Abstract
Background and purpose:
Apoptosis is a fundamental process required for neuronal development but also occurs in most of the common neurodegenerative disorders. In an attempt to obtain an anti-apoptotic neuroprotective compound from natural products, we isolated the diterpenoids, pinusolide and 15-MPA, from B. orientalis and investigated their neuroprotective activity against staurosporine (STS) -induced neuronal apoptosis. In addition, we determined the anti-apoptotic mechanism of these compounds in rat cortical cells.
Experimental approach:
Primary cultures of rat cortical cells injured by STS were used as an in vitro assay system. Cells were pretreated with pinusolide or 15-MPA before exposure to STS. Anti-apoptotic activities were evaluated by the measurement of cytoplasmic condensation and nuclear fragmentation. The levels of cellular peroxide, malondialdehyde (MDA) and [Ca2+]i , as well as the activities of superoxide dismutase (SOD) and caspase-3/7, were measured.
Key results:
Pinusolide and 15-MPA, at a concentration of 5.0 ìM, reduced the condensed nuclei and rise in [Ca2+]i that accompanies apoptosis induced by 100 nM STS. Pinusolide and 15-MPA also protected the cellular activity of SOD, an antioxidative enzyme reduced by STS insult. Furthermore, the overproduction of reactive oxygen species and lipid peroxidation induced by STS was significantly reduced in pinusolide and 15-MPA treated cells. In addition, pinusolide and 15-MPA inhibited STS-induced caspase-3/7 activation.
Conclusions and Implications:
These results show that pinusolide and 15-MPA protect neuronal cells from STS-induced apoptosis, probably by preventing the increase in [Ca2+]i and cellular oxidation caused by STS, and indicate that they could be used to treat neurodegenerative diseases.
Keywords: apoptosis, pinusolide, 15-methoxypinusolidic acid, Biota orientalis, staurosporine, neuroprotective activity
Introduction
Apoptosis plays an important homeostatic role in several cellular processes in the development of the immune and nervous systems (Oppenheim, 1991). However, apoptosis may also contribute to various pathological conditions (Bredesen, 1995; Mattson et al., 1998). In the central nervous system, apoptosis has been associated with many common neurodegenerative disorders; for example, those occurring after acute insults to the CNS, during ischaemia or trauma, as well as in chronic degenerative conditions including Alzheimer's and Parkinson's diseases (Coley and Puttfarcken, 1993; Thompson, 1995). Therefore, the identification of compounds that inhibit apoptosis is an important step towards the development of effective treatment strategies for neurodegenerative disorders.
Pathological features of apoptosis include morphological and biochemical attributes distinct from the process of necrosis. The morphological features are associated with a variety of biochemical changes in the cell death signalling pathway, such as increased intracellular [Ca2+]i, generation of free radicals and ATP, cytochrome c translocation and caspase activation. Staurosporine (STS), an alkaloid produced by Streptomyces staurospores, is a potent inhibitor of protein kinase and an established agent known to induce apoptosis (Falcieri et al., 1993). STS-induced neuronal apoptosis includes a number of characteristic signalling pathways, for example. an increase in the intracellular concentrations of calcium and of reactive oxygen species, the release of cytochrome c and activation of caspase (Falcieri et al., 1993; Bertrand et al., 1994; Jarvis et al., 1994). Thus, we have attempted to find anti-apoptotic neuroprotective compounds from natural products using STS-injured primary cultures of rat cortical cells as an in vitro screening system.
In our screening system, we have found that the methanolic extract of Biota orientalis (L.) Endl. (Cupressaceae) leaves showed significant neuroprotective activity against STS-induced neurotoxicity. The leaves of B. orientalis have been used in Oriental medicine for the treatment of gout, rheumatism, diarrhoea, and chronic tracheitis (Zhu et al., 2004). We have previously shown that the diterpenoids, pinusolide and its derivative, 15-methoxypinosolidic acid (15-MPA), isolated from B. orientalis, protect against glutamate-induced neurotoxicity (Koo et al., 2002). In the present study, we investigated the effect of pinusolide and 15-MPA on apoptosis induced by STS in primary cultures of rat cortical cells and further elucidated the mechanism of their anti-apoptotic effects.
Methods
Cell culture
Primary cultures of mixed cortical cells containing both neuronal and glial cells were prepared from 17 to 19-day-old foetal Sprague–Dawley rats as described previously (Kim et al., 1998). Briefly, the trypsin (0.25%)-dissociated cortical cells were plated on multi-well culture plates (Corning, NY, USA) coated with collagen (0.1 mg ml−1) at a density of 1 × 106 cells per well and poly-L-lysine (10 μg ml−1) at a density of 2 × 105 cells per well, respectively. The cortical cells were grown in DMEM containing 10% heat-inactivated foetal bovine serum with penicillin (100 IU ml−1) and streptomycin (100 μg ml−1) at 37°C in a humidified atmosphere of 95% air-5% CO2. Cultures were allowed to mature for 11 days before being used for experiments. Our mixed cortical cultures consisted of approximately 70∼75% cells immunopositive for neuron-specific enolase and 25∼30% cells immunopositive for glial fibrillary acidic protein as determined by immunocytochemical staining methods (Kim et al., 2004). All experiments were performed with the Ethical Approval of Seoul National University.
Neurotoxicity
Test compounds were dissolved in DMSO (final culture concentration, 0.1%);. the solvent had no effect on cell viability of control and staurosporine-treated cells at the concentration used (Kim et al., 2004). Eleven-day-old cortical cell cultures were pretreated with pinusolide or 15-MPA at concentrations of 1.0 and 5.0 μM for 1 h and then exposed to 100 nM STS. After a further 18 h incubation, the cultures were assessed for neurotoxicity.
Cytoplasmic condensation and nuclear fragmentation
For evaluation of cytoplasmic condensation and nuclear fragmentation of cortical cells, Hoechst 33342 staining was used (Earnshaw, 1995). Cultures grown on glass coverslips were rinsed three times with phosphate-buffered saline (PBS, pH 7.4) and fixed with 4% paraformaldehyde for 20 min at 37°C. The cultures were washed three times with PBS and then permeabilized with ethanol/acetic acid (19:1, v/v) for 15 min at −20°C. The cells were stained with 0.1 μg ml−1 Hoechst 33342 for 20 min at room temperature. Images of Hoechst 33342-stained cells were acquired under a confocal laser scanning microscope (395 nm excitation and 420 nm barrier filter) with a × 40 oil immersion objective. We counted the number of cells showing nuclear fragmentations on cultures grown in three independent wells (100 cells assessed per well) using chambered coverglass multi-well plate (Lab-Tek, NUNC). Statistical significance was determined by one-way ANOVA.
Measurement of intracellular calcium
The [Ca2+]i content was quantified by fluorescence imaging of the cell-permeable calcium indicator dye, fluo-3 AM (Molecular probes, Eugene, OR, USA). Briefly, cultures grown on chambered coverglass were incubated in the presence of 0.1 μM fluo-3 AM for 30 min. The fluorescence photomicrographs were acquired using a confocal laser scanning microscope (488 nm excitation and 510 nm barrier filter) with × 40 oil immersion objective.
Measurement of cellular peroxide
The relative level of free radicals, that is peroxide, in cultured cells was measured with the oxidation-sensitive compound, 2,7-DCF-DA by the method of Goodman et al. (1996). Cultures were loaded with DCF-DA (50 μM, 50 min-incubation) followed by three washes in HBSS. DCF fluorescence was then determined after 3 h incubation by measuring light emitted at 530 nm of exciting cells with light at 485 nm.
Measurement of lipid peroxidation
The level of MDA, an intermediate product of lipid peroxidation, was determined by the modified thiobarbituric acid reactive substance (TBARS) fluorescence method (Goodman et al., 1996). To determine the amount of malondialdehyde (MDA), cultured cells were collected with 1 ml of ice-cold 0.5 mM PBS and then homogenized. Cell suspensions were added to a solution of 0.3 ml of 10% trichloroacetic acid (TCA), and then 0.15 ml of TBARS reagent (0.335% 2-thiobarbituric acid in 50% glacial acetic acid) was added. The solution was incubated at 90°C for 60 min, and the fluorescence was measured at 553 nm with an excitation wavelength of 515 nm. The levels of MDA were quantified using a standard curve of 1,1,3,3-tetraethoxypropane, and expressed as nanomol of MDA per milligram of protein.
Assay for SOD activity
Cells were collected in 0.1 M. phosphate buffer (pH 7.4) and homogenized. The homogenate was centrifuged for 30 min at 3000 g at 4°C and the supernatant (cytosolic and mitochondrial fractions) was used for the measurement of superoxide dismutase (SOD) activity. The activity of SOD was determined according to the method of McCord and Fridovich (1969) by xanthine-xanthine oxidase reaction.
Assay for caspase-3/7 activity
Caspase-3/7 activity was measured with the Apo-ONE homogeneous assay kit using a synthetic fluorometric substrate for caspase, Z-DEVD-Rhodamine 110. After treatment with STS for the time indicated, 100 μl of homogeneous caspase-3/7 reagent (buffer+substrate) was added to each well of the cultured neurons in 96-well plates. The 96-well plate was incubated at room temperature and assayed on a Cytofluor II multiwell fluorescence spectrometer (excitation 485 nm, emission 520 nm).
Protein assay
Protein content was measured by the method of Lowry et al. (1951) with bovine serum albumin as a standard.
Materials
Supplements for cell culture and other reagents used in the study were obtained from Sigma (St Louis, MO, USA). Caspase 3/7 assay kit was purchased from Promega (MA, USA). All other chemicals were of the highest purity available. Pinusolide and 15-MPA were isolated from the barks of B. orientalis as previously reported and their purities were higher than 95.0% (Koo et al., 2002).
Statistical analysis
Statistical significance was determined by one-way ANOVA and, if significant, group means were compared by post hoc analysis using Tukey multiple comparison of means test. Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Results
Pinusolide and 15-MPA prevented apoptosis induced by STS
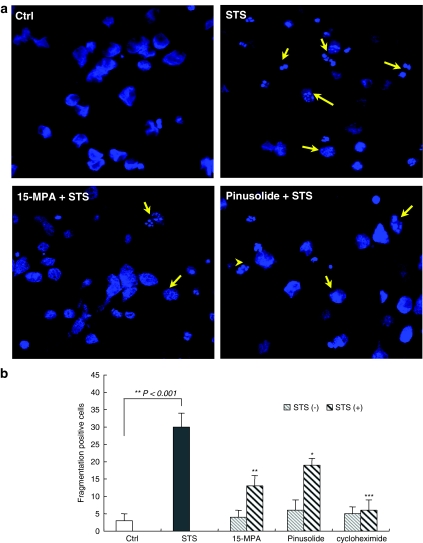
We evaluated the neuroprotective activity of pinusolide and 15-MPA, isolated from B. orientalis, on cultured cortical neurons exposed to pro-apoptotic STS (Figure 1). Generally, cells undergoing apoptosis exhibit characteristic morphological features such as cell shrinkage, membrane blebbing, formation of apoptotic bodies, chromatin condensation and nuclear fragmentation (Bredesen, 1995). In our system, cytoplasmic condensation and nuclear fragmentation were observed in STS-injured cortical cells in the fluorescence photomicrograph, observed with a confocal microscope using Hoechst dye 33342 (Figure 2). However, 15-MPA and pinusolide significantly decreased these characteristic morphological changes of apoptosis induced by STS whereas no effect was seen with the control cells. These results suggest that pinusolide and 15-MPA protect these cells against STS-induced apoptosis.
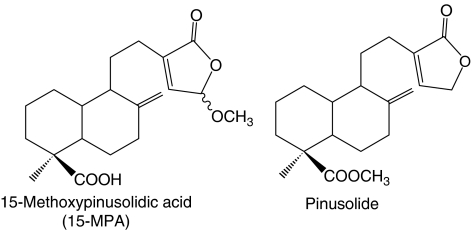
Figure 1.
Structure of 15-methoxypinusolidic acid (15-MPA) and pinusolide isolated from a methanolic extract of B. orientalis leaves.
Figure 2.
The effect of 15-MPA and pinusolide on cytoplasmic condensation and nuclear fragmentation in STS-injured neuronal cells. (a) The images of Hoechst 33342 fluorescence were acquired under a confocal microscope (395 nm excitation and 420 nm barrier filter) with × 40 oil immersion objective. Cortical cells were pretreated for 1 h with 5 μM 15-MPA or pinusolide and were then exposed to 100 nM of STS for 18 h. (b) Quantification of data representing the shape and intensity of Hoechst 33342 fluorescence in each cell. Arrows indicate cytoplasmic condensation, apoptotic body and nuclear fragmentation. Cycloheximide, a protein synthesis inhibitor, was used as a positive control for STS-induced apoptosis. Mean value of the STS-treated cultures are significantly different (P<0.001) from the value of the control cultures. *P<0.05, **P<0.01, ***P<0.001 vs STS-treated cultures.
Pinusolide and 15-MPA significantly reduced the elevation of [Ca2+]i induced by STS
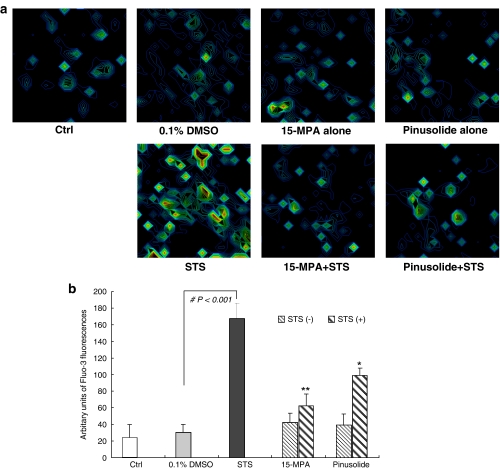
An elevation of [Ca2+]i, mediated by its release from the intracellular store and influx, has been implicated as a pro-apoptotic second messenger involved in both triggering apoptosis and regulating death-specific enzymes in various apoptotic pathways (Nicotera et al., 1994; McConkey and Orrenius, 1997; Prehn et al., 1997; Nicotera and Orrenius, 1998). Therefore, the effect of pinusolide and 15-MPA on the [Ca2+]i increase induced by STS was evaluated by use of a confocal laser scanning microscope using fluo-3 AM, a calcium specific indicator dye. As shown in Figure 3, [Ca2+]i in STS-treated cells was increased approximately 7.0 fold compared to that of control cells. However, pretreatment with pinusolide or 15-MPA significantly blocked the [Ca2+]i increase induced by STS (Figure 3). At the concentration of 5.0 μM, pinusolide and 15-MPA blocked [Ca2+]i increase by 38 and 68%, respectively, as compared with that of STS-injured control cells. These results suggest that the neuroprotective effect of pinusolide and 15-MPA is associated with Ca2+ signalling, a critical event in the STS-induced apoptotic pathway.
Figure 3.
Effect of 15-MPA and pinusolide on the [Ca2+]i increase in STS-injured rat cortical cells. (a) 3D pseudo fluorescence images of calcium indicator dye fluo-3 under a confocal microscope (488 nm excitation and 510 nm barrier filter) represent the intensity of Ca2+ in living cortical cells. Cultures were pretreated for 1 h with 5 μM 15-MPA or 5 μM pinusolide and were then exposed to 100 nM STS for 6 h. Cells grown on chambered coverglass were incubated in the presence of 0.1 μM fluo-3 AM for 30 min and then washed twice with HBSS. (b) The graph represents the quantification of the intensity of fluo-3 staining in each cell. Values are the mean±s.d. of determinations made in cells each of cultures (30 cells assessed). Mean value of the STS-treated cultures are significantly different (#P<0.001) from the value of the vehicle-treated cultures. *P<0.05, **P<0.01 vs STS-treated cultures.
Effect of pinusolide and 15-MPA on ROS overproduction and lipid peroxidation induced by STS insult
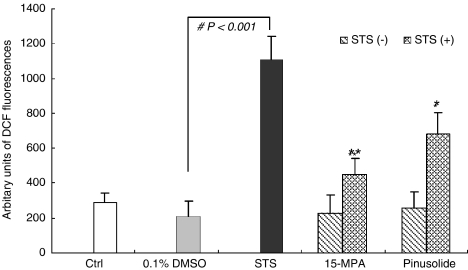
The sustained elevation of [Ca2+]i induced by STS results in ROS generation which induces oxidative stress and the apoptotic signal pathway (Keller et al., 1998; Richter, 1993; Schultz et al., 1996). Moreover, superoxide anion, a major ROS generated in mitochondria, can interact with nitric oxide (NO) to form peroxynitrite, another detrimental free radical. Thus, we evaluated the effect pinusolide and 15-MPA on cellular ROS using the specific fluorescence dyes, DCF-DA. Under our experimental conditions, exposure of cortical cells to 100 nM STS for 18 h increased the cellular ROS levels about four times compared to control cultures. However, treatment with pinusolide or 15-MPA at concentrations of 1.0 and 5.0 μM, significantly reduced ROS production provoked by STS (Figure 4).
Figure 4.
Effect of 15-MPA and pinusolide on cellular ROS level in primary cultures of STS-injured rat cortical cells. The relative content of intracellular peroxide was determined using the fluorescent dye DCF-DA. The values shown are means±s.d. of three experiments (3–4 cultures per experiment). Mean value of the STS-treated cultures are significantly different (#P<0.001) from the value of the vehicle-treated cultures. *P<0.05, **P<0.01 vs STS-injured cells.
We also evaluated the effect of pinusolide and 15-MPA on membrane lipid peroxidation by measuring the production of MDA. Under our experimental conditions, the level of MDA in primary cultures of rat cortical cells exposed to 100 nM STS increased threefold compared to that of control cultures. Interestingly, treatment with 5.0 μM 15-MPA inhibited the production of MDA almost completely in STS-injured cells; the level was retained near that of control cells (Table 1).
Table 1.
Effects of 15-MPA and pinusolide on the membrane lipid peroxidation and SOD activity in STS-induced apoptosis
| TBARSa (nmol mg−1 protein) | SODb (U mg−1 protein) | |
|---|---|---|
| Control | 0.998±0.191 | 40.93±9.41 |
| STS | 3.434±0.487 | 12.01±5.63 |
| 15-MPA+STS | ||
| 1 μM | 1.953±0.508* | 32.68±5.01*** |
| 5 μM | 1.166±0.439** | 41.57±6.68*** |
| Pinusolide+STS | ||
| 1 μM | 2.642±0.415 | 19.60±3.16* |
| 5 μM | 1.965±0.397* | 24.63±7.01** |
Control represents the value for cultures not exposed to STS.
The TBARS intensities of cell homogenate were measured by using a fluorescence spectrometer with an excitation wavelength at 515 nm and an emission wavelength at 553 nm.
The supernatants were used to assay for SOD activity which was based on the ability of SOD enzyme to compete with ferricytochrome C for superoxide anions generated by the xanthine oxidase system. Data are expressed as the mean±s.d. of three independent experiments. Mean value of the STS-treated cultures are significantly different (P<0.001) from the value of the control cultures.
P<0.05
P<0.01
P<0.001 vs STS-treated cultures.
Effect of pinusolide and 15-MPA on the reduction in SOD activity in response to STS
Mammalian brains have mechanisms that can protect against the damage caused by oxidative stress. These include glutathione and antioxidative enzymes such as SOD, glutathione peroxidase, catalase and glutathione disulphide reductase (Sampath et al., 1994; Yu, 1994). As pinusolide and 15-MPA significantly inhibited overproduction of ROS, we determined whether 15-MPA and pinusolide affected this antioxidative defense system. Treatment with 15-MPA or pinusolide almost completely inhibited the reduction in SOD activity induced by STS at a concentration of 5.0 μM (Table 1). From these results and those above, we suggest that pinusolide and 15-MPA inhibit the overproduction of ROS by preserving SOD activity from attenuation by STS.
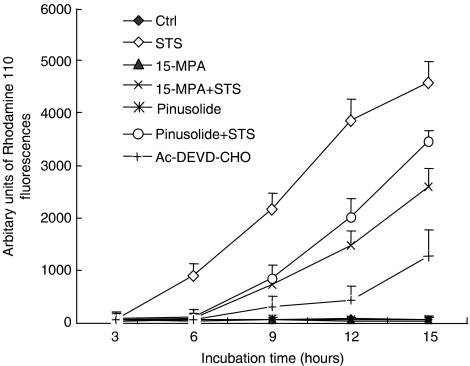
Pinusolide and 15-MPA decreased activation of caspase-3/7
The elevation of [Ca2+]i and ROS production, and subsequent release of cytochrome c from mitochondria can lead the activation of the caspase cascade (Kluck et al., 1997; Gross et al., 1999; Martin et al., 2002). Hence, we examined the effect of pinusolide and 15-MPA on the activation of caspase-3/7 in neuronal apoptosis induced by STS. In our system, caspase-3/7 activity was increased after STS insults. However, treatment with pinusolide or 15-MPA efficiently reduced the increase in caspase-3/7 activity induced by STS (Figure 5).
Figure 5.
Effect of 15-MPA and pinusolide on caspase 3/7 activation in STS-induced apoptosis. The caspase-3/7 activity was measured with the Apo-ONE homogeneous assay kit. Caspase-3/7 activation in cortical cultures was quantified at the end point of the incubation times for 12 h following exposure to 100 nM STS. The fluorescence intensities of cleaved rhodamine 110 products were measured on a fluorescence spectrometer (excitation 485 nm, emission 520 nm). Data are expressed as the means±s.d. of three separate cultures.
Discussion
The pathological features of apoptosis include a variety of characteristics distinguishable from necrosis. The morphological features are associated with various biochemical changes in the apoptotic signalling pathway that are time-dependent. These include increases in levels of [Ca2+]i and oxygen radicals, followed by activation of caspases, and the resulting typical apoptotic morphology; plasma membrane blebbing, chromatin condensation and formation of apoptotic bodies. Thus, we tried to elucidate the anti-apoptotic mechanism of pinusolide and 15-MPA in STS-injured neuronal cultures by evaluating their effects on some of these biochemical changes. The increase in [Ca2+]i and cellular accumulation of ROS are general phenomenon that occur in STS-induced neuronal apoptosis and during apoptosis induced by other stimuli (Prehn et al., 1997; Aaron et al., 1998). With regard to the roles of the [Ca2+]i increase and ROS production in apoptosis, it has been postulated that an increase in [Ca2+]i is involved in the generation of ROS. However, ROS can also affect calcium homeostasis, therefore, it is possible that both ROS and Ca2+ act synergistically to affect apoptotic cell death (Richter, 1993; Kluck et al., 1997). Previous studies have shown that antioxidants exert a protective effect on STS-induced apoptosis in many cell types. In addition, the increase in [Ca2+]i can also be partially prevented by the presence of antioxidants, suggesting a role for ROS in the STS-induced rise in [Ca2+]i (Kruman et al., 1997; Prehn et al., 1997; Gil et al., 2003). In the present study, treatment with pinusolide or 15-MPA significantly inhibited the increase in [Ca2+]i and ROS accumulation induced by STS in the primary cultured rat cortical cells (Figures 3 and 4). Measurement of the direct free radical scavenging activity of 15-MPA and pinusolide, using the DPPH assay, showed that neither compounds directly scavenged free radicals in our study (data not shown). Therefore, it is possible that pinusolide and 15-MPA inhibit ROS production by stabilizing Ca2+ homeostasis which, in turn, prevent further ROS production; an effect that does not involve the scavenging of free radicals.
This decrease in ROS production in STS-injured cortical cells induced by pinusolide and 15-MPA could also result from their preservation of SOD. Oxidative stress is caused by the overproduction of ROS such as superoxide anion radical (O2−.), peroxynitrite and hydroxyl radical. These cause cellular structural and functional dysfunction by attacking chemical bonds in cellular lipids, proteins, and nucleic acids (Bromont et al., 1989). Other detrimental free radicals are NO and peroxynitrite, which can directly induce apoptosis in many types of cultured cells including neurons (Bromont et al., 1989; Beckman and Crow, 1993; Keller et al., 1998). The accumulation of superoxide anion radicals is prevented by its conversion to hydrogen peroxide, a process catalyzed by Cu/ZnSOD in cytoplasm and MnSOD in mitochondria (Yu, 1994). Previous studies reported that the expression of SOD plays an important role in protecting cells from ROS in neuronal apoptotic cell death (Greenlund et al., 1995). Mitochondrial MnSOD prevents neuronal apoptosis and reduces ischaemic brain injury by suppression of peroxynitrite production, lipid peroxidation and mitochondrial dysfunctions (Keller et al., 1998). In our culture system, pretreatment of the primary cultured rat cortical cells with pinusolide and 15-MPA completely inhibited the decrease in SOD activity induced by STS (Table 1). Therefore, the reduction in the rise in [Ca2+]i and ROS production by 15-MPA and pinusolide might also be exerted via its protective effect on SOD activity.
The elevation of [Ca2+]i and ROS production can result in the activation of the caspase cascade (Lebel et al., 1992; Kluck et al., 1997; Gross et al., 1999; Martin et al., 2002). The caspase cascade, therefore, is the later stage of apoptosis and can be prevented by Ca2+ modulators and antioxidants, whereas general caspase inhibitors do not affect Ca2+ influx and ROS production. In our studies, pinusolide and 15-MPA strongly inhibited the activation of caspase-3/7 after STS exposure (Figure 5). Therefore, inhibition of caspase-3/7 might be achieved as a consequence of a reduction in the rise of [Ca2+]i and ROS overproduction.
In conclusion, we suggest that pinusolide and 15-MPA significantly attenuate STS-induced neuronal apoptosis in primary cultures of rat cortical cells via stabilization of [Ca2+]i homeostasis and suppression of oxidative stress. On the basis of the present study, the anti-apoptotic effect of 15-MPA and pinusolide may provide a potential therapeutic approach for preventing and/or treating neurodegenerative diseases. Further studies must be carried out to explore this possibility.
Acknowledgments
This research was supported by a grant (MK103KV010024-06K2201-02410) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, The Republic of Korea.
Abbreviations
- DPPH
1,1-diphenyl-2-picryl-hydrazyl
- MDA
malondialdehyde
- 15-MPA
15-methoxypinusolidic acid
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STS
staurosporine
- TBARS
thiobarbituric acid reactive substance
Conflict of interest
The authors state no conflict of interest.
References
- Aaron JK, Elke P, Jochen H. Staurosporine-induced apoptosis of cultured rat hippocampal neurons involves caspase-1-like proteases as upstream initiators and increased production of superoxide as a main downstream effector. J Neurosci. 1998;18:8186–8197. doi: 10.1523/JNEUROSCI.18-20-08186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Transac. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- Bertrand R, Solary E, O'Connor P, Kohn KW, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211:314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- Bredesen DE. Neuronal apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- Bromont C, Marie C, Bralet J. Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke. 1989;20:918–924. doi: 10.1161/01.str.20.7.918. [DOI] [PubMed] [Google Scholar]
- Coley JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;269:30761–30764. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Falcieri E, Martelli AM, Bareggi R, Cataldi A, Ccocco L. The protein kinase inhibitor staurosporine induces morphological changes typical of apoptosis in MOLT-4 cells without concomitant DNA fragmentation. Biochem Biophys Res Comm. 1993;193:19–25. doi: 10.1006/bbrc.1993.1584. [DOI] [PubMed] [Google Scholar]
- Gil J, Almeida S, Oliveira CR, Rego AC. Cytosolic and mitochondrial ROS in staurosporine-induced retinal cell apoptosis. Free Rad Biol Med. 2003;5:1500–1514. doi: 10.1016/j.freeradbiomed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Greenlund LJS, Deckwerth TL, Johnson EM. Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed cell death. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- Gross A, Mcdonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Develop. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res. 1994;54:1707–1714. [PubMed] [Google Scholar]
- Keller JN, Kondy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Sung SH, Kang SY, Koo KA, Kim SH, MA CJ. Aristolactam BII of Saururus chinensis attenuates glutamate-induced neurotoxicity in rat cortical cultures probably by inhibiting nitric oxide production. Planta Med. 2004;70:391–396. doi: 10.1055/s-2004-818964. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim SR, Markelonis GJ, Oh TH. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells form glutamate-induced neurodegeneration. J Neurosci Res. 1998;53:426–432. doi: 10.1002/(SICI)1097-4547(19980815)53:4<426::AID-JNR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Koo KA, Sung SH, Kim YC. A new neuroprotective pinusolide derivative from the leaves of Biota orientalis. Chem Pharm Bull. 2002;50:834–836. doi: 10.1248/cpb.50.834. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo O, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1997;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lebel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Martin O, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Nat Acad Sci USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Comm. 1997;239:357–366. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymatic fuction for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Nicotera P, Orrenius S. The role of calcium in apoptosis. Cell Calcium. 1998;23:173–180. doi: 10.1016/s0143-4160(98)90116-6. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Zhivotovsky B, Orrenius S. Nuclear calcium transport and the role of calcium in apoptosis. Cell Calcium. 1994;16:79–88. doi: 10.1016/0143-4160(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Prehn JH, Jordan J, Ghadge GD, Preis E, Galindo MF, Roos RP, et al. Ca2+ and reactive oxygen species in staurosporine-induced neuronal apoptosis. J Neurochem. 1997;68:1679–1685. doi: 10.1046/j.1471-4159.1997.68041679.x. [DOI] [PubMed] [Google Scholar]
- Richter C. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett. 1993;325:104–107. doi: 10.1016/0014-5793(93)81423-w. [DOI] [PubMed] [Google Scholar]
- Sampath D, Jackson GR, Werrbach-Perez K, Perez-Polo R. Effects of nerve growth factor on glutathione peroxidase and catalase in PC12 cells. J Neurochem. 1994;62:2476–2479. doi: 10.1046/j.1471-4159.1994.62062476.x. [DOI] [PubMed] [Google Scholar]
- Schultz JB, Weller M, Klockgether T. Potassium deprivation induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity and reactive oxygen species. J Neurosci. 1996;16:4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathologenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:239–262. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Ling YW, Kong D, Yang C, Zhang X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol. 2004;93:133–140. doi: 10.1016/j.jep.2004.03.037. [DOI] [PubMed] [Google Scholar]