Abstract
Background and purpose:
Ivabradine, a specific and use-dependent I f inhibitor, exerts anti-ischaemic activity purely by reducing heart rate. The aim of this work was to characterize its effect on the predominant HCN channel isoform expressed in human sino-atrial nodes (hSAN), to determine its kinetics in HCN channels from multicellular preparations and rate-dependency of its action.
Experimental approach:
RT-PCR analysis of the four HCN channel isoforms was carried out on RNAs from hSAN. Patch-clamp and intracellular recordings were obtained from CHO cells stably expressing hHCN4 and isolated SAN, respectively. Beating rate of rat isolated atria was followed using a transducer.
Key results:
hHCN4 mRNAs were predominant in hSAN. Ivabradine induced a time-dependent inhibition of hHCN4 with an IC50 of 0.5 μM. In rabbit SAN, ivabradine progressively reduced the frequency of action potentials: by 10% after 3 h at 0.1 μM, by 14% after 2 h at 0.3 μM and by 17% after 1.5 h at 1 μM. After 3h, ivabradine reduced the beating rate of rat right atria with an IC30 of 0.2 μM. The onset of action of ivabradine was use-dependent rather than time-dependent with slower effects than caesium, an extracellular I f blocker. Ivabradine 3 μM decreased the frequency of action potentials in SAN from guinea-pig, rabbit and pig by 33%, 21% and 15% at 40 min, respectively.
Conclusions and implications:
The use-dependent inhibition of hHCN4 current by ivabradine probably contributes to its slow developing effect in isolated SAN and right atria and to its increased effectiveness in species with rapid SAN activity.
Keywords: ivabradine, S 16257, pacemaker, sino-atrial action potentials, human HCN4 channel, patch-clamp, RT–PCR
Introduction
Ivabradine (3-(3-{[((7S)-3,4-Dimethoxybicyclo[4,2,0]octa-1,3,5-trien-7-yl)methyl]methylamino}propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one, hydrochloride) (S 16257, Procoralan) is a pure heart rate (HR) lowering agent that acts by inhibiting specifically If, one of the main ionic currents involved in the regulation of HR in the sino-atrial node (SAN) (Bois et al., 1996; Vilaine et al., 2003b). Ivabradine represents an attractive putative therapeutic agent, as increased heart rate plays a major role in coronary artery disease, not only as a trigger of most of the ischaemic episodes, by increasing myocardial oxygen consumption and by reducing the diastolic time of myocardial perfusion, but also as an independent predictor of mortality (Diaz et al., 2005). The anti-anginal and anti-ischaemic efficacy of ivabradine, demonstrated in patients with chronic stable angina (Borer et al., 2003), is at least as potent as that of a β-blocker (Tardif et al., 2005).
If, the pharmacological target of ivabradine, is a hyperpolarization-activated depolarizing current generated in the pacemaker cells within the SAN located in the right atria. This inward ionic current is partly responsible for the slow diastolic depolarization phase that determines the firing rate of spontaneous action potentials propagated to the whole heart for the initiation of the heartbeat (Accili et al., 2002). The amplitude of this current determines the slope of the diastolic depolarization phase and thereby the HR. The molecular basis of If and its related equivalent in non cardiac cells Ih have been characterized by cloning a family of ionic channels (Ludwig et al., 1998; Santoro et al., 1998; Ishii et al., 1999) known as HCN, which stands for hyperpolarization-activated cyclic nucleotide-gated channels (Ludwig et al., 1998). Four isoforms have been identified, HCN1–4 (Ludwig et al., 1999a). Their homomeric currents, studied in heterologous expression systems, show typical characteristics of pacemaker currents, activation on hyperpolarization, current carried by Na+ and K+ and , modulation by cAMP and sensitivity to caesium (Cs) (Baruscotti and DiFrancesco, 2004). However, the role of each of these isoforms in generating If in SAN cells has not been definitively elucidated. Their expression profile has been evaluated in mouse and rabbit SAN (Shi et al., 1999; Moosmang et al., 2001), but it has not been characterized in human SAN.
Detailed patch-clamp studies in rabbit SAN cells have shown that the drug blocks If channels in a use-dependent way and that it interacts with the channels from the intracellular side (Bois et al., 1996). More recently, also in SAN cells, ivabradine has been shown exclusively to be an open channel blocker, indicating that it cannot reach its binding site when the channels are closed (Bucchi et al., 2002). Moreover, the authors observed that its blocking effect is current-dependent and is attenuated during very long hyperpolarized pulses (more than 20 s of hyperpolarization) (Bucchi et al., 2002).
The aim of this work was to characterize the pharmacological target of ivabradine further in human SAN and to study its kinetics of action on different preparations. A comparison with Cs was also done and the possible consequences of ivabradine's properties discussed.
Methods
Experiments were performed in accordance with the recommendations of the French Accreditation of Laboratory Animal Care. Pigs were purchased from Frenelles EARL (Frenelles-Boisemont, France) and all other animals (rats, guinea-pigs and rabbits) were purchased from Charles River Laboratories (L'Arbresle, France).
RT–PCR analysis of HCN isoforms in human SAN
RNAs of sinus node tissue from 4 male patients (3 Caucasians and one Hispanic, 72.2±6.6 years old, all dead after non-cardiac disease) were purchased from Analytical Biological Services Inc. (Wilmington, DI, USA) and analysed separately. The PCR primer pairs used for the amplification of HCN channel mRNAs are described in Table 1. Four micrograms of sinus node total RNAs were reverse transcribed in a final volume of 15 μl (RT) using the First Strand cDNA synthesis kit (Amersham Biosciences, Pantin, France). Then, 2 μl of the RT sample were amplified by PCR using the protocol obtained with the kit purchased from Qiagen, in the presence of Q solution and the specific primers. The PCR reactions (100 μl final volume) were performed in a thermocycler Perkin-Elmer 2400 using the following amplifications steps: 94°C 3 min/94°C 40 s, 60°C 40 s, 72°C 1 min (40 cycles)/72°C 5 min. Amplification products were then analysed on agarose gel. The mRNAs from human brain (Takara BioEurope/Clontech, Saint Germain en laye, France) were used as positive control, as the presence of the four HCN isoforms has been described previously in this tissue (Monteggia et al., 2000).
Table 1.
Characteristics of the specific PCR primer pairs used for the amplification of HCN channel mRNAs
| Accession number (GenBank) | Names | Sequences | Positions | Size (pb) of PCR fragments | Number of intron in amplified region | Size (pb) of intron |
|---|---|---|---|---|---|---|
| hHCN1 | ||||||
| AF488549 | F1769 | 5′-GCCTTTGAGACAGTTGCCATTG-3′ | 1769–1791 | 597 | 1 | 4278 |
| AF488549 | B2365 | 5′-GGTCAGGTTGGTGTTGTGAAGC-3′ | 2365–2387 | 597 | 1 | 4278 |
| AF488549 | F2344 | 5′-GCTTCACAACACCAACCTGACC-3′ | 2344–2366 | 338 | — | — |
| AF488549 | B2682 | 5′-AATCGTGGCTTTTCTGCGTCTG-3′ | 2682–2704 | 338 | — | — |
| hHCN2 | ||||||
| AF065164 | F1067 | 5′-CCGCTACATCCATCAGTGGGAG-3′ | 1067–1089 | 519 | 3a | 5910 |
| AF065164 | B1586 | 5′-GCTGTCCTCGTCAAACATCTTGC-3′ | 1586–1609 | 519 | 3a | 5910 |
| AF065164 | HCN25Bielb | 5′-CGCCTGATCCGCTACATCCAT-3′ | 1059–1080 | 280 | 2a | 3834 |
| AF065164 | HCN23Bielb | 5′-AGTGCGAAGGAGTACAGTTCACT-3′ | 1265–1288 | 280 | 2a | 3834 |
| hHCN3 | ||||||
| AF488550 | F1574 | 5′-TCAATGCTGTGCTTCAGGAGTTC-3′ | 1574–1597 | 587 | 1 | 443 |
| AF488550 | B2161 | 5′-GAGGGTTGGGAGGCTGAGAGTG-3′ | 2161–2183 | 587 | 1 | 443 |
| AF488550 | F164 | 5′GCTCCAGCCTACGGTCAACAAG-3′ | 164–186 | 314 | 1 | 4542 |
| AF488550 | B478 | 5′ATCTCAGCACCCTCCTCCACCA-3′ | 478–500 | 314 | 1 | 4542 |
| hHCN4 | ||||||
| AJ0238850 | F3166 | 5′-AAAGTCCAGCACGACCTCAACTC-3′ | 3166–3189 | 396 | — | — |
| AJ0238850 | B3562 | 5′-AGGATGAAGACGGTGTGTCCAC-3′ | 3562–3584 | 396 | — | — |
| AJ0238850 | HCN45Bielb | 5′-CCCGCCTCATTCGATATATTCAC-3′ | 2170–2193 | 280 | 1 | 11092 |
| AJ0238850 | HCN43Bielb | 5′-GAGCGCGTAGGAGTACTGCTTC-3′ | 2381–2403 | 280 | 1 | 11092 |
Abbreviations: PCR, polymerase chain reaction; HCN, hyperpolarization-activated cyclic nucleotide-gated channel.
Presence of a pseudo HCN2 gene containing no intron in the human genome.
Primers used by the research group of M Biel (Ludwig et al., 1999b).
Recordings of pacemaker current in a CHO cell line stably expressing hHCN4
Chinese hamster ovary (CHO) cells were stably co-transfected with the cDNA of hHCN4 and the gene for the resistance to geneticin by the Lipofectamine method. The geneticin-resistant clone was selected according to its immunofluorescence level, assessed using monoclonal antibodies directed against hHCN4 channels (Alomone labs Ltd, Jerusalem, Israel) and with patch-clamp experiments. Cells were cultured at 37°C with 5% CO2 in Ham-F12 medium supplemented with 10% decomplemented foetal calf serum, 1% penicillin–streptomycin and 1% geneticin. They were used for patch-clamp experiments in 35 mm Petri dishes at low density, at passages 1–8, 1 or 2 days after trypsinization.
Patch-clamp recordings were conducted in a modified Tyrode solution containing (in mM): NaCl 110, KCl 30, MgCl2 0.5, CaCl2 1.8, 4-(2-hydroxyethyl)-1-piperazineethyl-sulphonic acid (HEPES) 5, (pH adjusted to 7.4 with NaOH). Cells were superfused at the controlled temperature of 32 or 35±1°C as further detailed in the text, with a fast changing perfusion system. Borosilicate glass pipettes (2–7 MΩ) were filled with a solution containing (in mM): KCl 130, NaCl 10, MgCl2 0.5, ethylene glycol bis(β-aminoethylether)-N,N,N′,N′ tetraacetic acid (EGTA) 1, HEPES 5 (pH adjusted to 7.2 with KOH). Recordings obtained using a RK-400 patch-clamp amplifier (Bio-Logic, Claix, France) and filtered at 1 kHz were stored at 5 kHz further analysed using pClamp 8.0 software (Molecular Devices Corporation, Sunnyvale, CA, USA). Leak substraction by the P/N method was performed only for the half-activation voltage measurement. In all experiments, the holding potential was −30 mV and the hyperpolarization-activated current was measured as the difference between the current at the beginning and at the end of the pulse. One concentration per cell of ivabradine (from 0.1 to 100 μM) was applied. Run-down of the hHCN4 current (around 15% after 12 min) was not subtracted. Data represented the percent of control current inhibited at steady-state block. Half-maximum effective concentration (IC50) was calculated using a curve-fitting software (GraphPadPrism 4.01) according to the equation:
where Y is the percent of hHCN4 block, X the concentration and nH the Hill coefficient.
Intracellular recordings of spontaneous APs in isolated sino-atrial node
Male Hartley guinea-pigs (550–650 g) and New Zealand male rabbits (2–5 kg) were anaesthetized with sodium pentobarbital (30 mg kg−1 intraperitoneally (i.p.) or intravenously (i.v.) respectively). Large-White pigs (male or female, 25–30 kg) were anaesthetized with an intramuscular injection of a mixture of Tiletamine and Zolazepam (20 mg kg−1). After exsanguination, the heart was rapidly removed and immersed in an oxygenated physiological solution, containing (in mM): NaCl 130, KCl 5.6, NaH2PO4 0.6, MgCl2 1.1, CaCl2 1.8, NaHCO3 20 and glucose 11 (4°C, pH 7.4±0.1).
The SAN preparation, including the intercaval region, the crista terminalis and a small part of the contractile atria, was surgically isolated and pinned to the bottom of an experimental chamber supplied with physiological solution at 5 ml min−1 (36±0.5°C, 95% O2–5% CO2). After 2 h of recovery, transmembrane potentials were recorded with glass microelectrodes filled with 3 M KCl (25–35 MΩ) connected to a high input impedance amplifier. The signal was digitalized and analysed using specific software (Clovis, Clod sarl, France). Spontaneous APs from pacemaker cells were recorded for 10–20 min in control conditions.
In a first set of experiments, one concentration of ivabradine (0.1, 0.3 or 1 μM) or Cs chloride (3 mM) was added and spontaneous action potentials firing rate (APs min−1) was measured every 5 min for 1.5–4 h. In addition, changes in AP amplitude and duration (APA and APD50, respectively), maximal diastolic and take-off potentials (maximal diastolic potential (MDP) and take-off potential (TOP), respectively) and diastolic depolarization rate (DDR) were determined at the end of the exposure to ivabradine.
In a second set of experiments, SAN of guinea-pig and pig were exposed to ivabradine at 3 μM for 40 min and the firing rate of the spontaneous APs was measured every 5 min.
Spontaneous beating rates of rat isolated right atria
Male Wistar rats (350–400 g) were anaesthetized with sodium pentobarbital (30 mg kg−1, i.p.). Right atria was carefully removed and immersed in physiological solution at 35°C containing (in mM): NaCl 120.3, KCl 4.8, KH2PO4 1.0, MgSO4 1.3, CaCl2 2.5, NaHCO3 24.2, glucose 11.1, ethylenediamine tetra aceticacid (EDTA) 0.026 (pH 7.4, 95% O2–5% CO2). Changes in isometric tension were recorded with IT1-25 transducer (EMKA Technologies, Paris, France). Atria were stretched to a resting tension of 0.4 g. Spontaneous beating frequencies were measured using IOX software (EMKA Technologies, France). After a stabilization period, one concentration of ivabradine (0.056, 0.1, 0.18, 0.3 or 0.56 μM) or Cs chloride 3 mM was added and atrial beating rate (b.p.m.) was measured every 10 min for 3 h. IC30 values are presented as the negative log molar concentration required to produce a 30% reduction of the initial beating rate.
Drugs
Ivabradine was provided by Technologie Servier (Orléans, France) as powder form. It was dissolved daily in distilled water and added to the physiological solution. Cs chloride was obtained from Sigma Chemical Co., (Saint Quentin, Fallavier, France) and was directly dissolved in the physiological solutions.
Data analysis
All results in the text, tables and figures are expressed as mean±s.e.m. for n experiments. Statistical analysis was performed with either a two-way analysis of variances (ANOVA) or Student's t-test for unpaired data. Differences were considered significant when P-value was less than 0.05.
Results
HCN channels mRNA expression in human SAN
Representative results of non-quantitative reverse transcriptase-polymerase chain reaction (RT–PCR) analysis carried out on total RNA from human sino-atrial node (hSAN), using specific PCR primer pairs for the amplification of HCN channels mRNAs, are presented in Figure 1. No traces of genomic DNA (gDNA) were found in the mRNA extracts from hSAN, as no product was amplified from the non-reversed-transcript mRNAs, and no contamination of samples was noted when mRNAs were replaced by water (RT− and H2O, respectively,Figure 1). Human brain mRNAs were used as positive controls for HCN isoforms. In the human SAN, of the four HCN channels isoforms, only mRNAs from hHCN1, hHCN2 and hHCN4 were found (RT+;Figure 1). The hHCN1 and hHCN2 were transcripted at low levels while hHCN4 was the major isoform expressed. Similar results were observed in the four batches of hSAN from patients who had died from non cardiovascular diseases.
Figure 1.
RT–PCR analysis of HCN channel isoforms in (hSAN). RT–PCRs with SAN RNA from one patient (RT+) or without reverse transcription of mRNA (RT−), compared to human brain, was performed using two different primer pairs specific for hHCN isoforms. The internal controls correspond to exchange of the mRNA by H2O or by gDNA in RT–PCR. The size of PCR fragments, indicated on the right of the figure, was estimated with ΦX174 DNA fragments. Similar results were obtained with 3 others batches of hSAN.
Inhibition of pacemaker current in hHCN4-CHO
Stably transfected CHO cells displayed a slow inward current activated upon hyperpolarization of the membrane (Figure 2a). Its voltage dependence measured at 32°C with 7 s hyperpolarizing pulses from −40 to −130 mV (each increased by 10 mV), followed by a test pulse at −130 mV, showed a voltage of half-activation of −77.8±0.6 mV (n=3). This current activated by repetitive pulses to −130 mV for 4 s, followed by a deactivation pulse at +20 mV for 0.8 s, applied every 6 s at 32±1°C, was reduced by ivabradine, as illustrated in Figure 2b with 0.3 μM. Addition of ivabradine resulted in a progressive decrease of the current elicited at each pulse in a concentration-dependent manner (Figure 2c), with an IC50 value of 0.54 μM (confidence interval at 95%: 0.45–0.65 μM) and a Hill coefficient of 0.87±0.07 (Figure 2d). This inhibition of the hHCN4 current was time-dependent, with a slowest kinetic of block for the lowest concentrations (Figure 2c). For example, the steady-state block was obtained after about 4 min at 30 μM, whereas about 12 min were required at 0.3 μM.
Figure 2.
Time- and concentration-dependent inhibition of hHCN4 current in stably transfected CHO cells by ivabradine. (a) Voltage-dependence of hHCN4 inward current elicited by 7 s hyperpolarizing pulses from −40 to −130 mV with increments of 10 mV and followed by a test pulse at −130 mV led to a voltage of half-activation of −77.8 mV±0.6. (b) Sample traces of whole-cell hHCN4 current elicited by trains of activating–deactivating (−130 mV for 4 s, +20 mV for 0.8 s) voltage steps, applied every 6 s at 32±1°C in control solution and after exposure to ivabradine 0.3 μM. (c) Time- and use-dependent inhibition of hHCN4 current by ivabradine (0.1–100 μM) (the arrow indicates the beginning of the application). (d) Concentration-dependent inhibition of hHCN4 current with an IC50 value of 0.54 μM (95% confidence interval of 0.45–0.65) and a Hill coefficient of 0.87±0.07. The number of experiments is indicated above each point of the curve.
Reduction of the frequency of spontaneous action potentials in rabbit SAN
The basal spontaneous firing rate of rabbit SAN preparations was similar in the different groups before the applications of ivabradine or vehicle (Table 2) and exposure to the vehicle induced non-significant changes in action potentials (APs) frequency even after 4 h (2.9±2.6 APs min−1; +1.3±1.2%). Ivabradine reduced the pacemaker rate of firing in a concentration-dependent way (Table 2 and Figure 3): by −9.9±1.1% after 3 h at 0.1 μM, by −14.0±1.4% after 2 h at 0.3 μM and by −17.3±0.6% after 1.5 h at 1 μM; an increase in AP duration and changes in threshold AP were associated with these effects of ivabradine. As illustrated in the original tracings shown in Figure 3b–d, the reduction of the rate of firing of APs induced by ivabradine resulted from a decrease in the slope of diastolic depolarisation with no change in the maximal rate of rise of AP (dV/dt max, Table 2). Depending on the cell, the reduction in the diastolic depolarisation rate was sometimes associated with more electronegative take-off potentials (at the higher tested concentrations, as an example,Table 2). Significant decreases in the frequency of APs were obtained after 20, 15 and 5 min of exposure to 0.1, 0.3 and 1 μM ivabradine, respectively. Maximal effect was achieved more rapidly at higher concentrations (Figure 3) (after 165, 115 and 75 min for 0.1, 0.3 and 1 μM, respectively).
Table 2.
Effects of prolonged applications of ivabradine on AP parameters of rabbit sino-atrial node preparations
| Rate | APA | dV/dt max | MDP | TOP | DDR | APD50 | |
|---|---|---|---|---|---|---|---|
| Control | 211±6 | 74.4±3.2 | 5.3±0.9 | −64.1±1.9 | −51.7±1.7 | 84.8±9.7 | 82.5±3.1 |
| Vehicle | 214±7 | 76.9±5.1 | 7.4±1.9 | −66.0±2.4 | −51.4±1.8 | 101.3±8.9 | 82.2±1.9 |
| Control | 221±3 | 72.2±4.1 | 5.7±1.4 | −63.1±2.5 | −50.9±4.0 | 115.2±8.7 | 83.4±3.4 |
| Ivabradine 0.1 μM | 199±1* | 73.4±0.7 | 4.3±0.8 | −64.3±1.7 | −52.4±2.7 | 62.0±13.4* | 94.0±4.4* |
| Control | 228±7 | 78.0±3.0 | 10.5±1.8 | −65.9±1.9 | −51.0±1.5 | 103.7±10.3 | 77.1±2.8 |
| Ivabradine 0.3 μM | 196±5* | 88.6±2.8 | 18.1±5.4 | −73.9±1.9 | −64.2±2.5* | 40.1±2.6* | 82.2±3.5* |
| Control | 224±8 | 67.2±3.0 | 3.9±1.1 | −61.1±2.6 | −52.3±3.4 | 91.2±7.9 | 82.4±3.4 |
| Ivabradine 1 μM | 185±7* | 78.2±1.8* | 3.7±1.3 | −67.0±2.6* | −59.0±3.5* | 42.0±6.9* | 92.4±3.3* |
Abbreviations: APA, action potential amplitude (mV); APD50, action potential duration at 50% of total repolarization (ms); DDR, diastolic depolarization rate (mV s−1); MDP, maximal diastolic potential (mV); dV/dt max, maximal upstroke velocity (V s−1); Rate, spontaneous pacemaker rate (APs min−1); TOP, take-off potential (mV).
Spontaneous APs recorded at the beginning of the experiments (control) and at the end of exposure to ivabradine, that is 3 h for 0.1 μM and vehicle, 2 h for 0.3 μM and 1.5 hours for 1 μM. Values are expressed as means±s.e.m. for five experiments per groups.
P⩽0.05 vs vehicle group (Student's unpaired t-test preceded by a Welch test when needed).
Figure 3.
Time-dependent effects of ivabradine at 0.1, 0.3 and 1 μM on the spontaneous firing rate of rabbit sino-atrial node tissue. Left panels: original traces of spontaneous action potentials after exposure to ivabradine are indicated by arrows, after 3 h of vehicle (a), 3 h of 0.1 μM (b), 2 h of 0.3 μM (c) and 1.5 h of 1 μM (d), and are compared to the respective control trace at the beginning of the experiment. Right panel: mean values±s.e.m. of APs rate expressed in percent change from basal values, for five experiments per groups. P<0.05: statistically significant differences from the vehicle from 15 min at 0.1 and 0.3 μM and from 5 min at 1 μM (two-way ANOVA test with repeated measures on factor time).
Reduction of spontaneous beating rate of rat isolated atria
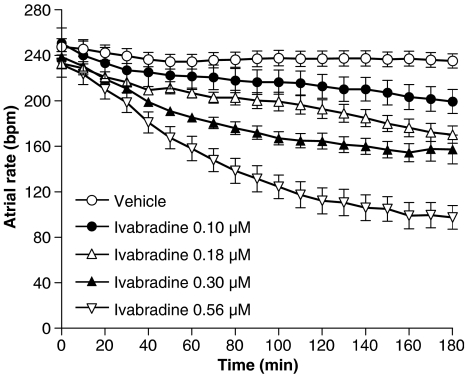
Basal beating rates of rat isolated atria were similar in the different groups and changes induced by the vehicle over 3 h were 5.2% at the maximum. Ivabradine induced a progressive concentration-dependent reduction in this beating rate (Figure 4). The plateau phase occurred between 140 and 180 min for the two higher concentrations (Figure 4). After 180 min, the inhibitory effect varied from −20.2±2.4% at 0.1 μM to −56.2±4.3% at 0.56 μM. IC30 values obtained after 120 and 180 min of incubation with ivabradine were 0.28 μM (confidence interval at 95%: 0.23–0.33 μM) and 0.19 μM (confidence interval at 95%: 0.15–0.24 μM), respectively.
Figure 4.
Time-dependent effects of four concentrations of ivabradine on the spontaneous beating rate of rat isolated right atria. Data are expressed as mean±s.e.m., in b.p.m, for five independent experiments from groups treated with ivabradine at 0.1 , 0.18, 0.3 and 0.56 μM. P<0.05: statistically significant differences from the vehicle from 150 min at 0.1 μM, 60 min at 0.18 μM, 30 min at 0.3 μM and 10 min at 0.56 μM (two-way ANOVA , followed with a Dunnett test).
Use-dependent effects of ivabradine and comparison with Cs
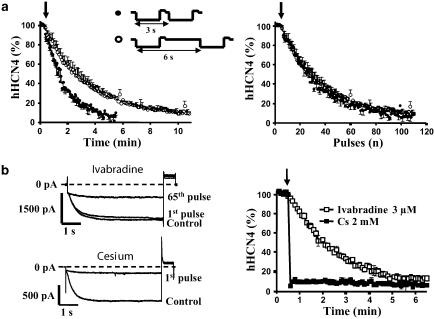
In order to evaluate the contribution of use-dependent inhibition to the progressive effects of ivabradine, the effects of 3 μM ivabradine on hHCN4 current were studied using a similar pulsing protocol, a 2 s pulse at −110 mV followed by a 0.6 s pulse at +20 mV, applied every 3 s (0.33 Hz, n=2) or every 6 s (0.16 Hz, n=2), both at 35±1°C. Comparison of the kinetics of block showed that the time required to reach the steady-state block was two times less when the protocol was applied every 3 vs 6 s (Figure 5a, left panel). Plotting the kinetics of the blocking effects against the number of pulses showed that the traces could be superimposed for both frequencies (Figure 5a, right panel).
Figure 5.
(a) Use-dependence of the block of hHCN4 current in CHO cells by 3 μM ivabradine: the same protocol (−110 mV for 2 s followed by a 0.6 s pulse at +20 mV at 35±1°C) is applied every 3 s (n=2) or every 6 s (n=2). The kinetics of block are expressed as a function of time (left) or pulse number (right), showing a same relationship to the pulse number (i.e. channel openings) with both protocols. The arrows indicated the beginning of the application of ivabradine. (b) Comparison of the time course of inhibition of hHCN4 current by ivabradine 3 μM and Cs 2 mM. Pulses at −110 mV for 5 s followed by +20 mV for 0.6 s were applied every 6 s at 35±1°C. The current was inhibited in a use-dependent manner by ivabradine (n=4) and in a time-independent way by Cs (n=3) leading to a reduction of about 90% at steady-state for both compounds. The steady-state block was obtained at the first pulse for Cs, while 65 pulses were needed for ivabradine as shown with typical current traces in the left panels.
A comparison of the kinetics of inhibition of HCN4 current induced by ivabradine and Cs, an extracellular blocker, was also performed in the same conditions, with a 5 s pulse at −110 mV followed by a 0.6 s deactivating pulse at +20 mV applied every 6 s at 35±1°C. Whereas ivabradine 3 μM (n=4) evoked a use-dependent decrease of the current elicited at each pulse, and needed about 60 pulses to reach a steady-state reduction of 87.5±2.5%, 2 mM Cs induced a maximum block at the first pulse (90.9±0.5%, n=3) that remained constant over the next 60 pulses (6 min) (Figure 5b).
The time course for the effects of ivabradine and Cs were also compared in rat right atria and rabbit SAN preparations. Preliminary experiments have shown that Cs 3 mM induced an inhibition of about 30% of the spontaneous beating rate of rat atria. Furthermore, in SAN preparations, 2 mM Cs induced only a small reduction in the firing rate of the APs (9±1% after 90 min, n=3),whereas 5 mM Cs reduced by 21% the frequency after 40 min but had marked non-specific effects on AP repolarization (data not shown). Therefore, the concentration of Cs chosen was 3 mM and its effects were compared to those of ivabradine at 0.3 μM on rat atria and 1 μM in rabbit SAN tissue, concentrations that induce equivalent reductions of spontaneous frequency in each of these tissues. As shown in Figure 6, Cs at 3 mM induced a very rapid decrease in the beating rate of both atria (−34.8±1.6% after 10 min) and SAN (−9.3±1% after 5 min). This effect was maintained for 180 and 90 min for atria and SAN, respectively. In contrast, and as previously shown in Figures 3 and 4, ivabradine induced a progressive reduction in rate, not significantly different from control after 10 min for atria and 5 min for SAN, and maximal at 180 min for atria (−35.6±5.9%) and 90 min for SAN (−17.3±0.6%). These maximal effects were similar to those induced by Cs 3 mM.
Figure 6.
Comparison of kinetics of reduction in pacemaker activity induced by ivabradine and Cs chloride. (a) Time-dependent effects of 3 mM Cs and 0.3 μM ivabradine on the spontaneous beating rate of rat isolated right atria during 3 h period of exposure. Data are expressed as percent change from basal values of beating rate for four independent experiments for each group. (b) Time-dependent effects of Cs 3 mM and ivabradine at 1 μM on the spontaneous firing rate of rabbit sino-atrial node tissue, during an exposure period of 1.5 h. Data are expressed as percent change from basal values of APs rate, for three experiments for Cs treated group and five experiments for the other groups (same as in Figure 4). *P<0.05: statistically significant differences between the two treated groups (Student's unpaired t-test preceded by a Welch test when needed). For clarity other statistical results are not indicated on the graphs.
Reduction of the rate of spontaneous APs of sino-atrial nodes from different species
The effects of ivabradine on the frequency of spontaneous APs of SAN were also studied in guinea-pig and pig; they have a higher and lower heart rate in vivo than rabbit, respectively. In this series of experiments, basal rate of APs was 193±7, 208±7 and 90±5 APs min−1, for rabbit (n=8), guinea-pig (n=8) and pig (n=7) SAN preparations, respectively. Exposure to ivabradine 3 μM for 40 min reduced the pacemaker firing rate by 21±3% (−41±6 APs min−1) in rabbit SAN vs 33±2% (−68±6 APs min−1) and 15±2% (−13±2 APs min−1) for guinea-pig and pigs, respectively (Figure 7, right panel). As illustrated in Figure 7 in all species tested (left panels), ivabradine decreased the slope of the diastolic depolarization.
Figure 7.
Effects of a 40 min exposure to ivabradine at 3 μM on the spontaneous firing rate of sino-atrial node tissue from different species. Left panels: original traces of spontaneous APs from rabbit (a), guinea-pig (b) and pig (c) pacemaker cells after exposure to ivabradine are indicated by arrows and are compared to the respective control trace at the beginning of the experiment. Right panels: time-dependent reduction in the rate of the APs from basal values, for n experiments for each group (n=7 for pig and n=8 for the other groups). *P<0.05: statistically significant differences between indicated groups (Student's unpaired t-test preceded by a Welch test when needed).
Discussion
Ivabradine is a specific heart rate reducing agent (Thollon et al., 1994, 1997; Vilaine et al., 2003a, 2003b) effective and well tolerated in the treatment of chronic stable angina pectoris (Borer et al., 2003; Tardif et al., 2005). It is now well known that its pharmacological target is the cardiac ‘pacemaker' If current (Bois et al., 1996; Bucchi et al., 2002). However, some questions about the molecular basis of this current still remain. Actually, four isoforms of HCN channels have been identified and cloned by different groups (Ludwig et al., 1998; Santoro et al., 1998; Ishii et al., 1999). This diversity could also be increased by the possibility that each isoform can co-assemble in heterotetramers (Ulens and Tytgat, 2001), a process that has been suggested to be physiologically relevant (Much et al., 2003; Brewster et al., 2005). Previous studies revealed that HCN4 and HCN2 are expressed in human ventricles and atria, but the authors did not investigate the SAN (Ludwig et al., 1999b).
In the present work, we demonstrated, using a RT–PCR technique, that the HCN4 isoform is the predominant transcript in human SAN rather than HCN2 and HCN1, suggesting that it could be the major molecular basis underlying the If current. This result fits well with the observation of sinus node bradycardia in members of a large family with a mutation in the gene coding for the pacemaker HCN4 ion channel (Milanesi et al., 2006). Studies performed in rabbit (Shi et al., 1999), mouse (Moosmang et al., 2001) and dog (Zicha et al., 2005) also showed that HCN4 is the dominant isoform expressed in the SAN. Furthermore, mice lacking HCN4 channels are not viable after about 10 embryonic days, but a reduction of If was observed, by 85% on average, in the cardiac pacemaker cells of these mice together with a slow heart rate (Stieber et al., 2003). HCN2 knock out mice only displayed cardiac sinus dysrhythmia but no bradycardia or modification in the control of heart rate by the autonomic nervous system (Ludwig et al., 2003). This also indicates that HCN4 channels are essential for the generation of pacemaker activity in the heart.
Our present results show that ivabradine inhibited the homomeric hHCN4 current in CHO cells with an IC50 of 0.54 μM. IC50 values previously reported for the inhibition of native If in isolated SAN cells from rabbit were 2.8 μM (Bois et al., 1996) and 1.5 μM (Bucchi et al., 2002). This lower IC50 on hHCN4 could be explained either by intrinsic properties of HCN4 vs native If channel or by differences in experimental conditions. The calculated Hill coefficient of 0.87, a value close to that reported in the two studies on native If current: 0.96 and 0.8 (Bois et al., 1996; Bucchi et al., 2002, respectively), suggests that one molecule of ivabradine interacts with each hHCN4 channel.
It was also demonstrated that the inhibition of native If current by ivabradine developed slowly during repetitive trains of activating/deactivating voltage steps, until steady-state block was reached (Bois et al., 1996; Bucchi et al., 2002). This feature is characteristic of a ‘use-dependent' block, which is explained by the high affinity of the drug for the open configuration of the channels (Bucchi et al., 2002). Thus, the kinetics of the effect of ivabradine could result from use-dependency alone or in association with the time required to reach its intracellular binding site. In the first case the availability of the open channels during time is the only limiting parameter while, in the second one, a time-dependent component is involved. To answer this question, experiments with a similar electrophysiological protocol applied at two different frequencies were carried out. The two kinetics of block showed a superimposed relationship with the pulse number and not with the time, which led to the conclusion that the kinetics of ivabradine inhibition of hHCN4 current is related to the availability of open channels during the activating-deactivating pulses. Consequently, a long application time is required to obtain the steady-state block (about 12 min at 0.3 μM in our experimental conditions) and this block is reached more rapidly with an increased pulse frequency.
At the multicellular level, ivabradine progressively slows the diastolic depolarization rate in sino-atrial node pacemaker cells leading to a prolonged diastolic period and thus to a decrease in the frequency of the spontaneous APs (Thollon et al., 1994) and to a reduction in atrial beating rate, as confirmed in this study. One to three hours were required to obtain a stable reduction in APs firing rate at low concentrations of ivabradine (0.1–1 μM) while, in the previous studies, it was achieved after about 20 min with concentrations in the micromolar range (Thollon et al., 1994). Similarly, prolonged applications of 3 h were needed to reach maximal reduction of spontaneous beating rate of rat isolated right atria with ivabradine at 0.1–0.6 μM. In these conditions, the calculated IC30 value was shifted to 0.17 μM. Actually, the time required to obtain the full effect of ivabradine on a multicellular preparation was much longer than the time needed for inhibition of hHCN4 current in CHO cells. This difference could be partly explained by the longer availability of the open channels during the 5 s hyperpolarizing pulses protocol, which does not occur in pacemaker cells during SAN physiological activity, where f-channels are poorly recruited during the short diastole at a less hyperpolarized membrane potential. In order to analyse the possible implication of use-dependency in the kinetics of inhibition, we compared the time-course effects of ivabradine with that of an immediate extracellular blocker of f-channels. Cs was used in spite of its low specificity for If and its possible voltage-dependent activity (DiFrancesco et al., 1986), as it blocks If channels in a non-use-dependent manner. Indeed, 2 mM Cs induced almost complete inhibition of the hHCN4 current (90%) at the first hyperpolarizing pulse, when about 60 pulses were required for an equivalent inhibition with 3 μM ivabradine. Cs also exerted a very rapid reduction in spontaneous firing rate of isolated SAN preparation and right atria (5–10 min), whereas 90–180 min were needed to obtain a similar effect with ivabradine. Together, these results suggest that the slow kinetics of action of ivabradine could be related to its use-dependent blocking mechanism. Even if extrapolations from in vitro results to in vivo need to be treated with caution, it can be reasonably speculated that this property of ivabradine is likely to be responsible for the lag time between the plasma peak concentration and the maximal heart rate reduction described in human in clinical studies (Ragueneau et al., 1998). This delay may be related to the progressive inhibition observed in vitro at equivalent concentration of ivabradine.
As a result of the use-dependent block, the reduction in pacemaker activity induced by ivabradine must be more important at higher firing rate as already suggested (Bucchi et al., 2002), and also for other positively charged use-dependent If blockers (Goethals et al. 1993; Van Bogaert and Pittoors, 2003; Stieber et al., 2004). We have compared the effects of ivabradine in preparations obtained from species with different heart rates. In vivo (data not shown), conscious guinea-pig heart rate is intermediate between rat and rabbit (250 vs 300 and 200 b.p.m., respectively) and pigs have a heart rate closer to that of humans (around 90 b.p.m.). These differences that are partly related to intrinsic properties of pacemaker activity also occur in isolated SAN (basal frequency of 193, 208 and 90 APs min−1, in rabbit, guinea-pig and pig, respectively). In fact, it is now clear that the spontaneous beating rate of the SAN reflects the heart rate measured in vivo (Ono et al., 2003; Satoh, 2003). In these different species, If current, activated by hyperpolarization in isolated SAN cells, was found to contribute to the diastolic depolarization in the pacemaker range (−45 to −65 mV) (Satoh, 2003), even though the large variation in the frequencies of the spontaneous beating rates between the species might be due, at least in part, to differences in repolarizing potassium channels, especially IKr and IKs (Ono et al., 2003). Using a concentration previously shown to exert maximal decrease in the frequency of the spontaneous beating of rabbit SAN (3 μM) (Thollon et al., 1994), we demonstrated that the reduction of pacemaker APs firing rate is stronger in guinea-pig than in rabbit and that these effects are more marked than in pig, suggesting a possibly greater contribution of If current to pacemaker activity in the small species. In the swine SAN, ivabradine reduced the spontaneous firing of APs by approximately 15% (−13 APs), an effect that can be achieved in humans at therapeutic doses (Ragueneau et al., 1998; Borer et al., 2003; Tardif et al., 2005).
In conclusion, HCN4 is the main isoform expressed in hSAN and ivabradine blocked this channel in a purely use-dependent way with an IC50 of 0.54 μM. This use-dependence is consistent with slowly developing effect of ivabradine in vitro in SAN preparations and right atria. Although increasing pulsing frequency improves the kinetics of inhibition of hHCN4 current by ivabradine in CHO cells, the direct link between the greater efficiency in species with higher basal beating rate and the use-dependency of ivabradine remains to be demonstrated.
Acknowledgments
We are grateful to Pr Dr Martin BIEL (München, Germany) for the gift of hHCN4 cDNA, to Sophie-Pénélope GUENIN, Véronique PIERRE and Christine PETIT for technical assistance. We thank Karine IMBERDIS for expert assistance in statistical analysis.
Abbreviations
- AP
action potential
- b.p.m.
beats per minute
- CHO
Chinese hamster ovary
- Cs
Caesium
- HCN
hyperpolarization-activated cyclic nucleotide-gated channel
- HR
heart rate
- If
funny current
- Ih
hyperpolarization-activated current
- SAN
sino-atrial node
Conflict of interest
The authors are employees of Servier group which has developed ivabradine.
References
- Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. doi: 10.1152/physiologyonline.2002.17.1.32. [DOI] [PubMed] [Google Scholar]
- Baruscotti M, DiFrancesco D. Pacemaker channels. Ann NY Acad Sci. 2004;1015:111–121. doi: 10.1196/annals.1302.009. [DOI] [PubMed] [Google Scholar]
- Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–1057. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer JS, Fox K, Jaillon P, Lerebours G, for the ivabradine Investigators Group Antianginal and antiischemic effects of ivabradine, an If inhibitor, in stable angina. A randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchi A, Baruscotti M, DiFrancesco D. Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J Gen Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarizing-activated current (If) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethals M, Raes A, Van Bogaert PP. Use-dependent block of the pacemaker current If in rabbit sinoatrial node cells by zatebradine (UL-FS 49). On the mode of action of sinus node inhibitors. Circulation. 1993;88:2389–2401. doi: 10.1161/01.cir.88.5.2389. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem. 1999;274:12835–12839. doi: 10.1074/jbc.274.18.12835. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Hofmann F, Biel M. Structure and function of cardiac pacemaker channels. Cell Physiol Biochem. 1999a;9:179–186. doi: 10.1159/000016315. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999b;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial Sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006;354:151–157. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, et al. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- Ono K, Shibata S, Iijima T. Pacemaker mechanism of porcine sino-atrial node cells. J Smooth Muscle Res. 2003;39:195–204. doi: 10.1540/jsmr.39.195. [DOI] [PubMed] [Google Scholar]
- Ragueneau I, Laveille C, Jochemsen R, ResplandY G, Funck-Brentano C, JailloN P. Pharmacokinetic–pharmacodynamic modeling of the effects of ivabradine, a direct sinus node inhibitor, on heart rate in healthy volunteers. Clin Pharmacol Ther. 1998;64:192–203. doi: 10.1016/S0009-9236(98)90153-9. [DOI] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, et al. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Satoh H. Sino-atrial nodal cells of mammalian hearts: ionic currents and gene expression of pacemaker ionic channels. J Smooth Muscle Res. 2003;39:175–193. doi: 10.1540/jsmr.39.175. [DOI] [PubMed] [Google Scholar]
- Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, et al. Distribution and prevalence of hyperpolarization-activated cation channels (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85:e1–e6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- Stieber J, Herrmann S, Fiel S, Löster J, Feil R, Biel M, et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA. 2003;100:15235–15240. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber J, Hofmann F, Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc Med. 2004;14:23–28. doi: 10.1016/j.tcm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Ford I, Tebdera M, Bourassa MG, Fox K, for the INITIATIVE Investigators Efficacy of ivabradine, a new selective If inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- Thollon C, Bidouard JP, Cambarrat C, Lesage L, Reure H, Delescluse I, et al. Stereospecific in vitro and in vivo effects of the new sinus node inhibitor (+)-S 16257. Eur J Pharmacol. 1997;339:43–51. doi: 10.1016/s0014-2999(97)01364-2. [DOI] [PubMed] [Google Scholar]
- Thollon C, Cambarrat C, Vian J, Prost JF, Peglion JL, Vilaine JP. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Van Bogaert PP, Pittoors F. Use-dependent blockade of cardiac pacemaker current (If) by cilobradine and zatebradine. Eur J Pharmacol. 2003;478:161–171. doi: 10.1016/j.ejphar.2003.08.083. [DOI] [PubMed] [Google Scholar]
- Vilaine JP, Bidouard JP, Lesage L, Reure H, Peglion JL. Anti-ischemic effects of ivabradine, a selective heart rate-reducing agent, in exercise-induced myocardial ischemia in pigs. J Cardiovasc Pharmacol. 2003a;42:688–696. doi: 10.1097/00005344-200311000-00016. [DOI] [PubMed] [Google Scholar]
- Vilaine JP, Thollon C, Villeneuve N, Peglion JL. Procoralan, a new selective If current inhibitor. Eur Heart J suppl. 2003b;5:G26–G35. [Google Scholar]
- Zicha S, Fernandez-Velasco M, Lonardo G, L'heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res. 2005;66:472–481. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]