Abstract
Background and purpose:
5-HT4 receptor agonists are used therapeutically to treat disorders of reduced gastrointestinal motility. Since such compounds are evaluated in guinea-pigs, we cloned, expressed and pharmacologically characterized the guinea-pig 5-HT4 and human 5-HT4(b) splice variant, which share 95% homology. The functional properties of guinea-pig 5-HT4(b) receptors were compared with native receptors in guinea-pig colon.
Experimental approach:
Membrane radioligand binding and whole cell cAMP accumulation assays were used to determine the affinities, potencies and intrinsic activities (IA). Contraction of the guinea-pig distal colon longitudinal muscle myenteric plexus preparation (LMMP) was monitored to evaluate functional activity.
Key results:
pKi values for guinea-pig and human recombinant receptors, and guinea-pig striatum 5-HT4 receptors, were in agreement, as were the potency and IA values for guinea-pig and human 5-HT4 receptors expressed at a similar density (∼0.2 pmol mg−1 protein). Tegaserod was a potent (pEC50=8.4 and 8.7, respectively), full agonist at both guinea-pig and human 5-HT4 receptors. In contrast, in the LMMP preparation, tegaserod was a potent, partial agonist (pEC50=8.2; IA=66%).
Conclusions and implications:
Close agreement between the pharmacological properties of guinea-pig and human 5-HT4 receptors support the use of guinea-pig model systems for the identification of 5-HT4 receptor therapeutics. However, the mechanisms underlying the different agonist properties of tegaserod in recombinant and isolated tissue preparations, and the extent to which these impact the clinical efficacy of tegaserod as a prokinetic agent, remain to be determined.
Keywords: gastrointestinal, 5-hydroxytryptamine, 5-HT4, tegaserod, adenylate cyclase, gastroprokinetic
Introduction
The neurotransmitter 5-hydroxytryptamine (5-HT) mediates a broad range of physiological and behavioural responses both centrally and peripherally (Saxena, 1995; Barnes and Sharp, 1999). All classes of the extensive 5-HT receptor family, except for the ligand-gated 5-HT3 receptor, are members of the seven transmembrane-spanning G protein-coupled family of receptors (Hoyer et al., 2002; Reeves and Lummis, 2002). These receptors modulate signal transduction pathways via stimulation or inhibition of adenylyl cyclase, modulation of cytosolic calcium concentration or activation of multiple alternative downstream effectors, including ERK1/2 (Pauwels, 2000; Norum et al., 2003).
The 5-HT4 receptor is thought to signal principally, but not exclusively, via Gs coupling to activation of adenylyl cyclase (Gerald et al., 1995; Pindon et al., 2002). Peripherally, activation of sinoatrial 5-HT4 receptors has a chronotropic effect on heart rhythm (Hegde and Eglen, 1996; Krobert et al., 2005; De Maeyer et al., 2006). In the digestive tract, activation of 5-HT4 receptors initiates a coordinated and complex prokinetic response that has been extensively studied and well described (Gershon, 1999). 5-HT4-mediated responses include modulation of cholinergic neurotransmission in the human proximal stomach (Leclere and Lefebvre, 2002) and increased colonic contractile responses in human, rat, dog and guinea-pig (Grider et al., 1998; Sakurai-Yamashita et al., 1999; Prins et al., 2000a, 2000b; Leclere et al., 2005). Contraction and potentiation of the electrical field twitch response in the guinea-pig ileum (Kajita et al., 2001) and colon (Jin et al., 1999) have also been described, as has facilitation of fast excitatory synaptic transmission (Galligan et al., 2003). In the rat oesophagus, 5-HT4 receptors mediate relaxation of smooth muscle (Leung et al., 1996; Goldhill et al., 1997).
Direct activation of 5-HT4 receptors has been demonstrated to relieve symptoms of several disease states, including functional bowel disorders, such as constipation-predominant irritable bowel syndrome and chronic constipation (Sanger, 1996; Alaradi and Barkin, 2002). Although the therapeutic potential of 5-HT4 agonists in the treatment of these disorders is accepted (Emmanuel et al., 2002; Lacy and Yu, 2002; Camilleri, 2004; Johanson, 2004), opportunities still exist for the development of novel agents with improved efficacy and/or tolerability profiles. Preclinical evaluations of novel 5-HT4 agonists include the use of human recombinant 5-HT4 receptor systems (Hinschberger et al., 2003). However, these systems have a number of limitations. Thus, questions surrounding the influence of receptor expression levels (Krobert et al., 2005), identity of relevant signal transduction pathways (Pindon et al., 2002) and desensitization mechanisms (Barthet et al., 2005), for example, prompt evaluation of novel agents in isolated tissue preparations, in particular from dog (Prins et al., 2000a), guinea-pig (Beattie et al., 2004) and/or human (Leclere et al., 2005) digestive tract. Guinea-pig tissue is readily accessible and guinea-pig pharmacological models are used routinely for the evaluation of 5-HT4 receptor selective agonists. Indeed, on the basis of work in guinea-pig isolated ileum, tegaserod has been described as a partial agonist (Buchheit et al., 1991). However, to date, although the guinea-pig 5-HT4 receptor has been cloned, there has been no comprehensive evaluation of the pharmacological properties of this recombinant receptor. The aims of the present study were to characterize the pharmacological properties of the guinea-pig recombinant 5-HT4 receptor in human embryonic kidney 293 (HEK293) cell stable transfectants, and compare these with native 5-HT4 receptors. A two-step approach was undertaken, in which binding affinities at the recombinant 5-HT4 receptor were compared with those at the native receptor in striatal membrane preparations, whereas functional comparisons were made with native guinea-pig 5-HT4 receptors, using the guinea-pig isolated colon longitudinal muscle myenteric plexus (LMMP) preparation. Although guinea-pig striatum has been used extensively to determine ligand-binding affinities (e.g. Grossman et al., 1993), to the best of our knowledge, there are no in vitro functional studies using guinea-pig striatum. In contrast, the use of guinea-pig colon preparations to profile 5-HT4 receptor ligands is well established (e.g. Leung et al., 1996). It should be noted, however, that there is an excellent correlation between the binding affinities for a range of agonist and antagonist ligands at 5-HT4 receptors in striatal and ileal/colon membrane preparations (Uchiyama-Tsuyuki et al., 1996). In addition, in the present study, the properties of the guinea-pig 5-HT4 receptor were compared directly with the prevalent human 5-HT4(b) receptor splice variant, with which it shares ∼95% identity, as well as high similarity within the C-terminus.
Methods
5-HT4 receptor cloning and expression
Guinea-pig and human recombinant 5-HT4 and 5-HT4(b) receptors were cloned by reverse transcription-polymerase chain reaction (RT-PCR) from guinea-pig striatum and a mixed ileum and jejunum human cDNA library, respectively. The sequence of the forward and reverse primers encoding the guinea-pig 5-HT4 receptor were 5′-GTCTAGATGGACAAACTTGATGCTAATGTGAG-3′ and 5′-CTCGAGTTACTAAGTGTCAATGGGCTGAGCAGCCACCAAAGGAGAACTTG CTGCAGGG-3′ and were designed using the published sequence provided in Genbank (van den Wyngaert et al., 1997, Genbank Accession Y13585). The forward and reverse primers encoding the human 5-HT4(b) receptor were 5′-ATGGACAAACTTGATGCTAATGTG-3′ and 5′-CTTCTGGGTCATTGTCCCAGG-3′, respectively. The final guinea-pig and human 5-HT4 receptor constructs were fully sequenced in both the forward and reverse directions to confirm both the identity and the integrity of each sequence. 5-HT4 receptor cDNA was subcloned into mammalian expression vectors and transfected into HEK293 cells by Ca2+-phosphate-mediated uptake. Cells were grown in Dulbecco's modified Eagles medium (DMEM) and incubated in a 5% CO2 humidified incubator at 37°C. Single colonies were generated by dilution cloning, under geneticin selection. Clones expressing 5-HT4 receptors were identified using 5-HT4 receptor selective agonists to stimulate adenylyl cyclase in a whole-cell cyclic adenosine monophosphate (cAMP) accumulation assay (see below). Corresponding receptor densities were determined in saturation-binding experiments using [3H]GR113808, as described below. HEK293 cells stably transfected with guinea-pig 5-HT4 or human 5-HT4(b) recombinant receptors, respectively, were cultured in DMEM supplemented with D-glucose (4500 mg l−1), 10% foetal bovine serum and 100 U of penicillin-(100 μg) streptomycin ml−1 and geneticin (800 μg ml−1) in a 5% CO2, humidified incubator (37°C).
mRNA isolation and RT-PCR
Adult male, Dunkin-Hartley guinea-pigs (200–300 g, Harlan, Indiana) were killed by CO2 asphyxiation and thoracotamy, in accordance with the Theravance Institutional Animal Care and Use Committee guidelines and the principles of laboratory animal care prescribed by the National Institute of Health. The oesophagus, duodenum, ileum, colonic submucosal plexus (SMP) and distal colon LMMP were harvested and stored in RNAlater RNA Stabilization Reagent at −20°C, before mRNA extraction. Unless otherwise noted, all steps of the extraction were performed at 4°C. Tissue samples were normalized by weight following isolation and microdissection of segments from each region of the digestive tract. Tissue samples were predisrupted using a hand-held homogenizer and then homogenized using QIAshredder columns. Total RNA was isolated using the RNeasy Protect Mini Kit and treated with the DNA-free kit to remove any remaining genomic DNA. Guinea-pig 5-HT4 cDNA was transcribed using the ThermoScript RT-PCR System and oligo dT primers in the presence of 3–5 μg RNA. PCR reactions were performed in a final volume of 50 μl containing 100–500 ng cDNA template, 0.5 μM primers, 0.2 mM each deoxynucleoside triphosphate, 1 × PCR buffer, 1.5 mM MgCl2 and 5 U Platinum Taq Polymerase. cDNA templates were denatured for 2 min at 95°C and amplified as follows: 95°C, 30 s; 55°C, 45 s; 72°C, 45 s; 42 cycles. A final extension was performed at 72°C for 7 min. The sequences of the forward and reverse primers encoding the C-terminus of the guinea-pig 5-HT4 receptor were 5′-GCCTTCCTTATCATCCTGTGCTGTG-3′ and 5′-CTCGAGTTACTAAGTGTCAATGGGCTGAGCAGCCACCAAAG-3′, respectively. Amplified PCR products, generated over the linear range of amplification, were visualized on a 1.2% agarose gel.
Cell membrane preparation
At 20–22 h before harvest, HEK293 cells stably transfected with guinea-pig 5-HT4 or human 5-HT4(b) receptor cDNA were washed twice and then cultured in serum-free DMEM. Cells were harvested by gentle mechanical agitation and then centrifugation (1200 g, 5 min). The pellets were resuspended in 50 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulphonic acid N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) (HEPES), pH 7.4 and homogenized with a polytron disrupter (setting 19.2 × 10 s) on ice. The resultant homogenates were centrifuged (1200 g, 5 min), the pellets discarded and the supernatants centrifuged (40 000 g 20 min). The pellets were washed once by resuspension in 50 mM HEPES (pH 7.4) and centrifugation (40 000 g for 20 min). The final pellets were resuspended in 50 mM HEPES (pH 7.4) and aliquots stored at −80°C, until required.
Membranes were also prepared from guinea-pig striatum, ileum and proximal colon. Tissue pieces were harvested, as described above. Membranes were then prepared according to the protocol described for recombinant HEK293 cells.
5-HT4 receptor radioligand binding
5-HT4 receptor radioligand membrane binding assays were conducted as described previously (Bender et al., 2000). Briefly, membranes were incubated with [3H]GR113808 in 50 mM Tris-HCl buffer pH.7.4. Nonspecific radioligand binding was defined using GR113808 (1 μM). Competition binding studies, to determine compound affinity, were conducted with increasing concentrations of unlabelled ligand (10 pM–30 μM) and at a fixed concentration of 0.15 nM [3H]GR113808. Following a 60 min incubation at 22°C (a period sufficient to reach equilibrium), the membranes were harvested by rapid filtration over Whatman GF/B filters and bound radioactivity quantitated by liquid scintillation spectroscopy.
Whole-cell cAMP accumulation studies
Whole-cell cAMP accumulation studies were conducted using HEK293 cells stably transfected with guinea-pig 5-HT4 or human 5-HT4(b) recombinant receptors, respectively, and the Flashplate Adenylyl Cyclase Activation Assay System, as described previously (Pindon et al., 2002).
Cells were cultured as described above. Cells were grown to 60–80% confluency and, at 20–22 h before harvest, washed twice and then cultured in serum-free DMEM. To harvest the cells, the media were aspirated and the cells were incubated for 5 min at room temperature with Versene. The cells were lifted from the flask by gentle mechanical agitation, suspended in pre-warmed (37°C) Dulbecco's phosphate-buffered saline, and then harvested by centrifugation at 1200 g for 5 min. The supernatant was discarded and the pellet was resuspended in pre-warmed (37°C) ‘stimulation buffer' provided with the Flashplate kit. Cells were diluted to a concentration of 5 × 105 cells ml−1 in pre-warmed (37°C) ‘stimulation buffer' and preincubated at 37°C for 10 min. cAMP accumulation assays were performed with increasing concentrations of test compound and 5-HT (10 pM–100 μM). Cell suspension (50 μl) was added to each well of the Flashplate for a final assay volume of 100 μl. The cells were incubated, with shaking, at 37°C for 15 min. After the incubation period, a direct radioimmunoassay, using [125I]cAMP, was performed by the addition of 100 μl of ice-cold ‘detection buffer' to each well, according to the manufacturer's instructions. The plates were sealed and incubated at 4°C, overnight. Bound radioactivity was quantified by scintillation proximity spectroscopy using the Topcount (Perkin-Elmer, Boston, MA, USA). The amount of cAMP produced was extrapolated from a cAMP standard curve.
Guinea-pig isolated distal colon LMMP preparation
Adult, male, Dunkin-Hartley guinea-pigs (200–300 g, Harlan, Indiana) were killed by CO2 asphyxiation and thoracotamy, in accordance with the Theravance Institutional Animal Care and Use Committee guidelines. The colon was removed, and placed in Krebs–Henseleit physiological buffer, containing (in mM): KCl 4.7, KH2PO4 1.2, MgSO4 anhydrous 1.2, NaCl 118.1, D-glucose 11.1, NaHCO3 25.0, CaCl2 2.6, ondansetron 0.003 (to block 5-HT3 receptors), methysergide 0.001 (to block 5-HT1 and 5-HT2 receptors) and indomethacin 0.001 (to inhibit prostaglandin synthesis). The colon was cut into 5 cm lengths, the contents gently removed and each segment then placed on a 10 ml pipette. An incision was made along the length of the colon with a scalpel blade, and the longitudinal muscle was then peeled off carefully using the tip of a thumb. Each longitudinal muscle strip was then mounted, under a tension of 1 g, in a 10 ml tissue bath filled with Krebs–Henseleit buffer. The bathing solution was aerated continuously with 95% O2/5% CO2 and maintained at 37°C. During the next 45 min, tissues were washed three times (at 0, 15 and 30 min after mounting) and tension re-applied to 1 g as necessary. The tissues were then challenged with 5-HT at a concentration (0.3 μM) previously established to evoke a maximal contractile response. Once the contraction had reached its maximum, tissues were washed four times every 2 min and once more 10 min later. An additional priming challenge of 5-HT (0.3 μM) was made 15 min later. Following further washing, before and after a third 5-HT (0.3 μM) challenge to confirm the maximal contractile response had been determined, a cumulative concentration–effect curve to 5-HT (0.0001–3 μM) or test compound (0.0001–10 μM) was constructed. Responses were allowed to reach a maximum before addition of the next, ascending concentration. Contractile responses were normalized to the primed 5-HT (0.3 μM) response in each tissue.
Data analysis
Binding data were analysed by nonlinear regression analysis using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) and a three-parameter model for one-site competition. pKi (negative decadic logarithm of Ki) values for test compounds were calculated from the best-fit IC50 values, and the Kd value of the radioligand, using the Cheng-Prusoff equation (Cheng and Prusoff, 1973): Ki=IC50/(1+[L]/Kd) where [L] is concentration radioligand. Data are expressed as mean±s.d. cAMP accumulation data were analysed by nonlinear regression analysis with the GraphPad Prism Software package using the three-parameter sigmoidal dose-effect model (slope constrained to unity). Potency data are presented as pEC50 values (negative decadic logarithm of the effective concentration producing 50% of the maximum response; mean±s.e.m.). To determine the IA, relative to 5-HT, the maximum compound-evoked response (minus basal) was expressed as a percentage of the maximum response evoked by 5-HT (minus basal), assayed in parallel on the same plate. LMMP contractile responses were analysed by nonlinear regression analysis with GraphPad Prism Software using the three-parameter sigmoidal dose–effect model (slope constrained to unity) to derive the mean potency (pEC50±s.e.m.) and IA (as a percentage of the 5-HT maximum response).
Materials
The mixed ileum and jejunum human cDNA library was purchased from Clontech (Mountain View, CA, USA). Tissue culture reagents, ThermoScript RT-PCR System and taq polymerase were purchased from Invitrogen (Carlsbad, CA, USA). The RNAlater RNA Stabilization Reagent, QIAshredder columns and the RNeasy Protect Mini Kit were purchased from Qiagen (Valencia, CA, USA). The DNA-free kit was purchased from Ambion (Austin TX, USA). HEPES, Tris-HCl, KCl KH2PO4, MgSO4, NaCl, D-glucose, NaHCO3, CaCl2, 5-HT and 5-methoxytryptamine (5-MeOT) were purchased from Sigma-Aldrich (St Louis, MO, USA). [3H]GR113808 was purchased from Amersham Biosciences (Newark, NJ, USA). The Flashplate Adenylyl Cyclase Activation Assay System was purchased from Perkin-Elmer (Boston MA, USA). Tegaserod and ondansetron were purchased from Apin Chemicals and Sequoia Research Products (Oxfordshire, UK), respectively. GR113808 ([1-2[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate), RS39604 (1-[4-amino-5-chloro-2-(3,5-dimethoxybenzyloxy)phenyl]-3-[1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]-1-propanone hydrochloride), SB203186 (1-piperidinylethyl1H-indole-3-carboxylate), RS67506 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-(1-n-butyl-4-piperidinyl)-1-propanone, RS67333 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-butyl-4-piperidinyl]-1-propanone), RS23597-190 (3-(piperdine-1-yl)-propyl-4-amino-5-chloro-2-methoxy benzoate hydrochloride), RS56812 (N-(quinuclidin-3-yl)-2-(1-methyl-1H-indol-3-yl)-2-oxoacetamide), methysergide and indomethacin were purchased from Tocris Cookson (Ellisville, MO, USA). Piboserod, TS951 (N-[endo-8-(3-hydroxypropyl)-8-azabicyclo[3.2.1]oct-3-yl]-1-isopropyl-2-oxo-1,2-dihydro-3-quinolinecarboxamide), prucalopride, cisapride, BIMU-8 (endo-N-(8-methyl-8-azabicyclo[3.2.1]oct-3-yl)-2,3-dihydro-(1-methyl)ethyl-2-oxo-1H-benzimidazole-carboxamide HCl), mosapride, ML10302 (2-(1-piperidinyl)ethyl-4-amino-5-chloro-2-methoxybenzoate) and metoclopramide were prepared by the Theravance Inc. Medicinal Chemistry department for research purposes.
For radioligand-binding and cAMP accumulation studies, 5-HT4 ligands were prepared at 10 mM in dimethyl sulfoxide (DMSO), diluted to 400 μM with 50 mM HEPES (pH 7.4) at 25°C, containing 0.1% bovine serum albumin, and then serially diluted in the same buffer. For examining contractile responses in the LMMP preparation compounds were prepared at 10 mM in DMSO and then serially diluted in water.
Results
Distribution of guinea-pig 5-HT4 receptors in the digestive tract
A guinea-pig 5-HT4 receptor variant was cloned by RT-PCR from guinea-pig striatum. DNA sequence analysis confirmed the identity of this receptor and that the sequence shared the highest C-terminal identity with the human 5-HT4(b) receptor.
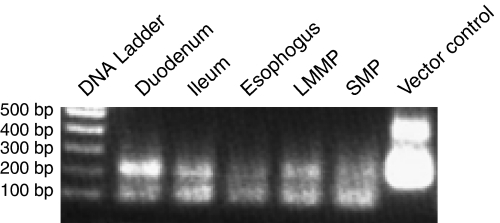
Using RT-PCR, the distribution pattern of this 5-HT4 receptor variant was examined in various tissue regions isolated from the guinea-pig digestive tract. Guinea-pig 5-HT4 receptor mRNA transcripts were detected in all regions examined, specifically the duodenum, ileum, oesophagus, LMMP and colonic SMP (Figure 1). The apparent highest and lowest levels of 5-HT4 receptor mRNA were detected in the duodenum and oesophagus, respectively.
Figure 1.
Analysis of 5-HT4 receptor cDNA fragments isolated from representative regions of guinea-pig digestive tract. The DNA markers are shown in the far left lane. The amplified fragments corresponding to the C-terminus of the 5-HT4 receptor can be visualized as the upper 0.2 kb band.
The presence of saturable, specific [3H]GR113808 binding sites in membranes prepared from guinea-pig isolated ileum and proximal colon, respectively, confirmed the presence of 5-HT4 receptor protein in these tissues. [3H]GR113808 bound to a single population of sites in both ileum and colon with pKD values of 10.6 and 10.4 and corresponding Bmax values of 37 and 28 fmol mg−1 protein, respectively (n=2). For comparison, the pKD and Bmax values for [3H]GR113808 were 10.31±0.14 and 147±26 fmol mg−1 protein (n=6), respectively, in membranes prepared from guinea-pig striatum.
Pharmacological characterization of guinea-pig recombinant 5-HT4 receptors
Stable transfectants expressing 5-HT4 receptors at both low (gp 5-HT4-clone 1, Bmax=0.21±0.03 pmol mg−1 protein) and high (gp 5-HT4-clone 2, Bmax=2.54±1.18 pmol mg−1 protein) receptor densities were used for pharmacological studies. [3H]GR113808 bound with high affinity (pKD=10±0.48 and 10.18±0.29, respectively), to a single population of sites in membranes prepared from both gp 5-HT4-clone 1 and gp 5-HT4-clone 2.
The Bmax value in the gp 5-HT4 – clone 1 was similar to that observed in guinea-pig striatum. Therefore, membranes prepared from this clone were used in [3H]GR113808 competition binding studies to profile the binding affinities of a panel of 5-HT4 receptor selective agonists and antagonists. As can be seen from Table 1, 5-HT4 receptor selective antagonists, for example GR113808 and SB203186, bound with high affinity (pKi ⩾9.9) to guinea-pig recombinant 5-HT4 receptors. The 5-HT4 receptor selective agonists, for example tegaserod, TS-951 and cisapride, bound with medium to high affinity (pKi ⩾7.7), whereas metoclopramide and 5-MeOT exhibited low affinity (pKi ⩽6.9).
Table 1.
Binding affinities (pKi) for a range of 5-HT4 ligands at native 5-HT4 receptors in guinea-pig striatal membranes and recombinant guinea-pig 5-HT4 and human 5-HT4(b) receptors stably expressed in HEK293 cells
| Compound | gp 5-HT4/HEK293 | gp striatal membranes | h 5-HT4(b)/HEK293 |
|---|---|---|---|
| pKi | pKi | pKi | |
| (mean±s.d.) | (mean±s.d.) | (mean±s.d.) | |
| GR113808 | 10.3±0.2 | 10.4±0.1 | 10.5±0.1 |
| RS39604 | 10.1±0.5 | 9.8±0.1 | 10.1±0.1 |
| SB203186 | 9.9±0.2 | 9.3±0.2 | 9.4±0.1 |
| RS67506 | 9.4±0.1 | 8.9±0.1 | 9.0±0.1 |
| RS67333 | 8.7±0.1 | 8.7±0.1 | 9.0±0.1 |
| RS23597–190 | 8.6±0.1 | 8.3±0.1 | ND |
| Tegaserod | 8.4±0.2 | 7.7±0.3 | 8.1±0.1 |
| TS951 | 8.1±0.1 | 7.5±0.2 | 7.6±0.1 |
| Prucalopride | ND | 7.7±0.1 | 7.5±0.1 |
| Cisapride | 7.7±0.1 | 7.4±0.2 | 7.4±0.1 |
| BIMU-8 | 7.6±0.2 | 6.9±0.2 | 7.2±0.1 |
| Mosapride | 7.4±0.2 | 7.0±0.1 | 7.2±0.1 |
| 5-HT | 7.4±0.2 | 7.2±0.2 | 6.9±0.2 |
| RS56812 | 7.2±0.1 | 6.7±0.2 | 7.1±0.1 |
| 5-MeOT | 6.9±0.1 | 6.4±0.1 | 6.4±0.1 |
| Metoclopramide | 6.7±0.2 | 6.3±0.4 | 6.7±0.1 |
Abbreviations: 5-HT, 5-hydroxytryptamine; 5-MeOT, 5-methoxytryptamine; ND, not determined.
Competition binding experiments were as described in the Methods section. Data are given as the mean±s.d. from at least three independent experiments.
The functional properties of guinea-pig recombinant 5-HT4 receptors were characterized using whole-cell cAMP accumulation studies. To examine the influence of receptor density on the potency and IA of a range of 5-HT4 receptor selective ligands, these studies were conducted using both gp 5-HT4 – clone 1 and gp 5-HT4 – clone 2.
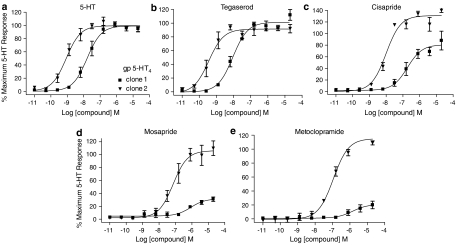
Basal cAMP accumulation in 5-HT4 transfectants (gp 5-HT4 – clone 1 and gp 5-HT4 – clone 2) and untransfected HEK293 cells was not significantly different (data not shown). 5-HT evoked a concentration-dependent increase in whole-cell cAMP accumulation in both gp 5-HT4 – clone 1 and gp 5-HT4 – clone 2 (Figure 2a) with corresponding pEC50 values of 7.9±0.3 (n=11) and 9.0±0.3 (n=5), respectively (Table 2). A similar increase in potency of one log unit, or greater, was observed for all 5-HT4 receptor agonists in gp 5-HT4 – clone 2, relative to gp 5-HT4 – clone 1 (Table 2, Figure 2b–e). However, the rank order of potency was unchanged and, was as follows: tegaserod >5-HT >cisapride >mosapride >metoclopramide (Table 2). The rank order of IA (expressed as a percentage of the maximal 5-HT response) in the lower receptor density clone, gp 5-HT4 – clone 1, was as follows: tegaserod (106%) >cisapride (76%) ⩾prucalopride (66%) >metoclopramide (24%) >mosapride (22%) (Table 2). In comparison, all the compounds tested were full agonists (IA>90%) in the higher receptor density clone, gp 5-HT4 – clone 2 (Table 2, Figure 2a–e). Notably, metoclopramide and mosapride were weak partial agonists (IA <24%) in the lower receptor density clone, gp 5-HT4 – clone 1, but behaved as full agonists in the higher receptor density clone, gp 5-HT4 – clone 2 (Figure 2d and e, respectively).
Figure 2.
Agonist-evoked cAMP accumulation in HEK293 cells expressing 5-HT4 receptors at a low (clone 1) and high (clone 2) receptor density, respectively. Data points (mean and s.e.m. (vertical lines)) from at least three separate experiments were used to generate curves for 5-HT (a), tegaserod (b), cisapride (c), mosapride (d) and metoclopramide (e) that were normalized to the maximum 5-HT-evoked response.
Table 2.
The mean potency (pEC50) and IA values determined in cAMP accumulation assays for guinea-pig 5-HT4 and the human 5-HT4(b) receptors expressed in HEK293 cells and in the guinea-pig isolated colon LMMP preparation
| Compound |
gp 5-HT4 /HEK293 – clone 1 |
gp 5-HT4/HEK293 – clone 2 |
h 5-HT (4b) /HEK293 |
gp isolated LMMP |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEC50 | IA | n | pEC50 | IA | n | pEC50 | IA | n | pEC50 | IA | n | |
| mean±s.e.m. | mean (95% CI) | Mean±s.e.m. | mean (95% CI) | Mean±s.e.m. | mean (95% CI) | Mean±s.e.m. | mean (95% CI) | |||||
| Tegaserod | 8.4±0.1 | 106 (99–111) | 11 | 9.4±0.1 | 95 (99–111) | 5 | 8.7±0.1 | 127 (107–135) | 6 | 8.2±0.1 | 66 (61–72) | 3 |
| Prucalopride | 7.9±0.1 | 66 (25–107) | 3 | ND | ND | 8.2±0.1 | 95 (88–102) | 6 | 7.5±0.1 | 93 (87–100) | 14 | |
| 5-HT | 7.9±0.1 | 100 | 11 | 9.0±0.1 | 100 | 5 | 8.4±0.1 | 100 | 6 | 8.0±0.1 | 98 (94–102) | 20 |
| Cisapride | 6.9±0.1 | 76 (63–89) | 11 | 8.0±0.1 | 121 (104–138) | 5 | 7.3±0.1 | 102 (92–107) | 6 | 7.0±0.1 | 74 (69–80) | 10 |
| Mosapride | 6.3±0.1 | 22 (15–30) | 11 | 7.1±0.1 | 105 (85–125) | 5 | 6.7±0.1 | 35 (24–47) | 6 | 5.4±0.1 | 37 (32–42) | 3 |
| Metoclopramide | 5.9±0.2 | 24 (15–32) | 6 | 7.0±0.2 | 90 (78–102) | 5 | 5.9±0.1 | 39 (0–78) | 3 | 5.5±0.1 | 84 (76–92) | 8 |
Abbreviations: CI, confidence interval; 5-HT, 5-hydroxytryptamine; HEK293, human embryonic kidney 293; IA, intrinsic activity; LMMP, longitudinal muscle myenteric plexus; ND, not determined.
Values represent mean±s.e.m. or 95% CI and the number of individual experiments.
Comparison between the pharmacological properties of guinea-pig recombinant and native 5-HT4 receptors
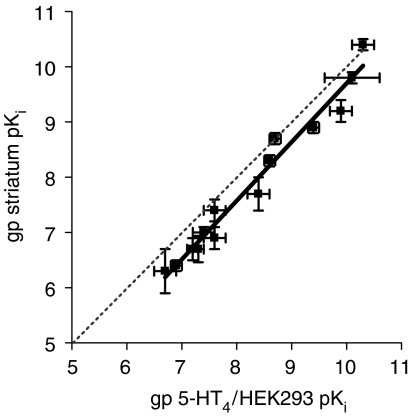
[3H]GR113808 radioligand membrane binding and conventional isolated tissue bath methodologies were used to characterize the pharmacological properties of guinea-pig native 5-HT4 receptors. The binding affinities of a panel of 5-HT4 receptor selective agonists and antagonists for guinea-pig native 5-HT4 receptors in striatal membranes were in very close agreement (r2=0.95, slope=0.92±0.05; P<0.0001) with the corresponding values for guinea-pig recombinant 5-HT4 receptors (Table 1; Figure 3).
Figure 3.
Correlation plot for a range of 5-HT4 receptor agonist and antagonist pKi values at recombinant and native guinea-pig 5-HT4 receptors determined using [3H]GR113808 competition binding assays. The solid line represents a linear regression through the points, and the dashed line represents unity. Data represent mean±s.d. of 3–12 independent experiments.
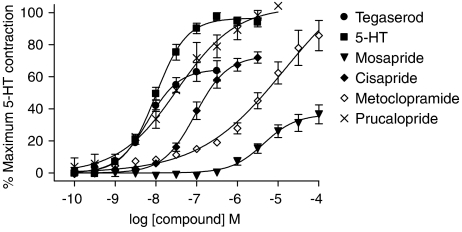
The functional activity of guinea-pig native 5-HT4 receptors was evaluated using the guinea-pig isolated distal colon LMMP preparation. The guinea-pig isolated colon LMMP preparation has been used widely as a bioassay for 5-HT4 agonist and antagonist activity (Wardle and Sanger, 1993; Leung et al., 1996; Beattie et al., 2004). 5-HT, and a panel of 5-HT4 receptor selective agonists, produced a concentration-dependent contraction of the LMMP preparation, with a range of potency and IA values (Figure 4a, Table 2). The rank order of potency (pEC50) for this panel of compounds was as follows: tegaserod (8.2)⩾5-HT (8.0)>prucalopride (7.6) >cisapride (7.0) >metoclopramide (5.5)=mosapride (5.4). The rank order of IA values, relative to 5-HT, was as follows: prucalopride (93%)>metoclopramide (84%) >cisapride (74%)>tegaserod (66%)>mosapride (37%). The contribution of 5-HT4 receptor activation to tegaserod-evoked responses in the guinea-pig distal colon LMMP preparation was confirmed by pretreatment with the 5-HT4 receptor selective antagonist piboserod. Piboserod attenuated tegaserod-evoked contractions with an apparent pKb=9.09 (n=2, 9.05 and 9.12).
Figure 4.
Concentration–response curves to tegaserod, 5-HT, prucalopride, cisapride, metoclopramide and mosapride in the guinea-pig isolated colon LMMP. Values are expressed as the mean±s.e.m (vertical lines) change in tension, as a percentage of the primed, maximum 5-HT (0.3 μM) response in the same tissue.
Comparison between the pharmacological properties of guinea-pig and human recombinant 5-HT4 receptors
There is 95% similarity between the sequences of the human 5-HT4 receptors and the guinea-pig 5-HT4 receptor cloned in this study, up to, but not including, the divergent C-terminus described for the human 5-HT4 receptor isoforms. The significant similarity, 93% between the C-terminus (from amino acid 358 onwards), of the guinea-pig 5-HT4 receptor described here and the human 5-HT4(b) receptor splice variant, suggests that the 5-HT4(b) receptor represents the human orthologue. Therefore, the pharmacological properties of guinea-pig 5-HT4 and human 5-HT4(b) recombinant receptors, both stably expressed in HEK293 cells, were compared directly.
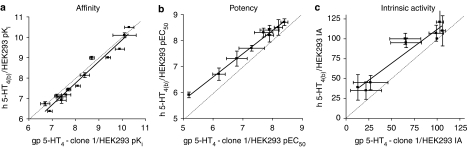
The binding affinities of a panel of 5-HT4 receptor selective agonists and antagonists for human 5-HT4(b) (Bmax=0.34±0.03 pmol mg−1 protein) and guinea-pig 5-HT4 recombinant receptors were in very close agreement (r2=0.96, slope=1.06±0.06; P<0.0001) (Table 1; Figure 5a). Furthermore, there was a very good correlation between the potency (r2=0.99, slope=0.89±0.03; P<0.0001) (Table 2; Figure 5b) and IA (r2=0.91, slope=0.85±0.1; P<0.0001) (Table 2; Figure 5c) values of a range of 5-HT4 receptor selective ligands for the elevation of cAMP levels in guinea-pig 5-HT4 and human 5-HT4(b) receptor transfectants.
Figure 5.
Correlation plots for a range of 5-HT4 receptor agonist and antagonist pKi, pEC50 and IA values at recombinant guinea-pig 5-HT4 and human 5-HT4(b) receptors determined using [3H]GR113808 competition binding assays and cAMP accumulation assays, respectively. The solid lines represent a linear regression through the points, and the dashed lines represent unity. Data represent mean±s.d. of 3–12 independent experiments.
Discussion
The present study has demonstrated that the pharmacological properties of guinea-pig native and recombinant 5-HT4 receptors are very similar. Thus, there was a strong correlation between the binding affinities for a range of 5-HT4 receptor selective agonists and antagonists at 5-HT4 receptors in guinea-pig striatal membranes and the guinea-pig recombinant 5-HT4 receptor. Furthermore, the potency values of a panel of 5-HT4 receptor selective agonists for contraction of guinea-pig isolated colon LMMP preparations and in whole-cell cAMP accumulation assays, using HEK293 cells stably transfected with the recombinant receptor, were similar. The results of the present study confirm that 5-HT4 receptor density can influence the apparent potency and IA of 5-HT4 receptor agonists, as has been reported previously for other classes of G-protein-coupled receptor (Kenakin, 2002). These observations emphasize the importance of characterizing the functional activity of compounds of interest in recombinant systems expressing 5-HT4 receptors at densities that are believed to best approximate physiological levels. By extension, these studies also highlight the value of profiling at least subsets of compounds in relevant target tissues, whenever possible.
A wide variety of methodologies, ranging from functional analyses in isolated tissue preparations to immunohistochemical studies, have been used to describe the distribution of 5-HT4 receptors in mouse, rat, guinea-pig, dog and human digestive tract (Cohen et al., 1994; Sakurai-Yamashita et al., 1999; Prins et al., 2000a; Liu et al., 2005). In the present study, 5-HT4 mRNA transcripts were found ubiquitously in the guinea-pig digestive tract. The corresponding density of [3H]GR113808 binding sites in the ileum and proximal colon was ∼30 fmol mg−1 protein. This is consistent with values of 45 (Yoshikawa et al., 1998) or 12 (Uchiyama-Tsuyuki et al., 1996) fmol mg−1 protein, obtained previously for [3H]GR113808 binding to guinea-pig ileum. In humans, there has been no direct measurement of 5-HT4 receptor densities in the digestive tract. However, using autoradiographic techniques, 5-HT4 receptor binding sites have been identified in both the myenteric plexus and muscle layers of human colon (Sakurai-Yamashita et al., 1999). Interestingly, in the present study, 5-HT4 receptor mRNA transcripts were detected in guinea-pig oesophageal tissue, consistent with recent studies demonstrating 5-HT4 receptor immunoreactivity in the muscularis mucosae of the guinea-pig oesophagus (Poole et al., 2006).
The 10 5-HT4 receptor isoforms that have been identified in human tissues exhibit differential tissue distribution patterns (Medhurst et al., 2001; Vilaro et al., 2002; Brattelid et al., 2004) and the functional significance of these isoforms, which differ (with the exception of 5-HT4(h)) in the C terminal tail region is unclear. There is no evidence for an influence of C-terminal splice variant identity on 5-HT4 receptor binding affinity (e.g. Brattelid et al., 2004; Krobert et al., 2005). However, subtle differences in agonist potency and efficacy, receptor constitutive activity, signal transduction pathways and receptor desensitization properties have been reported for the different splice variants (Claeysen et al., 1999; Bender et al., 2000; Pindon et al., 2002; Mialet et al., 2003). For the guinea-pig, although a sequence encoding a single 5-HT4 receptor variant was submitted to Genebank in 1997, to the best of our knowledge, there has been no publication describing the recombinant receptor. The present study has focused on this variant, which has close amino-acid sequence similarity to the human 5-HT4(b) receptor splice variant and probably represents the guinea-pig orthologue of this receptor. It is hypothesized that in the guinea-pig this is the most abundant form of the receptor. However, a more thorough cloning strategy using primers encoding the specific C-terminal of each receptor variant would be required to definitively rule out the presence of alternative variants.
The pharmacological and functional properties of the guinea-pig recombinant 5-HT4 receptor, in general, were very similar to those of guinea-pig native receptors. As expected, the pKi values for a range of 5-HT4 receptor selective agonists and antagonists at the recombinant receptor were similar to those at native 5-HT4 receptors in guinea-pig striatal (present study; Uchiyama-Tsuyuki et al., 1996) and ileal (Uchiyama-Tsuyuki et al., 1996) membrane preparations.
The functional properties of guinea-pig recombinant 5-HT4 receptors were characterized using whole-cell cAMP accumulation studies on two different clones: (i) gp 5-HT4 – clone 2, for which the 5-HT4 receptor density (∼3 pmol mg−1 protein) was of a similar magnitude to that reported for several clones that have been used in the characterization of human 5-HT4 receptor splice variants (Bach et al., 2001; Pindon et al., 2002; Krobert et al., 2005) and (ii) gp 5-HT4 – clone 1, for which the 5-HT4 receptor density was at least 10-fold lower (∼0.2 pmol mg−1 protein) and proposed to be closer to physiological levels. Thus, although the 5-HT4 receptor density in gp 5-HT4 – clone 1 is 5- to 10-fold higher than that in guinea-pig isolated ileum or proximal colon, it is very similar to that in guinea-pig striatal membranes.
The rank order of 5-HT4 receptor agonist potency, tegaserod >5-HT=prucalopride >cisapride >metoclopramide=mosapride, was similar for the lower receptor density clone and the guinea-pig isolated colon LMMP preparation (present study; Mine et al., 1997). Tegaserod was a full agonist (relative to 5-HT) at the guinea-pig recombinant receptor, in contrast to prucalopride, and the benzamide analogues, cisapride and mosapride, which were partial agonists (IA values=22–76%). Cisapride and mosapride are also 5-HT4 receptor partial agonists (IA values=37–74%) in isolated tissue preparations from several species including guinea-pig (present study; Bach et al., 2001). In the present study, the potency and IA of mosapride (pEC50 <5.4 and IA <37%, respectively) were similar at both the recombinant guinea-pig 5-HT4 receptor and in the guinea-pig LMMP preparation. The present findings suggest that receptor density significantly influences the functional properties of a range of 5-HT4 agonists at the guinea-pig 5-HT4 receptor. There was a significant (5- to 10-fold) increase in agonist potency in the higher receptor density cell line, gp 5-HT4 – clone 2, relative to that in gp 5-HT4 – clone 1, although the rank order of agonist potency was unchanged. In addition, all the agonists tested were full agonists (relative to 5-HT) in gp 5-HT4– clone 2. Therefore, consistent with previous observations at the human 5-HT4(a) and 5-HT4(b) receptors (Pindon et al., 2002), the partial agonist cisapride behaved as full agonist in the higher receptor density clone.
Several authors have noted a similar dependence of 5-HT4 agonist potencies and/or IA values on human 5-HT4 receptor density (Bach et al., 2001; Krobert et al., 2005). For example, a receptor density-dependent increase in agonist efficacy is thought to underlie the different IA values for prucalopride at human 5-HT(4a, b, c and i) receptor splice variants (Krobert et al., 2005). These observations are consistent with the operational model of agonism (Black and Leff, 1983). Thus, at a higher density of transfected receptors there is amplification of the receptor-induced signals, that is spare receptors (Bruheim et al., 2003).
It is well established that tegaserod is a potent, partial agonist at 5-HT4 receptors in rodent isolated tissue preparations (Buchheit et al., 1991; Beattie et al., 2004). In contrast, tegaserod was a full agonist at the recombinant guinea-pig 5-HT4 receptor, when expressed at either low or high receptor density. Tegaserod is also a potent, full agonist at human 5-HT4(a) and 5-HT4(b) receptor splice variants (Pindon et al., 2002). Further investigation is warranted to understand the apparent discrepancies between the isolated tissue and recombinant data. Recently, De Maeyer et al. (2006), using the operational model of agonism, demonstrated that the efficacy of tegaserod is in fact similar (equivalent τ-values) to that of 5-HT in porcine atrium and stomach in vitro preparations. The potency of tegaserod, however, was lower than expected and the authors attributed this to poor tissue penetration and/or partial precipitation, because of low solubility. Other factors which also may influence the tegaserod response in the recombinant and isolated tissue preparations are differential receptor binding kinetics, receptor reserve, splice variant identity, stimulus-response coupling efficiency, activation of alternate signalling pathways and/or receptor desensitization. However, such factors might be expected to influence the response to other 5-HT4 receptor agonists unless phenomena like agonist-directed trafficking of the stimulus response occurs. To date, there is no evidence that agonist-directed trafficking of 5-HT4 receptors, as has been previously described for the 5-HT2(a) and 5-HT2(c) receptors (Berg et al., 1998), occurs. Alternatively, tegaserod may interact with non-5-HT4 receptors in the tissue preparations, thereby influencing the magnitude of 5-HT4 receptor-mediated responses. However, the contribution of 5-HT4 receptors to the tegaserod-evoked contractile responses in digestive tract tissues has been confirmed using 5-HT4 receptor selective antagonists such as piboserod (present study; Beattie et al., 2004). Collectively, these data suggest that tegaserod is a full agonist at 5-HT4 recombinant receptors and additional factors underlie its apparent poor efficacy in tissue preparations.
Finally, the present study has demonstrated that the pharmacological and functional properties of the guinea-pig recombinant 5-HT4 receptor are very comparable to those of human recombinant 5-HT4(b) receptor splice variants (Mialet et al., 2000; Pindon et al., 2002). Given the potential influence of receptor density on the functional properties of human (Bach et al., 2001; Bruheim et al., 2003; Krobert et al., 2005) and/or guinea-pig (present study) 5-HT4 receptors, two clones that expressed the respective receptors at a similar density (Bmax=0.21±0.03 and 0.34±0.03 pmol mg−1 protein, respectively) were selected for the comparison in the present study. There was a strong correlation between the agonist potency values for the human and guinea-pig recombinant receptors and very good agreement between the corresponding IA values, with the exception of prucalopride and cisapride, which behaved as full agonists in the human 5-HT4(b) recombinant cell line. A number of investigators have evaluated the pharmacological properties of 5-HT4 receptors in human isolated tissue preparations (Tam et al., 1995; Prins et al., 2000b; Leclere et al., 2005). In spite of the significant limitations associated with use of such tissue preparations there was a reasonable correlation with the guinea-pig recombinant data.
In conclusion, the present study has demonstrated that ligand binding affinities and functional properties at the guinea-pig recombinant 5-HT4 receptor are similar to those at guinea-pig native receptors, in striatal and colonic tissue, respectively. Furthermore, the guinea-pig recombinant 5-HT4 receptor and human 5-HT4(b) receptor splice variant share very similar pharmacological properties. Collectively, these data suggest that pharmacological models using the guinea-pig recombinant 5-HT4 receptor and/or isolated colon preparations represent relevant, and important, tools for the identification and/or characterization of novel 5-HT4 receptor agonists. Such agents may offer improved efficacy for the treatment of disorders of reduced gastrointestinal motility. The pharmacological rationale for the different agonist profiles of tegaserod and the extent to which this may influence the clinical efficacy of tegaserod as a prokinetic agent require elucidation.
Abbreviations
- DMEM
Dulbecco's modified Eagle medium
- HEK293
human embryonic kidney 293
- IA
intrinsic activity
- LMMP
longitudinal muscle myenteric plexus
- 5-MeOT
5-methoxytryptamine
Conflict of interest
The authors state no conflict of interest.
References
- Alaradi O, Barkin JS. Irritable bowel syndrome: update on pathogenesis and management. Med Princ Pract. 2002;11:2–17. doi: 10.1159/000048654. [DOI] [PubMed] [Google Scholar]
- Bach T, Syversveen T, Kvingedal AM, Krobert KA, Brattelid T, Kaumann AJ, et al. 5HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:146–160. doi: 10.1007/s002100000299. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barthet G, Gaven F, Framery B, Shinjo K, Nakamura T, Claeysen S, et al. Uncoupling and endocytosis of 5-HT4 receptors. Distinct molecular events with different GRK2 requirements. J Biol Chem. 2005;280:27924–27934. doi: 10.1074/jbc.M502272200. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Smith JA, Marquess D, Vickery RG, Armstrong SR, Pulido-Rios T, et al. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol. 2004;143:549–560. doi: 10.1038/sj.bjp.0705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender E, Pindon A, van Oers I, Zhang YB, Gommeren W, Verhasselt P, et al. Structure of the human serotonin 5-HT4 receptor gene and cloning of a novel 5-HT4 splice variant. J Neurochem. 2000;74:478–489. doi: 10.1046/j.1471-4159.2000.740478.x. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Maayani S, Goldfarb J. Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann NY Acad Sci. 1998;861:104–110. doi: 10.1111/j.1749-6632.1998.tb10180.x. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc London Ser B. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Brattelid T, Kvingedal AM, Krobert KA, Andressen KW, Bach T, Hystad ME, et al. Cloning, pharmacological characterisation and tissue distribution of a novel 5-HT4 receptor splice variant, 5-HT4(i) Naunyn Schmiedebergs Arch Pharmacol. 2004;369:616–628. doi: 10.1007/s00210-004-0919-4. [DOI] [PubMed] [Google Scholar]
- Bruheim S, Krobert KA, Andressen KW, Levy FO. Unaltered agonist potency upon inducible 5-HT7(a) but not 5-HT4(b) receptor expression indicates agonist-independent association of 5-HT7(a) receptor and Gs. Receptor Channel. 2003;9:107–116. [PubMed] [Google Scholar]
- Buchheit KH, Gamse R, Pfannkuche HJ. SDZ 205–557, a selective antagonist at 5-HT4 receptors in the isolated guinea pig ileum. Eur J Pharmacol. 1991;200:373–374. doi: 10.1016/0014-2999(91)90601-l. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Advances in pharmacological treatments of IBS. J Pediatr Gastroenterol Nutr. 2004;39:S766–S767. doi: 10.1097/00005176-200406003-00024. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- Cohen ML, Susemichel AD, Bloomquist W, Robertson DW. 5-HT4 receptors in rat but not guinea pig, rabbit or dog esophageal smooth muscle. Gen Pharmacol. 1994;25:1143–1148. doi: 10.1016/0306-3623(94)90130-9. [DOI] [PubMed] [Google Scholar]
- De Maeyer JH, Prins NH, Schuurkes JA, Lefebvre RA. Differential effects of 5-HT4 receptor agonists at gastric versus cardiac receptors: an operational framework to explain and quantify organ specific behaviour. J Pharmacol Exp Ther. 2006;317:955–964. doi: 10.1124/jpet.106.101329. [DOI] [PubMed] [Google Scholar]
- Emmanuel AV, Roy AJ, Nicholls TJ, Kamm MA. Prucalopride, a systemic enterokinetic, for the treatment of constipation. Aliment Pharmacol Ther. 2002;16:1347–1356. doi: 10.1046/j.1365-2036.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Pan H, Messori E. Signaling mechanism coupled to 5-HT4 receptor-mediated facilitation of fast synaptic transmission in the guinea-pig ileum myenteric plexus. Neurogastroenterol Motil. 2003;5:523–529. doi: 10.1046/j.1365-2982.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- Gerald C, Adham N, Kao HT, Olsen MA, Laz TM, Schechter LE, et al. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO J. 1995;14:2806–2815. doi: 10.1002/j.1460-2075.1995.tb07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13:15–30. [PubMed] [Google Scholar]
- Goldhill J, Porquet MF, Angel I. Post-synaptic 5-HT4 receptor modulation of tachykinergic excitation of rat oesophageal tunica muscularis mucosae. Eur J Pharmacol. 1997;323:229–233. doi: 10.1016/s0014-2999(97)00046-0. [DOI] [PubMed] [Google Scholar]
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-HT4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- Grossman CJ, Kilpatrick GJ, Bunce KT. Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br J Pharmacol. 1993;3:618–624. doi: 10.1111/j.1476-5381.1993.tb13617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS, Eglen RM. Peripheral 5-HT4 receptors. FASEB J. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- Hinschberger A, Butt S, Lelong V, Boulouard M, Dumuis A, Dauphin F, et al. New benzo[h][1,6]naphthyridine and azepino[3,2-c]quinoline derivatives as selective antagonists of 5-HT4 receptors: binding profile and pharmacological characterization. J Med Chem. 2003;46:138–147. doi: 10.1021/jm020954v. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- Johanson JF. Review article: tegaserod for chronic constipation. Aliment Pharmacol Ther. 2004;20:20–24. doi: 10.1111/j.1365-2036.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- Kajita S, Ito C, Kawamura R, Yasuda S, Isobe Y, Fukushima K. Pharmacological characterization of a novel 5-HT4 receptor agonist, TS-951, in vitro. Pharmacology. 2001;63:8–16. doi: 10.1159/000056107. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Brattelid T, Levy FO, Kaumann AJ. Prucalopride is a partial agonist through human and porcine atrial 5-HT4 receptors: comparison with recombinant human 5-HT4 splice variants. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:473–479. doi: 10.1007/s00210-005-1068-0. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Yu S. Tegaserod: a new 5-HT4 agonist. J Clin Gastroenterol. 2002;34:27–33. doi: 10.1097/00004836-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Leclere PG, Lefebvre RA. Presynaptic modulation of cholinergic neurotransmission in the human proximal stomach. Cloning and characterization of a novel human 5-HT4 receptor variant that lacks the alternatively spliced carboxy terminal exon. Br J Pharmacol. 2002;135:135–142. doi: 10.1038/sj.bjp.0704471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclere PG, Prins NH, Schuurkes JA, Lefebvre RA. 5-HT4 receptors located on cholinergic nerves in human colon circular muscle. Neurogastroenterol Motil. 2005;17:366–375. doi: 10.1111/j.1365-2982.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- Leung E, Pulido-Rios MT, Bonhaus DW, Pekins LA, Zeitung KD, Hsu SA, et al. Comparison of 5-HT4 receptors in guinea-pig colon and rat oesophagus: effects of novel agonists and antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:145–156. doi: 10.1007/BF00178714. [DOI] [PubMed] [Google Scholar]
- Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:1148–1163. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Lezoualc'h F, Fischmeister R, Middlemiss DN, Sanger GJ. Quantitative mRNA analysis of five C-terminal splice variants of the human 5-HT4 receptor in the central nervous system by TaqMan real time RT-PCR. Brain Res Mol Brain Res. 2001;90:125–134. doi: 10.1016/s0169-328x(01)00095-x. [DOI] [PubMed] [Google Scholar]
- Mialet J, Berque-Bestel I, Eftekhari P, Gastineau M, Giner M, Dahmoune Y, et al. Isolation of the serotoninergic 5-HT4(e) receptor from human heart and comparative analysis of its pharmacological profile in C6-glial and CHO cell lines. Br J Pharmacol. 2000;129:771–781. doi: 10.1038/sj.bjp.0703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mialet J, Fischmeister R, Lezoualc'h F. Characterization of human 5-HT4(d) receptor desensitization in CHO cells. Br J Pharmacol. 2003;138:445–452. doi: 10.1038/sj.bjp.0705061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida N, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997;283:1000–1008. [PubMed] [Google Scholar]
- Norum JH, Hart K, Levy FO. Ras-dependent ERK activation by the human G(s)-coupled serotonin receptors 5-HT4(b) and 5-HT7(a) J Biol Chem. 2003;278:3098–3104. doi: 10.1074/jbc.M206237200. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ. Diverse signaling by 5-hydroxytryptamine (5-HT) receptors. Biochem Pharmacol. 2000;60:1743–1750. doi: 10.1016/s0006-2952(00)00476-7. [DOI] [PubMed] [Google Scholar]
- Pindon A, van Hecke G, van Gompel P, Lesage AS, Leysen JE, Jurzak M. Differences in signal transduction of two 5-HT4 receptor splice variants: compound specificity and dual coupling with Gαs- and Gαi/o-proteins. Mol Pharmacol. 2002;61:85–96. doi: 10.1124/mol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Prins NH, Akkermans LM, Lefebvre RA, Schuurkes JA. 5-HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol. 2000a;131:927–932. doi: 10.1038/sj.bjp.0703615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NH, Shankley NP, Welsh NJ, Briejer MR, Lefebvre RA, Akkermans LM, et al. An improved in vitro bioassay for the study of 5-HT4 receptors in the human isolated large intestinal circular muscle. Br J Pharmacol. 2000b;129:1601–1608. doi: 10.1038/sj.bjp.0703254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, et al. Identification of neurons that express 5-HT4 receptors in intestine. Cell Tissue Res. 2006;3:413–422. doi: 10.1007/s00441-006-0181-9. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Lummis SC. The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel. Mol Membrane Biol. 2002;19:11–26. doi: 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yamashita Y, Yamashita K, Kanematsu T, Taniyama K. Localization of the 5-HT4 receptor in the human and the guinea pig colon. Eur J Pharmacol. 1999;383:281–285. doi: 10.1016/s0014-2999(99)00642-1. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. 5-Hydroxytryptamine and functional bowel disorders. Neurogastroenterol Motil. 1996;8:319–331. doi: 10.1111/j.1365-2982.1996.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Saxena PR. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol Ther. 1995;66:339–368. doi: 10.1016/0163-7258(94)00005-n. [DOI] [PubMed] [Google Scholar]
- Tam FS, Hillier K, Bunce KT, Grossman C. Differences in response to 5-HT4 receptor agonists and antagonists of the 5-HT4-like receptor in human colon circular smooth muscle. Br J Pharmacol. 1995;115:172–176. doi: 10.1111/j.1476-5381.1995.tb16335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama-Tsuyuki Y, Saitoh M, Muramatsu M. Identification and characterization of the 5-HT4 receptor in the intestinal tract and striatum of the guinea pig. Life Sci. 1996;59:2129–2137. doi: 10.1016/s0024-3205(96)00569-3. [DOI] [PubMed] [Google Scholar]
- van den Wyngaert I, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W, et al. Cloning and expression of a human serotonin 5-HT4 receptor cDNA. J Neurochem. 1997;69:1810–1819. doi: 10.1046/j.1471-4159.1997.69051810.x. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Domenech T, Palacios JM, Mengod G. RT-PCR distribution in human brain and periphery of multiple 5-HT4 receptor variants. Neuropharmacology. 2002;42:60–73. doi: 10.1016/s0028-3908(01)00154-x. [DOI] [PubMed] [Google Scholar]
- Wardle KA, Sanger GJ. The guinea-pig distal colon – a sensitive preparation for the investigation of 5-HT4 receptor-mediated contractions. Br J Pharmacol. 1993;4:1593–1599. doi: 10.1111/j.1476-5381.1993.tb14006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Yoshida N, Mine Y, Hosoki K. Affinity of mosapride citrate, a new gastroprokinetic agent, for 5-HT4 receptors in guinea pig ileum. Jpn J Pharmacol. 1998;77:53–59. doi: 10.1254/jjp.77.53. [DOI] [PubMed] [Google Scholar]