Abstract
Background and purpose:
Selective oestrogen receptor (ER) modulators (SERMs) are of great value in the treatment of breast cancer and osteoporosis. The aim of this study was to characterize pharmacologically a new class of SERMs synthesized based on the core structure of raloxifene.
Experimental approach:
Competitive receptor binding and luciferase-based reporter methods were used to study the bioactivities of raloxifene analogues, followed by efficacy determination in breast cancer cell proliferation assay. ER antagonist effects were investigated in female rats by measuring uterine and mammary gland growth, using wet weight, BrdU incorporation and terminal end bud (TEB) as indicators.
Key results:
Five analogues, belonging to two different structural series and display higher binding affinities for ERα than ERβ were functionally evaluated. One such analogue, Y134, exhibited potent antagonist activity at ERs in CV-1 cells cotransfected with plasmids containing ERα or ERβ and oestrogen-response element-driven luciferase. The estimated IC50 value was 0.52 nM for ERα and 2.94 nM for ERβ, comparable to that of raloxifene. Little cytotoxicity was observed at Y134 concentrations below 10 μM. Y134 suppressed oestrogen-stimulated proliferation of ER-positive human breast cancer MCF-7 and T47D cells. At an identical dose, administered to ovariectomized rats, Y134 was more effective than raloxifene at arresting oestrogen-induced outgrowth of TEB and mammary gland DNA synthesis, but their inhibitory effects on the uterus were comparable.
Conclusions and Implications:
Y134 is a potent ER antagonist with better mammary gland selectivity than raloxifene and shows potential for development as a new SERM for therapeutic use.
Keywords: nuclear receptor, oestrogen, mammary gland, uterus
Introduction
Although hormone replacement therapy (HRT) with oestrogens alleviates postmenopausal symptoms and is effective in treating osteoporosis and cardiovascular diseases, the most common concern is an increased risk of breast cancer, endometrial hyperplasia and venous thrombosis (Beral et al., 1999; Steffen and Carnes, 2000; Rosendaal et al., 2002). A similar situation applies to tamoxifen – an oestrogen receptor (ER) antagonist widely used as an adjuvant treatment for breast cancer. However, its weak oestrogen-like activity in the uterus was found to be associated with an increased incidence of endometrial carcinoma after long-term administration.
The concern has led to continued efforts to find new compounds with improved efficacy and tissue selectivity. Selective oestrogen receptor modulators (SERMs) (Ariazi et al., 2006) were initially developed as anti-oestrogens for the treatment of breast cancer, but their unusual properties, such as supporting maintenance of bone density and promoting healthy blood lipid profile, have expanded to the treatment and prevention of other diseases as well (Siddiqui et al., 2005). Raloxifene (RAL) was approved by the Food and Drug Administration (FDA) in 1997 for treating osteoporosis in postmenopausal women. It exerts ER agonist effects on the bone and blood lipids, whereas in the endometrium and breast it acts as an antagonist (Francucci et al., 2005; Ohmichi et al., 2005). Recent studies involving a fairly large population demonstrated impressive benefits of this drug in prevention of breast cancer (Vogel et al., 2002, 2006; Sporn et al., 2004).
ER is a member of the nuclear receptor super family and consists of two subtypes, α and β. Upon binding to its endogenous ligand, 17β-oestradiol (E2), ER forms homodimers or heterodimers between the subtypes to recruit co-activator(s) and activate transcription (Klinge et al., 2004). Based on this mechanism, a variety of in vitro assay systems can be devised to examine bioactivities of potential ER modulators.
Working with medicinal chemists, a number of novel RAL analogues were designed and synthesized in an attempt to discover novel tissue-selective ER antagonists. Following initial characterization relative to their ER binding affinities (Ji et al., 2005; Yang et al., 2005), a group of five was selected for sequential in vitro and in vivo evaluation using a variety of bioassays, as described in the present study. As a result of the present study, a novel SERM, Y134 ([6-hydroxy-2-(4-hydroxy-phenyl)-benzo[b]thiophen-3-yl]-[4-(4-isopropylpiperazin-1-yl)-phenyl]-methanone), was identified, which may pave the way for preclinical development of a new drug to combat breast cancer.
Methods
Chemicals
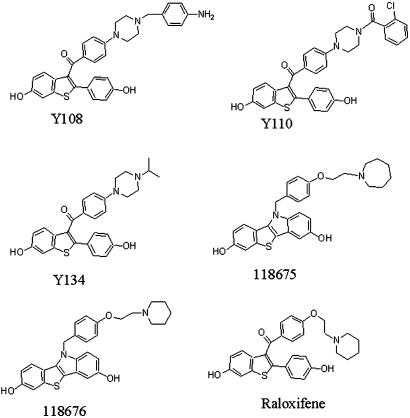
RAL analogues were designed and synthesized as previously described (Ji et al., 2005; Yang et al., 2005). Of which, Y108, Y110, Y134, 118675 and 118676 belong to two different structural series (Figure 1). The compounds (purity 95% minimum) were dissolved in dimethyl sulphoxide (DMSO) and stored at −20°C before use.
Figure 1.
Structures of RAL and its analogues.
Reagents
RAL, 17β-oestradiol (E2), 5-bromo2′deoxy-uridine (BrdU) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) were obtained from Sigma (St Louis, MO, USA). T4 DNA ligase, restriction enzymes and buffers used for construction of plasmids were bought from Takara (Dalian, Liaoning, China). Dulbecco's modified Eagle's medium (DMEM), RPMI1640 medium, bovine foetal serum (BFS) and charcoal/dextran-treated BFS (CDT-BFS) were purchased from Hyclone (Logan, UT, USA). Fugene 6 transfection reagent was the product of Roche (Indianapolis, IN, USA) and Steady-Glo luciferase kit of Promega (Madison, WI, USA). AlamarBlue was obtained from Biosource International (Camarillo, CA, USA) and anti-BrdU mouse monoclonal IgG1 from Becton Dickinson (San Jose, CA, USA). Full-length nuclear receptors of human origin, including ERα, ERβ, androgen receptor (AR), progesterone receptor (PR), glucocorticoid receptor (GR) and mineral corticoid receptor (MR), used in the binding assay, were produced by Bac-to-Bac baculovirus expression system (Invitrogen, Carlsbad, CA, USA) following the manufacture's instructions.
Plasmids
Human ERβ (pSG5-hERβ) plasmid was kindly provided by Dr Jan-Åke Gustafsson of Karolinska Institutet (Stockholm, Sweden) and a luciferase reporter plasmid (ERE-MMTV-Luc) by Dr Donald McDonnell of Duke University (Durham, NC, USA). The insect expression vector for human ERα (EX-A0322-I01) was purchased from FulenGen (Guangzhou, China), based on which the ERα mammalian expression vector was constructed. Briefly, a primer pair (forward:
5′-CCGGAATTCGCCACCATGACCATGACCCTCCACACCAAAG-3′; reverse:
5′-CCGCTCGAGTCAGACTGTGGCAGGGAAAC-3′; HPLC purity), with restriction sites for XhoI and EcoRI, was synthesized by Shanghai Sangon Biological Engineering & Technology and Service Co., Ltd (Shanghai, China). ERα gene in EX-A0322-I01 was amplified by PCR with the primer pair under the following conditions: 95°C for 4 min of initial denaturation, 22 cycles of denaturation at 95°C for 45 s, annealing at 71°C for 45 s, extension at 72°C for 2 min 20 s, and a final extension at 72°C for 10 min. The reaction mixture contained 50 ng template DNA (EX-A0322-I01), 10 × Pyrobest Buffer II (Mg2+ plus, 10 mM; Takara) 5 μl, each of the oligonucleotide primer (20 μM) 1 μl, dNTP (2.5 mM each) 4 μl, 5 U μl−1 Pyrobest DNA polymerase (Takara) 0.25 μl and H2O 37.75 μl to give a final volume of 50 μl. The amplified products were analysed by electrophoresis and the corresponding ERα gene fragments were purified by a gel extraction kit (Omega Bio-tek Inc., Doraville, GA, USA). They were then digested with XhoI and EcoRI restriction enzymes and inserted into pcDNA3.1(+) expression vector (Invitrogen) to construct pcDNA3.1-hERα. The selected colony was analysed by DNA sequencing and confirmed to be the right ERα gene (NM_000125).
Receptor-binding assay
Receptor-binding assay was performed as previously described (Wu et al., 2005). The assay buffer consists of 10% glycerol (v/v), 25 mM NaH2PO4, 0.5 mM MgCl2, 1 mM DTT, 1 mM EDTA, 5 mM zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 2 μg ml−1 aprotinin and 100 μM leupeptin. An appropriate amount of respective nuclear receptor protein extract (stock concentrations: ERα 10.5 mg ml−1, ERβ 10.2 mg ml−1, AR 12.6 mg ml−1, PR 12.8 mg ml−1, GR 13.1 mg ml−1 and MR 12.8 mg ml−1) was loaded into each well of Isoplate containing the assay buffer, followed by addition of [3H]E2 (4 μl, 5 nM), [3H]dihydrotestosterone (DHT, 5.5 μl, 5 nM), [3H]progesterone (4 μl, 5 nM) and [3H]dexamethasone (4.5 μl, 5 nM), respectively. All the radioisotopes were purchased from Amersham Biosciences (Buckinghamshire, UK). Various concentrations of cold ligands were added thereafter (2.5 μl) to give a final volume of 100 μl well−1. The plates were sealed and incubated overnight at 4°C. In total, 25 μl hydroxyapatite (HA) (25%, v/v) was added to each well the next morning and the plates were gently agitated twice for 5 min each. Following centrifugation for 3 min at 1200 g, the supernatant was decanted and 100 μl assay buffer added to each well. This washing procedure was repeated twice before adding 150 μl scintillation liquid (PerkinElmer, Boston, MA, USA), gently agitating the plates to resuspend HA and counting with a MicroBeta counter (PerkinElmer). Ki values were calculated from IC50 using the equation of Cheng and Prusoff (Khan et al., 1994).
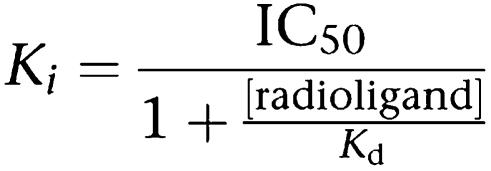 |
Co-transfection assay
Transient co-transfection assay was performed with Fugene 6 according to the manufacturer's instructions. CV-1 cells were cultured in the presence of DMEM supplemented with 10% CDT-FBS and seeded 24 h before transfection in 6 cm dish (6 × 105 cells per dish). Reporter plasmid (ERE-MMTV-Luc) (2 μg) and plasmid containing ERα (pcDNA3.1-hERα) or ERβ (pSG5-hERβ) were introduced simultaneously into cells with a ratio of 5 to 1. Cells were transfected for 8 h, and harvested with 0.05% trypsin and 0.02% EDTA before reseeding onto a 96-well microtitre plate (8000 cells per well). ERα expression was detected by Western blot analysis, corresponding to a molecular weight of 67 kDa. Transfected cells were incubated for 24 h with or without various concentrations of control or test compounds. For antagonist assay, test samples were added 30 min before E2. Cell extracts were prepared and the luciferase activity expressed was determined in a Wallac 1420 multilabel counter (VICTOR2, PerkinElmer) using a Steady-Glo luciferase kit from Promega. Before luciferase activity measurement, treated cells were reacted with alamarBlue (Biosource) (Hamid et al., 2004) for 4 h and fluorescence intensity was monitored at 540 nm excitation wavelength and 590 nm emission wavelength on a FlexStation 384II (Molecular Devices, Sunnyvale, CA, USA). The relative luciferase activity was normalized against cell viability (% growth) assessed with alamarBlue in the same well.
Cell proliferation assay
MCF-7 cells derived from human breast cancer were maintained in RPMI1640 medium supplemented with 10% BFS and 0.01 mg ml−1 bovine insulin. T47D cells were maintained in RPMI1640 supplemented with 10% BFS. The medium was changed to RPMI1640 with 10% CDT-BFS 48 h before assaying. MCF-7 cells were seeded at a density of 2000 cells per well onto 96-well microtitre plates and incubated at 37°C overnight. For antagonist assay, 1.0 nM E2 and various concentrations of test compounds were added to the medium (changing on the third day) and incubated for 6 days. Colouration substrate, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), was added to the medium followed by further incubation for 3 h. The medium was then displaced by 150 μl DMSO and absorbance at 560 nm, with a 690 nm reference, measured. IC50 value was calculated as the concentration of ligand required to give half-maximal inhibition of cell proliferation (Wang and Lou, 2004). The proliferation assay for T47D cells was conducted in the same manner except that the cell density used was 3000 per well and E2 concentration was 10 nM. The ER-negative cell line (MDA-MB-231; Harnagea-Theophilus and Miller, 1998) maintained in Leibovitz's L-15 medium containing 10% BFS was used as control. Cells (1500 per well) were seeded onto 96-well microtitre plates and incubated with 10 nM E2 and various concentrations of test compounds for 3 days followed by the above MTT measurement. For agonist assays on these cell lines, no E2 was incubated with RAL or Y134.
Animals and treatment
Four-week old female Sprague–Dawley rats were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China) and housed at 22°C under a standard 12 h light/12 h dark cycle with food and water available ad libitum. They were allowed to adapt to the new environment for at least 3 days. Ovariectomy was carried out (bilateral dorsal surgery) with a parallel sham group, which underwent the surgical procedure without removal of ovaries (control). Following a 4-week recovery period, rats were randomly divided into six groups with seven rats per group: (1) sham-operated group received vehicle alone; (2) ovariectomized group received vehicle alone; (3) ovariectomized animals received subcutaneously injected E2 (20 μg kg−1 per day × 3); (4) ovariectomized rats received a combination of E2 (as above) and an oral dose of RAL (3 mg kg−1 per day × 3); (5) ovariectomized animals received a lower oral dose of Y134 (1 mg kg−1 per day × 3); and (6) ovariectomized rats received a higher oral dose of Y134 (3 mg kg−1 per day × 3). RAL and Y134 were given in 0.5% sodium carboxymethyl cellulose (aqueous suspension), whereas E2 was formulated in sesame oil. On day 4, BrdU in saline was administered intraperitoneally 4 h (100 mg kg−1) and 1 h (50 mg kg−1) before the animals were killed and the mammary tissue and uterus were removed.
Mammary gland terminal end bud outgrowth assay
Mammary tissue was mounted onto a glass slide and treated with Carnoy's fix solution (100% ethanol:chloroform:glacial acetic acid=6:3:1). After overnight fixation, the sample was immersed in 70% ethanol for 15 min and fat tissue removed by incubating the slide in acetone for 30 min, three times in sequence. Re-hydration was performed by treatment with 100% ethanol for 30 min and then 95% ethanol for an additional 30 min. Mammary glands were stained with 1% acetic carmine for 2 h, dehydrated for 1 h each with 70% ethanol and 95% ethanol, and then overnight with 100% ethanol. The preparation was cleared in xylene for 15 min before examination (Bocchinfuso et al., 2000). The stained mammary tissue was photographed and the terminal end bud (TEB) counted from randomly selected five fields under an XTL-3400Z stereo microscope ( × 30; Shanghai Zhou Shan Fine Optical Instruments Co., Ltd, Shanghai, China). Positive score was given when counted rami were more than four and percentage of the positive TEB was calculated based on an average reading of five fields.
BrdU incorporation assay
Uterine and mammary gland tissue DNA syntheses were analysed using an immunohistochemistry. Longitudinal tissue sections (5 μm) were mounted onto poly-L-lysine-coated glass slides and immunohistochemical staining of incorporated BrdU was performed as previously described (Edwards et al., 1998). Briefly, the sections were treated to remove the paraffin and re-hydrated. After being heated in the microwave for 10 min in 10 mM sodium citrate (pH=6.0), the sections were washed twice in PBS for 5 min each at room temperature. For the mammary gland, tissue sections were digested with proteinase K (10 μg ml−1 in 50 mM Tris buffer, pH 7.5; Sangon) at 37°C for 15 min. Samples were blocked by incubating with 10% bovine serum albumin (BSA; blocking solution) in phosphate-buffered saline (PBS) for 3 h at room temperature. Incubation of samples (1 h) with mouse anti-BrdU monoclonal antibody (IgG1) at a dilution of 1:50 in the blocking solution was completed at room temperature. Samples were then washed twice with PBS for 4 min each and reacted with 1:100 FITC-conjugated goat anti-mouse IgG antibodies in the blocking solution for 1 h at room temperature. After two washes with PBS (4 min each), DAPI (4,6-diamidino-2-phenylindole, 0.5 μg ml−1 in PBS) was added onto the slides that were washed again after 10 min. They were then mounted with Slowfade Antifade Medium (Invitrogen) and examined using an epiflourescence microscope ( × 400; Olympus, Tokyo, Japan). Raw data were recorded in a blind manner with a minimum of five fields and the labelling index represents the FITC-stained vs all cells counted in each field × 1000.
Statistical analysis
Statistical analysis with Student's t-test was performed using GraphPad Prism software (GraphPad, San Diego, CA, USA) and data are presented as means±s.e. mean. The criterion for significance was a probability of less than 0.05.
Results
Receptor-binding properties
A total of 88 compounds were designed and synthesized based on the core structure of RAL. Among them, Y108, Y110, Y134, 118675 and 118676 were selected for further evaluation in cell-based functional assays due to their relatively higher binding affinities for ER. Y108, Y110 and Y134 are all benzothiophenes containing a piperazine side chain without modification of the core structure of RAL, whereas 118675 and 118676 represent a change from the benzothiophene core to benzothio[3,2-b]indole (Figure 1). As shown in Table 1, this group of compounds appeared to be more selective for ERα than ERβ with Y134 showing the most significant difference (121.1-fold) in terms of receptor-binding affinity. Except for Y108, 118675 and 118676 that displayed some binding properties to AR, they possessed little or no cross-reactivity with other steroid receptors.
Table 1.
ER-binding affinities and steroid receptor cross-reactivities of raloxifene and its analogues
| Compound | ERα Ki (nM) | ERβ Ki (nM) | Selectivity [β]/[α] | AR Ki (nM) | PR Ki (nM) | GR Ki (nM) | MR Ki (nM) |
|---|---|---|---|---|---|---|---|
| Raloxifene | 0.24 | 6.30 | 25.9 | 2768 | NA | NA | NA |
| Y108 | 0.14 | 4.74 | 33.1 | 283.3 | 6550 | NA | NA |
| Y110 | 0.57 | 1.07 | 1.9 | NA | NA | NA | NA |
| Y134 | 0.09 | 11.31 | 121.1 | NA | NA | NA | NA |
| 118675 | 1.11 | 2.42 | 2.2 | 57.15 | NA | NA | NA |
| 118676 | 0.95 | 3.67 | 3.9 | 99.93 | NA | NA | NA |
Abbreviations: AR, androgen receptor; ER, oestrogen receptor; GR, glucocorticoid receptor; MR, mineral corticoid receptor; NA, not active, PR, progesterone receptor.
Transcriptional activities on ER
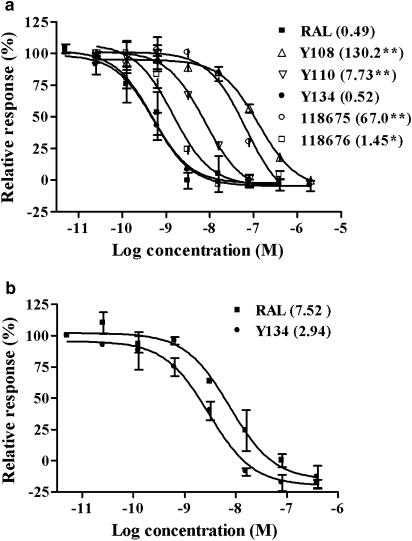
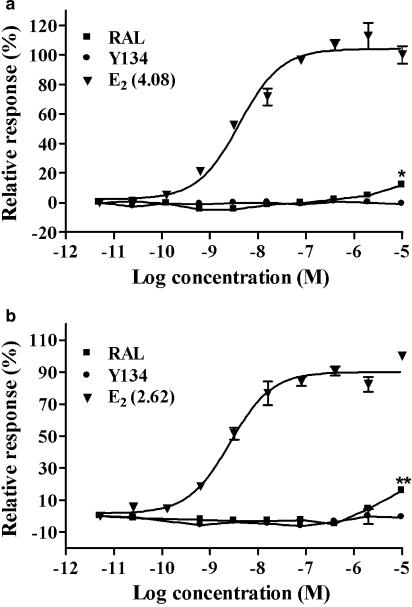
We first examined the antagonist effects of the five compounds in a reporter gene assay where plasmids containing ERα or ERβ and ERE-MMTV-Luc were transiently co-transfected in CV-1 cells. All the five analogues exhibited various degrees of ERα antagonist activities with Y134 being the most potent (IC50=0.52 nM; Figure 2a). Further assessment with ERβ confirmed that Y134 was also highly effective for this subtype (IC50=2.94 nM; Figure 2b). The preferential specificity for ERα over ERβ is in agreement with the activities observed in the binding assay (Table 1). In addition, Y134 showed little effect on the CV-1 cell viability in the range of concentrations studied (up to10 μM) with alamarBlue (data not shown), implying that the above antagonist activity is not a consequence of cytotoxicity. As seen in the binding assay, no cross-reactivity was detected for Y134 in cells transfected with AR, PR, GR and MR (data not shown). When tested for agonist effects, E2 increased both ERα- and ERβ-mediated-luciferase activities in a concentration-dependent manner; Y134 did not show any agonist effect at all at the concentrations investigated (Figure 3), whereas RAL elicited weak but statistically significant ERα (1.69-fold increase, P<0.05; Figure 3a) as well as ERβ (1.72-fold increase, P<0.01; Figure 3b) agonist responses at high concentrations (1−10 μM). However, when tested in MCF-7 cells transiently transfected with the luciferase reporter gene and expressing ER (ERE-MMTV-Luc), neither RAL nor Y134 displayed any agonist activity at the highest concentration (10 μM; data not shown), suggesting that the observed effect of RAL is restricted to CV-1 cells.
Figure 2.
The antagonist effects of RAL and its analogues on ERα- and ERβ-mediated transcriptional activities. (a) CV-1 cells co-transfected with ERα (pcDNA3.1-hERα) and a luciferase reporter gene plasmid (ERE-MMTV-Luc) was treated with various concentrations of the compounds as listed. 17β-Oestradiol (E2) was added 30 min later to give a final concentration of 10 nM (EC80). The data presented are the percentage of the activity of each compound relative to that induced by E2 at EC80. Values in parentheses indicate respective IC50 (nM) for each compound. Results represent an average of three independent measurements. (b) Experiments were performed as above, except that the cells were transfected with pSG5-hERβ. RAL, raloxifene. *P<0.05, **P<0.01 compared to RAL treatment.
Figure 3.
Transcriptional activity induced by 17β-oestradiol (E2) and RAL. (a) CV-1 cells co-transfected with pcDNA3.1-hERα and a luciferase reporter gene plasmid (ERE-MMTV-Luc) were treated with various concentrations of E2, raloxifene (RAL) and Y134. The data are displayed as percentage of the maximum response to E2. (b) Experiments were performed as above, except that the cells were transfected with pSG5-hERβ. Values in parentheses indicate respective EC50 (nM) for E2. Results represent an average of three independent experiments. *P<0.05, **P<0.01 compared between RAL and Y134 treatments.
Effects on cell proliferation
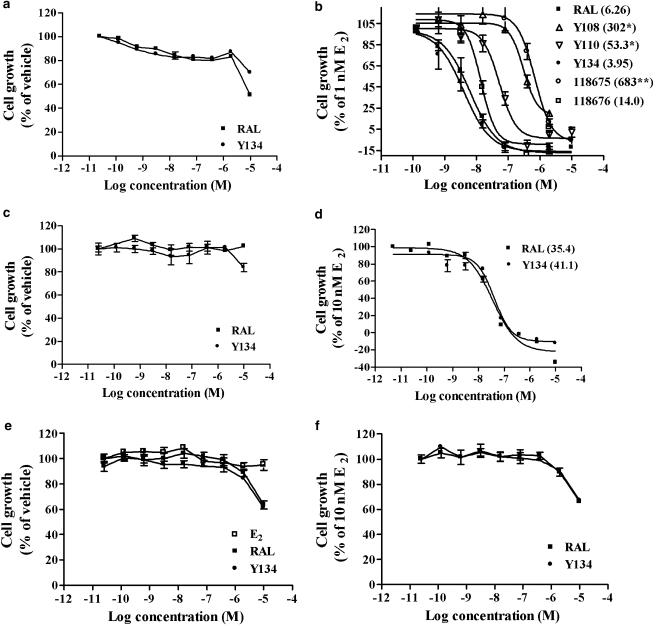
The human breast cancer cell line, MCF-7, was cultured in medium containing 1.0 nM E2 and various concentrations of test compounds for 6 days. As shown in Figure 4b, all five analogues produced a marked suppression of oestrogen-stimulated MCF-7 cell proliferation as determined by MTT. IC50 values varied between 3.95 and 683 nM with Y134 being the most effective of the five analogues. The antagonist activity of Y134 was also observed in another breast cancer cell line T47D (Figure 4d). In both cell lines, Y134 and RAL exhibited a comparable inhibitory effect on cell proliferation (P>0.05). This effect was not observed in ER-negative breast cancer cells, MDA-MB-231, except some cytotoxicity was seen at high concentrations (1–10 μM; Figure 4f). This indicates that the inhibitory effects of Y134 and RAL are specific for ERs. Furthermore, no ER agonist activity was recorded for either Y134 or RAL in all of the three cell lines, but cytotoxicity was detected at 10 μM in most cases (Figure 4a, c and e).
Figure 4.
Effects of RAL and its analogues on ER-expressing breast cancer cell (MCF-7 and T47D) proliferation. Oestrogen-negative breast cancer cell line (MDA-MB-231) was used as a control. (a, c and e) Agonist effects of raloxifene (RAL) and Y134 on MCF-7, T47D and MDA-MB-231 cell growth relative to vehicle control (100%) in the absence of oestrogen, respectively. (b, d and f) Demonstrate antagonist effects of RAL and the analogue(s) on MCF-7, T47D and MDA-MB-231 cell growth relative to 17β-oestradiol (E2; 1 nM for MCF-7 and 10 nM for T47D and MDA-MB-231 cells; 100%). Values in parentheses indicate respective IC50 (nM) for each compound. Results represent an average of three independent measurements. *P<0.05, **P<0.01 compared to RAL treatment.
Inhibition on the mammary gland
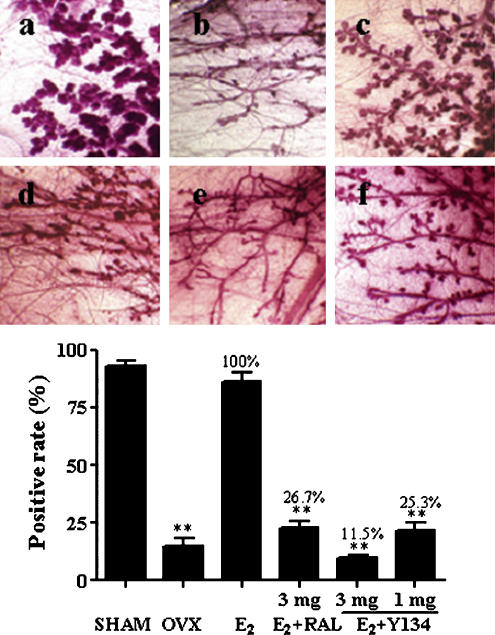
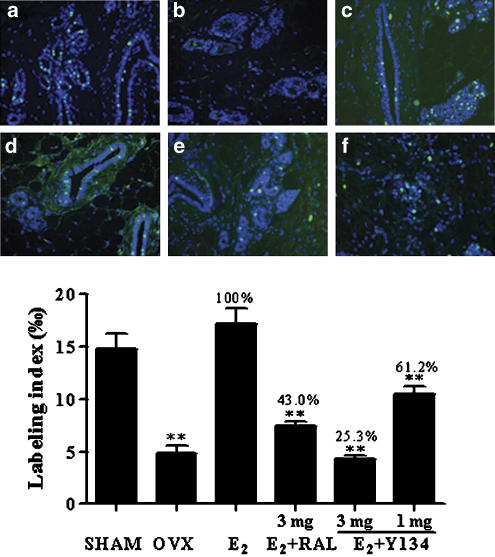
The outgrowth of rat mammary gland TEB is regulated by E2 (Raafat et al., 1999; Bocchinfuso et al., 2000). Ovariectomized rats were supplied with exogenous E2 (20 μg kg−1 day−1) in conjunction with a daily oral dose of Y134 (1 and 3 mg kg−1 day−1). Following 3 days treatment, E2-stimulated acinar development and associated gland structural changes, in response to Y134, were evaluated using the mammary gland whole-mounts method (Bocchinfuso et al., 2000). As shown in Figure 5 (upper panel), after ovariectomy, the gland became a simple tree branch-like structure with little TEB or acini; the addition of E2 could completely restore the acinar and TEB structures to the level of sham group; Y134 abolished the effect exerted by E2 in a dose-dependent manner. Compared with RAL, Y134 was more potent as it produced a similar attenuation of the stimulant effect of E2 at a dose three times less than that of RAL (Figure 5, lower panel). This observation is in agreement with mammary tissue DNA synthesis studies, in which E2-stimulated BrdU incorporation was more profoundly suppressed by Y134 than RAL at the same dose (3 mg; Figure 6).
Figure 5.
Effects of raloxifene (RAL) and Y134 on mammary gland terminal end bud (TEB) outgrowth. Ovariectomized rats were treated with 17β-oestradiol (E2) in conjunction with a daily oral dose of either RAL or Y134. The upper panel shows: (a) sham-operated group given vehicle only (negative control); (b) ovariectomized rats given vehicle only; (c) ovariectomized rats supplemented with E2 alone; (d) ovariectomized rats administered both E2 and RAL (3 mg kg−1 day−1); (e) ovariectomized rats administered both E2 and Y134 (3 mg kg−1 day−1) and (f) ovariectomized rats administered both E2 and Y134 (1 mg kg−1 day−1). The mammary glands were dissected out, fixed, stained and photographed as described in Methods (magnification × 30). The lower panel represents a quantitative analysis of the above observation by counting TEB from five fields, randomly selected, under a stereo microscope. Positive score was given when rami counted were more than four and percentage of the positive TEB calculated are based on an average reading of five fields. SHAM, sham-operated; OVX, ovariectomized. **P<0.01 compared between OVX and E2, E2 and E2+RAL, E2 and E2+Y134, and E2+RAL and E2+Y134 (3 mg), respectively.
Figure 6.
Effects of raloxifene (RAL) and Y134 on mammary gland DNA synthesis. Ovariectomized rats were treated with 17β-oestradiol (E2) in conjunction with a daily oral dose of either RAL or Y134. The upper panel shows: (a) sham-operated group given vehicle only (negative control); (b) ovariectomized rats administered vehicle only; (c) ovariectomized rats supplemented with E2 alone; (d) ovariectomized rats administered both E2 and RAL (3 mg kg−1 day−1); (e) ovariectomized rats administered both E2 and Y134 (3 mg kg−1 day−1) and (f) ovariectomized rats administered both E2 and Y134 (1 mg kg−1 day−1). The mammary glands were collected and longitudinal sections made for immunohistochemistry at autopsy. Raw data were generated in a blind manner with a minimum of five fields, and the BrdU labelling index (low panel) represents the FITC-stained vs all cells counted in each field × 1000 (magnification, × 400). SHAM, sham-operated; OVX, ovariectomized. **P<0.01 compared between OVX and E2, E2 and E2+RAL, E2 and E2+Y134, and E2+RAL and E2+Y134 (3 mg), respectively.
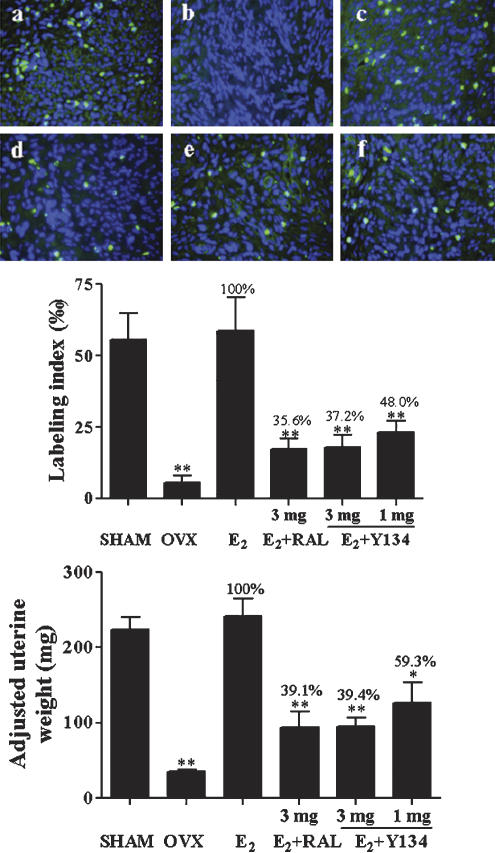
Suppression on the uterus
Both uterine wet weight and DNA synthesis were measured after the above treatment regime. Figure 7 shows that Y134 significantly inhibited uterine cell proliferation induced by E2 in a dose-dependent manner as determined by wet weight and fluorescence-labelled BrdU incorporation. In this case, no difference was observed between Y134 and RAL in terms of efficacy.
Figure 7.
Effects of raloxifene (RAL) and Y134 on uterine tissue growth as measured by DNA synthesis and wet weight. Sham-operated (SHAM, a) animals received vehicle treatment. Ovariectomized (OVX) rats were administered with vehicle (OVX, b), 17β-oestradiol alone (E2, c) or E2 in conjunction with a daily oral dose of either RAL (d) or Y134 (3 or 1 mg kg−1 day−1, e and f, respectively). Uteri were collected, weighed and longitudinal sections made for immunohistochemistry at autopsy. Raw data (upper panel) were generated in a blind manner with a minimum of five fields, and the BrdU labelling index (middle panel) represents the FITC-stained vs all cells counted in each field × 1000 (magnification, × 400). The lower panel depicts the wet uterine weight adjusted to 100 g of body weight after treatment. The level of significance was calculated by comparison with E2 group (*P<0.05; **P<0.01).
Discussion
A series of complementary in vitro and in vivo assays were applied in this study to qualify the lead compound, Y134, as a choice for further preclinical evaluation. Five analogues, modified either at the basic side chain (BSC) (Gao et al., 1999) or in the core structure of RAL, were selected based on their relatively higher binding affinities with ER (at the lower nM range); following cross-reactivity check with AR, PR, GR and MR, they were subjected to co-transfection assays to determine their pharmacological properties, that is, agonists or antagonists; thereafter, their effects on MCF-7 and T47D breast cancer cell proliferation were investigated. In every case, Y134 either outperformed its counterparts or displayed similar effects in terms of potency, efficacy and specificity. It was thus chosen for in vivo assessment and it was found that Y134 showed a better selectivity in the mammary gland than the uterus in comparison with RAL. This unique attribute would certainly be useful in the development of a novel treatment for breast cancer based upon ER antagonism.
Although numerous efforts have been made to modify the structure of RAL, its core structure and BSC are thought to be crucial to its pharmacological effects (Brzozowski et al., 1997; Shiau et al., 1998). In the Y134 series, benzothiophenes are characteristic of a piperazine side chain without modification of the RAL core structure. When the benzothiophene core was changed to benzothio[3,2-b]indole (e.g., 118675 and 118676), the binding affinity to ERα was somewhat reduced compared to RAL leading to a poor ER subtype selectivity (Table 1). Meanwhile, a reasonably good IC50 value for 118676 in CV-1 cells transfected with ERα (Figure 2a) did not fully translate into a potent inhibitory effect on the MCF-7 cell proliferation, confirming the complexity of ER function modulation. A detailed analysis of the structure–activity relationship of these two series of RAL analogues has been described previously (Ji et al., 2005; Yang et al., 2005).
It is noteworthy that a simple side chain replacement, that is, an isopropyl piperazine in the BSC instead of an aminoethyoxy group, resulted in full antagonism in both the ERα and β co-transfection assays (Figure 3) accompanied by a better mammary gland selectivity (Figures 5 and 6), without compromising the potency, efficacy or specificity of RAL. Because the IC50 values of Y134 and RAL were very similar (Figures 2 and 4), it is possible that the effect of Y134 is achieved through mechanisms other than alterations in ligand–receptor interaction, such as inhibition of the formation of transcriptional machinery complex (Shibata et al., 1997). However, this mechanism can be ruled out as both compounds have been found to be equally effective at restoring bone mineral density in ovariectomized mice (Yang et al., 2005).
There are some discrepancies relating to the potency values measured by receptor binding compared to those obtained from co-transfection assays (Table 1; Figure 2). It is thought that the results from the receptor-binding assay should accord with those from the cell-based assay. Nevertheless, the former is a molecular level assay that is more stable and less affected by experimental conditions. In contrast, the outcome of the cell-based co-transfection assay can be influenced by many factors. As a result, discrepancies in potency measurements between the two assay systems often exist and, in the present study, the differences are in general within the same log concentration range. Furthermore, as the backbone of ERα and ERβ plasmid constructs used here is different, this may alter assay performance due to variations in receptor expression and associated activation machinery. In our case, the detection window of ERβ co-transfection assay was smaller than that of ERα (data not shown), thereby partially accounting for the discrepancies in the observed receptor selectivity. It is noted that EC50 values measured for E2 by different reporter gene methods varied considerably (Gaido et al., 1999; Legler et al., 1999). Therefore, our conclusion for selectivity assessment is largely based on the receptor-binding affinities.
In order to further examine the weak agonist effect of RAL seen in the CV-1 cells (Figure 3), ER-expressing MCF-7 cells were transiently transfected with ERE-MMTV-Luc and luciferase activity was measured. At a RAL concentration (10 μM) that produced an agonist effect in the CV-1 cells, no similar action was observed (data not shown). This result concords with the finding that neither RAL nor Y134 stimulated MCF-7 and T47D cell proliferation (Figure 4a and c), and suggests that the agonist effect of RAL is restricted to the CV-1 cells co-transfected with ER and the reporter gene. In fact, the existence of agonist behavior for RAL at high concentrations has been debated for some time. For example, Shiau et al. (1996) found that RAL showed slight agonist activity for ERα in an ERE-reporter gene assay. Barkhem et al. (1998) also noted this phenomenon. However, Jisa et al. (2001) reported some contradictory results. In their yeast model, RAL exerted no agonist activity. Clearly, this feature of RAL is somewhat related to the nature of the experimental system used, such as cell types, as seen in this study.
In the mammary gland, Y134 exhibited about a two-fold better efficacy than RAL in blocking E2-stimulated DNA synthesis (Figure 6) and TEB outgrowth (Figure 5) with distinct morphological changes that represent the state of E2 deficiency (Raafat et al., 1999). This observation is supported by the finding that Y134 is highly selective for ERα as opposed to ERβ (Table 1) and previous studies in the literature showing that (1) ERα is abundant after ovariectomy and (2) ERβ is not essential for mammary growth and differentiation (Bocchinfuso et al., 2000; Shyamala et al., 2002). Finally, both compounds were equally effective in the uterus: E2-induced increases in uterine wet weight and fluorescence-labelled BrdU incorporation (as an indicator for DNA synthesis) were significantly curtailed following oral administration (Figure 7). As the differentiated effects of Y134 on the mammary gland and uterus were studied in the same animal, and under the same experimental conditions, the tissue selectivity revealed in the present study is genuine.
In summary, Y134 is a chemically modified version of RAL, it has a higher binding affinity to ERα, acts as a full antagonist in CV-1 cells transfected with either ERα or ERβ, and preferentially targets the mammary gland, while preserving other bioactivities of the parent compound. Its dual actions, that is, agonist in the bone and antagonist in the reproductive organs, may offer some new insights into the development of novel SERMs.
Acknowledgments
We are indebted to Mr Gao Jie, Mr Xin Hui, Mr Qunyi Li and Ms Lingyun Mu for technical assistance, and to Dr Dale E Mais for a critical review of the manuscript. This work was supported by grants from the Ministry of Science and Technology of China (2004CB518902) and the National Natural Science Foundation (30371653 and 20302009).
Abbreviations
- BrdU
5-bromo2′deoxy-uridine
- BSC
basic side chain
- BFS
bovine foetal serum
- CDT-BFS
charcoal/dextran-treated BFS
- RAL
raloxifene
- Y134
[6-hydroxy-2-(4-hydroxy-phenyl)-benzo[b]thiophen-3-yl]-[4-(4-isopropylpiperazin-1-yl)-phenyl]-methanone
Conflict of interest
The authors state no conflict of interest.
References
- Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:195–216. [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Beral V, Banks E, Reeves G., Appleby P. Use of HRT and the subsequent risk of cancer. J Epidemiol Biostat. 1999;4:191–210. [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Edwards JP, Zhi L, Pooley CL, Tegley CM, West SJ, Wang MW, et al. Preparation, resolution, and biological evaluation of 5-aryl-1, 2-dihydro-5H-chromeno[3,4-f]quinolines: potent, orally active, nonsteroidal progesterone receptor agonists. J Med Chem. 1998;41:2779–2785. doi: 10.1021/jm980190c. [DOI] [PubMed] [Google Scholar]
- Francucci CM, Romagni P, Boscaro M. Raloxifene: bone and cardiovascular effects. J Endocrinol Invest. 2005;28:85–89. [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, et al. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology. 1999;140:5746–5753. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- Gao H, Katzenellenbogen JA, Garg R, Hansch C. Comparative QSAR analysis of estrogen receptor ligands. Chem Rev. 1999;99:723–744. doi: 10.1021/cr980018g. [DOI] [PubMed] [Google Scholar]
- Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. Comparison of alamarBlue™ and MTT assays for high through-put screening. Toxicol In Vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Harnagea-Theophilus E, Miller MR. Acetaminophen alters estrogenic responses in vitro: stimulation of DNA synthesis in estrogen-responsive human breast cancer cells. Toxicol Sci. 1998;46:38–44. doi: 10.1006/toxs.1998.2531. [DOI] [PubMed] [Google Scholar]
- Ji Q, Gao J, Wang J, Yang C, Hui X, Yan X, et al. Benzothieno[3,2-b]indole derivatives as potent selective estrogen receptor modulators. Bioorg Med Chem Lett. 2005;15:2891–2893. doi: 10.1016/j.bmcl.2005.03.111. [DOI] [PubMed] [Google Scholar]
- Jisa E, Dornstauder E, Ogawa S, Inoue S, Muramatsu M, Jungbauer A. Transcriptional activities of estrogen receptor alpha and beta in yeast properties of raloxifene. Biochem Pharmacol. 2001;62:953–961. doi: 10.1016/s0006-2952(01)00731-6. [DOI] [PubMed] [Google Scholar]
- Khan IM, Yaksh TL, Taylor P. Ligand specificity of nicotinic acetylcholine receptors in rat spinal cord: studies with nicotine and cytisine. J Pharmacol Exp Ther. 1994;270:159–166. [PubMed] [Google Scholar]
- Klinge CM, Jernigan SC, Mattngly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J Mol Endocrinol. 2004;33:387–410. doi: 10.1677/jme.1.01541. [DOI] [PubMed] [Google Scholar]
- Legler J, Van den Brink CE, Brouwer A, Murk AJ, Van der Saag PT, Vethaak AD, et al. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicol Sci. 1999;48:55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Tasaka K, Kurachi H, Murata Y. Molecular mechanism of action of selective estrogen receptor modulator in target tissues. Endocr J. 2005;52:161–167. doi: 10.1507/endocrj.52.161. [DOI] [PubMed] [Google Scholar]
- Raafat AM, Hofseth LJ, Li S, Bennett JM, Haslam SZ. A mouse model to study the effects of hormone replacement therapy on normal mammary gland during menopause: enhanced proliferative response to estrogen in late postmenopausal mice. Endocrinology. 1999;140:2570–2580. doi: 10.1210/endo.140.6.6634. [DOI] [PubMed] [Google Scholar]
- Rosendaal FR, Vessey M, Rumley A, Daly E, Woodward M, Helmerhorst FM, et al. Hormonal replacement therapy, prothrombotic mutations and the risk of venous thrombosis. Br J Haematol. 2002;116:851–854. doi: 10.1046/j.0007-1048.2002.03356.x. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Shiau SP, Glasebrook A, Hardikar SD, Yang NN, Hershberger CL. Activation of the human estrogen receptor by estrogenic and antiestrogenic compounds in Saccharomyces cerevisiae: a positive selection system. Gene. 1996;179:205–210. doi: 10.1016/s0378-1119(96)00345-9. [DOI] [PubMed] [Google Scholar]
- Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, et al. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80:137–148. doi: 10.1016/s0960-0760(01)00182-0. [DOI] [PubMed] [Google Scholar]
- Siddiqui NI, Rahman S, Mia AR, Shamsuzzaman AK. Evaluation of hormone replacement therapy. Mymensingh Med J. 2005;14:212–218. [PubMed] [Google Scholar]
- Sporn MB, Dowsett SA, Mershon J, Bryant HU. Role of raloxifene in breast cancer prevention in postmenopausal women: clinical evidence and potential mechanisms of action. Clin Ther. 2004;26:830–840. doi: 10.1016/s0149-2918(04)90127-0. [DOI] [PubMed] [Google Scholar]
- Steffen KA, Carnes M. Hormone replacement therapy in prevention of morbidity and mortality in the older woman: part III: potential adverse effects of hormone replacement therapy. J Am Med Dir Assoc. 2000;1:117–121. [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Wolmark N. The study of tamoxifen and raloxifene: preliminary enrollment data from a randomized breast cancer risk reduction trial. Clin Breast Cancer. 2002;3:153–159. doi: 10.3816/CBC.2002.n.020. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Lou YJ. Proliferation-stimulating effects of icaritin and desmethylicaritin in MCF-7 cells. Eur J Pharmacol. 2004;504:147–153. doi: 10.1016/j.ejphar.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Wu B, Gao J, Wang MW. Development of a complex scintillation proximity assay for high-throughput screening of PPARγ modulators. Acta Pharmacol Sin. 2005;26:339–344. doi: 10.1111/j.1745-7254.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- Yang C, Xu G, Li J, Wu X, Liu B, Yan X, et al. Benzothiophenes containing a piperazine side chain as selective ligands for the estrogen receptor alpha and their bioactivities in vivo. Bioorg Med Chem Lett. 2005;15:1505–1507. doi: 10.1016/j.bmcl.2004.12.074. [DOI] [PubMed] [Google Scholar]