Abstract
Background and purpose:
Maintained penile erection depends on the absence of α-adrenoceptor (α-AR) activation and so can be facilitated by α-blockers. This study seeks the α1-AR subtypes involved in order to inform the pro-erectile consequences of subtype selective blockade.
Experimental approach:
Wire myography was used with dorsal (nutritional supply) and cavernous (erectile inflow) penile arteries; standard α-AR-selective agonists and antagonists were employed to classify responses.
Key results:
In both penile arteries noradrenaline (NA) and phenylephrine (PE, α1-AR agonist) caused concentration-dependent contractions. Sensitivity to NA was increased by NA uptake blockers, cocaine (3 μM) and corticosterone (30 μM). PE responses were antagonised by phentolamine (non-selective α-AR: dorsal pKB 8.00, cavernous 8.33), prazosin (non-subtype-selective α1-AR: dorsal 8.60, cavernous 8.41) and RS100329 (α1A-AR selective: dorsal 9.03, cavernous 8.80) but not by BMY7378 (α1D-AR selective: no effect at 1-100 nM) or Rec15/2615 (α1B-AR selective: no effect at 1-100 nM). Schild analysis was straightforward in cavernous artery, indicating that PE activates only α1A-AR. In dorsal artery Schild slopes were low, though α1A-AR was still indicated. Analysis using UK 14,304 and rauwolscine indicated an α2-AR component in dorsal artery that may account for low slopes to α1-AR antagonists.
Conclusions and implications:
Penile arteries have a predominant, functional α1A-AR population with little evidence of other α1-AR subtypes. Dorsal arteries (nutritional supply) also have α2-ARs. Thus, α-AR blockers with affinity for α1A-AR or α2-AR would potentially have pro-erectile properties; the combination of these perhaps being most effective. This should inform the design of drugs to assist/avoid penile erection.
Keywords: penile, dorsal artery, cavernous artery, adrenergic, α1-adrenoceptor subtypes
Introduction
Penile tumescence is brought about by engorgement of the erectile tissues with blood, and this is caused by vasodilatation of the inflow arteries coupled with relative vasoconstriction of outflow veins. Pharmacological interest has centred on vasodilators and vasoconstrictors for their respective effects to improve tumescence, where this is desired to improve sexual performance, or decrease tumescence, where the erection is in a pathologically prolonged state known as priapism. Drugs to treat this condition have been targeted at manipulation of the physiological regulation of penile arterial tone. The concept is to modify the vasodilator nitrergic and vasoconstrictor adrenergic mechanisms that respectively cause and terminate tumescence. On the adrenergic side, α-blockers such as phentolamine have long been known to cause priapism as a side effect when used for other therapeutic purposes, and this has been exploited to treat erectile dysfunction by intra-penile injection (Chin et al., 1998). Conversely, vasoconstrictor α-adrenoceptor (α-AR) agonists such as phenylephrine (PE) can be used to treat priapism, including that to an overdose of phentolamine (Dougherty et al., 2006). The concept of vasoconstrictor α2-ARs has also been mooted as an explanation for the pro-erectile effects of yohimbine originating from traditional medicine, and yohimbine is still marketed (non-medically) for this effect.
There are three subtypes of α1-ARs, and α-blockers that are in clinical use (to treat benign prostatic hyperplasia, hypertension and, although currently discontinued, heart failure) vary in their subtype selectivity. It is therefore of relevance to know which subtypes are involved in vasoconstriction of penile blood vessels in order to avoid priapism, or to cause penile erection according to the desired effect. However, there are currently different opinions on the matter. Two contributing factors are the poor selectivity of antagonists used to define functional responses and the use of strips of erectile tissue rather than preparations of arteries per se.
Penile vasculature and erectile tissue are densely innervated by adrenergic nerves (Tamura et al., 1995), which cause contraction of the smooth muscle of corpus cavernosum trabecular tissue and penile arteries to initiate and maintain detumescence. Contraction of human and animal erectile tissue and vasculature has been shown to be predominantly mediated by α1-ARs (Saenz de Tejada et al., 1989; Traish et al., 1995; Recio et al., 1997; Sato and Kawatani, 2002). Expression of mRNA for all three α1-AR subtypes, α1A, α1B and α1D, has been demonstrated in human (Dausse et al., 1998; Goepel et al., 1999), rabbit (Peng et al., 1998) and rat (Veronneau-Longueville et al., 1998) corpus cavernosum tissue. Few studies, however, have attempted to identify α1-AR subtypes involved in adrenergic contractions of the erectile tissue and the results, so far, are contradictory. One of the main events leading to the initiation of an erection is an increase in arterial inflow to the erectile tissue. This relies greatly on vascular control of the main inflow arteries, the dorsal and cavernous penile arteries. Most studies so far have concentrated on the investigation of contractile responses of strips of penile corpus cavernosum, which contain a variety of cell and tissue types that incorporate many different and potentially conflicting pathways, and so may not faithfully represent the properties of inflow arteries. However, this is the only evidence available on α1-AR subtypes in the erectile tissues. In summary, in rabbit and rat, major mRNA expression is suggested to be α1B-AR (Peng et al., 1998), but functional receptors to be either the α1B-AR (Furukawa et al., 1996) or the α1A-AR (Furukawa et al., 1996; Tong and Cheng, 1997; Peng et al., 1998).
The aim of the present study was to characterize α1-ARs underlying PE-induced contractions in rabbit isolated genital arteries. To clarify the mechanisms of adrenergic contraction important to normal sexual function, arteries central to the erectile response were investigated. Dorsal arteries primarily supply the glans penis and prepuce while having an additional role in the supply of blood to the corpus cavernosum. The dorsal artery functions to maintain an uninterrupted supply of nutrients and oxygen to the penile tissue. Meanwhile, cavernous arteries comprise the main arterial inflow to cavernous tissue, inflow that is greatly increased during sexual arousal. During the flaccid state, an increased level of sympathetic nerve activity maintains contraction of the cavernous arteries and trabecular tissue and prevents onset of the erectile response (reviewed by Andersson and Wagner, 1995). Therefore, an understanding of the vascular function of both dorsal and cavernous arteries is critical to our understanding of sexual function in general. Both dorsal and cavernous artery preparations were studied to gain an insight into functional mechanisms of the penile tissue.
Methods
Tissues
Penile tissue was obtained from male New Zealand White rabbits (2.5–3.5 kg) killed by an overdose of pentobarbitone (Euthatal, Rhône Merieux, UK) injected into the ear marginal vein. Experiments were carried out in compliance with UK legislation. Further tissue dissections were carried out in ice-cold physiological saline solution, composition (mM) NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4·H2O 1.2, KH2PO4 1.2, NaHCO3 24.9, glucose 11.1, EDTA 0.023 and pH 7.3. Dorsal and cavernous penile arteries, internal lumen diameter 181±1 μm (n=33) and 150±2 μm (n=35), respectively (measured under conditions of zero pressure), were isolated from the erectile tissue. Arterial rings, approximately 2 mm in length, were mounted on two 40 μm wires attached to a wire myograph (DMT, Copenhagen, SV, Denmark) to allow isometric tension recordings. The optimal isometric tension for maximal contraction in response to PE (10 μM) was predetermined using a range of resting tensions from 0 to 1 g and set at 0.25 g for all experiments. Vessels were bathed in physiological saline solution at 37°C and oxygenated with 95% O2, 5% CO2.
Protocols
Vessels were allowed to equilibrate under resting tension for 30 min following which a reproducible maximal contraction to noradrenaline (NA) (10 μM) was determined before experimental protocols were begun. The mean NA maximal contraction for each vessel was used to compare experimental responses. Endothelium integrity was confirmed by assessing the ability of acetylcholine (ACh) (3 μM) to relax NA (10 μM) pre-contracted vessels.
During the evaluation of antagonists, first curves to PE were performed with an absence of antagonist in all preparations. PE was added cumulatively in half-log increments from 1 nM to 300 μM. Vessels were incubated with an antagonist for 30 min before the second PE concentration–response curves (CRCs). The antagonists prazosin, RS 100329, Rec 15/2615, BMY 7378 and rauwolscine were tested at concentrations of 1–100 nM, concentrations known to be selective for the appropriate adrenoceptor subtypes and in line with published data. Owing to a lack of effect at concentrations of 1 and 10 nM in the dorsal arteries, phentolamine was tested at an additional concentration of 1 μM. All protocols included a parallel control vessel that was studied in the absence of antagonist during both the first and second curves.
Drugs
All drugs used were of analytical grade and were purchased from Sigma-Aldrich, ACh, NA, PE, prazosin, phentolamine, 5-methylurapidil, BMY 7378 (8-(2-[4-{2-methoxyphenyl}-1-piperazinyl]ethyl)8-azaspiro[4,5]decane-7,9-dione dihydrochloride), corticosterone; Roche Bioscience (Palo Alto, CA, USA), RS 100329 (N-[(2-trifluoroethoxy)phenyl],N′-(3-thyminylpropyl) piperazine hydrochloride) and L-NAME (Nω-nitro-L-arginine methyl ester hydrochloride); Tocris (Bristol, UK), rauwolscine and UK 14 304 (5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline); or manufactured in house, Rec 15/2615 (1-(4-amino-6,7-dimethoxy-2-guinazolinil)-4-[2-[2-metoxy-6-(1-methylethyl)phenoxy]acetyl]piperazine hydrochloride). Stock solutions were made from powder form using de-ionized H2O with the exception of NA, which was dissolved in 23 μM EDTA, and all further dilutions were made in de-ionized H2O, and UK 14 304 and Rec 15/2615, which were made up as stock solutions in dimethylsulphoxide.
Data analysis
Data, acquired using Chart v4.1.2 (PowerLab, ADInstruments Ltd, Chalgrove, Oxfordshire, UK) were analysed using Prism v3.0 software (GraphPad Software Inc., San Diego, CA, USA) and expressed as mean±s.e.m. Where shown, n indicates the number of animals used. Contractions are expressed either as force in g, a percentage of the vessel response to NA (10 μM), or a percentage of the maximum contraction (Emax) achieved during a CRC. Agonist responses were expressed using pEC50 values; the inverse log of the effective concentration producing 50% of the maximum response. In cases where an antagonist caused a parallel shift of an agonist curve, a Schild plot of log(concentration ratio – 1) versus log(antagonist concentration) was constructed, and a pA2 was calculated from the x-intercept of this plot. If the slope of the Schild plot was equal to 1, then pA2=pKB and was indicative of competitive binding. Where a pA2 could not be calculated, a pKB was calculated instead using the equation
where pKB is the negative logarithm of the dissociation constant KB and [B] is the concentration of antagonist. Individual pKB values were determined for each experiment at each antagonist concentration. Mean±s.e.m. of total data is quoted to provide a pKB value for a specific tissue.
Hill slopes, pEC50 values and maximal responses were compared using a one-way analysis of variance with a Bonferroni post-test. Statistical significance was taken as P<0.05.
Results
Agonist profiles
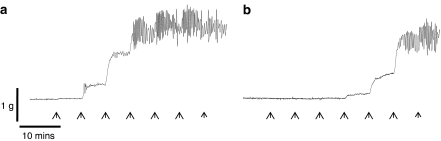
The α1-AR agonist PE and the non-selective α-AR agonist NA both caused concentration-dependent contractions in dorsal and cavernous arteries from rabbit penile tissue (Table 1). In dorsal arteries, rhythmic activity was often observed in response to agonist stimulation (Figure 1). Where rhythmic activity occurred, the mean tone of the vasoconstriction response was calculated, using Chart v4.1.2 software, over a period of activity where a stable maximum and minimum tone was evident.
Table 1.
Agonist profile in the dorsal and cavernous arteries
|
Dorsal artery |
Cavernous artery |
|||||
|---|---|---|---|---|---|---|
| N | Emax (g) (s.e.m.) | pEC50 (s.e.m.) | n | Emax (g) (s.e.m.) | pEC50 (s.e.m.) | |
| NA | 6 | 2.01 (0.04) | 5.85 (0.06) | 6 | 0.96g (0.03) | 6.14f (0.08) |
| NA plus L-NAME | 6 | 2.47c (0.08) | 6.57c (0.08) | 5 | 1.06 (0.07) | 6.28 (0.15) |
| NA plus uptake blockers | 6 | 1.84 (0.11) | 6.75c (0.19) | 5 | 1.15 (0.01) | 6.84a (0.04) |
| PE | 6 | 1.56b (0.03) | 5.70 (0.03) | 6 | 0.93g (0.06) | 6.12g (0.01) |
| PE plus L-NAME | 6 | 2.02d (0.08) | 6.20d (0.09) | 5 | 1.14 (0.08) | 6.18 (0.22) |
| UK 14 304 | 6 | 0.81c (0.18) | 8.14c (0.10) | 7 | 0.44c (0.07) | 8.04c (0.06) |
| UK 14 304 plus L-NAME | 6 | 1.78e (0.18) | 8.01 (0.13) | 5 | 0.53 (0.13) | 8.00 (0.09) |
Abbreviations: L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; NA, noradrenaline; PE, phenylphrine.
Summary of Emax and pEC50 of NA, PE and UK 14 304 (controls and in the presence of L-NAME or NA uptake blockers).
P<0.05
P<0.01
P<0.001 versus NA control
P<0.001 versus PE control
P<0.01, versus UK 14 304 control
P<0.05
P<0.001 versus dorsal artery.
Figure 1.
Non-equilibrium activity observed during a CRC in dorsal penile arteries. (a) Agonist-induced, rhythmic activity was observed more often in dorsal arteries; in this example, the agonist was PE (0.3–300 μM, arrows). (b) Rhythmic activity remained in the presence of prazosin (0.1 μM).
Maximal contractions to NA in dorsal arteries exceeded 2 g force with responses to NA>PE (P<0.01). In the dorsal artery, responses to NA (10 μM) demonstrated an initial transient contraction before decreasing to a sustained plateau. This fall was unaffected by the inclusion of NA uptake blockers, cocaine (3 μM, uptake 1 blocker) and corticosterone (30 μM, uptake 2 blocker) in the bathing medium. Uptake blockers did, however, increase the sensitivity, but not the Emax, of NA responses in both dorsal (P<0.001) and cavernous (P<0.05) arteries. In the presence of L-NAME (100 μM), maximal contractions to NA and PE were significantly increased in dorsal arteries (P<0.001).
In cavernous arteries, maximal contractions to NA and PE were of a similar magnitude and were approximately 50% of NA-induced responses in dorsal arteries (P<0.001). Responses were generally well maintained over a 6-min period. In cavernous arteries, maximal contractions to NA and PE were unaffected (P>0.05) by incubation with L-NAME (100 μM).
α1-AR subtypes in the dorsal artery
Two sequential control curves with no antagonist were created in each individual experiment (i.e. time controls). In dorsal arteries, no significant change in sensitivity to PE from first (pEC50 5.74±0.06) to second (pEC50 5.65±0.09) CRC was observed (P>0.05, n=27). The mean PE Emax decreased 18% from 1.86±0.02 g to 1.52±0.01 g (P<0.001, n=27). This decrease occurred uniformly across all second curves, with or without antagonist pre-incubation, regardless of concentration, and so it was not considered indicative of antagonism. However, because of this decrease in Emax, the effect of antagonists was investigated by comparison of second phenylephrine concentration–response curves (PE CRCs) using paired preparations with an absence or presence of antagonist.
To determine the α1-AR subtype/s mediating vasoconstriction in dorsal arteries, non-subtype- and subtype-selective α1-AR antagonists were tested against responses to the α1-AR selective agonist PE (Table 2). Phentolamine, a non-selective α-AR antagonist, caused a parallel rightward shift of the PE CRC with pKB of 8.00±0.16 (n=10). Schild plot analysis for phentolamine gave a pA2 of 8.32 (95% confidence interval (CI) 7.34 to 10.31) with a slope not significantly different from unity (0.72±0.19).
Table 2.
Antagonist profile in the dorsal artery
| Antagonist | n | pKB (s.e.m.) | pA2 (95% CI) | Schild slope (s.e.m.) |
Concentration ratio (s.e.m.) |
|||
|---|---|---|---|---|---|---|---|---|
| 1 nM | 10 nM | 100 nM | 1 μM | |||||
| Phentolamine | 10 | 8.00 (0.16) | 8.32 (7.34–10.31) | 0.72 (0.19) | 0.80 (0.18) | 4.75 (3.75) | 21.97 (14.56) | 191.39 (161.27) |
| Prazosin | 7 | 8.60 (0.16) | 9.71 (8.13–19.94) | 0.41 (0.16) | 2.79 (0.97) | 8.63 (2.39) | 21.84 (7.42) | ND |
| RS 100329 | 6 | 9.03 (0.07) | 9.22 (8.82–9.84) | 0.43 (0.11) | 2.44 (0.04) | 9.79 (2.48) | 14.94 (6.21) | ND |
| Rec 15/2615 | 4 | ND | ND | ND | 1.03 (0.28) | 1.50 (1.18) | 1.01 (0.19) | ND |
| BMY 7378 | 6 | ND | ND | ND | 1.91 (0.67) | 1.02 (0.38) | 1.47 (0.50) | ND |
| Rauwolscine | 4 | ND | ND | ND | 2.86 (1.49) | 3.53 (1.47) | 3.18 (1.34) | ND |
Abbreviations: CI, confidence interval; ND, not determined; PE CRC, phenylephrine concentration–response curves.
Summary of pKB's, pA2's, Schild slopes and concentration ratios of α 1- and α2-AR antagonists versus PE. Where antagonists did not cause a rightward shift of the PE CRC, pKB, pA2 and Schild plots could not be determined.
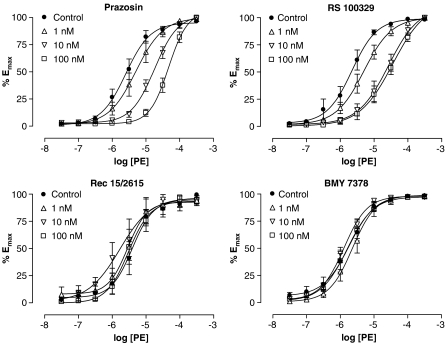
The non-subtype-selective α1-AR antagonist prazosin caused a parallel rightward shift of the PE CRC (Figure 2). Schild analysis of the data demonstrated a shallow regression slope of 0.41±0.16 while pKB calculation demonstrated a progressive decrease in potency with increasing concentrations: 9.34±0.29 at 1 nM (n=4), 8.64±0.23 at 10 nM (n=7) and 8.14±0.17 at 100 nM (n=7).
Figure 2.
PE-induced vasoconstriction in the dorsal artery in the absence or presence of 1, 10 or 100 nM prazosin (non-selective antagonist, n=7), RS 100329 (α1A-AR antagonist, n=6), Rec 15/2615 (α1B-AR antagonist, n=4) or BMY 7378 (α1D-AR antagonist, n=6). A rightward shift of the PE CRC was observed at 1 and 10 nM RS 100329, but no further rightward shift occurred at 100 nM. All PE response data were taken from second CRCs in the absence or presence of antagonist.
An α1A-AR selective antagonist RS 100329 (Williams et al., 1999) caused a parallel rightward shift of the PE curve at concentrations of 1 and 10 nM (Figure 2). RS 100329 has been shown to be a potent antagonist at α1A-ARs with a pA2/pKB of between 9.2 and 9.6 (Williams et al., 1999; Choppin et al., 2001; Cleary et al., 2003). Pre-incubation with 100 nM RS 100329 caused no further rightward movement of the PE response in dorsal arteries, and a pKB of 9.03±0.07 (n=6) was determined from 1 and 10 nM antagonist data. A Schild plot of the total data demonstrated a relationship with a shallow slope, 0.43±0.11, which was skewed by data obtained using 100 nM RS 100329. Excluding these data gave a slope not significantly different from unity, 0.74±0.11, with an apparent pA2 of 9.22 (95% CI 8.82 – 9.84).
The α1B-AR selective antagonist Rec 15/2615 (Testa et al., 1997) caused no shift of the PE CRC at concentrations of 1 to 100 nM (Figure 2). Concentration ratios were between 1.01±0.19 and 1.50±1.18 (n=4) demonstrating that the antagonist was ineffective at the concentrations tested.
The α1D-AR selective antagonist BMY 7378 produced no shift of the PE CRC in the dorsal artery at concentrations of 1 to 100 nM (Figure 2). Concentration ratios lay between 1.02±0.38 and 1.91±0.67 (n=6). BMY 7378 is a well-characterized α1D-AR selective compound that has been shown to be potent at α1D-ARs in various tissues with pA2/pKB's of 8.3 to 9.6 (Kenny et al., 1995; Satoh et al., 1999; Daly et al., 2002; Tanoue et al., 2002; Deighan et al., 2005). Therefore, 100 nM should be expected to produce a significant shift of approximately 50-fold of an α1D-AR-mediated response even at the insensitive end of this spectrum.
α1-AR subtypes in the cavernous artery
As in dorsal arteries, no significant change in sensitivity to PE between the first (pEC50 6.25±0.01) and second (pEC50 6.21±0.02) CRCs was observed in cavernous arteries (P>0.05, n=26). A significant decrease in mean Emax (P<0.001, n=26) of 6% from 1.02±0.01 to 0.96±0.01 g occurred to a uniform extent across all second curves, including those with antagonist pre-incubation. Again, the effect of antagonist incubation on PE CRCs was determined by a comparison of second CRCs in the absence or presence of antagonist.
Phentolamine caused a parallel rightward shift of the PE curve in cavernous arteries with a pKB of 8.33±0.09 (n=8, Table 3). Schild analysis of the data gave a pA2 of 8.55 (95% CI 8.09–9.12) with a slope close to unity of 0.81±0.08. This indicates an effect at α-ARs.
Table 3.
Antagonist profile in the cavernous artery
| Antagonist | n | pKB (s.e.m.) | pA2 (95% CI) | Schild slope (s.e.m.) |
Concentration ratio (s.e.m.) |
|||
|---|---|---|---|---|---|---|---|---|
| 1 nM | 10 nM | 100 nM | 1 μM | |||||
| Phentolamine | 8 | 8.33 (0.09) | 8.55 (8.09–9.12) | 0.81 (0.08) | 1.35 (0.09) | 5.48 (1.42) | 22.21 (6.23) | 170.78 (83.18) |
| Prazosin | 8 | 8.41 (0.15) | 8.42 (7.66–9.85) | 0.99 (0.25) | 0.65 (0.16) | 6.76 (3.11) | 52.77 (19.21) | ND |
| RS 100329 | 7 | 8.80 (0.09) | 8.98 (8.5–9.61) | 0.83 (0.10) | 2.13 (0.33) | 10.22 (3.14) | 45.85 (6.70) | ND |
| Rec 15/2615 | 4 | ND | ND | ND | 1.06 (0.47) | 2.14 (0.72) | 3.00 (2.06) | ND |
| BMY 7378 | 8 | ND | ND | ND | 0.86 (0.33) | 1.83 (0.69) | 1.30 (0.53) | ND |
| Rauwolscine | 4 | ND | ND | ND | 1.13 (0.22) | 1.17 (0.08) | 1.36 (0.45) | ND |
Abbreviations: CI, confidence interval; ND, not determined; PE, phenylphrine; PE CRC, phenylephrine concentration–response curves.
Summary of pKB's, pA2's, Schild slopes and concentration ratios of α 1- and α2-AR antagonists versus PE. Where antagonists did not cause a rightward shift of the PE CRC, pKB, pA2 and Schild plots could not be determined.
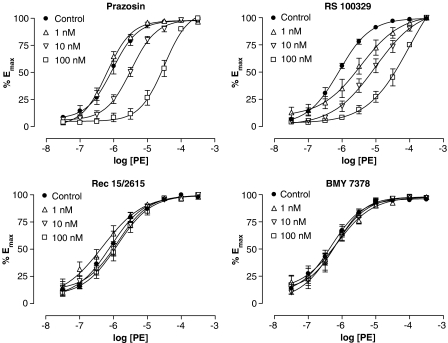
The non-subtype selective α1-AR antagonist prazosin was shown to cause a parallel rightward shift of the PE CRC with a pKB of 8.41±0.15 (n=8, Figure 3). The corresponding Schild plot gave a pA2 of 8.42 (95% CI 7.66–9.85) with a slope that was close to unity, 0.99±0.25, confirming the competitive nature of prazosin in this tissue.
Figure 3.
PE-induced vasoconstriction in the cavernous artery in the absence or presence of 1, 10 or 100 nM prazosin (non-selective antagonist, n=7–8), RS 100329 (α1A-AR antagonist, n=5–6), Rec 15/2615 (α1B-AR antagonist, n=3–4) or BMY 7378 (α1D-AR antagonist, n=6). All PE response data were taken from second CRCs in the absence or presence of antagonist.
The α1A-AR selective antagonist RS 100329 caused a parallel rightward shift of the PE curve with a pKB of 8.80±0.09 (n=7, Figure 3). A Schild plot of the data demonstrated a relationship with a slope close to unity, 0.83±0.10, giving a pA2 of 8.98 (95% CI 8.51–9.61).
As with the dorsal arteries, the α1B-AR selective antagonist Rec 15/2615 (n=4) and the α1D-AR selective antagonist BMY 7378 (n=8) caused no shift of the PE curve in cavernous arteries (Figure 3). Concentration ratios ranged from 1.06±0.47 to 3.00±2.06 for Rec 15/2615 and from 0.86±0.33 to 1.83±0.69 for BMY 7378.
α2-AR responses in the dorsal artery
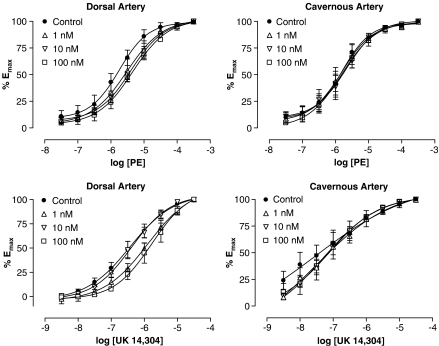
In a follow-up to the observed shallow Schild slopes of PE responses in the dorsal arteries, investigations were made into the involvement of α2-ARs. Rauwolscine produced small rightward shifts of the PE CRC in dorsal arteries but the change in EC50 was not significant (P>0.05, Figure 4). There was no shift in cavernous arteries (Figure 4).
Figure 4.
PE (top) or UK 14 304-induced (bottom) vasoconstriction in the dorsal (n=4–7) and cavernous (n=4) arteries in the absence or presence of 1, 10 or 100 nM rauwolscine (non-selective α2-AR antagonist). All agonist response data were taken from second CRCs in the absence or presence of antagonist.
Both dorsal and cavernous arteries demonstrated vasoconstrictor responses to the α2-AR agonist UK 14 304 that were smaller in magnitude and greater in potency than responses to NA (P<0.001, n=6–7, Table 1). Similar to previous results using NA and PE, incubation with L-NAME (100 μM) significantly increased maximal responses to UK 14 304 in the dorsal (P<0.01, n=6), but not cavernous (P>0.05, n=5) arteries.
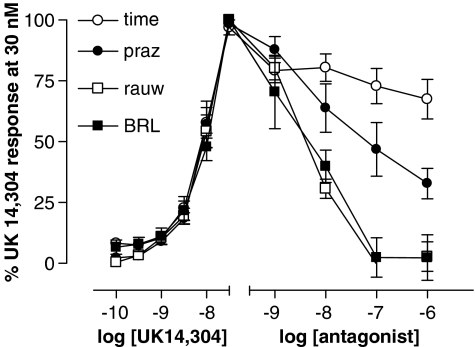
CRCs to UK 14 304 were not significantly shifted at the EC50 by rauwolscine (1 to 100 nM) in either penile artery (P>0.05, Figure 4). However, responses were smaller in the presence of 1 and 100 nM rauwolscine at some concentrations of agonist, particularly in the dorsal artery (0.1–1 μM, P<0.05). This trend was investigated further by assessing the ability of antagonists to decrease UK 14 304-induced vasoconstriction. Following the establishment of UK 14 304-induced tone during a partial CRC (0.1–30 nM), antagonists were added cumulatively in log steps from 1 nM to 1 μM (Figure 5). Rauwolscine significantly reversed UK 14 304-induced tone, causing a maximal reduction in tone of 97±6% (n=5) compared to a 37±5% (n=4) loss of tone with time (P<0.001). In addition, the α2A-AR antagonist BRL 44408 was demonstrated to be equipotent to rauwolscine at causing a maximal, 97±7% (n=8), reduction in UK 14 304-induced tone (P<0.01). The non-selective α1-AR antagonist prazosin demonstrated a non-significant trend in decreasing UK 14 304-induced tone by 67±6% (P>0.05, n=5). These results indicate that an α2-AR-mediated response can be demonstrated in the dorsal artery. A similar analysis applied to cavernous artery showed the same trends but did not reach statistical significance (data not shown).
Figure 5.
UK 14 304-induced (0.1–30 nM) vasoconstriction in the dorsal artery followed by addition of 1–1000 nM rauwolscine (rauw, non-selective α2-AR, n=5), prazosin (praz, non-selective α1-AR, n=5) or BRL 44408 (BRL, α2A-AR selective, n=6) compared to a decrease in tone with the time control (time, n=4).
Discussion
Dorsal and cavernous arteries demonstrated contractile responses to both NA and PE, an α1-AR selective agonist, with sensitivities in the normal range for adrenergically innervated rabbit arteries (Dunn et al., 1991; Naghadeh, 1996). Sensitivity to standard antagonists phentolamine and prazosin was also within the normal range for rabbit arteries (Naghadeh, 1996). However, there are some detailed pharmacological issues that need to be explained. In the dorsal artery, prazosin did not show straightforward characteristics of competitive antagonism of PE responses; affinity estimates decreased with increasing concentrations of prazosin and the corresponding Schild plot had a shallow slope. In addition, pKB estimates for prazosin in both dorsal and cavernous arteries were lower than 9; considered to be an indicator of a low-affinity α1-AR (Flavahan and Vanhoutte, 1986; Ford et al., 1997). However, low-affinity estimates for prazosin versus PE are not unusual in rabbit vasculature: estimated pA2's of 8 in the lateral saphenous vein (Naghadeh, 1996), 8.7 in the mesenteric and carotid arteries, 8.8 in the thoracic aorta (Muramatsu et al., 1990) and 8.7 in the pulmonary artery (Docherty, 1988) have been demonstrated previously. Therefore, quantitatively, the α1-AR/α2-AR agonist and antagonist pharmacology of these arteries is consistent with that of other rabbit arteries.
To characterize α1-AR subtype/s involved in PE responses, three α1-AR-subtype selective antagonists were tested. In the cavernous artery, pharmacological analysis of these antagonists was clearcut. RS 100329, selective for the α1A-AR subtype (Williams et al., 1999), inhibited PE responses potently and competitively. The high potency and Schild compliance of RS 100329 indicated that there is no need to invoke participation from other α1-AR subtypes. In addition, antagonists selective for the α1B-AR subtype (Rec 15/2615) and the α1D-AR subtype (BMY 7378) were ineffective when tested against PE. Rec 15/2615 has been previously shown to be selective for the α1B-AR subtype (Testa et al., 1997) and has been used in two recent in vivo studies of adrenoceptor pharmacology of genital tissues (Sironi et al., 2000; Kim et al., 2002) in rabbits, rats and dogs. Its lack of effect in the cavernous arteries argues against an involvement of the α1B-AR subtype. BMY 7378 is a tried and tested α1D-AR antagonist and was used at concentrations of 1–100 nM, covering its known affinity for the α1D-AR (pKB ∼8.7, Kenny et al., 1995; Satoh et al., 1999; Daly et al., 2002; Tanoue et al., 2002; Deighan et al., 2005) while avoiding its affinity at the α1A-AR (pKB ∼6.6; Lachnit et al., 1997; Zacharia et al., 2004; Deighan et al., 2005). As no effect on the PE response was observed over this range of concentrations, it would be reasonable to conclude that the α1D-AR subtype is not the predominant functional subtype in cavernous penile arteries.
Pharmacological analysis of the dorsal artery was a little more complicated. Similar to the cavernous artery, the potency order of antagonists pointed to α1A-AR, but the low Schild slopes did not confirm competitive antagonism. Both NA, and to a lesser extent, PE (Brown et al., 1988), at high concentrations can act at α2-ARs. Rabbit penile tissue, specifically corpus cavernosum strips (Gupta et al., 1998), has been demonstrated to possess functional post-junctional α2-ARs in addition to the predominant α1-ARs. In the present study, analysis with the α2-AR agonist UK 14 304 and the α2-AR antagonist rauwolscine demonstrated the presence of α2-ARs in the dorsal arteries. Additional support for the hypothesis of a mixed α1- and α2-AR population in the dorsal arteries comes from a greater maximum response to NA, a non-selective adrenoceptor agonist, than to the α1-AR selective agonist PE; suggesting an additive effect of responses mediated by α2-ARs. Determining subtypes of α2-ARs using selective antagonists is more difficult than for α1-ARs. However, one compound, BRL 44408, believed to be a relatively selective antagonist for the α2A-AR (Uhlen et al., 1995) was as potent as rauwolscine against UK 14 304, favouring the presence of α2A-AR in the dorsal artery.
A further complicating factor in the dorsal, but not cavernous, artery was that blockade of nitric oxide synthase by L-NAME potentiated responses to NA, PE and UK 14 304. This indicated either constitutive or agonist-induced release of endothelial nitric oxide in the course of the construction of agonist CRCs; further suggesting that the conditions for equilibrium responses to PE were compromised in this artery. In functional terms, this is an interesting issue as nitrergic and adrenergic influences are considered to be physiologically antagonistic in erectile arteries, and both are areas of therapeutic interest. The present data show that the dorsal artery is more subject to nitrergic influence than is the cavernous artery, even in the absence of specific nitrergic activation. In a comparison with cavernous artery and arteries from female erectile tissue, dorsal artery showed endothelium-mediated vasodilatation that was almost exclusively mediated by nitric oxide whereas the other vessels had a substantial endothelial-derived hyperpolarizing factor (EDHF) component. The interaction between nitric oxide metabolism and adrenergic mechanisms may, therefore, be of particular importance in regulating the nutritional supply to erectile tissue (Morton et al., 2007).
The physiological significance of these findings would be to suggest that the predominant functional receptor in the penile arteries is the α1A-AR. The presence of α1A-ARs in erectile tissues is supported by two studies in cavernous tissue strips (Tong and Cheng, 1997; Peng et al., 1998) and by an in vivo study (Sironi et al., 2000). However, this is the first study to demonstrate the presence of functional α1A-ARs in isolated genital arteries. Increased sympathetic activity and concomitant release of NA during detumescence and flaccidity could lead to activation of α1A-ARs and vasoconstriction of cavernous arteries thereby decreasing arterial inflow. While this study has concentrated on responses to exogenous agonists, the physiological validity of our conclusions could be further investigated using a study of nerve-induced responses. Responses to NA (10 μM) demonstrated that over a 6-min period, vasoconstriction was well maintained in cavernous but not dorsal arteries. In addition, dorsal arteries had a lower sensitivity to NA (P<0.05) and PE (P<0.001) than cavernous arteries. Less complete vasoconstriction of dorsal arteries, because of lower sensitivity to agonists and less well-maintained vasoconstriction, would allow a residual blood flow for nutritional and oxygenation purposes without initiation of an erectile response.
NA responses were significantly potentiated by the combination of uptake blockers, cocaine and corticosterone in both dorsal and cavernous arteries; demonstrating that NA uptake mechanisms were active in these tissues. This would be particularly relevant when in relation to erectile dysfunction brought about by illegal drug abuse with pro-adrenergic compounds, such as cocaine and indirect sympathomimetics related to amphetamine. It would be expected that acute potentiation of transmission by cocaine would lead to erectile dysfunction owing to an increased sensitivity of tissues to NA. However, long-term cocaine use has been reported to cause priapism (Dougherty et al., 2006). Chronic dosage of cocaine may lead to failure of adrenergic transmission either through depletion of NA stores in adrenergic neurons or downregulation of α1A-ARs, each compromising the ability of the erectile tissues to oppose vasodilatation with consequent erection.
Pharmacological antagonism of penile α1A-ARs could be utilized to aid the erectile response in male erectile dysfunction sufferers by reducing vasoconstriction of the penile arteries, in particular, the cavernous artery. Dorsal and cavernous arteries are central to normal sexual function. Maintained vasoconstriction of the cavernous artery during the flaccid state is vital to prevent initiation of an erectile response. Consequently, attenuation of this vasoconstriction is equally important when an erection is required. A more subtle control of the vasoconstrictor/vasodilator state of the dorsal arteries is fundamental in preserving the viability of erectile tissues. Hence, knowledge of the contractile pathways affecting the function of these arteries is vital to allow the selection of potential pharmacological targets for therapies aimed at the treatment of sexual dysfunction. Regarding selectivity of antagonists for facilitating erection, the ideal combination indicated by the current data would be a combination of α1A- and α2-AR antagonism, a selectivity profile for which we know of no compound. However, the non-selective α-blocker phentolamine would achieve both actions, perhaps explaining why it remains the drug of choice for intracavernous injection, despite the availability of more selective compounds. The same argument would apply to yohimbine, which is only marginally selective for α2- over α1-AR and would be likely to block both at an effective dose. Of course, the similarity of the pharmacological profile of the penile arteries to those of all other arteries carries the risk of cardiovascular and other autonomic side effects on systemic administration.
In conclusion, this study demonstrated that the predominant α1-AR subtype involved in vasoconstriction of both dorsal and cavernous penile arteries to exogenous PE was the α1A-AR. No evidence was found for the involvement of α1B- or α1D-AR subtypes; clarifying an area of investigation previously complicated by conflicting evidence for all three α-AR subtypes and improving the basis from which to study pharmacological targets in the treatment of erectile dysfunction. The effects of the selective α1-AR antagonists, taken together with the clear effect of the non-selective (between α1-AR and α2-AR) antagonist phentolamine and the antagonism of UK 14 304 by rauwolscine and BRL 44408, suggest that dorsal arteries have a mixed receptor population composed of α1A and α2 (potentially the α2A-AR subtype)-ARs.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council and Pfizer Global Research and Development. JSM was funded by a CASE Postgraduate Scholarship.
Abbreviations
- α-AR
α-adrenoceptor
- ACh
acetylcholine
- CRC
concentration–response curve
- CI
confidence interval
- NA
noradrenaline
- PE
phenylephrine
Conflict of interest
The authors state no conflict of interest.
References
- Andersson K-E, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Brown CM, McGrath JC, Midgley JM, Muir AG, O'Brien JW, Thonoor CM, et al. Activities of octopamine and synephrine stereoisomers on alpha-adrenoceptors. Br J Pharmacol. 1988;93:417–429. doi: 10.1111/j.1476-5381.1988.tb11449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MTM, Lee SM, Dinsmore WW. Priapism induced by intracavernosal vasoactive intestinal polypeptide and phentolamine mesylate. Sexual Dysfunction. 1998;1:49–51. [Google Scholar]
- Choppin A, Blue DR, Jr, Hegde SS, Gennevois D, McKinnon SA, Mokatrin A, et al. Evaluation of oral Ro70-0004/003, an alpha1A-adrenoceptor antagonist, in the treatment of male erectile dysfunction. Int J Impot Res. 2001;13:157–161. doi: 10.1038/sj.ijir.3900653. [DOI] [PubMed] [Google Scholar]
- Cleary L, Vandeputte C, Docherty JR. Investigation of postjunctional α1- and α2-adrenoceptor subtypes in vas deferens from wild-type and α2A/D-adrenoceptor knockout mice. Br J Pharmacol. 2003;138:1069–1076. doi: 10.1038/sj.bjp.0705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, et al. A knockout approach indicates a minor vasoconstrictor role for vascular α1B-adrenoceptor in mouse. Physiol Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- Dausse J-P, Leriche A., Yablonsky F. Patterns of messenger RNA expression for alpha1 adrenoceptor subtypes in human corpus cavernosum. J Urol. 1998;160:597–600. [PubMed] [Google Scholar]
- Deighan C, Methven L, Naghadeh MM, Wokoma A, Macmillan J, Daly CJ, et al. Insights into the functional roles of α1-adrenoceptor subtypes in mouse carotid arteries using knockout mice. Br J Pharmacol. 2005;144:558–565. doi: 10.1038/sj.bjp.0706089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JR. No evidence for more than one type of alpha 1-adrenoceptor in rabbit pulmonary artery. J Auton Pharmacol. 1988;8:327–332. doi: 10.1111/j.1474-8673.1988.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Dougherty CM, Richard AJ, Carey MJ.Priapism 2006. Kreplick LW, Talavera F, Sinert R, Halamka J and O'Connor RE (eds)eMedicine
- Dunn WR, Daly CJ, McGrath JC, Wilson VG. The effects of nifedipine on α2-adrenoceptor-mediated contractions in several isolated blood vessels from the rabbit. Br J Pharmacol. 1991;103:1493–1499. doi: 10.1111/j.1476-5381.1991.tb09816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan NA, Vanhoutte PM. α1-Adrenoceptor subclassification in vascular smooth muscle. Trends Pharmacol Sci. 1986;7:347–349. [Google Scholar]
- Ford APDW, Daniels DV, Chang C-H, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Chess-Williams R, Uchiyama T. Alpha 1B adrenoceptor subtype mediating the phenylephrine-induced contractile response in rabbit corpus cavernosum penis. Jpn J Pharmacol. 1996;71:325–331. doi: 10.1254/jjp.71.325. [DOI] [PubMed] [Google Scholar]
- Goepel M, Krege S, Price DT, Michelotti GA, Schwinn DA, Michel MC. Characterisation of alpha-adrenoceptor subtypes in the corpus cavernosum of patients undergoing sex change surgery. J Urol. 1999;162:1793. [PubMed] [Google Scholar]
- Gupta S, Moreland RB, Yang S. The expression of functional postsynaptic α2-adrenoceptors in the corpus cavernosum smooth muscle. Br J Pharmacol. 1998;123:1237–1245. doi: 10.1038/sj.bjp.0701739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny BA, Chalmers DH, Philpott PC, Naylor AM. Characterisation of an α1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NN, Min K, Huang Y-H, Goldstein I, Traish A. Biochemical and functional characterisation of alpha-adrenergic receptors in the rabbit vagina. Life Sci. 2002;71:2909–2920. doi: 10.1016/s0024-3205(02)02162-8. [DOI] [PubMed] [Google Scholar]
- Lachnit WG, Tran AM, Clarke DE, Ford APDW. Pharmacological characterisation of an α1A-adrenoceptor mediating contractile responses to noradrenaline in isolated caudal artery of rat. Br J Pharmacol. 1997;120:819–826. doi: 10.1038/sj.bjp.0700983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JS, Jackson VM, Daly CJ, McGrath JC.Endothelium-dependent relaxation in rabbit genital resistance arteries is predominantly mediated by EDHF in females and nitric oxide in males J Urol 2007. in press [DOI] [PubMed]
- Muramatsu I, Ohmura T, Kigoshi S, Hashimoto S, Oshita M. Pharmacological subclassification of α1-adrenoceptors in vascular smooth muscle. Br J Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghadeh MM.Vasomodulator mechanisms in rat carotid artery and in vessels from an experimental model of heart failure 1996. PhD Thesis, University of Glasgow
- Peng M, Zhang Y, Xue Z, Han Q. Alpha 1-adrenoceptor subtypes in rabbit penile corpus cavernosum. Zhonghua Wai Ke Za Zhi. 1998;36:497–499. [PubMed] [Google Scholar]
- Recio P, Lopez PG, Fernandez JL, Garcia-Sacristan A. Pharmacological characterisation of adrenoceptors in horse corpus cavernosum penis. J Auton Pharmacol. 1997;17:191–198. doi: 10.1046/j.1365-2680.1997.00457.x. [DOI] [PubMed] [Google Scholar]
- Saenz De Tejada I, Kim N, Lagan I, Krane RJ, Goldstein I. Regulation of adrenergic activity in penile corpus cavernosum. J Urol. 1989;142:1117–1121. doi: 10.1016/s0022-5347(17)39009-2. [DOI] [PubMed] [Google Scholar]
- Sato M, Kawatani M. Effects of noradrenaline on cytosolic concentrations of Ca2+ in cultured corpus cavernosum smooth muscle cells of the rabbit. Neurosci Lett. 2002;324:89–92. doi: 10.1016/s0304-3940(01)02579-4. [DOI] [PubMed] [Google Scholar]
- Satoh M, Enomoto K, Takayanagi I, Koike K. Analysis of α1-adrenoceptor subtypes in rabbit aorta and arteries: regional difference and co-existence. Eur J Pharmacol. 1999;374:229–240. doi: 10.1016/s0014-2999(99)00340-4. [DOI] [PubMed] [Google Scholar]
- Sironi G, Columbo D, Poggesi E, Leonardi A, Testa R, Rampin O, et al. Effects of intracavernous administration of selective antagonists of α1-adrenoceptor subtypes on erection in anaesthetised rats and dogs. J Pharmacol Exp Ther. 2000;292:974–981. [PubMed] [Google Scholar]
- Tamura M, Kagawa S, Kimura K, Kawanishi Y, Tsuruo Y, Ishimura K. Coexistence of nitric oxide synthase, tyrosine hydroxylase and vasoactive intestinal polypeptide in human penile tissue – a triple histochemical and immunohistochemical study. J Urol. 1995;153:530–534. doi: 10.1097/00005392-199502000-00077. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Angelico P, Poggesi E, Taddei C, Sironi G, et al. Pharmacological characterisation of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, Part II. J Pharmacol Exp Ther. 1997;281:1284–1293. [PubMed] [Google Scholar]
- Tong YC, Cheng JT. Subtyping of α1-adrenoceptors responsible for the contractile response in the rat corpus cavernosum. Neurosci Lett. 1997;228:159–162. doi: 10.1016/s0304-3940(97)00388-1. [DOI] [PubMed] [Google Scholar]
- Traish AM, Netsuwan N, Daley J, Padma-Nathan H, Goldstein I, Saenz De Tejada I. A heterogeneous population of alpha1 adrenergic receptors mediates contraction of human corpus cavernosum smooth muscle to norepinephrine. J Urol. 1995;153:222–227. doi: 10.1097/00005392-199501000-00081. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Mucenience R, Rangel N, Tiger G, Wikberg JE. Comparison of the binding activities of some drugs on alpha 2A, alpha 2B and alpha 2C-adrenoceptors and non-adrenergic imidazoline sites in the guinea pig. Pharmacol Toxicol. 1995;76:353–364. doi: 10.1111/j.1600-0773.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Jardin A, Benoit G, Giuliano F. Expression of alpha 1 adrenoceptor subtypes in rat corpus cavernosum. Int J Impot Res. 1998;10:187–194. doi: 10.1038/sj.ijir.3900348. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, Gever JR, et al. In vitro α1-adrenoceptor pharmacology of Ro 70-0004 and RS-100329, novel α1A-adrenoceptor selective antagonists. Br J Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharia J, Hillier C, MacDonald A. Pharmacological characterisation of α1-adrenoceptors in mouse isolated femoral small arteries. Eur J Pharmacol. 2004;503:155–163. doi: 10.1016/j.ejphar.2004.09.046. [DOI] [PubMed] [Google Scholar]