Abstract
Background and purpose:
Orexin (OX) receptors induce Ca2+ elevations via both receptor-operated Ca2+ channels (ROCs) and the "conventional" phospholipase C (PLC)–Ca2+ release–store-operated Ca2+ channel (SOC) pathways. In this study we assessed the ability of these different Ca2+ influx pathways to amplify OX1 receptor signalling to PLC in response to stimulation with the physiological ligand orexin-A.
Experimental approach:
PLC activity was assessed in CHO cells stably expressing human OX1 receptors.
Key results:
Inhibition of total Ca2+ influx by reduction of the extracellular [Ca2+] to 1 μM effectively inhibited the receptor-stimulated PLC activity at low orexin-A concentrations (by 93% at 1 nM), and this effect was gradually reduced by higher orexin-A concentrations. A similar but weaker inhibitory effect (84% at 1 nM) was obtained on depolarization to ∼0 mV, which disrupts most of the driving force for Ca2+ entry. The inhibitor of the OX1 receptor-activated ROCs, tetraethylammonium chloride (TEA), was somewhat less effective than the reduction in extracellular [Ca2+] at inhibiting PLC activation, probably because it only partially blocks ROCs. The partial inhibitor of both ROCs and SOCs, Mg2+, and the SOC inhibitors, dextromethorphan, SKF-96365 (1-[β-(3-(4-methoxyphenyl)propoxy)-4-methoxyphenethyl]-1H-imidazole HCl) and 2-APB (2-aminoethoxydiphenyl borate), inhibited PLC activity at low concentrations of orexin-A, but were not as effective as TEA.
Conclusions and implications:
Both ROCs and SOCs markedly amplify the OX1 receptor-induced PLC response, but ROCs are more central for this response. These data indicate the crucial role of ROCs in orexin receptor signalling.
Keywords: orexin, hypocretin, receptor, G-protein-coupled receptor, calcium, calcium influx, phospholipase C
Introduction
Phospholipase C (PLC) is an enzyme responsible for the hydrolysis of the membrane phospholipid phosphatidylinositol bisphosphate (PIP2) to inositol trisphosphate (IP3) and diacylglycerol, two important intracellular messengers. IP3 activates IP3 receptors leading to the release of Ca2+ from the endoplasmic reticulum and subsequently an increase in the intracellular level of Ca2+, another important intracellular messenger. The other messenger, diacylglycerol, is known to activate, for example, protein kinase C and transient receptor potential (TRP) channels (Tesfai et al., 2001; Jung et al., 2002). At present, there are six known subfamilies of PLC, β, γ, δ, ɛ, ζ and η, giving a total of 13 different isoforms of the enzyme (reviewed by Katan (2005). These are to some extent regulated by different signals depending on the subfamily of the enzyme. For example, PLCβ is activated by G-protein-coupled receptors (GPCRs) via Gαq or Gβγ-subunits and PLCγ is activated primarily by tyrosine kinases. Ca2+ binding is an obligatory requirement for PLC activity, and whereas some of the isoforms show significant activity at resting cytosolic Ca2+ levels, some are strongly simulated by Ca2+ elevations (Allen et al., 1997; reviewed by Rhee, 2001; Kouchi et al., 2004). Recent studies have shown that an increase in Ca2+ has a positive feedback effect on GPCR-mediated PLC activity in different types of intact cells (Lund et al., 2000; Young et al., 2003; Thore et al., 2005).
Orexin/hypocretin receptors, OX1R and OX2R, belong to the GPCR superfamily. Orexin receptors are expressed in different areas of the brain, where they are thought primarily to regulate sleep and wakefulness and energy homeostasis. They are also expressed in the periphery of the body, where their functions are less clear (reviewed by Kukkonen et al., 2002). Their ligands are the peptides orexin-A and -B, which are also found in the brain and in the periphery (reviewed by Kukkonen et al., 2002). It has previously been shown that stimulation of OX1R heterologously expressed in CHO (Chinese hamster ovary) cells results in an increase of intracellular Ca2+ (Sakurai et al., 1998; Lund et al., 2000). This Ca2+ increase seems owing to different sources depending on the concentration of the ligand. At low orexin-A concentrations, the Ca2+ increase is primarily triggered by activation of receptor-operated Ca2+ channels (ROCs), and this Ca2+ influx somehow enhances/allows PLC activation leading to Ca2+ release from the endoplasmic reticulum and additional influx through store-operated Ca2+ channels (SOCs) (Lund et al., 2000; Kukkonen and Akerman, 2001; Larsson et al., 2005). At high concentrations of orexin-A (high degree of receptor activation), PLC is activated independently of ROCs (Lund et al., 2000; Kukkonen and Akerman, 2001). It has been suggested that Ca2+, independently of whether it arises through ROCs or some other Ca2+ channels, acts upstream from the targets of OX1R signalling (PLC, adenylyl cyclase, ERK (extracellular signal-regulated kinase)), somehow enabling the coupling of OX1R to these pathways (Ammoun et al., 2006). Thus, OX1R-stimulated PLC activity might be affected by Ca2+ influx in a complex manner, firstly, at a level close to the receptor and secondly, by PLC itself, and that both ROCs and SOCs could play a part in this process. Previous studies on the specific roles of ROCs and SOCs have been hampered by lack of effective and selective inhibitors of ROCs (Kukkonen and Akerman, 2001), but recently more selective substances have been identified (Larsson et al., 2005). In this study, we have used these channel inhibitors and other techniques to reduce Ca2+ influx so as to evaluate the importance of ROC and SOC activity in PLC regulation by OX1R. The results show that both ROCs and SOCs markedly amplify OX1R-induced PLC activity, especially at the low levels of OX1R activity (=low concentrations of orexin-A), but that the impact of ROCs is more profound, most likely owing to their more central role in OX1R signalling.
Methods
Test systems used
CHO-OX1 cells, expressing human OX1 receptors, have been described previously (Lund et al., 2000). CHO cells were grown in Ham's F-12 medium (Gibco, Paisley, UK) supplemented with 100 U ml−1 penicillin G (Sigma Chemical Co., St Louis, MO, USA), 80 U ml−1 streptomycin (Sigma), 400 μg ml−1 geneticin (G418; Gibco) and 10% (v/v) foetal calf serum (Gibco) at 37°C in 5% CO2 in an air ventilated humidified incubator in 260 ml plastic culture flasks (75 cm2 bottom area; Greiner Bio-One GmbH, Frickenhausen, Germany). For the total inositol phosphate generation experiments, the cells were cultivated on 24-well plates (1.77 cm2 well bottom area; Greiner Bio-One).
For transient transfection with human M1 muscarinic cholinoceptors, CHO-OX1-cells were grown on 24-well plates to 40–50% confluence (Holmqvist et al., 2005). The wells were washed with phosphate-buffered saline (PBS), and OPTI-MEM (Gibco) was added. The cells were transfected with pcDNA3.1-hM1 (see below) using Lipofectamine reagent (Invitrogen Corp., Carlsbad, CA, USA). After 5 h, this medium was replaced with fresh Ham's F-12 medium with all the usual supplements (see above). Experiments were performed 48 h after transfection. Transfection efficiency was 40–70%, determined by measuring the expression of pEGFP-C1 (see below).
Measurements made
Measurement of total inositol phosphate generation
Membrane phosphoinositides were prelabelled by incubating the cells with 3 μCi ml−1 [3H]-inositol for 18 h in culture medium after which the cells were washed and incubated in TES-buffered medium (TBM; composition in mM: NaCl 137, KCl 5, CaCl2 1, MgCl2 1.2, KH2PO4 0.44, NaHCO3 4.2, glucose 10 and TES [2-([2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino) ethane sulfonic acid] 20 adjusted to pH 7.4 with NaOH) at 37°C containing 10 mM LiCl (to inhibit inositol monophosphatase) for 10 min. For some experiments, instead of TBM, K+-TBM (all the Na+-salts replaced with corresponding K+-salts), tetraethylammonium (TEA)-TBM (70 mM of the NaCl in TBM replaced with TEA) or TBM with reduced Ca2+ concentration (either 1 μM (CaCl2 excluded) or 140 nM (CaCl2 excluded and 0.5 mM ethylene glycol-bis[β-aminoethyl ether]N,N,N′,N′-tetraacetic acid (EGTA) added)) was used, and for others, TBM was supplemented with ion channel inhibitors (please see figure legends for details). The cells were then stimulated with orexin-A or carbamoylcholine chloride (carbachol) for 20 min and the reactions were stopped by removal of the medium and addition of 0.4 M perchloric acid and freezing. After thawing, the samples were neutralized with 0.5 volumes of 0.36 M KOH+0.3 M KHCO3, the insoluble fragments were spun down, and the supernatants were subjected to anion exchange chromatography (AG 1-X8; Bio-Rad, Hercules, CA, USA) with H2O (for inositol), 5 mM Na2-tetraborate+60 mM NH4-formate (for glycerophosphoinositol), and 0.1 M formic acid+1 M NH4-formate (for inositol mono-, bis- and trisphosphates) (Berridge et al., 1983; Ammoun et al., 2006). The inositol phosphate fraction was dissolved in an appropriate volume of scintillation cocktail (Optiphase Hisafe 3, PerkinElmer, Boston, MA, USA) and analysed in a 1219 Rackbeta Liquid Scintillation Counter (LKB Wallac, Turku, Finland).
Ca2+ measurements
Ca2+ measurements were performed as microfluorometric imaging of individual CHO cells attached on glass coverslips as described previously (Ammoun et al., 2006). Briefly, the cells were loaded with 4 μM fura-2 acetoxymethyl ester for 20 min at 37°C in TBM+0.5 mM probenecid, rinsed once, and used immediately. TILLvisION version 4.01 imaging system (TILL Photonics GmbH, Gräfelding, Germany) with Nikon TE200 fluorescence microscope (× 20/0.5 air objective) was used for measurements. The cells were excited with 340- and 380-nm light from a xenon lamp through a monochromator and the emitted light collected through a 400-nm dichroic mirror and a 470-nm barrier filter with a high-resolution (1280 × 1024) cooled CCD camera. One 340 and one 380 reading were obtained each second and the ratio obtained after background subtraction.
Data analysis and statistical procedures
All the data are presented as mean±s.e.m. of at least three measurements performed in triplicate. Student's two-tailed t-test was used in all pairwise comparisons and analysis of variance, followed by Tukey's post-hoc test, and t-test with Bonferroni correction for multiple comparisons. Significances are as follows: NS (not significant), P>0.05; *P<0.05; **P<0.01; ***P<0.001. SigmaPlot 4.1 (Jandel Scientific, Corte Madera, CA, USA) was used for nonlinear curve-fitting.
Drugs, chemical reagents and other materials
Human orexin-A was from Neosystem (Strasbourg, France), [3H]-myo-inositol (PT6-271 TRK911) from Amersham Biosciences (Buckinghamshire, UK) and BaCl2 and MgCl2 from Merck AG (Darmstadt, Germany). 2-APB (2-aminoethoxydiphenyl borate) and SKF-96365 (1-[β-(3-[4-methoxyphenyl]propoxy)-4-methoxyphenethyl]-1H-imidazole HCl) were from Calbiochem (La Jolla, CA, USA), carbachol, EGTA, TEA and dextromethorphan from Sigma, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) and fura-2 acetoxymethyl ester from Molecular Probes (Eugene, OR, USA) and thapsigargin from RBI (Natick, MA, USA).
The plasmid construct pcDNA3.1-hM1 was from UMR cDNA Resource Center (http://www.cdna.org) and pEGFP-C1, used to identify transfection efficiency, was from Clontech (Palo Alto, CA, USA).
Results
Ca2+ influx is required for OX1R-mediated PLC stimulation at low concentrations of orexin-A
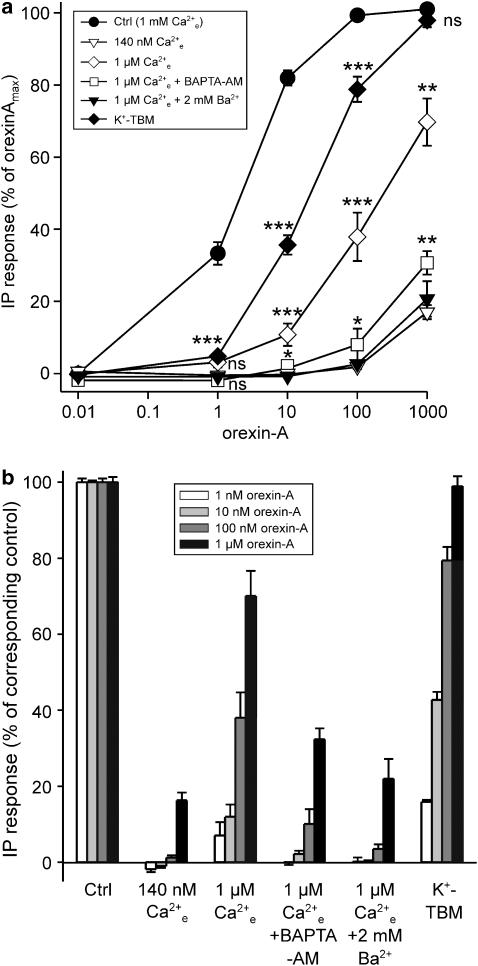
Stimulation of OX1R with orexin-A resulted in a robust inositol phosphate release, as previously demonstrated (Lund et al., 2000; Holmqvist et al., 2005). This amounted to 30.6±4.6 times the basal level at the saturating concentration (100 nM), with an EC50=2.9±0.7 nM (Figure 1a, ctrl).
Figure 1.
The effect of reducing the level of Ca2+ on OX1R-stimulated PLC activity. A [Ca2+]e of 1 μM was obtained in nominally Ca2+-free TBM (no CaCl2 added), and 140 nM by adding 0.5 mM EGTA to this medium. For BAPTA-AM treatment, the cells were preincubated with 30 μM BAPTA-AM for 20 min; 1 mM probenecid was included both in the preincubation and the experimental media to inhibit extrusion of free BAPTA. The data are presented as % of maximum orexin-A response (a) and normalized to the control orexin-A response at each orexin-A concentration (b). The significances are indicated for K+-TBM compared to control, for 1 μM [Ca2+]e compared to K+-TBM, and for 1 μM [Ca2+]e+BAPTA-AM compared to 1 μM [Ca2+]e alone; 1 μM [Ca2+]e+BAPTA-AM, 1 μM [Ca2+]e+2 mM Ba2+ and 140 nM [Ca2+]e are not significantly different from each other. *P<0.05; **P<0.01; ***P<0.001.
Our previous results suggest that OX1R-stimulated IP3 production is amplified by extracellular Ca2+ (probably via Ca2+ influx) at low concentrations of orexin-A (e.g. 1 nM) (Lund et al., 2000). At higher concentrations, IP3 production becomes gradually less dependent on Ca2+ influx (Lund et al., 2000, Johansson and Kukkonen, unpublished observations). Low concentrations of orexin-A primarily activate ROCs, whereas SOC activation is a secondary response, and only high concentrations of orexin-A activate SOCs without previous involvement of ROC activity (Kukkonen and Akerman, 2001; Ammoun et al., 2006). Therefore, PLC activity at different concentrations of orexin-A is likely to be dependent on the different influx pathways.
To test the importance of the Ca2+ influx from the extracellular side for PLC activity, the total influx was inhibited by different methods before stimulation with orexin-A. Upon removal of the extracellular Ca2+ by using the Ca2+ chelator EGTA (reduces the extracellular Ca2+ concentration [Ca2+]e to ∼140 nM), the generation of inositol phosphates was almost completely abolished for all the orexin-A concentrations tested (Figure 1a and b). Reduction of [Ca2+]e to approximately 1 μM is enough to abolish the Ca2+ response to low concentrations of orexin-A (Lund et al., 2000). A free Ca2+ concentration of about 1 μM was obtained in nominally Ca2+-free TBM (no CaCl2 or EGTA added); also in this medium, the impact on inositol phosphate production was marked, yet less dramatic than in 140 nM free Ca2+. Strong inhibition (⩾90%) of inositol phosphate production was seen with low OX1R stimulation (low concentrations of orexin-A, 1–10 nM), which successively recovered at higher orexin-A concentrations, although at 1 μM the response was still 25% lower than the control response (Figure 1a and b).
PLC activity should be affected by the intracellular (submembrane) Ca2+ concentration and its elevation upon Ca2+ influx, and not [Ca2+]e as such. Thus, the fact that 140 nM [Ca2+]e (EGTA added) reduces the orexin-A response much more than 1 μM (no EGTA) is probably owing to the ability of EGTA not only to remove the extracellular Ca2+ but also to ‘extract out' the intracellular Ca2+ during the long incubation required for the assay. This would effectively inhibit all PLC activity, which is essentially dependent on Ca2+ (reviewed by Rhee, 2001; Katan, 2005). To verify this possibility, we loaded the cells with 30 μM BAPTA-AM (20 min preincubation); 30 μM should effectively clamp the overall intracellular Ca2+ to the resting level (or even lower). When these cells were exposed to orexin-A in the presence of 1 μM [Ca2+]e, the inhibition of the PLC activity was as strong as that obtained in 140 nM [Ca2+]e. We further exposed the cells to 2 mM Ba2+ in the presence of 1 μM [Ca2+]e. This treatment, again, was as effective as EGTA or BAPTA-AM. It is likely that Ba2+, upon entering the cells (see e.g. Ammoun et al., 2006), replaces Ca2+ at PLC resulting in inactive PLC. Both the data with BAPTA-AM and Ba2+ support the view that the additional effect obtained with EGTA as compared to 1 μM [Ca2+]e depends on effects on intracellular targets.
Depolarization should theoretically inhibit Ca2+ influx by reducing the driving force for Ca2+ entry. Indeed, strong depolarization (to +40 mV), applied using patch-clamp electrodes, can fully block the Ca2+ response to low concentrations of orexin-A (Lund et al., 2000), while depolarization to ∼0 mV with high-K+ medium (K+-TBM) produces a strong but not as complete block (∼75% block) (Larsson et al., 2005). Using K+-TBM, we could see strong inhibition (>80%) of the PLC activity at 1 nM orexin-A, which successively disappeared and no inhibition was seen at 1 μM orexin-A. In principle, responses in 1 μM Ca2+ and K+-TBM should be equal (Figure 1), yet K+-TBM was less effective. Expression of voltage-gated Ca2+ channels might distort the results. However, this does not seem to be the case here as depolarization of CHO cells did not cause any measurable Ca2+ elevation (not shown, see also Lund et al., 2000; Larsson et al., 2005). Therefore, the decreased effectiveness of K+-TBM might be owing to its less than complete inhibition of orexin-A-induced Ca2+ elevation (Larsson et al., 2005).
Thus, these data show that Ca2+ influx is required for OX1R stimulation of PLC activity at low concentrations of orexin-A, whereas at higher concentrations, the response is less dependent on Ca2+ influx.
ROCs are more important for OX1R-mediated PLC stimulation than SOCs
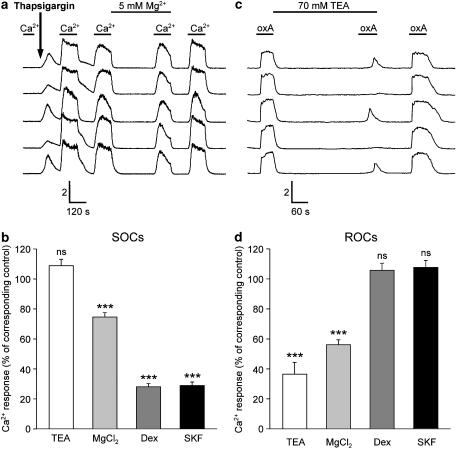
We next wanted to focus on the role of the different channel types activated on stimulation of OX1R, namely ROCs and SOCs, on PLC activity. For this we used pharmacological inhibitors TEA, Mg2+, dextromethorphan, SKF-96365 and 2-APB, identified in a previous study as suitable for separation of ROC and SOC activity in CHO cells (Larsson et al., 2005). The selectivity of these substances for ROCs over SOCs was evaluated using Ca2+ imaging (Figure 2). Thapsigargin is an irreversible inhibitor of the endoplasmic/sarcoplasmic reticulum Ca2+-ATPase (Serca), and it can be used to activate SOCs irreversibly (Figure 2a and b, see also Krjukova et al., 2004; Ammoun et al., 2006). ROC activity was tested by investigating the response to1 nM orexin-A, a response that is completely dependent on ROCs (Figure 2c and d, see also, e.g. Ammoun et al., 2006). TEA did not have any effect on the SOC activity, but it strongly, although only partially, inhibited ROCs. TEA does not depolarize CHO cells (Larsson et al., 2005) so this effect was not caused by a reduction in the driving force for Ca2+ entry. In contrast to TEA, dextromethorphan and SKF-96365 strongly inhibited SOCs but did not have any effect on ROCs. We have previously evaluated the effects of 2-APB on both SOCs and ROCs, and it has an inhibitory profile identical to dextromethorphan and SKF-96365 (see e.g. Ammoun et al., 2006). Mg2+ displayed significant though weak inhibition of the SOC-dependent influx and a stronger inhibition of ROCs. Thus, based on these data together with our previous data on 2-APB, we can classify the inhibitors as follows: TEA, a strong ROC inhibitor; MgCl2, a strong (though weaker than TEA) ROC inhibitor and also a weak SOC inhibitor; dextromethorphan, SKF-96365 and 2-APB, strong SOC inhibitors.
Figure 2.
The effects of some Ca2+ channel inhibitors on ROC- and SOC-dependent Ca2+-influx. (a and b) SOC-dependent influx was assessed by exposing the cells to 1 μM thapsigargin, an irreversible inhibitor of SERCA pumps, and thus also an activator of SOCs, and then exposing the cells to alternating cycles of TBM containing 1 mM (‘Ca2+') and 1 μM Ca2+ (minimum of 2 min of each medium) ((a) Krjukova et al., 2004; Ammoun et al., 2006). The cells were preincubated with the inhibitors TEA (70 mM), MgCl2 (Mg2+; 5 mM) and SKF-96365 (SKF; 10 μM) for 5 min and dextromethorphan (Dex; 100 μM) for 10 min in 1 μM [Ca2+]e min before applying 1 mM Ca2+. (c and d) ROC-dependent influx was assessed by exposing the cells to 1 nM orexin-A, a concentration at which the whole Ca2+ response is dependent on activation of ROCs. The cells were perfused with 1 mM Ca2+ throughout the experiment. Preincubation times for the inhibitors were the same as in (a). For both (a and b) and (c and d), the controls (absence of inhibitor), from which the inhibition was calculated, were from the same experiments. The significances for the inhibitors are indicated with respect to the controls normalized to 100%. The calibration bars indicate the ratio (ordinate) and time (abscissa) (a and c). ***P<0.001.
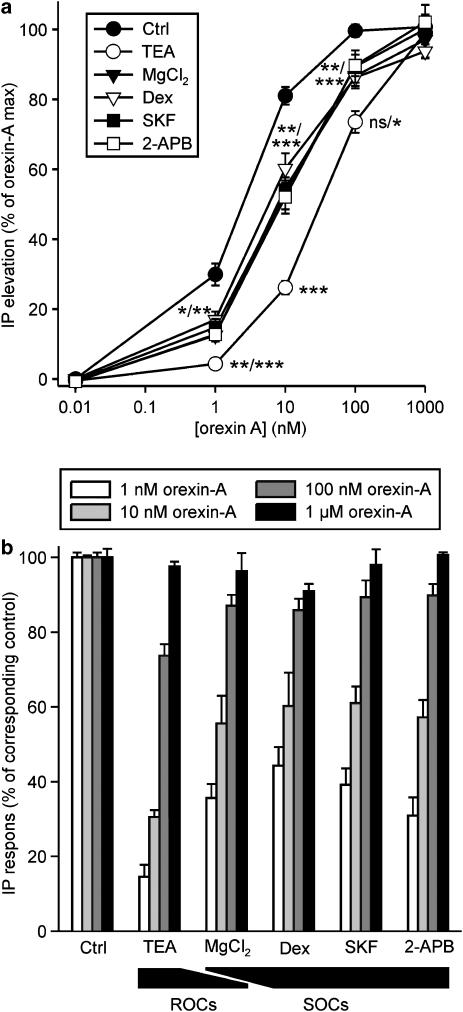
We next evaluated the effect of these inhibitors on OX1R-induced PLC activity. Inhibition of the ROCs with TEA was clearly the most effective treatment for reducing inositol phosphate liberation (Figure 3), being equally if not even more effective than K+-TBM (Figure 1), with an almost complete inhibition of the response to 1 nM orexin-A. All the other treatments, Mg2+, dextromethorphan, SKF-96365 and 2-APB, that either produce a complete block of SOCs or incomplete block of both ROCs and SOCs, were similarly effective but significantly less effective than TEA (Figure 3). This suggests that ROCs are required to trigger the PLC activity at low concentrations of orexin-A, whereas SOCs only have a further amplifying role, although even this effect is significant especially at low concentrations of orexin-A. Remarkably, the inhibitory effect of all of the compounds (Figure 3), as also that of K+-TBM (Figure 1), was strongest at 1 nM orexin-A and fully absent at 1 μM orexin-A.
Figure 3.
The effects of blocking ROC- and SOC-dependent Ca2+-influx on OX1R-stimulated PLC activity. CHO cells were kept in normal TBM (1 mM [Ca2+]e) and when indicated, preincubated with 70 mM TEA (5 min), 5 mM MgCl2 (Mg2+; 5 min), 100 μM Dex (10 min), 10 μM SKF-96365 (SKF; 5 min) or 20 μM 2-APB (5 min) before stimulation with orexin-A. The data are presented as % of maximum orexin-A response (a) and normalized to the control orexin-A responses at each orexin-A concentration (b). The significances are indicated for TEA compared to the other inhibitors and for the other inhibitors compared to control (Ctrl). The significances separated by a slash indicate some differences compared to different inhibitors. There were no significant differences in the inhibitory potencies of the inhibitors (Mg2+, Dex, SKF and 2-APB) other than for TEA. *P<0.05; **P<0.01; ***P<0.001.
SOC activity enhances M1 cholinoceptor-mediated PLC stimulation
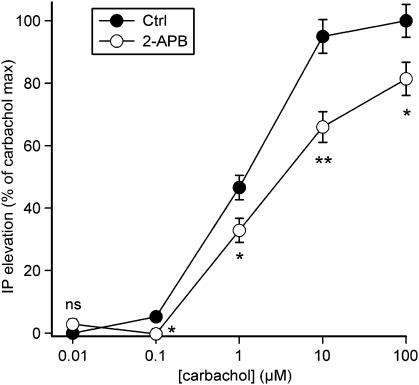
M1/M3/M5 muscarinic cholinoceptors are classical Gq-coupled receptors, which signal to Ca2+ elevations through the PLC-IP3 pathway in all expression systems, including CHO cells (reviewed by Caulfield, 1993). Yet, this PLC response can also be amplified by Ca2+ influx, putatively via SOCs (Wojcikiewicz et al., 1994; Kim et al., 1999; Thore et al., 2005). To confirm this and to compare this response with the OX1R-mediated response, we transiently transfected CHO cells with the human M1 receptor and measured carbachol-stimulated inositol phosphate generation in the absence and in the presence of the SOC blocker 2-APB. Carbachol stimulated inositol phosphate production, the maximum being 3.6±0.4 times the level under basal conditions and EC50=1.2±0.2 μM (Figure 4). In contrast to the OX1R response (Figure 3), 2-APB inhibited inositol phosphate production induced by all the carbachol concentrations tested (Figure 4).
Figure 4.
The effect of blocking SOC-dependent Ca2+-influx on human M1 muscarinic receptor-stimulated PLC activity. CHO cells were kept in normal TBM (1 mM [Ca2+]e) and where indicated, preincubated with 20 μM 2-APB for 5 min before stimulation with carbachol. The data are presented as % of maximum carbachol response. *P<0.05; **P<0.01.
Discussion and conclusions
PLCs are Ca2+-dependent enzymes, and for some isoforms, the EC50 value for Ca2+ lies in a region where the enzyme is likely to be significantly stimulated by receptor-mediated Ca2+ elevations (Allen et al., 1997; reviewed by Rhee, 2001; Kouchi et al., 2004). Indeed, Ca2+ release and store-operated Ca2+ influx pathways have been shown to stimulate PLC signalling in different cell types (Wojcikiewicz et al., 1994; Kim et al., 1999; Thore et al., 2005); this results in a positive feedback loop where stimulation of PLC activity induces, via for example Gαq, IP3-dependent Ca2+ release and therewith store-operated Ca2+ influx, which further enhances PLC signalling. The store-operated Ca2+ influx or the Ca2+ release itself are also thought to assist in the generation of oscillatory IP3 and Ca2+ signalling (Young et al., 2003; Kouchi et al., 2004; Thore et al., 2004). Also other types of Ca2+ elevation, for instance, that artificially induced using ionomycin, can stimulate PLC (Del Rio et al., 1994; Wojcikiewicz et al., 1994; Lund et al., 2000).
OX1Rs are known to induce marked Ca2+ influx responses (reviewed by Kukkonen and Åkerman, 2005), in addition to the more direct activation of PLC. Therefore, in this study we have studied the importance of different Ca2+ influx pathways for the signalling of OX1R to PLC. We have used different techniques to inhibit Ca2+ influx including Ca2+ removal/chelation, Ca2+ replacement, reduction in the driving force for Ca2+ entry and pharmacological blockers. All these techniques have their advantages and drawbacks, as in some degree discussed in the Results section. In summary, based on the experiments with nominally Ca2+-free medium, EGTA, BAPTA-AM, Ba2+ and depolarization (K+-TBM), we consider that nominally Ca2+-free medium is the most reliable method of producing virtually complete inhibition of Ca2+ influx with a minimum impact on basal intracellular Ca2+ levels. Depolarization should offer an equally effective and reliable treatment, but it is not possible to abolish the driving force for Ca2+ entry completely using K+-TBM (Larsson et al., 2005). Different pharmacological inhibitors are also used to inhibit Ca2+ channels. These inhibitors show poor selectivity for specific channels, but they can be useful for particular channels, especially when used in panels. In selection of inhibitors for ROCs and SOCs, we relied on previous studies with CHO cells both from our own (Kukkonen et al., 2001; Ammoun et al., 2006) and another laboratory (Larsson et al., 2005). In the latter study, SKF-96365 and 2-APB were identified as strong and selective blockers of SOCs. TEA and Mg2+ were relatively strong and selective blockers of OX1R-activated ROCs at concentrations of 70 mM and 5 mM, respectively, and dextromethorphan (100 μM) was a strong inhibitor of ROCs and it also showed significant inhibition of SOCs. Although we can verify most of these data in the present study, the selectivity profiles of these inhibitors, especially Mg2+ and dextromethorphan are clearly different from those obtained by Larsson et al. (2005). We have previously observed that orexin-A displays significantly higher potency in the CHO-OX1 cells used by Larsson et al. (2005) than those used in the present study. This may be an indication of partially different expression of components, for example, Ca2+ channel subunits. For instance, increased expression of TRPC3 would enhance the potency of TEA as compared to dextromethorphan (Larsson et al., 2005). Through generation of large quantities of single cell data we could also observe significant heterogeneity in the response of individual cells to inhibitors of ROCs but not SOCs (see Figure 2c); this suggests that expression of the channel components making up ROCs may be subjected to variations even within a single ‘line' of CHO-OX1 cells. Alternatively, this could reflect significantly higher receptor expression levels in the cells of Larsson et al. (2005), which could also lead to differential signal coupling.
The data show that OX1R-mediated PLC activity is strongly amplified by Ca2+ influx at low orexin-A concentrations, as assessed by removal of extracellular Ca2+ and reduction of the driving force for Ca2+ entry. We have previously shown that Ca2+ elevation as such, induced with ionomycin or thapsigargin, is a weak stimulant of PLC activity in CHO cells (Lund et al., 2000) and we verified this in the present study (data not shown). Thus, even though Ca2+ is itself a weak stimulant of PLC activity, it can strongly enhance PLC activity induced by stimulation of the orexin receptor. Both SOCs and ROCs contribute to this amplification. However, ROCs appear to be more important than SOCs as inhibition of ROCs (with TEA) causes a similar degree of inhibition as complete block of the Ca2+ influx, induced by reducing [Ca2+]e to 1 μM or depolarization (with K+-TBM). Furthermore, a very weak block of SOCs combined with a stronger block of ROCs (Mg2+) results in an inhibitory effect on PLC activity that is as potent as that induced by strong inhibitors of SOCs. This is not surprising as ROC activity is the primary response to orexin receptor activation at low concentrations of orexin-A (Lund et al., 2000; Larsson et al., 2005), and SOCs are only activated consequent to PLC activation. However, the effect of ROCs may be more complex than a simple amplification of PLC activity; we have recently suggested that cytosolic Ca2+ elevation via Ca2+ channel activity has a critical role in the coupling of OX1R to signal pathways such as adenylyl cyclase and ERK (Ammoun et al., 2006). Thus, ROC-mediated Ca2+ influx may also act, in addition to PLC, upstream of PLC, possibly at OX1Rs.
The role of Ca2+ release is more difficult to determine. As the treatments inhibit Ca2+ entry, they also inhibit PLC activity and IP3-dependent Ca2+ release at low orexin-A concentrations. Consequently, there is no Ca2+ release that could amplify PLC activity. It is yet possible that Ca2+ elevation through release from intracellular stores affects PLC activity under the normal signalling cycle. However, the contention that, for orexin signalling at low concentrations, ROC activity is the primary response giving rise to the other responses (Ammoun et al., 2006) should also be borne in mind. This view is strongly supported by the results of this study. As 2-APB was originally presented as an inhibitor of IP3 receptors, the effect obtained with it could originate from inhibition of Ca2+ release (and SOC activation). However, our previous studies suggest that 2-APB at 100 μM is – at best – a poor inhibitor of Ca2+ release in many cell types, including CHO cells (Kukkonen et al., 2001; see also Bootman et al., 2002; Ammoun et al., 2006) and that even this effect may relate to its ability to release Ca2+ itself at high concentrations (Missiaen et al., 2001). Also, 20 μM 2-APB has an equivalent effect on PLC as the other strong inhibitors of SOCs, dextremethorphan and SKF-96365. Therefore, it is assumed that 2-APB behaves as an SOC inhibitor in our system.
Muscarinic M1 receptor signalling to PLC was also affected by SOCs. Most interestingly, SOCs appeared to amplify muscarinic receptor signalling to PLC throughout the carbachol concentration range, in contrast to OX1Rs for which the inhibition reversed at high ligand concentrations. Based on previous studies, PLC is expected to be amplified by Ca2+ throughout the entire range of receptor activity (Willars and Nahorski, 1995; Kim et al., 1999), and therefore the behaviour of OX1R is considered to be unusual. It is possible that the much higher level of PLC activity obtained with OX1Rs leads to a bottle-neck at some stage of the signal cascade, for instance, through a rate-limiting access to PIP2, and that this gives an apparent lack of Ca2+ effect at high orexin-A levels despite the actual amplification. An alternative possibility is that activities of different PLC isoforms are engaged by muscarinic and orexin receptors. Orexin receptors interact with at least three different families of G-proteins with different efficacies (Randeva et al., 2001; Holmqvist et al., 2005; Magga et al., 2006), and it is possible that different PLC isoforms are also engaged at different receptor activation levels (different ligand concentrations). It has been previously shown that the regulation of PLC activity can be dramatically different for different GPCRs even in a single cell type (Young et al., 2003), and one factor affecting signalling in our CHO cells could also be the expression level of the respective receptors. However, it is noticeable that stimulation of OX1Rs causes maximum increase in PLC activity 5- to 10-fold higher than that induced on stimulation of muscarinic receptors, which are also strongly coupled to PLC (PLCβ). Therefore, it is likely that different PLC isoforms are implicated in OX1 and muscarinic receptor signalling. However, this and the actual isoforms involved remain to be determined.
In native neurons as well as in recombinant expression systems, orexin receptors activate non-selective cation influx pathways and putatively the reverse (Ca2+-elevating) mode of Na+/Ca2+ exchanger, and activation of voltage-gated Ca2+ channels is seen in both neurons and endocrine cells (reviewed by Kukkonen and Åkerman, 2005). Thus, orexin receptors have both (i) the ability and (ii) the propensity to connect to Ca2+ influx. We have suggested that this signalling is also (iii) necessary for OX1R signalling altogether (Ammoun et al., 2006). However, so far this has not been shown in native cell systems and, altogether, evidence for the mechanistic significance of this coupling in native systems is scarce. Signalling to Ca2+ channels could have an important role in effects such as receptor plasticicity, presynaptic facilitation and hormone release (reviewed by Kukkonen and Åkerman, 2005).
In conclusion, we have shown that OX1R signalling to PLC is very strongly amplified by the influx of Ca2+ induced via ROCs and SOCs. As this effect is most marked at low orexin-A concentrations, it is likely to represent a physiologically relevant signalling mechanism for the orexin receptor. The stronger impact on this effect induced by inhibition of ROCs, compared to SOCs, is probably owing to the more central role of ROCs in orexin receptor signalling. These results stress the importance of doing future studies on the mechanism of orexin receptor coupling to ROCs and evaluating the physiological significance of this system.
Acknowledgments
This work was supported by the European Union Contract QLG3-CT-2002-00826, The Swedish Research Council, the Göran Gustafsson Foundation, the Novo Nordisk Foundation, the Mary, Åke and Hans Ländall Foundation and Uppsala University.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester
- [Ca2+]e
extracellular Ca2+ concentration
- carbachol
carbamoylcholine chloride
- CHO
Chinese hamster ovary
- Dex
dextromethorphan
- EGTA
ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid
- GPCR
G-protein-coupled receptor
- ERK
extracellular signal-regulated kinase
- IP3
inositol trisphosphate
- OX1R
OX1 orexin receptor
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PLC
phospholipase C
- probenecid
p-(dipropylsulphamoyl)benzoic acid
- ROC
receptor-operated Ca2+ channel
- SERCA
endoplasmic/sarcoplasmic reticulum Ca2+-ATPase
- SKF-96365
1-(β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl)-1H-imidazole HCl
- SOC
store-operated Ca2+ channel
- TBM
TES-buffered medium
- TEA
tetraethylammonium chloride
- TES
2-([2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino) ethane sulphonic acid
Conflict of interest
The authors state no conflict of interest.
References
- Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. Regulation of inositol lipid-specific phospholipase cdelta by changes in Ca2+ ion concentrations. Biochem J. 1997;327:545–552. doi: 10.1042/bj3270545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, et al. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dawson RM, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- del Rio E, Nicholls DG, Downes CP. Involvement of calcium influx in muscarinic cholinergic regulation of phospholipase C in cerebellar granule cells. J Neurochem. 1994;63:535–543. doi: 10.1046/j.1471-4159.1994.63020535.x. [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Johansson L, Ostman M, Ammoun S, Akerman KE, Kukkonen JP. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Katan M. New insights into the families of PLC enzymes: looking back and going forward. Biochem J. 2005;391:e7–e9. doi: 10.1042/BJ20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Park TJ, Lee YH, Baek KJ, Suh PG, Ryu SH, et al. Phospholipase C-delta1 is activated by capacitative calcium entry that follows phospholipase C-beta activation upon bradykinin stimulation. J Biol Chem. 1999;274:26127–26134. doi: 10.1074/jbc.274.37.26127. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, et al. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Krjukova J, Holmqvist T, Danis AS, Akerman KE, Kukkonen JP. Phospholipase C activator m-3M3FBS affects Ca2+ homeostasis independently of phospholipase C activation. Br J Pharmacol. 2004;143:3–7. doi: 10.1038/sj.bjp.0705911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Akerman KE. Orexin receptors couple to Ca2+ channels different from store-operated Ca2+ channels. Neuroreport. 2001;12:2017–2020. doi: 10.1097/00001756-200107030-00046. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Åkerman KEO.Intracellular signal pathways utilized by the hypocretin/orexin receptors Hypocretins as Integrators of Physiological Signals 2005Springer Science Business Media: Berlin; 221–231.In: de Lecea L, Sutcliffe JG (eds) [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Akerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Lund PE, Akerman KE. 2-Aminoethoxydiphenyl borate reveals heterogeneity in receptor-activated Ca2+ discharge and store-operated Ca2+ influx. Cell Calcium. 2001;30:117–129. doi: 10.1054/ceca.2001.0219. [DOI] [PubMed] [Google Scholar]
- Larsson KP, Peltonen HM, Bart G, Louhivuori LM, Penttonen A, Antikainen M, et al. Orexin-A-induced Ca2+ entry: evidence for involvement of trpc channels and protein kinase C regulation. J Biol Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- Magga J, Bart G, Oker-Blom C, Kukkonen JP, Akerman KE, Nasman J. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol. 2006;71:827–836. doi: 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Callewaert G, De Smedt H, Parys JB. 2-Aminoethoxydiphenyl borate affects the inositol 1, 4, 5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29:111–116. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jcem.86.10.7921. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Tesfai Y, Brereton HM, Barritt GJ. A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem J. 2001;358:717–726. doi: 10.1042/0264-6021:3580717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore S, Dyachok O, Gylfe E, Tengholm A. Feedback activation of phospholipase C via intracellular mobilization and store-operated influx of Ca2+ in insulin-secreting beta-cells. J Cell Sci. 2005;118:4463–4471. doi: 10.1242/jcs.02577. [DOI] [PubMed] [Google Scholar]
- Thore S, Dyachok O, Tengholm A. Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J Biol Chem. 2004;279:19396–19400. doi: 10.1074/jbc.C400088200. [DOI] [PubMed] [Google Scholar]
- Willars GB, Nahorski SR. Quantitative comparisons of muscarinic and bradykinin receptor-mediated Ins (1, 4, 5)P3 accumulation and Ca2+ signalling in human neuroblastoma cells. Br J Pharmacol. 1995;114:1133–1142. doi: 10.1111/j.1476-5381.1995.tb13325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Tobin AB, Nahorski SR. Muscarinic receptor-mediated inositol 1, 4, 5-trisphosphate formation in SH-SY5Y neuroblastoma cells is regulated acutely by cytosolic Ca2+ and by rapid desensitization. J Neurochem. 1994;63:177–185. doi: 10.1046/j.1471-4159.1994.63010177.x. [DOI] [PubMed] [Google Scholar]
- Young KW, Nash MS, Challiss RA, Nahorski SR. Role of Ca2+ feedback on single cell inositol 1, 4, 5-trisphosphate oscillations mediated by G-protein-coupled receptors. J Biol Chem. 2003;278:20753–20760. doi: 10.1074/jbc.M211555200. [DOI] [PubMed] [Google Scholar]