Abstract
Background and purpose:
The present study investigated mechanisms underlying impaired endothelium-dependent vasodilatation elicited by elevating the intraluminal pressure in rat mesenteric small arteries.
Experimental approach:
Arterial segments (internal diameter 316±2 μm, n=86) were mounted in a pressure myograph. The effect of elevating pressure from 50 to 120 mmHg for 1 h before resetting it to 50 mmHg was studied on endothelium-dependent vasodilatation.
Key results:
In arteries constricted with U46619 in the presence of indomethacin, shear stress generated by flow, evoked vasodilatation that was abolished by an inhibitor of nitric oxide (NO) synthase, asymmetric dimethylarginine (1 mM), whereas acetylcholine-induced vasodilatation was unchanged. After elevation of intraluminal pressure for 1 h and then resetting it to 50 mmHg, vasodilatation induced by shear stress and the NO donor, S-nitrosopenicillamine was inhibited, while vasodilatation induced by a guanylyl cyclase activator, BAY 412272, and acetylcholine was unaltered. Superoxide levels sensitive to polyethylene glycol superoxide dismutase were increased in segments exposed to elevated pressure. A superoxide scavenger, tempol (300 μM), a general endothelin receptor antagonist, SB 217242 and the selective ETA receptor antagonist, BQ 123 preserved shear stress-evoked vasodilatation.
Conclusions and Implications:
The present study shows that transient exposure to an elevated intraluminal pressure selectively inhibits flow-evoked NO-mediated vasodilatation, probably through activation of endothelin receptors and increased formation of superoxide. In contrast, elevation of pressure did not affect the acetylcholine-evoked endothelium-derived hyperpolarizing factor type vasodilatation in mesenteric small arteries.
Keywords: endothelial dysfunction, flow-induced vasodilatation, nitric oxide, tempol, superoxide
Introduction
Endothelium-dependent vasodilatation induced by increased blood flow and receptor-specific agonists, such as acetylcholine and bradykinin, is reduced in various forms of human hypertension (Panza et al., 1990; Taddei et al., 1998) and in hypertensive animals (Koller and Huang 1994; Hoshino et al., 1998; Schnackenberg et al., 1998; Heitzer et al., 1999) and endothelial dysfunction in hypertensive patients appears to be independent of the aetiology (Rizzoni et al., 1996). Short-term increases in intraluminal pressure in vivo also impairs endothelial function (Wei et al., 1985; Debruyn et al., 1994). Moreover, increased transmural pressure inhibits the release of endothelium-derived relaxing factor (EDRF) in bioassays of canine carotid arteries (Rubanyi, 1988). In addition, in isolated skeletal arterioles (Huang et al., 1998) and human subcutaneous arteries (Paniagua et al., 2000), a transient increase in intraluminal pressure has been shown to impair the endothelium-dependent vasodilatation induced by increased blood flow and acetylcholine. These findings suggest that elevated intravascular pressure by itself is deleterious to endothelial function.
Several mechanisms may be involved in impaired endothelial function following elevated intravascular pressure. Elevated intravascular pressure may downregulate the smooth muscle guanylyl cyclase pathway and hence may reduce sensitivity for endothelium-derived nitric oxide (NO), as reduced sensitivity for NO donors and/or reduced smooth muscle guanylyl cyclase expression has been observed in arteries from hypertensive animals (Preik et al., 1996; Bauersachs et al., 1998; Kloss et al., 2000). Superoxide can also inactivate EDRF (Gryglewski et al., 1986). Scavenging of superoxide or inhibition of enzymatic sources of superoxide within the vascular wall has resulted in an improvement in the vasodilatator capacity of both patients with hypertension (Taddei et al., 1998) and hypertensive rats (Dobrian et al., 2001; Hamilton et al., 2002; Ulker et al., 2003), and this has been attributed to an increased bioavailability of NO (Schnackenberg et al., 1998). However, the impaired endothelium-derived hyperpolarizing factor (EDHF) type vasodilatation observed in arteries from hypertensive animals (Fujii et al., 1992; Bussemaker et al., 2003) has also been shown to be restored by scavenging of superoxide in hypertensive rats (Adeagbo et al., 2003). In rat isolated skeletal arterioles, elevated intravascular pressure through activation of protein kinase C leads to increased superoxide formation and impaired flow-evoked vasodilatation (Huang et al., 1998; Ungvari et al., 2004), suggesting that superoxide formation is due to high pressure per se. In addition to the superoxide, other contractile factor(s) may counteract endothelium-dependent vasodilatation. For example, in cat cerebral arteries, elevated pressure causes release of a transferable endothelial constrictor factor (Harder et al., 1989), and formation of contractile prostanoids counteracts both agonist and flow-evoked vasodilatation in arteries from hypertensive animals (see Vanhoutte et al., 2005). Elevated intravascular pressure has also been shown to upregulate endothelin-1 expression in the vascular wall (Hasdai et al., 1997; Lauth et al., 2000) and, in transgenic mice, overexpression of endothelin-1 was found to be associated with blunted endothelium-dependent vasorelaxation (Amiri et al., 2004).
In rat mesenteric small arteries, NO only has a very minor role in acetylcholine-induced relaxation, which is, instead, dependent on endothelium-derived hyperpolarizing factors (EDHF) (Edwards et al., 1998) or myoendothelial gap junction communication (Dora and Garland 2001; Mather et al., 2005). In contrast, we have recently found that in arteries constricted with a thromboxane analogue, U46619, flow-induced vasodilatation in the same preparation is mainly NO dependent (Thorsgaard et al., 2003). Thus, the rat mesenteric small arteries can be used to distinguish between the mechanisms involved in EDHF-type and NO-dependent vasodilatation. However, the extent to which acetylcholine- and flow-induced vasodilatations are impaired by the superoxide production, associated with elevated pressure, has not been elucidated. Therefore, in the present study, we investigated whether short periods of elevated intraluminal pressure are able to impair NO- and acetylcholine-induced vasodilatation by inducing superoxide formation.
Methods
Perfusion myography
Male Wistar rats (12 weeks) were killed with a blow to the head followed by decapitation, and the mesenteric vascular bed was isolated and placed in cold physiological salt solution (PSS, 4°C) with the following composition (mM): CaCl2 1.6, NaCl 119, KCl 4.7, glucose 5.5, MgSO4H2O 1.17, NaHCO3 25, KH2PO4 1.18 and ethylenediaminetetraacetic acid (EDTA) 0.026. The solution was constantly bubbled with 5% CO2 in air to maintain pH at 7.4 and to yield pO2 levels of 140±4 mm Hg (three independent measurements). Third-order mesenteric small arteries were dissected and cannulated on two micropipettes in the flow direction in a microvascular myograph (Danish Myotechnology, Aarhus, Denmark), equipped for measurements of pressure and generation of flow as previously described (Thorsgaard et al., 2003).
The middle of the vessel segment was viewed through an inverted microscope (magnification × 100), and its inner and outer diameters were measured by a video microscope technique. The signal from the CCD video camera module attached to the inverted microscope was fed to a frame grabber and then to a dimension-analysing programme (VesselView, Danish Myotechnology, Aarhus, Denmark), allowing continuous sampling of internal diameter and pressure, at 10 Hz. In addition, these signals were recorded continuously by a chart recorder (Servogor 400, Omni Instruments, Dundee, Scotland). Intraluminal hydrostatic pressures of both inlet and outlet to the vessel segment were measured by pressure transducers connected to the perfusion line. The protocol established for evaluation of impact of transient pressure increase on endothelium-dependent vasodilatation in human subcutaneous vessels was followed with some modifications (Paniagua et al., 2000). Thus, the mesenteric arteries were pressurized to 50 mm Hg. U46619 (10–40 nM) constricted the vessel to 60% of resting diameter and was added both intra- and extraluminally, as we have previously observed that intraluminal washout also contributes to the vasodilatation obtained by increasing flow (Thorsgaard et al., 2003). When constriction was stable, vasodilatation was induced by flow and acetylcholine. Flow was generated by suction with a peristaltic pump and flow adjusted to be is either 25–50 μl or 100–200 μl min−1, corresponding to shear stress levels, 8 and 24 dyne cm−2 in the mesenteric small arteries. The flow generated with the pump was controlled with a ball flow metre (FL-310 Omega Engineering, Atlanta, GA, USA). Shear stress was calculated from the following equation: τ=4 ηQ/πr3, where r is the internal vessel diameter, Q the average flow velocity, and η the viscosity. For each flow, responses were obtained for 300 s and in between the two responses to flow; the pump was stopped, allowing restoration of the preconstriction state. Acetylcholine was added in volumes of 10 μl to the preheated bath solution and the vessel segment exposed to the bath concentration for 300 s, before the concentration was increased by cumulative addition. Care was taken to provide stable bubbling and hence mixing of the drug in the bath.
An initial response was obtained to flow and acetylcholine, and U44619 was washed out by exchanging the bath solution 9–10 times. The vessel segment was allowed to rest for 30 min before it was either treated with vehicle, an NO synthase inhibitor, asymmetric dimethylarginine (ADMA, 1 mM), or exposed to an intraluminal pressure of 120 mm Hg for 60 min, and a second response to flow and acetylcholine was obtained at an intraluminal pressure of 50 mm Hg in U46619-contricted segments. In a second series of experiments, an initial response was obtained to flow and acetylcholine, U44619 was washed out, and the vessels were exposed to 120 mm Hg or kept at 50 mm Hg in the presence of a cell-permeable superoxide scavenger, tempol (300 μM), for 1 h. Then pressure was set to 50 mm Hg, and responses for flow and acetylcholine were repeated in U46619-contricted segments with vehicle or blockers present. A third series of experiments were performed to address the role of endothelin. Thus, vessels were exposed to 120 mm Hg for 60 min, pressure was lowered to 50 mm Hg, and the effects of the nonspecific endothelin receptor antagonist, SB 217242 (10 μM), and the ETA receptor antagonist, BQ 123 (3 μM), were evaluated with regard to responses to flow, acetylcholine and to the concentration–response curves for endothelin.
To determine whether the smooth muscle guanylyl cyclase pathway was altered by elevated pressure, separate experiments were performed, in which vasodilatation to the NO donor, S-nitroso-N-acetyl-D, L-penicillamine (SNAP) and a guanylyl cyclase activator, BAY 412272, was evaluated by exposing the vessel segment to increasing drug concentrations at 300 s intervals.
Evaluation of vascular superoxide
Dihydroethidium was used to localize superoxide production in the vascular wall. Dihydroethidium is freely permeable to cells and, in the presence of superoxide, dihydroethidium is oxidized to ethidium, which is trapped inside the cell by intercalation with DNA. Ethidium was excited at 488 nm with an emission spectrum of 610 nm. Isolated arteries were mounted in a pressure myograph as described above. The bath was heated, indomethacin added, and intraluminal pressure was raised to 50 or 120 mm Hg for 1 h. After 1 h, the vessel was immediately frozen in Tissue Tek embedding media with liquid nitrogen, and stored at −80°C. On the day of staining, frozen segments were sectioned (30 μm thick) and placed on glass slides. Each tissue section was incubated for 30 min in a light-protected humidified chamber with (1) phosphate-buffered saline (PBS) or, (2) dihydroethidium (2 μM) in PBS or, (3) polyethyleneglycol-superoxide dismutase (PEG-SOD, 100 U ml−1) in PBS or (4) dihydroethidium and PEG–SOD in PBS. Fluorescence was evaluated with a confocal microscope (ODYSSEY XL, Noran) equipped with a water immersion objective (× 60, numerical aperture 1.2, Nikon). Fluorescence images were quantified by using MATLAB extended with image-processing toolbox (www.mathworks.com). Laser settings were identical for all images obtained.
Drugs
The following drugs were purchased from Sigma-Aldrich (Brondby, Denmark): acetylcholine HCl, indomethacin, ADMA, BQ 123 (cyc(dTrp-dAsp-Pro-dVal-Leu), endothelin, U-46619 (9,11-di-deoxy-11α, 9α-epoxymethano prostaglandin F2α), 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (tempol), SNAP, dihydroethidium, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA). SB 217242 (enrasentan) was a gift from GlaxoSmithKline Pharma (Uxbridge, Middlesex, UK), BAY 412272 (5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine), a gift from Bayer Ltd, Wuppertal, Germany. U46619 was dissolved in 50% ethanol and further diluted in distilled water. Indomethacin was dissolved in 0.8% acetic acid and further dissolved in distilled water. Stock solutions of dihydroethidium, SNAP and BAY 412272 were dissolved in dimethylsulphoxide and further dilutions were made in distilled water. The other drugs were dissolved in distilled water. The final concentrations of solvents added to the organ bath did not influence tone in the preparations.
Calculations and statistical evaluation
All responses are expressed as mean±s.e.m., where n is the number of rats. Vasodilatation is expressed as changes in internal diameter, or area under the vasodilatation curve (AUC) was calculated and expressed as changes in internal diameter (μm) with time (s) (GraphPad Prism, San Diego, CA, USA) for each response to flow, acetylcholine and SNAP (Thorsgaard et al., 2003). Differences between means were compared with two-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
Effect of NO synthase inhibition and transient pressure elevation on responses to flow and acetylcholine
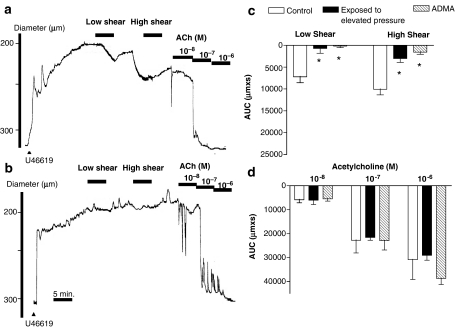
The arteries pressurized to 50 mm Hg expressed no basal tone and had an average diameter of 316±2 μm (n=86). U46619 constricted the arteries to 179±2 μm (n=86). Increasing flow resulted in low (7.7±0.3 dyne cm−2) and high (24.5±0.6 dyne cm−2) shear stress-evoked vasodilatation, dependent on the magnitude of shear stress applied (P<0.05, n=6, Figure 1a). Increasing pressure to 120 mm Hg for 1 h and then resetting pressure to 50 mm Hg markedly reduced vasodilatation to both low and high shear stresses (Figure 1b and c). Incubation with ADMA (1 mM) abolished vasodilatation evoked by low- and high-shear stresses (Figure 1c, Table 1). Acetylcholine-evoked vasodilatation was concentration dependent, and remained unchanged in the presence of ADMA and after an intraluminal pressure elevation to 120 mm Hg for 1 h (Figure 1a, b and d). Incubation with ADMA did not increase U46619 constriction compared to control preparations (Table 1).
Figure 1.
Diameter changes in (a) parallel time control where pressure was kept at 50 mm Hg and (b) preparations exposed to 120 mm Hg for 1 h and pressure reset to 50 mm Hg, before they were contracted with U46619 and dilated with low and high flows corresponding to a shear stress of 7 and 25 dynes cm−2, respectively, followed by addition of increasing concentrations of acetylcholine at 300 s intervals. Average changes in area under curve (AUC) in response to (c) flow and (d) acetylcholine in preparations kept at 50 mm Hg (control) in the presence of vehicle or ADMA (1 mM) and preparations where pressure was elevated to 120 mm Hg and then reset to 50 mm Hg (exposed to high pressure), before responses were measured in U46619-contracted preparations. The experiments were performed in the presence of indomethacin (3 μM). Differences in responses were evaluated by two-way analysis of variance: *P<0.05 vs time control. Vasodilatation responses were dependent on the magnitude of shear stress and acetylcholine concentration applied.
Table 1.
Absolute changes in vessel diameter (ΔD, μm) induced by U46619 (0.1 μM), high-shear stress (24.5±0.6 dyne cm−2), and acetylcholine (0.01 μM) in vessels kept at an intraluminal pressure of 50 mm Hg in the absence (control) or in the presence of ADMA (1 mM), or exposed to elevated pressure of 120 mm Hg for 1 h and then returned to 50 mm Hg, before responses were obtained in the absence (vehicle) and in the presence of tempol (300 μM), SB 217242 (10 μM), and BQ 123 (3 μM)
| n | U46619 (ΔD, μm) | Flow (ΔD, μm) | Acetylcholine (ΔD, μm) | |
|---|---|---|---|---|
| Kept at 50 mm Hg | ||||
| Control | 6 | 137±11 | 46±4 | 43±15 |
| ADMA | 5 | 139±14 | 10±3* | 42±11 |
| Exposed to elevated pressure (120 mm Hg for 1 h and then returned to 50 mm Hg) | ||||
| Vehicle | 10 | 123±4 | 17±3* | 45±10 |
| Tempol | 5 | 145±9 | 57±14† | 57±14 |
| SB 217242 | 7 | 116±5 | 48±8† | 26±10 |
| BQ 123 | 6 | 118±3 | 60±17† | 31±9 |
Abbreviation: ADMA, asymmetric dimethylarginine.
Values are means±s.e.m., where n is the number of arteries (one per animal) examined. Responses to flow and acetylcholine were measured 300 s after initiating the stimulation.
P<0.05, vs control.
P<0.05, vs vehicle-treated preparation.
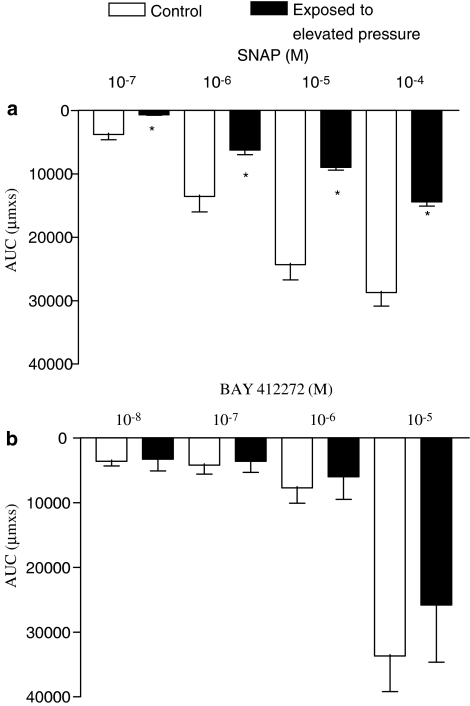
Vasodilatation evoked by the NO donor SNAP was concentration-dependent and was attenuated by an intraluminal pressure elevation to 120 mm Hg (Figure 2a). In contrast, an allosteric activator of soluble guanylyl cyclase, BAY 412272, induced vasodilatation, which was unaltered in preparations exposed to elevated pressure treatment (Figure 2b).
Figure 2.
Average vasodilatation measured as area under curve (AUC) in parallel control and pressure-treated preparations contracted with U46619 and dilated with (a) SNAP and (b) BAY 412272. The experiments were performed in the presence of indomethacin (3 μM). Results are expressed as mean and vertical lines show s.e.m. (n=6–8). Differences in responses were evaluated by two-way analysis of variance: *P<0.05 vs time control; vasodilatation responses were dependent on SNAP and BAY 412272 concentration applied.
Effect of tempol after transient pressure elevation
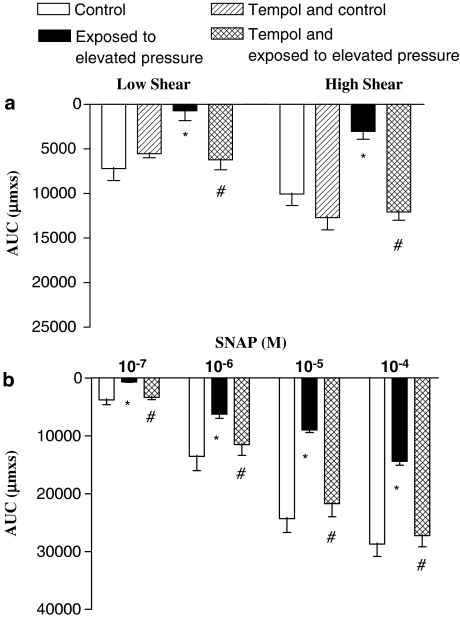
In control situations, where intraluminal pressure was kept at 50 mm Hg, tempol (300 μM) did not change shear stress-evoked vasodilatation, but in preparations exposed to the high-pressure treatment, vasodilatation was restored in the presence of tempol (Figure 3a, Table 1). Tempol also restored SNAP-induced vasodilatation in arteries exposed to the high-pressure treatment (Figure 3b). The magnitude of constriction induced by U46619 was not changed in the presence of tempol (Table 1). Tempol did not change acetylcholine-induced vasodilatation in rat mesenteric arteries (Table 1).
Figure 3.
Average vasodilatation measured as area under curve (AUC) in control and pressure-treated preparations contracted with U46619 and dilated with (a) flow, and (b) the NO-donor, SNAP in the absence and the presence of the superoxide scavenger, tempol (300 μM). The experiments were performed in the presence of indomethacin (3 μM). Results are expressed as mean and vertical lines which show s.e.m. (n=6–8). *P<0.05 vs time control. #P<0.05 vs elevated pressure treatment.
Assessment of vascular superoxide
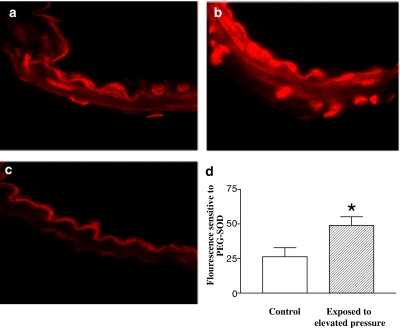
Dihydroethidium staining of sections from mesenteric small arteries exposed to a pressure of 50 mm Hg was mainly localized to the endothelium and adventitia, whereas it was more sparsely apparent in the media (Figure 4a). In arteries exposed to an elevated pressure treatment of 120 mm Hg, dihydroethidium staining was markedly increased in all the layers (Figure 4b). Incubation with (PEG–SOD) together with dihydroethidium reduced the fluorescence signals (Figure 4c), and only weak autofluorescence was observed in the inner elastic membrane of the arteries. Quantification of dihydroethidium fluorescence sensitive to PEG–SOD confirmed that superoxide formation was increased in preparations exposed to an elevated pressure treatment (Figure 4d).
Figure 4.
Dihydroethidium staining of mesenteric arteries is increased in vessels exposed to high pressure. Representative fluorescent photomicrographs of sections of rat mesenteric arteries exposed to an intraluminal pressure of 50 mm Hg (control) (a) and elevated pressure treatment to 120 mm Hg for 1 h (b). Addition of PEG–SOD lowered fluorescence signals (c). (d) Quantification of fluorescence levels sensitive to PEG–SOD show they are increased in vessels exposed to elevated pressure compared to low pressure. Results are mean and vertical lines show s.e.m. *P<0.05 vs time control.
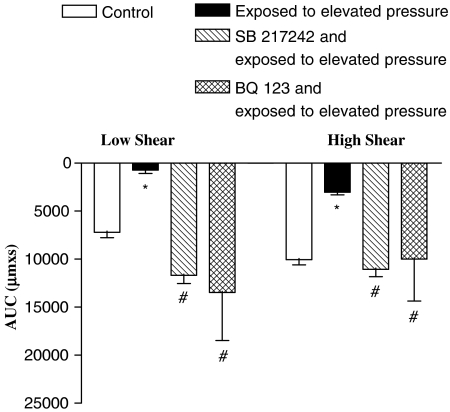
Effect of endothelin receptor antagonists on flow-evoked vasodilatation after high-pressure treatment
To determine whether formation of endothelin impairs shear stress-evoked vasodilatation, endothelin receptor antagonists were applied. Both the non-specific endothelin receptor antagonist, SB 217242 (10 μM), and the endothelin ETA receptor antagonist, BQ 123 (3 μM), prevented impairment of flow-dependent vasodilatation in arteries exposed to the high-pressure treatment (Figure 5). Moreover, SB 217242 and BQ 123 caused rightward shifts in the concentration–response curves for endothelin (data not shown). The vasoconstriction induced by U46619 was not changed in the presence of the endothelin receptor antagonists (Table 1).
Figure 5.
Incubation with either a general endothelin receptor antagonist, SB 217242, or ETA-selective receptor antagonist, BQ 123, prevented the impaired flow-dependent vasodilatation in arteries exposed to elevated intraluminal pressure. The experiments were performed in the presence of indomethacin. *P<0.05 vs time control. #P<0.05 vs elevated pressure treatment.
Discussion
The present study shows that in rat mesenteric small arteries an elevated pressure treatment for 1 h selectively inhibits flow-evoked NO-mediated vasodilatation, probably through activation of endothelin receptors and increased formation of superoxide. In contrast, guanylyl cyclase-dependent vasodilatation elicited by BAY 412272 and acetylcholine, mainly EDHF-type vasodilatation, was not affected by an elevation of the intraluminal pressure.
In previous studies, we have found that both flow and acetylcholine induce endothelium-dependent vasodilatation in rat mesenteric arteries (Thorsgaard et al., 2003). In the present study an inhibitor of NO synthase, ADMA, caused pronounced inhibition of flow-evoked vasodilatation, whereas acetylcholine-evoked vasodilatation remained unchanged even after the addition of a high concentration (1 mM) of ADMA. In previous studies, only the combination of ADMA and the calcium-activated K+ channel blockers, apamin and charybdotoxin, inhibited acetylcholine-induced vasodilatation (Thorsgaard et al., 2003). Therefore, these findings suggest that in U46619-constricted mesenteric small arteries, flow evokes NO-mediated vasodilatation, whereas acetylcholine mainly causes EDHF-dependent vasodilatation.
An elevation of the intraluminal pressure from 50 to 120 mm Hg for 1 h resulted in impaired flow-evoked NO-mediated vasodilatation in the present study. Our findings agree with those obtained in rat skeletal muscle arteries, where an impaired flow-evoked vasodilatation was observed in arteries exposed to a pressure elevation from 80 to 160 mm Hg for 30 min (Huang et al., 1998; Ungvari et al., 2003). Moreover, in the present study, the vasodilator response to the NO donor SNAP was inhibited in arteries exposed to elevated pressure treatment, whereas vasodilatation induced by a direct activator of guanylyl cyclase, BAY 412272, was unaltered. These findings suggest that reduced NO bioavailability rather than reduced guanylyl cyclase activation explains the inhibition of flow-evoked vasodilatation in rat mesenteric arteries exposed to elevated pressure. This is supported by the observations that a superoxide scavenger, tempol, prevented the inhibition of flow-evoked, NO-mediated vasodilatation, suggesting elevated pressure treatment leads to increased superoxide formation and decreased NO bioavailability. Our findings are in line with those from previous studies in models of hypertension in vivo (Fukui et al., 1997; Zalba et al., 2000) and in rat isolated skeletal arterioles (Ungvari et al., 2003), where elevated pressure was also shown to elicit arterial superoxide production by activation of NADPH oxidase resulting in impaired endothelial function. Furthermore, we observed that hydroethidium fluorescence signals sensitive to PEG–SOD were increased in arteries exposed to an elevated intraluminal pressure, indicating the presence of superoxide and providing an explanation for the attenuation of flow-evoked NO-mediated and NO donor-evoked vasodilatation in rat mesenteric small arteries.
Studies on cultured endothelial and vascular smooth muscle cells have demonstrated that both cell types are able to produce superoxide on exposure to cyclic strain (Hishikawa et al., 1997). Moreover, it has been suggested that exposure of isolated arteries to elevated pressure induces activation of NADPH oxidase and superoxide formation directly by causing an increase in smooth muscle calcium and activation of a protein kinase C-dependent mechanism in both the smooth muscle and endothelial cell layer (Ungvari et al., 2003, 2004). Although it is appreciated from the staining for superoxide (Figure 4b) that the increase in ethidium fluorescence appears most pronounced in the adventitial and endothelial cell layer of the mesenteric small arteries, superoxide was increased in all three layers of the arterial wall in the present study. These findings support the hypothesis that mechanosensitive pathways leading to formation of superoxide are present in all major cell types of the vascular wall of intact arteries.
Elevation of intraluminal pressure for 1 h did not affect acetylcholine-evoked EDHF-type vasodilatation in mesenteric small arteries in the present study. This is in contrast to the impaired agonist-induced vasodilatation observed in human subcutaneous arteries (Paniagua et al., 2000) and rat gracilis muscle arterioles exposed to elevated pressure (Huang et al., 1998). Discrepancies between these effects of elevated pressure on agonist-induced EDHF-type vasodilatation can probably be ascribed to the involvement of different mechanisms; epoxyeicosatrienoic acids are thought to mediate vasodilatation in rat skeletal arterioles and human subcutaneous arteries (Huang et al., 1998; Buus et al., 2000), whereas K+ and myoendothelial gap junctions have been suggested to mediate acetylcholine-evoked relaxation in rat mesenteric arteries (Edwards et al., 1998; Dora and Garland 2001; Mather et al., 2005). Therefore, the present study does not exclude the possibility that EDHF-type vasodilatation is impaired by elevated pressure in other vascular beds, but suggests exposure to elevated pressure selectively inhibits NO-mediated vasodilatation without affecting EDHF-type vasodilatation in mesenteric small arteries.
Endothelin receptors are coupled to protein kinase C, and elevated intraluminal pressure has been shown to upregulate endothelin-1 in human coronary arteries (Hasdai et al., 1997) and rabbit carotid arteries (Lauth et al., 2000). Also, recently overexpression of endothelin-1 in transgenic mice was shown to be associated with blunted endothelium-dependent vasorelaxation (Amiri et al., 2004). Moreover, endothelin-1 was proposed to play a role in deoxycorticosterone acetate-salt hypertensive and stroke-prone spontaneously hypertensive rat strains (Li et al., 2003) and although superoxide production does not account for chronic endothelin-induced hypertension, recent data provided evidence that chronic endothelin infusion increases vascular superoxide production (Elmarakby et al., 2003). Therefore, we investigated the effect of endothelin receptor antagonists and found that, a general ET antagonist, SB 217242, and an ETA-selective receptor antagonist, BQ 123, prevented the impaired flow-dependent vasodilatation in arteries exposed to elevated intraluminal pressure. These observations suggest that elevated pressure exposure impairs flow-evoked vasodilatation in rat small arteries by activating smooth muscle endothelin ETA receptors and increasing the formation of superoxide.
There is no spontaneous tone in arterial segments that are second- to third-order branches of the superior mesenteric artery (Thorsgaard et al., 2003). Therefore, a thromboxane analogue was used to raise tone, and this could influence the results obtained, as the processes leading to vasoconstriction evoked by the contractile drugs seem to play a role in the relaxations mediated by endothelium-derived relaxing factors (Plane and Garland 1996; Tomioka et al., 1999). Thus, the profile obtained in response to flow in the present study is different from the profile of the response obtained to flow in mesenteric arteries preactivated with phenylephrine (Liu et al., 2006). It is possible that the drug used to increase the tone of the preparation influences the release and/or smooth muscle effects of endothelium-derived factors. Moreover, U46619 has been found to cause marked inhibition of acetylcholine relaxation in mesenteric arteries in the presence of NOS blockade (Plane and Garland 1996), probably by inhibition of the small-conductance calcium-activated K+ channel, which is involved in EDHF-type relaxation (Crane and Garland 2004). However, in the present study only two to three contractions to U46619 were performed, and a comparison of the first and second concentration–response curves showed that flow- and acetylcholine-induced vasodilatations were unaltered.
In summary the present study shows that exposing isolated vessels to transient elevated pressure selectively inhibits flow-evoked NO-mediated vasodilatation, probably through activation of endothelin receptors and increased formation of superoxide. In contrast, acetylcholine EDHF-type vasodilatation is not affected by an elevation of intraluminal pressure in rat mesenteric small arteries.
Acknowledgments
We thank Helle Zibrandtsen and Kristina Litvin for technical assistance. This study was supported by grants from the Danish Heart Association (04-10-B116-A304-22197), Danish Research Council and Aarhus University Research Foundation.
Abbreviations
- ADMA
asymmetric dimethylarginine
- EDHF
endothelium-derived hyperpolarizing factor
- NO
nitric oxide
- PEG–SOD
polyethylene glycol superoxide dismutase
- SB 217242
enrasentan
- SNAP
S-nitroso-N-acetylpenicillamine
Conflict of interest
The authors state no conflict of interest.
References
- Adeagbo AS, Joshua IG, Falkner C, Matheson PJ. Tempol, an antioxidant, restores endothelium-derived hyperpolarizing factor-mediated vasodilation during hypertension. Eur J Pharmacol. 2003;481:91–100. doi: 10.1016/j.ejphar.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- Bussemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, et al. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Garland CJ. Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br J Pharmacol. 2004;142:43–50. doi: 10.1038/sj.bjp.0705756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyn VH, Nuno DW, Cappellibigazzi M, Dole WP, Lamping KG. Effect of acute hypertension in the coronary circulation – role of mechanical factors and oxygen radicals. J Hypertens. 1994;12:163–172. [PubMed] [Google Scholar]
- Dobrian AD, Schriver SD, Prewitt RL. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38:361–366. doi: 10.1161/01.hyp.38.3.361. [DOI] [PubMed] [Google Scholar]
- Dora KA, Garland CJ. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol. 2001;280:H2424–H2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. ETA receptor blockade attenuates hypertension and decreases reactive oxygen species in ETB receptor deficient rats. Hypertension. 2003;42:401. doi: 10.1097/01.fjc.0000166205.66555.40. [DOI] [PubMed] [Google Scholar]
- Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, et al. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth-muscle of the mesenteric-artery of spontaneously hypertensive rats. Circ Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, et al. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–762. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Harder DR, Sanchezferrer C, Kauser K, Stekiel WJ, Rubanyi GM. Pressure releases a transferable endothelial contractile factor in cat cerebral-arteries. Circ Res. 1989;65:193–198. doi: 10.1161/01.res.65.1.193. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Holmes DR, Garratt KN, Edwards WD, Lerman A. Mechanical pressure and stretch release endothelin-1 from human atherosclerotic coronary arteries invivo. Circulation. 1997;95:357–362. doi: 10.1161/01.cir.95.2.357. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RAK, et al. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Oemar BS, Yang Z, Luscher TF. Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ Res. 1997;81:797–803. doi: 10.1161/01.res.81.5.797. [DOI] [PubMed] [Google Scholar]
- Hoshino J, Nakamura T, Kurashina T, Saito Y, Sumino H, Ono Z, et al. Antagonism of ANG II type 1 receptors protects the endothelium during the early stages of renal hypertension in rats. Am J Physiol. 1998;275:R1950–R1957. doi: 10.1152/ajpregu.1998.275.6.R1950. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res. 1998;83:960–965. doi: 10.1161/01.res.83.9.960. [DOI] [PubMed] [Google Scholar]
- Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35:43–47. [PubMed] [Google Scholar]
- Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- Lauth M, Berger MM, Cattaruzza M, Hecker M. Elevated perfusion pressure upregulates endothelin-1 and endothelin B receptor expression in the rabbit carotid artery. Hypertension. 2000;35:648–654. doi: 10.1161/01.hyp.35.2.648. [DOI] [PubMed] [Google Scholar]
- Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, et al. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- Liu CL, Ngai CY, Huang Y, Ko WH, Wu M, He GW, et al. Depletion of intracellular Ca2+ stores enhances flow-induced vascular dilatation in rat small mesenteric artery. Br J Pharmacol. 2006;147:506–515. doi: 10.1038/sj.bjp.0706639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- Paniagua OA, Bryant MB, Panza JA. Transient hypertension directly impairs endothelium-dependent vasodilation of the human microvasculature. Hypertension. 2000;36:941–944. doi: 10.1161/01.hyp.36.6.941. [DOI] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential-hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Plane F, Garland CJ. Influence of contractile agonists on the mechanism of endothelium-dependent relaxation in rat isolated mesenteric artery. Br J Pharmacol. 1996;119:191–193. doi: 10.1111/j.1476-5381.1996.tb15970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preik M, Kelm M, Feelisch M, Strauer BE. Impaired effectiveness of nitric oxide-donors in resistance arteries of patients with arterial hypertension. J Hypertens. 1996;14:903–908. doi: 10.1097/00004872-199607000-00014. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, et al. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension. 1996;28:785–790. doi: 10.1161/01.hyp.28.5.785. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. Endothelium-dependent pressure-induced contraction of isolated canine carotid arteries. Am J Physiol. 1988;255:H783–H788. doi: 10.1152/ajpheart.1988.255.4.H783. [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Thorsgaard M, Lopez V, Buus NH, Simonsen U. Different modulation by Ca(2+)-activated K(+) channel blockers and herbimycin of acetylcholine- and flow-evoked vasodilatation in rat mesenteric small arteries. Br J Pharmacol. 2003;138:1562–1570. doi: 10.1038/sj.bjp.0705214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H, Hattori Y, Fukao M, Sato A, Liu MY, Sakuma I, et al. Relaxation in different-sized rat blood vessels mediated by endothelium-derived hyperpolarizing factor: importance of processes mediating precontractions. J Vasc Res. 1999;36:311–320. doi: 10.1159/000025659. [DOI] [PubMed] [Google Scholar]
- Ulker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41:534–539. doi: 10.1161/01.HYP.0000057421.28533.37. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress – involvement of protein kinase C-dependent NAD(P)H oxidase and local renin–angiotensin system. Am J Pathol. 2004;165:219–226. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Christman CW, Dewitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57:781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]