B, Schmidt PT, Kirchgessner AM, et al. Effect of peripherally administered ghrelin on gastric emptying and acid secretion in the rat. Regul Pept. 2005;131:59–65. doi: 10.1016/j.regpep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, et al. Ghrelin stimulates gastric emptying and hunger in normal weight humans. J Clin Endocrinol Metab. 2006;91:3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- Moechars D, Depoortere I, Moreaux B, De Smet B, Goris I, Hoskens L, et al. Altered gastrointestinal and metabolic function in the GPR39 – obestatin receptor – knockout mouse. Gastroenterology. 2006;131:1131–1141. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: Obestatin, ghrelin and adiponectin. Peptides. 2006;27:911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Poitras P, Polvino WJ, Rocheleau B. Gastrokinetic effect of ghrelin analog RC-1139 in the rat. Effect on post-operative and on morphine induced ileus. Peptides. 2005;26:1598–1601. doi: 10.1016/j.peptides.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Krueger JM. Obestatin alters sleep in rats. Neurosci Lett. 2006;404:222–226. doi: 10.1016/j.neulet.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- Trudel L, Bouin M, Tomasetto C, Eberling P, St-Pierre S, Bannon P, et al. Two new peptides to improve post-operative gastric ileus in dog. Peptides. 2003;24:531–534. doi: 10.1016/s0196-9781(03)00113-x. [DOI] [PubMed] [Google Scholar]
- Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- Wigglesworth M, Wolfe LA, Wise A.Orphan seven transmembrane receptor (7TMR) screening 2006. Proc., Ernst-Schering Foundation Symposium ‘GPCRs: From Deorphanisation to Lead Structure Identification' 2006 (in press) [DOI] [PubMed]
- Zhang JV, Ren P-G, Avsian-Kretchmer O, Luo C-W, Rauch R, Klein C, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]