Abstract
Background and Purpose:
Ephedrine and amphetamine can cause substantial increases in systemic arterial pressure. However, the role of endogenous noradrenaline release in mediating the pressor response to ephedrine is controversial. Studies using pharmacologic agents to decrease the synthesis, storage, and release of catecholamines have supported both a direct and an indirect mechanism of action for ephedrine. The purpose of the present study was to determine if endogenous noradrenaline release is required for cardiovascular responses to ephedrine and amphetamine using a genetic mouse model.
Experimental Approach:
Increases in systemic arterial pressure and heart rate in response to ephedrine and amphetamine were investigated and compared in dopamine β-hydroxylase knockout (Dbh -/-) mice that cannot synthesize noradrenaline. Dbh +/- littermates have normal noradrenaline and adrenaline tissue levels, and served as controls in all experiments.
Key Results:
In Dbh -/- mice the increases in systemic arterial pressure and heart rate in response to i.v. injections of ephedrine were not impaired whereas responses to amphetamine were markedly reduced, when compared with responses in Dbh +/- mice. The pressor response to tyramine was abolished whereas pressor responses to noradrenaline, phenylephrine, dopamine, and angiotensin II were similar in Dbh -/- and Dbh +/- mice.
Conclusions and Implications:
The present results in Dbh -/- mice provide support for the hypothesis that pressor responses to ephedrine are directly mediated whereas responses to amphetamine are dependent on the release of noradrenaline and suggest that Dbh +/- and Dbh -/- mice are useful for the study of direct and indirect mechanisms.
Keywords: noradrenaline, adrenaline, ephedrine, amphetamine, dopamine β-hydroxylase knockout mice, vasopressor responses
Introduction
Ephedrine, a commonly abused psychostimulant, has the potential to cause substantial increases in systemic arterial pressure, heart rate and cardiac output. However, the mechanism of the pressor response to ephedrine is uncertain. Ephedrine has been thought to elicit a pressor response through the release of noradrenaline from adrenergic terminals (Fleckenstein and Burn, 1953; Burn and Rand, 1958; DeMoraes and Carvalho, 1968; Kobayashi et al., 2003). It has been reported that the pressor response to ephedrine is reduced following treatment with 6-hydroxydopamine to destroy adrenergic nerve terminals (Kobayashi et al., 2003). However, the results of studies in isolated tissues and in vivo studies have suggested that cardiovascular responses to ephedrine can be directly mediated (Tye et al., 1967; Waldeck and Widmark, 1985; Bao et al., 1990; Kawasuji et al., 1996; Vansal and Feller, 1999;). Additionally, it has recently been demonstrated that pressor and vasoconstrictor responses to ephedrine are not inhibited following catecholamine depletion with reserpine and α-methyl-p-tyrosine (AMPT) (Liles et al., 2006). The psychostimulant amphetamine has cardiovascular effects similar to ephedrine. However, increases in systemic arterial pressure and heart rate in response to amphetamine have consistently been shown to be indirectly mediated (Florin et al., 1995; Sabol and Seiden, 1998; Yuan et al., 2002).
Owing to differences in results of studies on the mechanism of action of ephedrine using pharmacological agents to inhibit adrenergic function, the present study was carried out in a genetic mouse model to determine the role of noradrenaline release (Rapacciuolo et al., 2001; Cryan et al., 2004). The development of mice with targeted disruption of the gene for dopamine β-hydroxylase (Dbh), the enzyme that converts dopamine into noradrenaline, has provided a genetic model for investigating the role of endogenous noradrenaline release in physiological processes and vascular remodeling (Thomas et al., 1995; Zhang et al., 2004). Dbh knockout (Dbh −/−) mice have reduced tissue levels of noradrenaline and adrenaline, whereas their heterozygote littermates (Dbh +/−) mice have normal levels of Dbh protein, noradrenaline and adrenaline, and therefore serve as a control for studies in Dbh −/− mice (Thomas et al., 1998).
In view of divergent results of experiments using inhibitors of noradrenaline synthesis, storage and release, the present study was undertaken in Dbh −/− and Dbh +/− mice in order to determine if endogenous noradrenaline release is required for cardiovascular responses to ephedrine and amphetamine. In this study, the increases in systemic arterial pressure and heart rate in response to ephedrine and amphetamine were compared in Dbh −/− and Dbh +/− mice in order to ascertain the role of indirect and direct mechanisms in mediating pressor responses to these drugs of abuse.
Methods
Animals
All experimental procedures were carried out in accordance with protocols approved by the Tulane University Institutional Animal Care and Use committee. The Dbh −/− and Dbh +/− mice used in this study were created at the University of Pennsylvania from a hybrid line (129/SvCPJ and C57BL/6J) as described previously (Thomas and Palmiter, 1997). Dbh +/− littermates, which have normal noradrenaline and adrenaline tissue levels served as control mice in all experiments (Thomas et al., 1998). Dbh +/− mice had a baseline systemic arterial pressure of 88±10 mm Hg and a baseline heart rate of 486±16 b.p.m. Dbh −/− mice had a baseline systemic arterial pressure of 74±5 mm Hg and a baseline heart rate of 433±18 b.p.m. These baseline values are in agreement with other studies reporting baseline hemodynamic parameters in Dbh −/− mice, and are slightly lower when compared to control mice (Swoap et al., 2004).
In the present studies adult male mice aged 5–7 months were anesthetized with Inactin (Sigma Chemical Co., St Louis, MO, USA) (60 mg kg−1 i.p.) with supplemental doses given as needed to maintain a uniform level of anesthesia. The mice were placed on an isothermal pad to maintain body temperature. The trachea was cannulated with a short segment of polyethylene (PE) 90 tubing to maintain a patent airway and the mice breathed room air. A carotid artery was catheterized with PE10 tubing for the measurement of systemic arterial pressure and a jugular vein was catheterized with PE10 tubing for the i.v. administration of drugs. Systemic arterial pressure was measured with a Statham pressure transducer and recorded on a Grass Model 7D polygraph and heart rate was determined from the arterial pressure pulses. The data were digitized by a Biopac MP100 data acquisition system and stored on a PC.
High-performance liquid chromatography determination of tissue catecholamines
Following the completion of an experiment, the brain, heart, kidney and adrenal glands were removed and homogenized in a buffer of 0.1 M citrate, 10% ethanol and 250 mg/l sodium octyl sulfate (SOS) at pH 4. Tissues were stored in buffer at −80°C until analysis. An internal standard (IS) of 3,4-dihydroxybenzylamine hydrobromide (DHBA) was added in a final concentration of 1 ng/100 μl of the high-performance liquid chromatography (HPLC) injectate. The homogenate was centrifuged in a RC2B Sorvall centrifuge at 12 000 g and the supernatant was frozen at −80°C until assayed. Recoveries ranged from 75 to 90% in these and other studies. The chromatographic system consisted of Dual Rainin Rabbit HP pumps equipped with a self-washing piston pump heads (capable of maximum flows of 10 ml/min) that provided a flow rate of 1.5 ml/min. Automatic injection of samples was accomplished using an Alcott model 728 autosampler. This allowed us to run up to 40 samples overnight. The HPLC column was a Rainin microsorb (5 μ) C18 column (25 cm × 4.6 mm). A 1.5 cm × 4.6 mm guard column packed with microsorb C18 preceded the analytical column. Chromatographically separated monoamines were assayed by electrochemical detection utilizing an ESA model 5100 Coulchem multielectrode array. This electrode array consisted of a model 5020 guard cell and 5010 dual analytical electrode cell. The guard cell voltage was +0.4 V. The two analytical cells were set at −0.04 V (Detector 1) and +0.32 V (Detector 2). Noradrenaline, adrenaline, dopamine, 5-hydroxytryptamine (5HT; serotonin) and 5-hydroxyindole acetic acid (5HIAA) were determined in unknown and standard samples by comparison to retention times and integrated areas of peaks. Cochromatography of spiked standards of each compound were initially run with brain tissue in order to ascertain that peaks identified as monoamines were authentic.
Western blot analysis of Dbh protein in adrenal gland
Following the completion of an experiment, adrenal glands were collected from Dbh +/− and Dbh −/− mice and stored at −70°C. The glands were then homogenized (Polytron, Brinkmann Instruments, Westbury, NY, USA) in an ice-cold buffer containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.1 mg/ml leupeptin and 574 μM phenylmethylsulfonyl fluoride (Sigma; prepared in phosphate-buffered saline), incubated on ice for 45 min, centrifuged twice at 15 000 g at 4°C for 20 min and the supernatant was collected. Protein content of the homogenate was quantified colorimetrically using the BCA protein assay (Pierce, Rockford, IL, USA). For Western blot analysis, 12.5 μg of adrenal gland homogenate were loaded onto a 4–20% Tris-Glycine gel (ICN Biomedicals, Aurora, OH, USA). After electrophoresis, the protein was transferred onto a nitrocellulose membrane with 0.45 μm pore-size by electroelution. Immunodetection was performed with rabbit anti-DBH polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, 1:2500 dilution) and rabbit anti-β-tubulin polyclonal antibody (Santa Cruz Biotechnology Inc., 1:2500 dilution). The secondary antibody was horseradish peroxidase conjugated to goat anti-rabbit IgG (Santa Cruz Biotechnology Inc., 1:4000 dilution). The nitrocellulose membrane was then processed using enhanced chemiluminescence (ECL) Western blot detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA). About 2.5 μg of PC-12 cell lysate (Santa Cruz Biotechnology Inc.) were used as a Dbh positive control.
Statistical analysis
The hemodynamic data represent peak increases in mean systemic arterial pressure and peak increases in heart rate and are expressed as mean±s.e.m. The area under the curve is a time integrated value. Data were analyzed using paired and unpaired t-tests or analysis of variance (ANOVA) with a Fisher post-test. A P-value of less than 0.05 was used as the criterion for statistical significance (*=P<0.05).
Drugs
All drugs were obtained from Sigma (St Louis, MO, USA). L-Ephedrine sulfate, tyramine, D-amphetamine, angiotensin II, DL-noradrenaline, phenylephrine, dopamine, propranolol and phentolamine were dissolved in normal saline and were injected i.v. injection volumes were 20–100 μl.
Results
Responses to tyramine and noradrenaline in Dbh −/− knockout mice
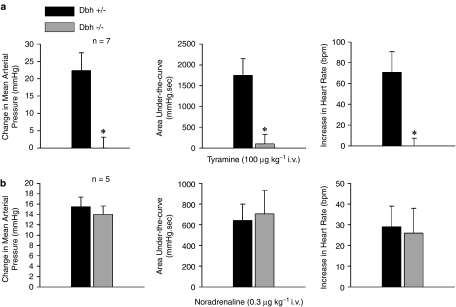
Increases in mean systemic arterial pressure and heart rate in response to i.v. injections of tyramine and noradrenaline were compared in Dbh −/− and Dbh +/− mice, and the data are presented in Figure 1. The increase in systemic arterial pressure and heart rate in response to tyramine (100 μg kg−1 i.v.) and the area under-the-curve of the pressor response were reduced in Dbh −/− mice when compared to responses in Dbh +/− mice (Figure 1a). The increase in systemic arterial pressure and heart rate in response to noradrenaline (0.3 μg kg−1 i.v.) and the area under-the-curve of the pressor response were not significantly different in Dbh −/− and Dbh +/− control mice (Figure 1b).
Figure 1.
Comparison of effects of i.v. injection of (a) tyramine and (b) noradrenaline on peak increases in mean systemic arterial pressure, the area under-the-curve of the pressor response, and the increase in heart rate in anesthetized Dbh +/− and Dbh −/− mice. Only increases in heart rate were analyzed. n Indicates number of experiments, * indicates P<0.05.
Responses to ephedrine and amphetamine in Dbh −/− knockout mice
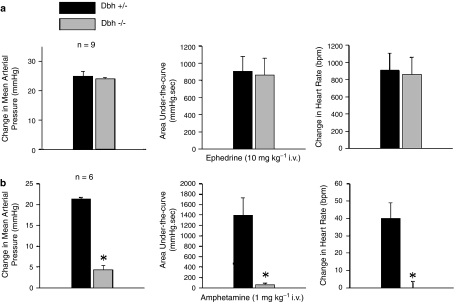
Increases in mean systemic arterial pressure and heart rate in response to i.v. injections of ephedrine and amphetamine were investigated in Dbh −/− and Dbh +/− mice, and the data are summarized in Figure 2. Injections of ephedrine (10 mg kg−1 i.v.) increased systemic arterial pressure and heart rate and the magnitude and area under-the-curve of the pressor and heart rate responses were not different from responses in Dbh −/− when compared to responses in Dbh +/− control mice (Figure 2a). The increase in systemic arterial pressure in response to amphetamine (1 mg kg−1 i.v.) and the area under-the-curve of the response was significantly smaller in the Dbh −/− mouse compared to responses in Dbh +/− control mice (Figure 2b). The increase in heart rate in response to amphetamine injection could not be demonstrated in Dbh −/− mice when compared with positive chronotropic responses in Dbh +/− control mice (Figure 2b).
Figure 2.
Comparison of the peak increases in mean systemic arterial pressure, the area under-the-curve of the pressor response, and the increase in heart rate in response to i.v. injections of (a) ephedrine and (b) amphetamine in anesthetized Dbh +/− and Dbh −/− mice. n Indicates number of experiments; only positive chronotropic responses were analyzed, * indicates P<0.05.
Effect of propranolol and phentolamine
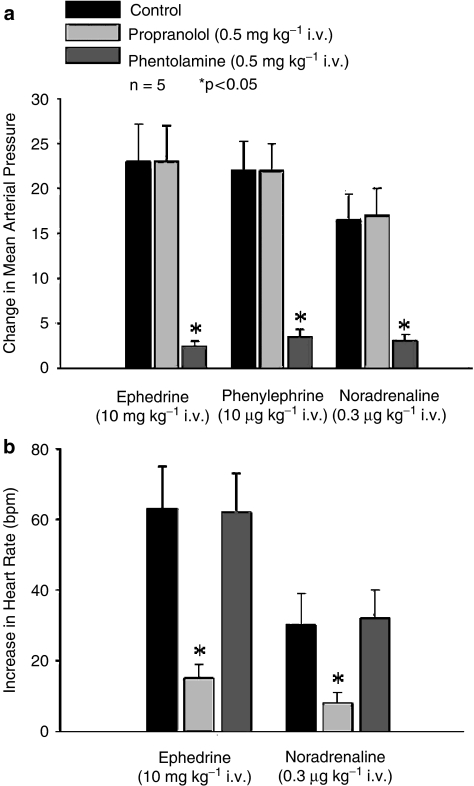
The effect of propranolol and phentolamine on increases in mean systemic arterial pressure and heart rate in response to the sympathomimetic agents was investigated in Dbh −/− mice, and these data are summarized in Figure 3. The increases in mean systemic arterial pressure in response to i.v. injections of ephedrine, phenylephrine and noradrenaline were not altered after administration of propranolol 0.5 mg kg−1 i.v. but were significantly decreased after administration of phentolamine 0.5 mg kg−1 i.v. (Figure 3a). The increase in heart rate in response to i.v. injections of ephedrine and noradrenaline were decreased significantly after administration of propranolol 0.5 mg kg−1 i.v. (Figure 3b). Phentolamine 0.5 mg kg−1 i.v. had no significant effect on increases in heart rate in response to ephedrine or noradrenaline (Figure 3b).
Figure 3.
The effect of phentolamine and propranolol (a) on the peak increases in mean systemic arterial pressure in response to i.v. injections of ephedrine, phenylephrine and noradrenaline in anesthetized Dbh −/− mice. The effect of phentolamine and propranolol (b) on the peak increase in heart rate following i.v. injections of ephedrine and noradrenaline. n Indicates number of experiments. Only peak increases in heart rate were analyzed and *indicates P<0.05.
Responses to dopamine, phenylephrine and angiotensin II in Dbh −/− knockout mice
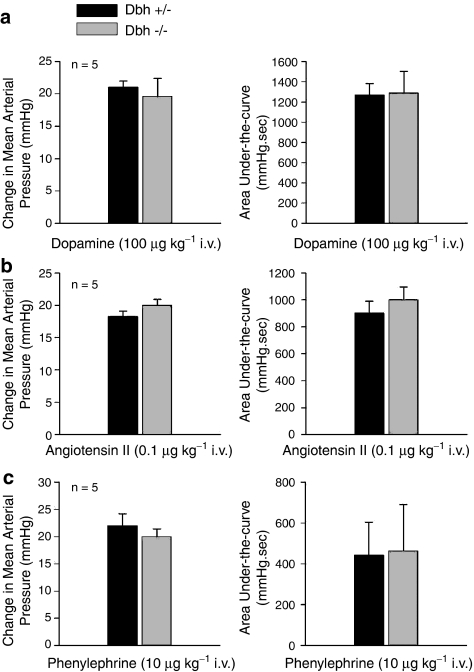
Pressor responses to i.v. injections of angiotensin II, phenylephrine and dopamine were compared in Dbh −/− and Dbh +/− mice, and these data are summarized in Figure 4. The increases in mean systemic arterial pressure in response to i.v. injections of angiotensin II, dopamine and phenylephrine were not different in magnitude or area under-the-curve in Dbh −/−and Dbh +/− mice (Figure 4a–c).
Figure 4.
Comparative effect of i.v. injections of (a) dopamine, (b) angiotensin II and (c) phenylephrine on the peak increases in mean systemic arterial pressure and the area under-the-curve of the pressor response in anesthetized Dbh +/− and Dbh −/− mice. n Indicates number of experiments.
Western blot analysis of Dbh protein
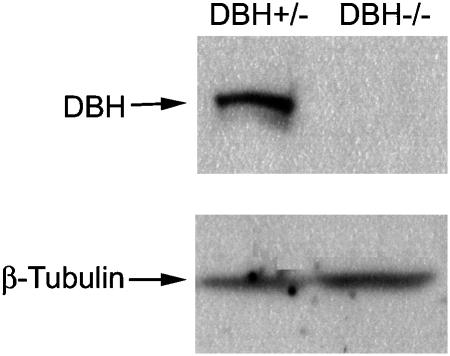
The expression of DBH protein was measured in the adrenal gland of Dbh +/− and Dbh −/− mice using Western blot analysis and these data are shown in Figure 5. Abundant Dbh protein expression was detected in Dbh +/− mice but not in Dbh −/− mice (Figure 5).
Figure 5.
Western blot analysis for the expression of Dbh in mouse adrenal gland. The adrenal glands of Dbh +/− and Dbh −/− mice were homogenized and the Dbh protein expression in mouse adrenal gland homogenate was analyzed using Western blotting. Lane 1: Dbh +/− mouse adrenal gland homogenate; Lane 2: Dbh −/− mouse adrenal gland homogenate; the analysis of β-tubulin was carried out to ensure that sample loading was similar in all lanes.
HPLC analysis of tissue catecholamine levels
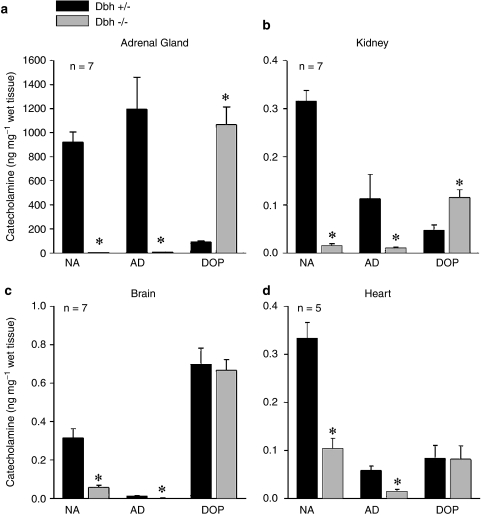
Catecholamine levels were measured at the end of the experiments and following noradrenaline injection. HPLC analysis shows that noradrenaline and adrenaline levels were significantly decreased in the brain, heart and adrenal gland of Dbh −/− mice when compared to levels in the Dbh +/− control mice (Figure 6a–d). Dopamine tissue levels were increased in the Dbh −/− mice when compared with levels in Dbh +/− mice (Figure 6a–d).
Figure 6.
Tissue levels of noradrenaline (NA), adrenaline (AD) and dopamine (DOP) in the (a) adrenal gland, (b) kidney, (c) brain and (d) heart of Dbh −/− and Dbh +/− control mice as measured by HPLC. All values are normalized to ng/mg of wet tissue n=8. *indicates P<0.05.
Discussion
New findings in this study are that increases in mean systemic arterial pressure in response to ephedrine were similar in Dbh −/− and Dbh +/− mice, whereas the pressor response to amphetamine was absent in Dbh −/− mice that do not synthesize noradrenaline. Phentolamine inhibited the pressor response to ephedrine and propranolol attenuated the increase in heart rate following ephedrine administration in Dbh −/− mice. These data provide support for the hypothesis that increases in systemic arterial pressure and heart rate in response to ephedrine are mediated by direct activation of α-receptors and of β-receptors and that these responses are not dependent on the presence of endogenous noradrenaline stores. The hypothesis that pressor responses to ephedrine are directly mediated by α and β-receptors is supported by experiments showing that i.v. injections of ephedrine produce similar increases in systemic arterial pressure and heart rate in Dbh −/− and Dbh +/− mice and that pressor responses to ephedrine were attenuated by phentolamine and increases in heart rate in response to ephedrine were inhibited by propronalol. The magnitude and the area under-the-curve of the pressor response to ephedrine were similar in Dbh −/− and Dbh +/− mice. In the present experiments pressor responses to tyramine could not be demonstrated in Dbh −/− mice whereas pressor responses to noradrenaline, phenylephrine, angiotensin II and dopamine in Dbh −/− mice were not different when compared with responses in Dbh +/− mice which served as control mice.
The results of studies on the mechanism of the pressor response to ephedrine using pharmacological agents to inhibit the synthesis, storage and release of catecholamines have been inconsistent (Kobayashi et al., 2003; Liles et al., 2006). Therefore, the present experiments were carried out in mice with targeted disruption of the Dbh gene that cannot synthesize noradrenaline (Thomas et al., 1995, 1997, 1998). This genetic model provided an alternative strategy for examining the role of endogenous noradrenaline in mediating responses to ephedrine and related amines. Previous studies in Dbh −/− mice have revealed that the direct trophic vascular actions of noradrenaline contribute to arterial wall remodeling and cardiac hypertrophy in vivo (Rapacciuolo et al., 2001; Zhang et al., 2004); however, little if anything is known about cardiovascular responses to indirect and direct acting sympathomimetic agents in Dbh −/− mice.
The results of the present study are the first to show that ephedrine has significant cardiovascular effects in Dbh −/− mice that have significantly reduced noradrenaline levels and demonstrate that pressor responses to amphetamine and tyramine are dependent on the presence of endogenous noradrenaline stores. Responses to ephedrine were not reduced in Dbh −/− mice whereas responses to amphetamine and tyramine were abolished in Dbh −/− mice. The Dbh −/− mice used in our study had undetectable expression of Dbh protein as measured by Western blot analysis and decreased levels of noradrenaline and adrenaline when compared to values in Dbh +/− mice. The Dbh −/− mice used had elevated levels of dopamine as measured by HPLC and this finding is in agreement with previous reports (Thomas et al., 1995).
It is possible that increased levels of dopamine in Dbh −/− mice may play a role in mediating the pressor response to ephedrine in Dbh −/− mice. This, however, seems unlikely since it has been reported that amphetamine is more readily taken up by the noradrenaline transporter and is more potent in releasing dopamine (Rothman et al., 2001, 2003). Moreover the observation that responses to tyramine could not be demonstrated in Dbh −/− mice suggest that indirect mechanisms are markedly impaired in Dbh −/− mice.
The observation that tissue catecholamine levels are not decreased in Dbh +/− mice is in agreement with the finding that endowment with a single copy of the Dbh gene does not lead to reduced noradrenaline and adrenaline synthesis in adult mice (Thomas et al., 1998).
The results of the present study show that responses to amphetamine and tyramine are diminished or absent in Dbh −/− mice and that baseline systemic arterial pressure is lower when compared to values in Dbh +/− mice. The decreased responses to amphetamine and tyramine could result from a decrease in baseline systemic arterial pressure, however, this seems unlikely since responses to noradrenaline, ephedrine, angiotensin II, phenylephrine and dopamine were similar in Dbh −/− and Dbh +/− mice.
It has been reported that tissue noradrenaline levels are very low or not detectable in Dbh −/− mice (Thomas et al., 1995, 1997, 1998). In Dbh −/− mice noradrenaline can only be synthesized from synthetic amino-acid precursors such as dihydroxy-phenylalanine (Thomas et al., 1995). In the present study decreased but significant levels of noradrenaline was found in cardiac tissue from Dbh −/− mice. The source of the noradrenaline is uncertain and cannot be accounted for by maternal transfer (Parvez and Parvez, 1974; Thomas et al., 1995). It is, however, possible that the noradrenaline in the heart results from the injection and uptake of the catecholamine in these experiments. However, the observation that tyramine does not increase systemic arterial pressure in Dbh −/− mice suggests that on a functional basis this cardiac store cannot be released by an indirectly acting sympathomimetic agent. The present data are consistent with previous studies showing that pressor responses to ephedrine are not diminished in rats that have undetectable cardiac levels of noradrenaline and markedly attenuated responses to tyramine after treatment with catecholamine depleting agents (Liles et al., 2006).
Chronic inhibition of the adrenergic system results in increased responses to exogenous adrenergic agonists (Taki et al., 2004). In the present study increased responses to noradrenaline and phenylephrine were not observed in Dbh −/− mice. The explanation for the absence of supersensitivity in Dbh −/− mice is uncertain and may be due to increased levels of dopamine in Dbh −/− mice, which could significantly stimulate both α- and β-receptors to maintain normal receptor number and function and thereby prevent the development of supersensitivity (Thomas et al., 1995).
The mechanism of the increase in systemic arterial pressure in response to dopamine involves both direct and indirect actions (Katai et al., 2004). Dopamine has been reported to both inhibit and enhance the release of noradrenaline and to activate α- and β-adrenoceptors and dopamine receptors (Goldberg 1972; Habuchi et al., 1997). In the present study responses to dopamine were similar in Dbh −/− and Dbh +/− mice. These results provide support for the hypothesis that responses to dopamine are not dependent on the presence of endogenous noradrenaline in in vivo experiments and are in agreement with studies using inhibitors to show that responses are directly mediated (Tsai et al., 1967; Mugelli et al., 1977; Katai et al., 2004). The results of experiments showing that responses to angiotensin II are similar in Dbh −/− and Dbh +/− mice are consistent with the results of previous studies using inhibitors of the adrenergic system in mice and rats (Champion et al., 1998; Bivalacqua et al 1999; Liles et al., 2006). It should, however, be pointed out that the present experiments were not designed to determine the mechanism of angiotensin II action and only single doses of the peptide were injected.
In conclusion, the results of studies in mice with targeted deletion of the Dbh gene provide evidence in support of the hypothesis that pressor and positive chronotropic responses to ephedrine are directly mediated by activation of α- and β-adrenoceptors, whereas responses to amphetamine are dependent on the release of noradrenaline. In the present study responses to tyramine could not be demonstrated in Dbh −/− mice whereas responses to noradrenaline, phenylephrine, angiotensin II and dopamine were similar in Dbh −/− and Dbh +/− mice that had normal catecholamine tissue levels. These data provide evidence in support of the hypothesis that responses to ephedrine and amphetamine are mediated by different mechanisms and suggest that Dbh −/− mice are useful for the study of direct and indirect adrenergic responses.
Abbreviations
- AII
angiotensin II
- DA
dopamine
- Dbh
dopamine β-hydroxylase
- HPLC
high performance liquid chromatography
- NA
noradrenaline
- PHE
phenylephrine
Conflict of interest
The authors state no conflict of interest.
References
- Bao JX, Wang B, Yang ZC. Effects of ephedrine on postsynaptic alpha-adrenoceptors and presynaptic beta-adrenoceptors in isolated guinea pig portal veins. Zhongguo Yao Li Xue Bao. 1990;11:130–133. [PubMed] [Google Scholar]
- Bivalacqua TJ, Dalal A, Champion HC, Kadowitz PJ. Role of AT(1) receptors and autonomic nervous system in mediating acute pressor responses to ANG II in anesthetized mice. Am J Physiol. 1999;277:E838–E847. doi: 10.1152/ajpendo.1999.277.5.E838. [DOI] [PubMed] [Google Scholar]
- Burn JH, Rand MJ. The action of sympathomimetic amines in animals treated with reserpine. J Physiol. 1958;144:314–336. doi: 10.1113/jphysiol.1958.sp006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion HC, Czapla MA, Kadowitz PJ. Responses to angiotensin peptides are mediated by AT1 receptors in the rat. Am J Physiol. 1998;274:E115–E123. doi: 10.1152/ajpendo.1998.274.1.E115. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci USA. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoraes S, Carvalho FV. Reciprocal pressor effects between amphetamine and some sympathomimetic amines. Pharmacology. 1968;1:129–134. doi: 10.1159/000135953. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A, Burn JH. The effect of denervation on the action of sympathomimetic amines on the nictitating membrane, Br. J Pharmacol. 1953;8:69–78. doi: 10.1111/j.1476-5381.1953.tb00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin SM, Kuczenski R, Segal DS. Effects of reserpine on extracellular caudate dopamine and hippocampus norepinephrine responses to amphetamine and cocaine: mechanistic and behavioral considerations. J Pharmacol Exp Ther. 1995;274:231–241. [PubMed] [Google Scholar]
- Goldberg MA. Inhibition of synaptosomal protein synthesis by neurotransmitter substances. Brain Res. 1972;39:171–179. doi: 10.1016/0006-8993(72)90793-7. [DOI] [PubMed] [Google Scholar]
- Habuchi Y, Tanaka H, Nishio M, Yamamoto T, Komori T, Morikawa J, et al. Dopamine stimulation of cardiac beta-adrenoceptors: the involvement of sympathetic amine transporters and the effect of SKF38393. Br J Pharmacol. 1997;122:1669–1678. doi: 10.1038/sj.bjp.0701574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katai R, Tsuneyoshi I, Hamasaki J, Onomoto M, Suehiro S, Sakata R, et al. The variable effects of dopamine among human isolated arteries commonly used for coronary bypass grafts. Anesth Analg. 2004;98:915–920. doi: 10.1213/01.ANE.0000107942.06422.75. [DOI] [PubMed] [Google Scholar]
- Kawasuji T, Koike K, Saito H. Chronotropic effects of optical isomers of ephedrine and methylephedrine in the isolated rat right atria and in vitro assessment of direct and indirect actions on beta 1-adrenoceptors. Biol Pharm Bull. 1996;19:1423–1428. doi: 10.1248/bpb.19.1423. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Endou M, Sakuraya F, Matsuda N, Zhang XH, Azuma M, et al. The sympathomimetic actions of l-ephedrine and d-pseudoephedrine: direct receptor activation or norepinephrine release. Anesth Analg. 2003;97:1239–1245. doi: 10.1213/01.ANE.0000092917.96558.3C. [DOI] [PubMed] [Google Scholar]
- Liles JT, Dabisch PA, Hude KE, Pradhan L, Varner KJ, Porter JR, et al. Pressor responses to ephedrine are mediated by a direct mechanism in the rat. J Pharmacol Exp Ther. 2006;316:95–105. doi: 10.1124/jpet.105.090035. [DOI] [PubMed] [Google Scholar]
- Mugelli A, Ledda F, Mantelli L, Torrini M, Maccioni T. Studies on the positive inotropic effect of dopamine in the guinea-pig heart, Naunyn. Schmiedebergs Arch Pharmacol. 1977;301:49–55. doi: 10.1007/BF00501263. [DOI] [PubMed] [Google Scholar]
- Parvez S, Parvez H. Placental transfer of 3H-epinephrine and its metabolites to the fetal heart during variable hormonal treatments. Hormone Res. 1974;5:321–330. doi: 10.1159/000178646. [DOI] [PubMed] [Google Scholar]
- Rapacciuolo A, Esposito G, Caron K, Mao L, Thomas SA, Rockman HA. Important role of endogenous norepinephrine and epinephrine in the development of in vivo pressure-overload cardiac hypertrophy, J. Am Coll Cardiol. 2001;38:876–882. doi: 10.1016/s0735-1097(01)01433-4. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Seiden LS. Reserpine attenuates D-amphetamine and MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis. Brain Res. 1998;806:69–78. doi: 10.1016/s0006-8993(98)00720-3. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh (−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- Taki N, Tanaka T, Zhang L, Suzuki F, Israilova M, Taniguchi T, et al. Alpha-1Dadrenoceptors are involved in reserpine-induced supersensitivity of rat tail artery. Br J Pharmacol. 2004;142:647–656. doi: 10.1038/sj.bjp.0705817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Tsai TH, Langer SZ, Trendelenburg U. Effects of dopamine and alpha-methyl-dopamine on smooth muscle and on the cardiac pacemaker. J Pharmacol Exp Ther. 1967;156:310–324. [PubMed] [Google Scholar]
- Tye A, Baldesberger R, LaPidus JB, Patil PN. Steric aspects of adrenergic drugs. VI. Beta adrenergic effects of ephedrine isomers. J Pharmacol Exp Ther. 1967;157:356–362. [PubMed] [Google Scholar]
- Vansal SS, Feller DR. Direct effects of ephedrine isomers on human beta-adrenergic receptor subtypes. Biochem Pharmacol. 1999;58:807–810. doi: 10.1016/s0006-2952(99)00152-5. [DOI] [PubMed] [Google Scholar]
- Waldeck B, Widmark E. The interaction of ephedrine with beta-adrenoceptors in tracheal, cardiac and skeletal muscles. Clin Exp Pharmacol Physiol. 1985;12:439–442. doi: 10.1111/j.1440-1681.1985.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA. Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J Neurochem. 2002;80:960–969. doi: 10.1046/j.0022-3042.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cotecchia S, Thomas SA, Tanoue A, Tsujimoto G, Faber JE. Gene deletion of dopamine beta-hydroxylase and alpha1-adrenoceptors demonstrates involvement of catecholamines in vascular remodeling. Am J Physiol Heart Circ Physiol. 2004;287:H2106–H2114. doi: 10.1152/ajpheart.00290.2004. [DOI] [PubMed] [Google Scholar]