Abstract
Background and purpose:
cGMP mediates nitrergic relaxations of intestinal smooth muscle, but several studies have indicated that cGMP-independent mechanisms may also be involved. We addressed this contention by studying the effect of ODQ and NS2028, specific inhibitors of soluble guanylate cyclase, on nitrergic relaxations of the mouse gut.
Experimental approach:
Mouse gastric fundus and small intestinal muscle preparations were mounted in organ baths to study relaxations to exogenous NO, NO donors and electrical field stimulation (EFS) of enteric nerves.
Key results:
In gastric fundus longitudinal muscle strips, ODQ and NS2028 abolished the L-nitroarginine-sensitive relaxations to EFS and the relaxations to NO and NO donors, glyceryl trinitrate (GTN), SIN-1 and sodium nitroprusside (SNP). EFS of intestinal segments and muscle strips showed L-nitroarginine-resistant relaxations, which were abolished by the purinoceptor blocker suramin. In the presence of suramin, ODQ and NS2028 abolished all relaxations to EFS in intestinal segments and strips. ODQ and NS2028 abolished the relaxations to exogenous NO and to the NO donors GTN, SIN-1 and SNP in circular and longitudinal intestinal muscle strips. Intestinal segments showed residual relaxations to NO and GTN.
Conclusions and Implications:
Our results indicate that relaxations to endogenous NO in the mouse gastric fundus and small intestine are completely dependent on cGMP. ODQ and NS2028 incompletely blocked nitrergic relaxations to exogenous NO in intact intestinal segments. However, it is unlikely that this is due to the involvement of cGMP-independent pathways because ODQ and NS2028 abolished all relaxations to endogenous and exogenous NO in intestinal muscle strips.
Keywords: cGMP, enteric nerves, guanylate cyclase, NANC, nitrergic neurotransmission, NO-cGMP pathway, nitric oxide, non-adrenergic non-cholinergic, NS 2028, ODQ
Introduction
Nitric oxide (NO), which is the most important non-adrenergic non-cholinergic (NANC) inhibitory neurotransmitter in the gut (for a recent review see Toda and Herman, 2005), relaxes smooth muscle by activating soluble guanylate cyclase thereby stimulating the formation of cyclic guanosine-3′,5′-monophopshate (cGMP). The major target of cGMP is protein kinase G, which modulates smooth muscle tone by interacting with myosin light chain kinase. However, previous studies on the NO-cGMP pathway have shown that NO can also induce smooth muscle relaxation through a cGMP-independent mechanism which, at least in vascular smooth muscle, may involve calcium-dependent potassium channels (Bolotina et al., 1994). cGMP-independent NO-mediated relaxations have also been demonstrated in smooth muscle preparations from airways (Zhou and Torphy, 1991; Perkins et al., 1998; Venugopalan et al., 1998; Janssen et al., 2000), myometrium and uterus (Kuenzli et al., 1996; Modzelewska et al., 1998) and the urinary tract (Garcia-Pascual et al., 2000; Costa et al., 2001).
In the gastrointestinal tract, cGMP-independent relaxations to NO have been observed in the lower oesophageal sphincter (Knudsen et al., 1992; Murray et al., 1992; Saha et al., 1993), stomach (Kitamura et al., 1993; Takakura and Muramatsu, 1999) and duodenum (Martins et al., 1995; Serio et al., 2003b). In the earlier studies, the lack of availability of selective inhibitors of guanylate cyclase may be responsible for an incomplete blockade of nitrergic relaxations. Methylene blue and cystamine, for instance, have been extensively used as modulators of NO-stimulated guanylate cyclase, but these compounds lack the specificity of the recently developed guanylate cyclase blockers such as ODQ (Hwang et al., 1998). The ODQ-sensitivity of nitrergic relaxations was subsequently demonstrated in a number of gastrointestinal preparations. However, ODQ-resistant relaxations, suggestive of the involvement of cGMP-independent pathways, have also been reported, for instance in the canine lower oesophageal sphincter, rat gastric fundus, guinea-pig, rat and hamster small intestine, mouse caecum and rat colon (Young et al., 1996; Goyal and He, 1998; Matsuyama et al., 1999; Tanovic et al., 2001; Daniel et al., 2002; Serio et al., 2003b; Van Crombruggen and Lefebvre, 2004; Vanneste et al., 2004). These discrepancies are intriguing and difficult to explain because the physiological significance of the putative cGMP-independent nitrergic pathway in the intestine is unknown.
Similar to vascular smooth muscle, it was suggested that NO-mediated cGMP-independent responses in the intestine involve the activation of Ca2+-dependent K+ channels. In longitudinal segments of the mouse small intestine, guanylate cyclase-independent relaxations to enteric nerve stimulation appear to be sensitive to apamin, a blocker of small conductance Ca2+-activated K+ channels (Martins et al., 1995; Serio et al., 2003b). Apamin-sensitive K+ channels, which are abundantly present in intestinal smooth muscle cells, mediate various physiological processes among which is purinergic NANC neurotransmission. Consequently, apamin is widely used as a tool to block the purinergic component of enteric inhibitory transmission. This underlines the importance of blocking these purinergic pathways when studying the mechanisms of nitrergic relaxations in the intestine.
The aim of this study was therefore to investigate, in detail, the guanylate cyclase dependency of nitrergic relaxations in the gastrointestinal tract by using two specific blockers of guanylate cyclase, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and 4H-8-bromo-1,2,4-oxadiazolo[3,4-d]benz[b](1,4)oxazin-1-one (NS 2028) (Garthwaite et al., 1995; Olesen et al., 1998). The effects of ODQ and NS 2028 were determined on relaxations induced by either endogenous or exogenous NO in isolated preparations of the mouse gastric fundus and small intestine. Endogenous NO release was induced by electrical stimulation of intramural NANC nerves. Exogenous NO was added either as NO in solution or as glyceryl trinitrate (GTN), SIN-1 and sodium nitroprusside (SNP), all widely used NO donors.
Methods
Tissue preparation
Swiss OF1 mice (25–30 g) were deprived of food for 24 h with free access to water before experimentation. Mice were anaesthetized with diethyl ether and exsanguinated from the carotid artery. The stomach and the small intestine were rapidly removed and put in ice-cold aerated Krebs–Ringer solution (containing in mM: NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5, NaCHO3 25, CaEDTA 0.026 and glucose 11.1). A ∼10 cm long segment of the jejunum, located ∼7-cm from the ligament of Treitz, was used for further preparation. All experimental procedures received approval of the Committee for Medical Ethics of the University of Antwerp.
Pharmacological studies: tissue preparation and isometric tension recording
The stomach was opened along the lesser curvature, the mucosa removed by sharp dissection and longitudinal muscle strips were prepared. The jejunal segment of ∼10 cm was gently flushed with Krebs–Ringer solution and cut in half. One segment of ∼5 cm was cut into 3–4 smaller longitudinal segments. The other jejunal segment of ∼5 cm was opened longitudinally along the mesenteric border; the mucosa was removed and muscle strips were cut either in the longitudinal or in the circular direction. A silk thread was attached at the upper and lower end of the segments and muscle strips after which they were mounted in organ baths (volume 5 ml) filled with Krebs–Ringer solution (37°C, aerated with 5% CO2/95% O2). The segments and muscle strips were carefully positioned in the organ bath between two platinum ring electrodes (distance in between rings: 10 mm, diameter of rings: 3 mm) that were mounted on a fixed Plexiglas rod. The lower end of the segments and muscle strip was fixed on the Plexiglas rod and the other end of the muscle strip was connected to a strain gauge transducer (Scaime transducers, Annemasse, France) for recording of isometric tension. After an initial equilibration period of 30 min, during which the tissues were washed every 10 min, the tissues were contracted with 0.1 μM carbachol. After washout of carbachol, the tissues were stretched (increments of 0.25 g) and when the basal tone of the muscle preparations was stabilized, 0.1 μM carbachol was added again. This procedure was repeated until the contraction to 0.1 μM carbachol was maximal. This point was taken as the point of optimal length-tension relationship (De Man et al., 2003). The tissues were then allowed to equilibrate for 60 min before starting the experiment. During the equilibration period, the preparations were washed every 15 min with fresh Krebs–Ringer solution containing atropine and guanethidine.
Experimental protocols
Once the tissues were optimally stretched, non-adrenergic non-cholinergic (NANC) conditions were obtained by adding atropine (1 μM) and guanethidine (3 μM) to the Krebs–Ringer solution. Atropine and guanethidine remained in the Krebs–Ringer solution throughout all further experiments. Preparations were precontracted with prostaglandin F2α (0.3 μM). Relaxations were induced either by electrical field stimulation (EFS, 1–8 Hz, 40 V, pulse width: 1 ms, pulse train: 10 s) of NANC nerves, or by addition of NO or the NO donors glyceryl trinitrate (GTN), 3-morpholinosydonimine-N-ethyl-carbamine (SIN-1) and sodium nitroprusside (SNP). The effects of the specific inhibitors of guanylate cyclase, ODQ (10 μM) and NS 2028 (10 μM), were investigated, either alone or in combination with the purinoceptor blocker suramin (200 μM). The incubation time for suramin, ODQ and NS 2028 was 15 min. NO was prepared by acidification of a NaNO2 solution as described previously (De Man et al., 2001). Briefly, a stock solution of 0.1 mM NaNO2 was prepared freshly in distilled water on the day of the experiment. Appropriate dilutions (10, 30 and 100 μM) of this stock solution were made. Thirty seconds before injection of NO in the organ bath, the appropriate NaNO2 solution (10, 30 or 100 μM) was adjusted to pH 3 with 5 mM HCl. A control solution of acidified distilled water did not affect the tension of the muscle strips.
Solutions and drugs
The following drugs were used: GTN, sodium nitrite (Merck, Darmstadt, Germany); sodium nitroprusside (SNP, Acros Organics, Geel, Belgium); NS 2028, ODQ (Tocris Bioscience, Bristol, UK); ATP sodium salt, atropine sulphate, carbachol, L-NOARG, SIN-1, suramin (Sigma-Aldrich, St Louis, MO, USA); prostaglandin F2α (Upjohn, Puurs); guanethidine monosulphate (Ciba Geigy, Basel, Switzerland). NS 2028 and ODQ were dissolved in 50% ethanol. The final concentration of ethanol in the organ bath did not exceed 0.1% and this did not affect the contractions and relaxations of the muscle preparations.
Presentation of results and statistical analysis
Relaxations were calculated as the maximal inhibitory response induced by electrical field stimulation (EFS), NO or the NO donors. Values are calculated as % inhibition of the prostaglandin F2α-induced contraction. Results are shown as mean±s.e.m. for the number (n) of mice indicated. For statistical analysis, Student's t-test for paired values or one-way ANOVA followed by Dunnett post hoc testing was used. P-values less than 0.05 were considered to be significant.
Results
Gastric fundus
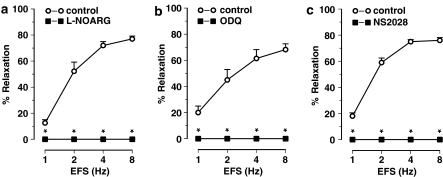
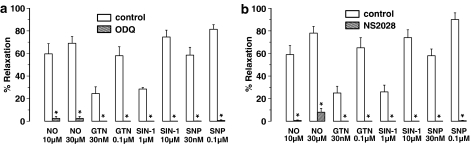
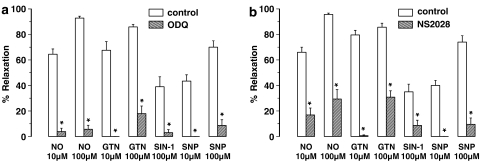
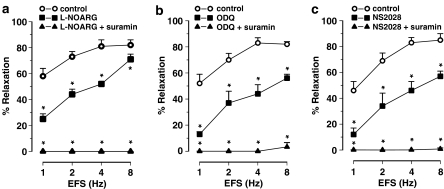
EFS (1–8 Hz) of longitudinal gastric fundus muscle strips induced transient and frequency-dependent NANC relaxations (Figure 1a), which were unaffected by the purinoceptor blocker suramin (Table 1) but abolished by the NO synthase blocker L-nitroarginine (L-NOARG) (Figure 2a). Relaxations to EFS were also abolished by ODQ or NS 2028 (Figures 1a, 2b and c). NO, GTN, SIN-1 and SNP induced concentration-dependent relaxations of gastric fundus muscle strips (Figure 3a). Relaxations to NO were acute and transient (Figure 1a) and unaffected by suramin (Table 1) whereas relaxations to GTN, SIN1- and SNP were more sustained. ODQ or NS 2028 virtually abolished all relaxations to NO, GTN, SIN-1 and SNP (Figure 3a and b). ODQ or NS 2028 did not affect the NO-independent relaxations to isoprenaline (Table 2).
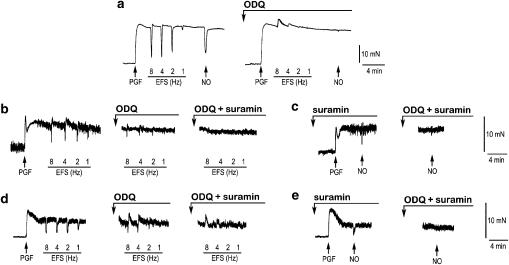
Figure 1.
Typical tracings of isolated preparations of the mouse gastrointestinal tract. (a) Tracing of a longitudinal muscle strip of the gastric fundus showing the effect of ODQ on NANC nerve-mediated relaxations to EFS (1–8 Hz) and on relaxations to exogenous NO (10 μM). Tracings of an intact jejunal segment (b) and a circular jejunal muscle strip (d) showing the effect of ODQ and ODQ plus suramin on NANC nerve-mediated relaxations to EFS (1–8 Hz). Tracings of another intact jejunal segment (c) and another circular jejunal muscle strip (e) showing the effect of ODQ (10 μM) on the relaxation to exogenous NO (10 μM), obtained in the presence of suramin to block purinergic responses.
Table 1.
Effect of the purinoceptor blocker suramin (200 μM) on relaxations to EFS (2 and 4 Hz) and NO (10 μM) in gastric fundus and circular jejunal strips and intact jejunal segments
|
Gastric fundus strips |
Jejunal segments |
Circular jejunal strips |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| EFS 2 Hz | EFS 4 Hz | NO | EFS 2 Hz | EFS 4 Hz | NO | EFS 2 Hz | EFS 4 Hz | NO | |
| Control (%) | 52±6 | 71±4 | 68±7 | 41±3 | 50±2 | 51±5 | 71±4 | 77±4 | 73±5 |
| Suramin (%) | 48±6 | 67±5 | 65±4 | 46±4 | 53±4 | 53±4 | 74±3 | 83±2 | 78±3 |
Abbreviations: EFS, electrical field stimulation; NO, Nitric oxide.
Relaxations are expressed as % relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=5 experiments. Student's t-test for paired observations did not show significant differences.
Figure 2.
Nerve-mediated relaxations induced by EFS (1–8 Hz) of the mouse gastric fundus and the effect of (a) L-NOARG (300 μM), (b) ODQ (10 μM) and (c) NS 2028 (10 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=5–6 experiments. *P⩽0.05, Student's t-test for paired observations.
Figure 3.
Muscle relaxations induced by NO, GTN, SIN-1 and SNP of the mouse gastric fundus and the effect of (a) ODQ (10 μM) and (b) NS 2028 (10 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=5–6 experiments. *P⩽0.05, Student's t-test for paired observations.
Table 2.
Effect of ODQ (10 μM) and NS 2028 (10 μM) on relaxations to isoprenaline (3–30 nM) in gastric fundus and circular jejunal muscle strips and in intact jejunal segments
|
Gastric fundus strips |
Jejunal segments |
Circular jejunal strips |
||||
|---|---|---|---|---|---|---|
| ISO 10 nM | ISO 30 nM | ISO 3 nM | ISO 10 nM | ISO 3 nM | ISO 10 nM | |
| Control (%) | 20±2 | 64±2 | 31±4 | 71±4 | 26±3 | 67±6 |
| ODQ (%) | 19±4 | 59±8 | 28±5 | 69±6 | 27±3 | 72±7 |
| Control (%) | 23±3 | 72±8 | 27±2 | 66±2 | 29±4 | 70±6 |
| NS 2028 (%) | 26±3 | 74±6 | 32±3 | 73±5 | 30±2 | 73±8 |
Abbreviation: ISO: isoprenaline.
Relaxations are expressed as % relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=4 experiments. Student's t-test for paired observations did not show significant differences.
Jejunum: intact intestinal segments
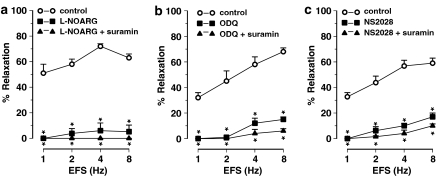
Intact jejunal segments relaxed in response to EFS (1–8 Hz) of NANC nerves. Relaxations to EFS were acute and tone quickly recovered after cessation of EFS (Figure 1b). The purinoceptor blocker suramin by itself had no effect on these relaxations (Table 1) whereas L-NOARG inhibited them (Figure 4a). Residual relaxations to EFS were observed in the presence of L-NOARG. The L-NOARG-resistant relaxations to EFS were of small amplitude and abolished by suramin (Figure 4a), indicating a purinergic component that is only apparent after blockade of the nitrergic component. Similar to L-NOARG, ODQ and NS 2028 inhibited the relaxations to EFS (Figure 4b and c), but small amplitude relaxations were still observed. In the presence of suramin, ODQ and NS 2028 almost completely inhibited the relaxations to EFS (Figures 1b, 4b and c).
Figure 4.
Nerve-mediated relaxations induced by EFS (1–8 Hz) of intact segments of the mouse jejunum and the effect of (a) L-NOARG (300 μM), (b) ODQ (10 μM) and (c) NS 2028 (10 μM) in the absence and presence of suramin (200 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=6–8 experiments. *P⩽0.05, one-way ANOVA followed by Dunnett post hoc test.
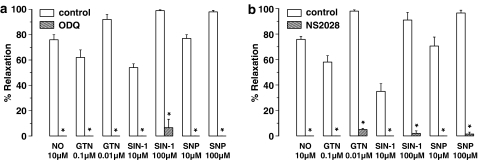
Suramin did not affect the relaxation in response to exogenous NO (Table 1), but to avoid any interference owing to purinergic signalling, all further experiments on exogenous NO were conducted in the presence of suramin. The jejunal segments relaxed on addition of NO or the NO donors GTN, SIN-1 and SNP (Figure 5). The relaxations to NO (Figure 1c) and GTN were acute and transient and resembled those to EFS. Relaxations to SNP were rapid in onset and recovered slowly. Relaxations to SIN-1 were slow both in onset and recovery. In the intact jejunal segments, ODQ almost completely blocked the relaxations to exogenous NO (Figure 1c) and those to the NO donors SIN-1, SNP and 10 μM GTN (Figure 5a). However, a significant residual relaxation to 100 μM GTN was still observed after ODQ treatment (Figure 5a). Similar to ODQ, NS 2028 significantly inhibited but did not abolish the relaxations to NO and the NO donors (Figure 5b). Sporadically, the relaxation to 100 μM SIN-1 (one out of six experiments) and to 100 μM NO (two out of six experiments) was reversed into a twitch contraction in the presence of ODQ. This was also observed with NS 2028: the relaxation to 100 μM SIN-1 and to 100 μM NO was reversed into a twitch contraction in two out of six tissues in each set of experiments. Contractions in response to either EFS, GTN or SNP were never observed in the presence of either ODQ or NS 2028. The guanylate cyclase blockers had no effect on the NO-independent relaxations to isoprenaline in the jejunal segments (Table 2).
Figure 5.
Relaxations induced by NO, GTN, SIN-1 and SNP of intact segments of the mouse jejunum and the effect of (a) ODQ (10 μM) and (b) NS 2028 (10 μM) in the presence of suramin (200 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=6–8 experiments. *P⩽0.05, Student's t-test for paired observations.
Jejunum: circular muscle strips
EFS of NANC nerves induced frequency-dependent relaxations of the circular jejunal muscle strips and these relaxation responses were rapid in onset and transient (Figure 1d). Suramin had no effect by itself on these relaxations (Table 1). L-NOARG inhibited the relaxations to EFS but did not abolish them: residual acute twitch relaxations were still observed in the presence of L-NOARG (Figure 6a). These L-NOARG-resistant relaxations to EFS were abolished by suramin (Figure 6a). Similar to L-NOARG, ODQ and NS 2028 only partially inhibited the relaxations to EFS of circular jejunal muscle strips (Figures 1d and 6b, c). However, in the presence of suramin, ODQ and NS 2028 abolished the relaxations to EFS (Figures 1d and 6b, c).
Figure 6.
Nerve-mediated relaxations induced by electrical field stimulation (EFS, 1–8 Hz) of circular muscle strips of the mouse jejunum and the effect of (a) L-NOARG (300 μM), (b) ODQ (10 μM) and (c) NS 2028 (10 μM) in the presence and absence of suramin (200 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=5 experiments. *P⩽0.05, one-way ANOVA followed by Dunnett post hoc test.
Suramin by itself did not affect the relaxations to exogenous NO (Table 1) but to avoid any interference of purinergic pathways, all experiments with exogenous NO and NO donors were conducted in the presence of suramin. In the presence of suramin, circular jejunal muscle strips readily relaxed to NO, GTN, SIN-1 and SNP (Figure 7). Relaxations to NO were rapid in onset and transient (Figure 1e) whereas relaxations to GTN, SNP and SIN-1 were rapid in onset but sustained. ODQ and NS 2028 abolished the relaxations to NO (Figure 1e) and those to GTN, SNP and SIN-1 (Figure 7a and b) without affecting the NO-independent relaxations to isoprenaline (Table 2). No contractions to NO or SIN-1, such as those sporadically seen in intact jejunal segments in the presence of ODQ or NS 2028, were ever observed in circular jejunal muscle strips.
Figure 7.
Relaxations induced by NO, GTN, SIN-1 and SNP of circular muscle strips of the mouse jejunum and the effect of (a) ODQ (10 μM) and (b) NS 2028 (10 μM) in the presence of suramin (200 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=5 experiments. *P⩽0.05, Student's t-test for paired observations.
Jejunum: longitudinal muscle strips
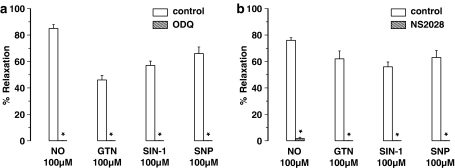
EFS (1–8 Hz) of longitudinal jejunal muscle strips had no clear relaxant effect. Only a minor relaxation was observed at EFS 8 Hz. This relaxation was abolished on addition of either L-NOARG (from 8.5±4.1 to 0±0%), ODQ (from 13±7 to 0±0%) or NS 2028 (from 8±3 to 0±0%). Longitudinal jejunal strips relaxed to exogenous NO and to GTN, SIN-1 and SNP (Figure 8). Relaxations to NO, GTN and SNP were rapid in onset and transient whereas those to SIN-1 developed slowly and were sustained. ODQ and NS 2028 abolished all these relaxations (Figure 8a and b). No contraction to NO or SIN-1 was ever observed in the presence of ODQ and NS 2028.
Figure 8.
Relaxations induced by NO, GTN, SIN-1 and SNP of longitudinal muscle strips of the mouse jejunum and the effect of (a) ODQ (10 μM) and (b) NS 2028 (10 μM). Relaxations are expressed as per cent relaxation of a prostaglandin F2α-induced contraction and shown as mean±s.e.m. for n=6 experiments. *P⩽0.05, Student's t-test for paired observations.
Discussion and conclusions
The possibility that NO-mediated relaxations in the intestine may be partially cGMP-independent is debatable. We therefore, studied the effect of the specific guanylate cyclase blockers ODQ and NS 2028 on relaxations to endogenous and exogenous NO in the mouse gastrointestinal tract.
In gastric fundus muscle strips, the NO synthase blocker L-NOARG abolished all NANC relaxations to EFS confirming the nitrergic nature of these responses (De Man et al., 2001). The effect of L-NOARG was completely mimicked by the guanylate cyclase blockers ODQ and NS 2028: ODQ and NS 2028 abolished all NANC nerve-mediated relaxations to EFS. In addition, ODQ and NS 2028 abolished the relaxations to exogenous NO. These results extend previous findings in the mouse and rat gastric fundus, where ODQ blocked the responses to NO and inhibited EFS-induced NANC relaxations to a similar degree as NO-synthase blockers (Lefebvre, 1998; Selemidis and Cocks, 2000). We also demonstrated that ODQ and NS 2028 completely inhibited the relaxations to three different NO donors (GTN, SIN-1 and SNP). From these results, it was concluded that relaxations induced by nitrergic nerve stimulation and by exogenously added NO in the mouse gastric fundus are completely dependent on cGMP.
We further studied nitrergic relaxations in the small intestine, in both intact segments and isolated muscle strips. The nerve-mediated NANC relaxations in mucosa-intact longitudinal segments of the small intestine were largely blocked by L-NOARG, indicating the involvement of neuronal NO in these responses. A small relaxation was still observed in the presence of L-NOARG and this residual relaxation was blocked by the purinoceptor blocker suramin. Interestingly, suramin by itself had no effect on the NANC relaxations to EFS. This indicates that the nerve-mediated purinergic relaxation is revealed only after blockade of the nitrergic response. This highlights the importance of blocking all non-nitrergic inhibitory pathways when studying the mechanisms of nitrergic neurotransmission. All further studies with ODQ and NS 2028 in the intestinal segments were therefore performed in the presence of suramin. In these conditions, the neurogenic NANC relaxations were almost completely blocked by ODQ or NS 2028. The intestinal segments readily relaxed to exogenously added NO and to the NO donors GTN, SIN-1 and SNP. ODQ and NS 2028 potently inhibited, but did not abolish, these relaxations. The effect of NS 2028 appeared to be less marked compared to that of ODQ.
To investigate whether cGMP-independent mechanisms may mediate these residual relaxations to NO, additional experiments were performed on isolated intestinal muscle strips. Muscle strips have the advantage that the mucosal layer can be removed, avoiding interference of mucosal substances with enteric neuromuscular signalling. Circular muscle strips of the mouse jejunum showed marked relaxations in response to NANC nerve stimulation. Blockade of NOS revealed acute non-nitrergic twitch-like relaxations that were more pronounced in the intestinal strips compared to intestinal segments. The non-nitrergic relaxations to EFS were abolished by suramin. This confirms the hypothesis that inhibitory NANC neurotransmission in the intestine involves purines, acting mainly on P2Y1 receptors as shown in mouse jejunum (De Man et al., 2003) and human colon (Gallego et al., 2006). It is important to note that, similar to intestinal segments, the pronounced nitrergic relaxation in response to NANC nerve stimulation masked the acute purinergic-mediated relaxation of the intestinal strips. Indeed, in the absence of L-NOARG, NANC relaxations to EFS were not affected by suramin. This finding was rather unexpected. Possibly, the purinergic response may come into play only after blockade of the nitrergic response. However, this does not accord with results from electrophysiological studies showing that EFS of enteric inhibitory NANC nerves induces an inhibitory junction potential (ijp), which is the electrical equivalent of the mechanical muscle relaxation that consists of a distinctive fast purinergic and slow nitrergic phase. The fast purinergic phase is also observed in the presence of NOS blockers (Crist et al., 1992; He and Goyal, 1993; Keef et al., 1993; Pluja et al., 1999; Xue et al., 1999; Serio et al., 2003a; Gallego et al., 2006). However, the distinctive fast and slow electrical phases may be difficult to differentiate when studying the mechanical muscle relaxation that is associated with the ijp. Therefore, an alternative explanation for the lack of effect of suramin alone is that the fast twitch-like purinergic relaxation to EFS is masked by the sustained nitrergic component of the NANC relaxation to EFS. A more detailed study in which the mechanical and electrical behaviour of the muscle can be studied simultaneously is needed to unravel this matter.
To avoid any interference of purinergic signalling, further studies on the cGMP-dependency of nitrergic pathways were performed in the presence of suramin. In these conditions, ODQ and NS 2028 completely abolished the relaxations to NANC nerve stimulation. In addition, the relaxations to NO and to the NO donors GTN, SIN-1 and SNP were potently inhibited or abolished by ODQ and NS 2028. These results strongly indicate that relaxations to the endogenously released nitrergic neurotransmitter and to exogenously added NO in circular jejunal muscle strips are mediated solely by guanylate cyclase. No evidence was obtained for the involvement of a cGMP-independent component in the relaxation of mouse jejunal circular muscle strips in response to endogenous and exogenous NO.
To substantiate these findings, we also studied nitrergic relaxations in longitudinal jejunal muscle strips. These strips did not readily relax in response to electrical stimulation of intrinsic NANC nerves: only a modest relaxation was observed at 8 Hz EFS. Stimulation at higher frequencies (16 Hz, results not shown) did not induce a more pronounced relaxation. These results are in contrast to those from a previous study in the mouse intestine, showing a pronounced EFS-induced NANC inhibitory response in mucosa intact longitudinal intestinal muscle strips (Ueno et al., 2004). However, in this study NANC inhibitory responses were measured as percentage inhibition of the spontaneous activity of the muscle strips, whereas in our experiments NANC relaxations were measured as the relaxant activity of a precontracted muscle strip. Similar weak relaxations to EFS have been observed previously in longitudinal muscle of the mouse small intestine (Young et al., 1996), and this accords with immunohistochemical evidence showing that nitrergic and purinergic inhibitory motor nerves preferentially innervate the circular and not longitudinal muscle layer of the small intestine in mice (Sang and Young, 1996) and other species (Costa et al., 1992; Wilhelm et al., 1998). Nevertheless, the relaxation to 8 Hz EFS was blocked by L-NOARG, thus nitrergic in nature, and also by ODQ and NS 2028. Similarly, Ueno et al., (2004) showed that ODQ strongly inhibited, although did not abolish, the EFS-induced NANC responses in longitudinal strips of the mouse small intestine. The ODQ-insensitive relaxation in the study of Ueno et al. (2004) may indicate the involvement of a non-nitrergic (possibly purinergic) neurotransmitter, because L-NOARG did not completely block the EFS-induced NANC relaxation either (Ueno et al., 2004). Longitudinal jejunal muscle strips readily relaxed to exogenous NO and to GTN, SIN-1 and SNP, although slightly higher concentrations had to be used to obtain similar (50–70%) degrees of relaxation to those in circular jejunal muscle strips. All relaxations to NO and NO donors were abolished by ODQ or NS 2028. These results indicate that nitrergic pathways, activated by endogenous or exogenous NO, are also completely cGMP-dependent in longitudinal jejunal muscle.
As outlined above, the effect of guanylate cyclase blockers on relaxations to exogenously added NO was less marked in intestinal segments compared to intestinal muscle strips. However, it is unlikely that this is owing to the involvement of cGMP-independent pathways, because ODQ and NS 2028 abolished the nitrergic relaxations in isolated intestinal muscle strips. Possibly, the penetration of the blockers into the tissue is hampered when intact segments are used. Using muscle strips from which the mucosa is removed, but which still contain the muscle layers and myenteric plexus, may overcome this problem thereby optimizing penetration of the drugs under study.
In some experiments, ODQ and NS 2028 unmasked a sharp twitch contraction to exogenous NO and SIN-1 in the intestinal segments. Such contractions were not observed with GTN or SNP or upon electrical stimulation of NANC nerves. Contractions to NO and NO donors have been observed previously in rat ileal intestinal muscle strips (Lefebvre and Bartho, 1997; Tanovic et al., 2001). A detailed study on the underlying mechanisms showed that these nitrergic contractions are Ca2+-dependent and apamin-sensitive (Lefebvre and Bartho, 1997) and, as also observed in our study, cGMP-independent. The physiological relevance of nitrergic contractions in the intestine is still not known but this phenomenon should not be overlooked when investigating the mechanisms of nitrergic relaxations.
In conclusion, we provided evidence that nitrergic relaxations induced by electrical stimulation of NANC nerves and by exogenously added NO in muscle strips from the mouse gastric fundus and small intestine are completely inhibited by the guanylate cyclase blockers ODQ and NS 2028. Our results therefore do not support the involvement of cGMP-independent pathways in nitrergic relaxations in the mouse stomach and small intestine.
Acknowledgments
This work was supported by Grant no. P5/20 of the Interuniversity Attraction Pole of the Belgian Federal Science Policy and by Grant no G.0200.05 from the Fund for Scientific Research - Flanders (FWO-Vlaanderen).
Abbreviations
- EFS
electrical field stimulation
- GTN
glyceryl trinitrate
- NS 2028
4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one)
- L-NOARG
L-nitroarginine
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- SIN-1
3-morpholinosydonimine-N-ethyl-carbamine
- SNP
sodium nitroprusside
- TTX
tetrodotoxin
Conflict of Interest
The authors state no conflict of interest.
References
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Costa G, Labadia A, Triguero D, Jimenez E, Garcia-Pascual A. Nitrergic relaxation in urethral smooth muscle: involvement of potassium channels and alternative redox forms of NO. Naun Schmiedebergs Arch Pharmacol. 2001;364:516–523. doi: 10.1007/s002100100480. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Pompolo S, Brookes SJ, Bornstein JC, Bredt DS, et al. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992;148:121–125. doi: 10.1016/0304-3940(92)90819-s. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel EE, Bowes TJ, Jury J. Roles of guanylate cyclase in responses to myogenic and neural nitric oxide in canine lower esophageal sphincter. J Pharmacol Exp Ther. 2002;301:1111–1118. doi: 10.1124/jpet.301.3.1111. [DOI] [PubMed] [Google Scholar]
- De Man JG, De Winter BY, Seerden TC, De Schepper HU, Herman AG, Pelckmans PA. Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br J Pharmacol. 2003;140:1108–1116. doi: 10.1038/sj.bjp.0705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man JG, Moreels TG, De Winter BY, Herman AG, Pelckmans PA. Pre- and postjunctional protective effect of neocuproine on the nitrergic neurotransmitter in the mouse gastric fundus. Br J Pharmacol. 2001;132:277–285. doi: 10.1038/sj.bjp.0703772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol. 2006;291:584–594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A, Labadia A, Costa G, Triguero D. Effects of superoxide anion generators and thiol modulators on nitrergic transmission and relaxation to exogenous nitric oxide in the sheep urethra. Br J Pharmacol. 2000;129:53–62. doi: 10.1038/sj.bjp.0703000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Goyal RK, He XD. Evidence for NO(°) redox form of nitric oxide as nitrergic inhibitory neurotransmitter in gut. Am J Physiol. 1998;275:1185–1192. doi: 10.1152/ajpgi.1998.275.5.G1185. [DOI] [PubMed] [Google Scholar]
- He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J Physiol. 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TL, Wu CC, Teng CM. Comparison of two soluble guanylyl cyclase inhibitors, methylene blue and ODQ, on sodium nitroprusside-induced relaxation in guinea-pig trachea. Br J Pharmacol. 1998;125:1158–1163. doi: 10.1038/sj.bjp.0702181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ, Premji M, Lu-Chao H, Cox G, Keshavjee S. NO(+) but not NO radical relaxes airway smooth muscle via cGMP-independent release of internal Ca(2+) Am J Physiol. 2000;278:L899–L905. doi: 10.1152/ajplung.2000.278.5.L899. [DOI] [PubMed] [Google Scholar]
- Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Lian Q, Carl A, Kuriyama H. S-nitrosocysteine, but not sodium nitroprusside, produces apamin-sensitive hyperpolarization in rat gastric fundus. Br J Pharmacol. 1993;109:415–423. doi: 10.1111/j.1476-5381.1993.tb13585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen MA, Svane D, Tottrup A. Action profiles of nitric oxide, S-nitroso-L-cysteine, SNP, and NANC responses in opossum lower esophageal sphincter. Am J Physiol. 1992;262:G840–G846. doi: 10.1152/ajpgi.1992.262.5.G840. [DOI] [PubMed] [Google Scholar]
- Kuenzli KA, Bradley ME, Buxton IL. Cyclic GMP-independent effects of nitric oxide on guinea-pig uterine contractility. Br J Pharmacol. 1996;119:737–743. doi: 10.1111/j.1476-5381.1996.tb15734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre RA. Influence of a selective guanylate cyclase inhibitor, and of the contraction level, on nitrergic relaxations in the gastric fundus. Br J Pharmacol. 1998;124:1439–1448. doi: 10.1038/sj.bjp.0701992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre RA, Bartho L. Mechanism of nitric oxide-induced contraction in the rat isolated small intestine. Br J Pharmacol. 1997;120:975–981. doi: 10.1038/sj.bjp.0700996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SL, De Oliveira RB, Ballejo G. Rat duodenum nitrergic-induced relaxations are cGMP-independent and apamin-sensitive. Eur J Pharmacol. 1995;284:265–270. doi: 10.1016/0014-2999(95)00348-o. [DOI] [PubMed] [Google Scholar]
- Matsuyama H, Thapaliya S, Takewaki T. Cyclic GMP-associated apamin-sensitive nitrergic slow inhibitory junction potential in the hamster ileum. Br J Pharmacol. 1999;128:830–836. doi: 10.1038/sj.bjp.0702851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewska B, Sipowicz MA, Saavedra JE, Keefer LK, Kostrzewska A. Involvement of K+ATP channels in nitric oxide-induced inhibition of spontaneous contractile activity of the nonpregnant human myometrium. Biochem Biophys Res Commun. 1998;253:653–657. doi: 10.1006/bbrc.1998.9844. [DOI] [PubMed] [Google Scholar]
- Murray JA, Du C, Ledlow A, Manternach PL, Conklin JL. Guanylate cyclase inhibitors: effect on tone, relaxation, and cGMP content of lower esophageal sphincter. Am J Physiol. 1992;263:G97–G101. doi: 10.1152/ajpgi.1992.263.1.G97. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Drejer J, Axelsson O, Moldt P, Bang L, Nielsen-Kudsk JE, et al. Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br J Pharmacol. 1998;123:299–309. doi: 10.1038/sj.bjp.0701603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins WJ, Pabelick C, Warner DO, Jones KA. cGMP-independent mechanism of airway smooth muscle relaxation induced by S-nitrosoglutathione. Am J Physiol. 1998;275:C468–C474. doi: 10.1152/ajpcell.1998.275.2.C468. [DOI] [PubMed] [Google Scholar]
- Pluja L, Fernandez E, Jimenez M. Neural modulation of the cyclic electrical and mechanical activity in the rat colonic circular muscle: putative role of ATP and NO. Br J Pharmacol. 1999;126:883–892. doi: 10.1038/sj.bjp.0702363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha JK, Hirano I, Goyal RK. Biphasic effect of SNP on opossum esophageal longitudinal muscle: involvement of cGMP and eicosanoids. Am J Physiol. 1993;265:G403–G407. doi: 10.1152/ajpgi.1993.265.2.G403. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- Selemidis S, Cocks TM. Nitrergic relaxation of the mouse gastric fundus is mediated by cyclic GMP-dependent and ryanodine-sensitive mechanisms. Br J Pharmacol. 2000;129:1315–1322. doi: 10.1038/sj.bjp.0703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mule F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol. 2003a;460:183–190. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- Serio R, Zizzo MG, Mule F. Nitric oxide induces muscular relaxation via cyclic GMP-dependent and -independent mechanisms in the longitudinal muscle of the mouse duodenum. Nitric Oxide. 2003b;8:48–52. doi: 10.1016/s1089-8603(02)00144-1. [DOI] [PubMed] [Google Scholar]
- Takakura K, Muramatsu I. Pharmacological comparison between the nitrergic responses produced by intramural nerve stimulation and exogenous NO-donors in rat gastric fundus. Jpn J Pharmacol. 1999;80:155–161. doi: 10.1254/jjp.80.155. [DOI] [PubMed] [Google Scholar]
- Tanovic A, Jimenez M, Fernandez E. Actions of NO donors and endogenous nitrergic transmitter on the longitudinal muscle of rat ileum in vitro: mechanisms involved. Life Sci. 2001;69:1143–1154. doi: 10.1016/s0024-3205(01)01198-5. [DOI] [PubMed] [Google Scholar]
- Toda N, Herman AG. Gastrointestinal function regulation by nitrergic efferent nerves. Pharmacol Rev. 2005;57:315–338. doi: 10.1124/pr.57.3.4. [DOI] [PubMed] [Google Scholar]
- Ueno T, Duenes JA, Zarroug AE, Sarr MG. Nitrergic mechanisms mediating inhibitory control of longitudinal smooth muscle contraction in mouse small intestine. J Gastrointest Surg. 2004;8:831–841. doi: 10.1016/j.gassur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Van Crombruggen K, Lefebvre RA. Nitrergic–purinergic interactions in rat distal colon motility. Neurogastroenterol Motil. 2004;16:81–98. doi: 10.1046/j.1365-2982.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- Vanneste G, Robberecht P, Lefebvre RA. Inhibitory pathways in the circular muscle of rat jejunum. Br J Pharmacol. 2004;143:107–118. doi: 10.1038/sj.bjp.0705918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan CS, Krautmann MJ, Holmes EP, Maher TJ. Involvement of nitric oxide in the mediation of NANC inhibitory neurotransmission of guinea-pig trachea. J Auton Pharmacol. 1998;18:281–286. doi: 10.1046/j.1365-2680.1998.18595.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Batori Z, Pasztor I, Gabriel R. NADPH-diaphorase positive myenteric neurons in the ileum of guinea-pig, rat, rabbit and cat: a comparative study. Eur J Morphol. 1998;36:143–152. [PubMed] [Google Scholar]
- Xue L, Farrugia G, Sarr MG, Szurszewski JH. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Johnson PJ, Stebbing MJ. Inhibitory transmission to the longitudinal muscle of the mouse caecum is mediated largely by nitric oxide acting via soluble guanylyl cyclase. J Auton Nerv Syst. 1996;61:103–108. doi: 10.1016/s0165-1838(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Zhou HL, Torphy TJ. Relationship between cyclic guanosine monophosphate accumulation and relaxation of canine trachealis induced by nitrovasodilators. J Pharmacol Exp Ther. 1991;258:972–978. [PubMed] [Google Scholar]