Abstract
Background and purpose:
Serum levels of tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) increase during an inflammatory response and have been reported to induce a negative inotropic effect on the myocardium. Alfentanil, an opioid analgesic often used in the critical care of patients with sepsis, has been shown to enhance ventricular contractility. This study characterised the effects of TNF-α and IL-1β on contraction and the Ca2+ transient and investigated whether depressed ventricular function was ameliorated by alfentanil.
Experimental approach:
Isolated rat ventricular myocytes were loaded with fura-2 and electrically stimulated at 1 Hz. Contraction and Ca2+ transients were measured after 60, 120 and 180 min incubations in TNF-α (0.05 ng ml−1) and IL-1β (2 ng ml−1). The effects of 10 μM alfentanil on contractility and Ca2+ transients of TNF-α and IL-1β treated cells were determined.
Key results:
After 180 min of TNF-α and IL-1β treatment, the amplitude of contraction, the Ca2+ transient and sarcoplasmic reticulum (SR) Ca2+ content were significantly reduced. Alfentanil significantly increased contraction of TNF-α and IL-1β treated cells via a small increase in the Ca2+ transient and a larger increase in myofilament Ca2+ sensitivity, effects that were not blocked by 10 μM naloxone, a broad spectrum opioid receptor antagonist.
Conclusions and implications:
TNF-α and IL-1β induce a significant negative inotropic effect on ventricular myocytes in a time dependent manner through disruption of SR Ca2+ handling and the Ca2+ transient. This negative inotropic effect was ameliorated by alfentanil, but this response may not be mediated via opioid receptors.
Keywords: heart, contraction, Ca2+ transient, tumour necrosis factor-α, interleukin-1β, alfentanil, naloxone
Introduction
A combination of pro-inflammatory cytokines has been implicated in contributing to ventricular dysfunction in a variety of cardiac conditions including reperfusion injury and myocardial depression associated with sepsis (Baumgarten et al., 2000). Evidence has emerged that both tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) play a major role in ventricular dysfunction in sepsis (Casey et al., 1993; Kumar et al., 1996; Stamm et al., 2001). Several previous studies have assessed the effects of individual cytokines on ventricular function. For example, at high concentrations, TNF-α induced a negative inotropic effect on isolated cardiac myocytes (Yokoyama et al., 1993; Goldhaber et al., 1996; Sugishita et al., 1999; Li et al., 2003) and the whole heart (Edmunds et al., 1999), although an initial positive effect has also been described (Murray and Freeman, 1996; Amadou et al., 2002). A depressant effect of TNF-α on Ca2+ transients in a time and concentration-dependent manner was observed by Yokoyama et al. (1993) and in addition a decrease in myofilament sensitivity to Ca2+ has been described (Goldhaber et al., 1996; Tavernier et al., 1998, 2001). Studies of the effects of IL-1β at relatively high concentrations have produced varied results. IL-1β has been shown to affect Ca2+ regulation via inhibition of sarcoplasmic reticulum (SR) Ca2+ release and an increase in Na+–Ca2+ exchanger activity but with no effect on the L-type Ca2+ current (Cao et al., 2003), whereas other groups have demonstrated inhibition of the L-type Ca2+ current by IL-1β (Schreur and Liu, 1997).
However, under conditions such as sepsis, it has been demonstrated that TNF-α and IL-1β act synergistically and together they depress ventricular contractility at much lower concentrations than is required individually (Kumar et al., 1996). The aim of the present study was to characterize the effects of exposure to a combination of low, clinically relevant concentrations of TNF-α and IL-1β, on contraction and Ca2+ transient amplitude in rat ventricular myocytes. Alfentanil (Alf), an opioid anaesthetic/analgesic used routinely in the sedation of patients with sepsis, has been shown previously to enhance ventricular contractility in normal cells (Graham et al., 2004) and therefore we tested whether Alf was able to improve or restore the depressed contractility induced following cytokine exposure.
The results of this study show that after 3 h of incubation with TNF-α and IL-1β, both contraction and the Ca2+ transient were significantly reduced. Addition of Alf partially reversed the cytokine-induced negative inotropic effect due to an increase in the Ca2+ transient and myofilament Ca2+ sensitivity, responses that persisted in the presence of naloxone (Nalox), a broad-spectrum opioid receptor antagonist, suggesting that these effects may not be mediated via opioid receptors.
Methods
Preparation of ventricular myocytes
The technique used to prepare rat ventricular myocytes has been described in detail previously (Harrison et al., 1992). Briefly, healthy adult male Wistar rats weighing 200–250 g were killed humanely using schedule 1 techniques sanctioned by the United Kingdom government Home Office and the local ethical review committee. The heart was excised rapidly and the aorta cannulated. The heart was perfused via the coronary arteries with a series of solutions based on a Ca2+-free ‘isolation solution' (composition below). The heart was perfused initially with isolation solution plus 750 μM CaCl2 and equilibrated with 100% oxygen to flush the coronary arteries of blood. Once the heart was beating regularly, the perfusate was changed to isolation solution containing 100 μM ethylene glycol bis(β-aminoethyl ether)- N,N,N′,N′-tetraacetic acid for 4 min. The heart was then perfused for 6 min with isolation solution supplemented with 1.2 mg ml−1 collagenase (type II; Worthington Biochemical Corp., Lakewood, NJ, USA), 0.1 mg ml−1 protease (type XIV; Sigma, Poole, Dorset, UK) and 80 μM CaCl2. The ventricles were cut from the heart, chopped finely and agitated gently in enzyme solution (supplemented with 1% bovine serum albumin (BSA)) for 5 min intervals. Dissociated cells were harvested by filtration at the end of each 5 min interval and the remaining tissue subjected to further enzyme treatment. Dissociated cells were centrifuged at 30 g for 60 s, resuspended in 750 μM CaCl2 solution and stored at room temperature until use.
Solutions and cytokine treatment
The isolation solution was composed of the following (in mM): NaCl 130; KCl 5.4; MgCl2 1.4; NaH2PO4 0.4; HEPES 5; glucose 10; taurine 20; creatine 10, pH 7.1 (NaOH) at 30°C. Normal tyrode solution (NT) contained (in mM): NaCl 140; KCl 5.4; MgCl2 1.2; NaH2PO4 0.4; HEPES 5; glucose 10; CaCl2 1, pH 7.4 (NaOH) at 30°C. TNF-α (Sigma; Poole, UK) was diluted to 0.05 ng ml−1 from a stock solution of 2.5 μg ml−1 in 10% BSA and IL-1β (2 ng ml−1) was diluted from a stock solution of 1 μg ml−1 (Sigma) in NT solution. 10 μM Alf (Rapifen; Janssen-Cilag, High Wycombe, UK) was prepared in NT solution from a 0.5 mg ml−1 stock solution as was 10 μM Nalox, from a stock solution of 5 mg ml−1.
Cells were incubated in either NT solution (control cells) or NT supplemented with TNF-α (0.05 ng ml−1) and IL-1β (2 ng ml−1) for 60, 120 or 180 min. These cytokine concentrations were chosen as they are close to levels measured in the serum of septic patients (e.g. Kumar et al., 1999) and have been used in previous studies assessing the inotropic effects of cytokine exposure on ventricular function (e.g. Kumar et al., 1996). Ten minutes before the end of each incubation, cells were loaded with fura-2 by gentle agitation of a 1 ml aliquot of cells with 3 μl of 1 mM fura-2 AM in dimethyl sulphoxide. Cells were centrifuged as before, the supernatant removed and the pellet resuspended in incubation solution. Fura-2-loaded cells were left for approximately. 15 min before use to allow for de-esterification of the dye to take place.
Cell length and Ca2+ transient measurement
Cells were transferred to a tissue chamber (volume <200 μl) attached to the stage of an inverted microscope (Diaphot; Nikon, Tokyo, Japan) and allowed to settle for several minutes. Control cells were superfused continuously with NT solution. TNF-α and IL-1β-treated cells were superfused with NT until they were contracting in a stable manner, at which point the superfusate was switched to a NT solution supplemented with TNF-α (0.05 ng ml−1) and IL-1β (2 ng ml−1). Solutions were delivered to the experimental chamber by magnetic drive gear metering pumps (Micropump; Concord, CA, USA) at 4.5 ml min−1 and solution level and temperature maintained by feedback circuits (Cannell and Lederer, 1986).
Cells were stimulated at a frequency of 1 Hz (stimulus duration 2 ms) via two platinum electrodes situated in the side of the tissue chamber. Rectangular-shaped cells with clear striations, which contracted in a stable manner and which were quiescent between stimuli were used for experimentation. Ventricular myocyte cell length was assessed optically using an Ionoptix cell edge detection system (IonOptix Corporation, Milton, MA, USA); cell length was digitized at 200 Hz.
To record the cytosolic Ca2+ transient, cells were excited alternately at 340 and 380 nm using a monochromator-based system (Cairn, Kent, UK) and emitted fluorescence detected at 510±40 nm. Fluorescence ratio (Fr) was digitized at 1 kHz using Ionoptix software.
Statistical analysis
Data are presented as mean±s.e.m. and unless otherwise stated, each data set was derived from cells isolated from a minimum of five animals. Statistical comparisons of multiple control and treated groups were performed using analysis of variance (ANOVA) with post hoc tests (Holme Sidak). Control Nalox and Alf data were compared using repeated measures ANOVA with post hoc Dunn's tests. Comparison of Alf-treated cells was performed using paired Student's t-tests. If data failed a normality test (Kolmogorov–Smirnov) an appropriate non-parametric test was carried out. Significant results, unless otherwise stated have a P value less than 0.05. Analysis was performed using SigmaStat (Jandel Scientific, Erkrath, Germany). All figures were prepared using Sigma Plot (Jandel Scientific).
Results
Effects of TNF-α and IL-1β on contraction and Ca2+ transient amplitude
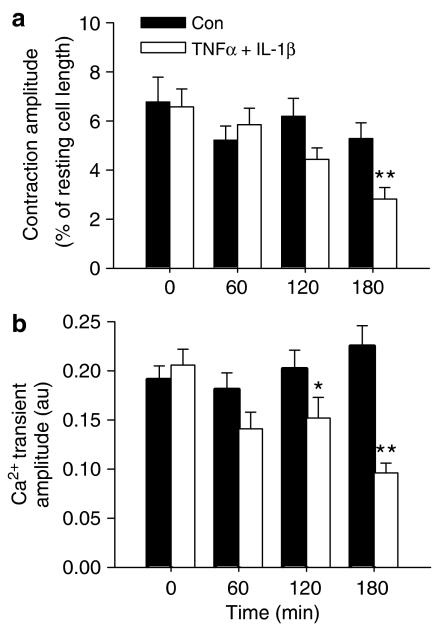
Incubation of ventricular myocytes in NT solution for up to 3 h had no significant effect on either contraction (expressed as % of resting cell length) or Ca2+ transient amplitude (Figure 1a and b). However, incubation of ventricular myocytes in NT supplemented with both TNF-α (0.05 ng ml−1) and IL-1β (2 ng ml−1) caused a time-dependent negative inotropic effect, which approached significance after 2 h (P=0.06 vs time control) and which became highly significant after 180 min. Similarly, Figure 1b shows a decrease in Ca2+ transient amplitude, which was just significant (P=0.044 vs time control) after 2 h but was reduced to a greater extent after 3 h incubation.
Figure 1.
Mean data (±s.e.m.) describing contraction amplitude (a) and Ca2+ transient amplitude (b) after 0, 60, 120 and 180 min incubation at 30°C in either NT solution (Con, n=16–22) or NT supplemented with TNF-α (0.05 ng ml−1) and IL-1β (2 ng ml−1, n=15–19). *P<0.05, **P<0.001 vs 0 min; two-way ANOVA.
To assess the impact of each cytokine individually, separate groups of cells were incubated for 3 h in either NT, NT+TNF-α (0.05 ng ml−1), or NT+IL-1β (2 ng ml−1). There were no significant effects of either cytokine on contraction or Ca2+ transient amplitude at the concentrations tested (n=13–15, from three animals).
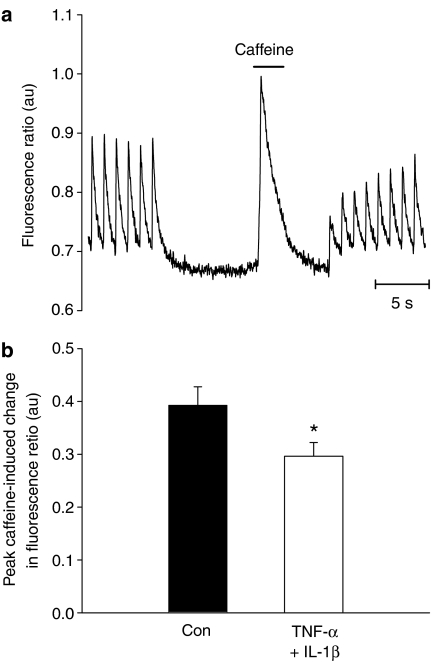
The decrease in the Ca2+ transient amplitude observed following 3 h incubation with TNF-α+IL-1β may result from a reduction in SR Ca2+ content. To assess this, fura-2-loaded ventricular myocytes were stimulated to steady state at 1 Hz, and stimulation then stopped for 5 s before 20 mM caffeine was applied rapidly to discharge SR Ca2+ content (Negretti et al., 1993; Fowler et al., 2005; see Figure 2a) The amplitude of the resulting caffeine-evoked Ca2+ transient was taken as a measure of SR Ca2+ content. Figure 2b illustrates that SR Ca2+ content was reduced by about 25% following 3 h of cytokine treatment, a decrease which would contribute to the decreased electrically evoked Ca2+ transient observed following TNF-α+IL-1β treatment.
Figure 2.
(a) an individual record of the protocol used to determine SR Ca2+ content is illustrated (see text for further details). (b) mean values (±s.e.m.) of the peak caffeine-induced Ca2+ transient in control (n=13) and treated (n=11) cells are shown. *P<0.05 vs control, t-test.
Effects of Alf on Ca2+ transient and contraction amplitude
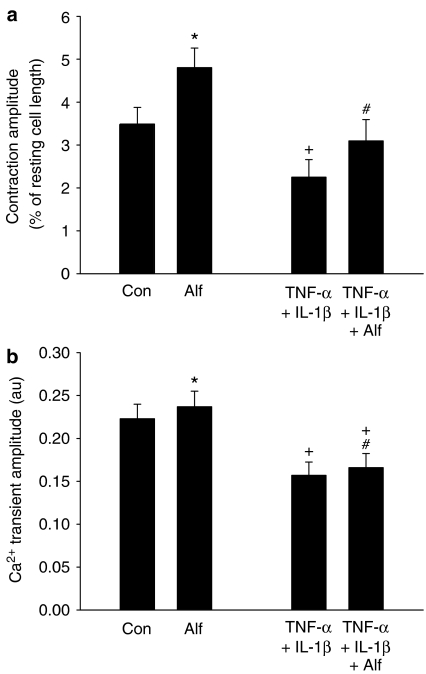
Opioid anaesthetics, such as Alf, are commonly used for the management of postoperative pain (Negishi et al., 2000). Alf has been reported to induce a positive inotropic effect under control conditions (Graham et al., 2004), which may have a beneficial effect in patients with cardio-depressive conditions such as septicaemia. Figure 3 illustrates fast time base records of cell length and Ca2+ transients in control and TNF-α and IL-1β-treated cells in the absence and presence of 10 μM Alf, a concentration close to the maximum clinically relevant dose and which led to a robust and reproducible increase in contractility in control cells. Figure 4 shows mean data for the effects of Alf on control and treated cells. As before, treatment with TNF-α and IL-1β for 3 h significantly reduced contraction magnitude compared to control cells. Alf enhanced contractility in both control and treated cells; in control cells contraction amplitude increased by about 30% and in treated cells a similar effect was seen. The contraction magnitude of cytokine-treated cells with Alf was not significantly different from the contraction magnitude of control cells.
Figure 3.
Representative fast time base records of contractions (a and b) and the associated Ca2+ transients (c and d) before and after addition of 10 μM Alf in control (a and c) and TNF-α+IL-1β-treated cells (b and d).
Figure 4.
(a) The mean values (±s.e.m.) of contraction amplitude in the absence and presence of Alf in control (n=17) and TNF-α+IL-1β-treated (n=21) cells are shown. *P<0.001 vs control, #P<0.001 vs TNF-α+IL-1β and +P<0.05 vs control. (b) Mean values (±s.e.m.) are shown for Ca2+ transient amplitude for control (n=17) and TNF-α+IL-1β-treated cells (n=21). A significant increase was induced by Alf in both control (*P=0.003) and treated cells (#P=0.003); however, the Ca2+ transient amplitude of TNF-α+IL-1β-treated cells with Alf was still significantly lower than control (+P<0.05).
Figures 3c and 4b illustrate that Alf caused a small, but reproducible increase of Ca2+ transient amplitude in control cells. In treated cells there was a small increase in Ca2+ transient amplitude; however, unlike contraction, the magnitude of the Ca2+ transient was still significantly depressed compared to control (Figure 4b).
Data from Figures 3 and 4 suggest that Alf may enhance myofilament sensitivity to Ca2+. To assess this, cell shortening was plotted against the cytosolic Fr. Figure 5a represents the relationship of cell length and Fr for an entire contraction–relaxation cycle. The gradient of the final phase of relaxation (Figure 5b), where the myofilaments come into quasi-equilibrium with Ca2+, can be used as an index of apparent myofilament Ca2+ sensitivity (Spurgeon et al., 1992). In control cells, myofilament Ca2+ sensitivity increased significantly in the presence of Alf and a similar increase occurred in treated cells. However, in the absence of Alf, no significant difference in myofilament Ca2+ sensitivity was observed between control and treated cells.
Figure 5.
(a) A plot of cell length against fura-2 Fr is shown, under control conditions and with 10 μM Alf in an untreated cell. In (b) is shown the linear regression of the final phase of relaxation; the gradient of the line gives an index of myofilament Ca2+ sensitivity. (c) Mean data (±s.e.m.) for myofilament Ca2+ sensitivity show no significant differences between control (n=17) and TNF-α+IL-1β-treated cells (n=21). Alf induced a significant increase in myofilament Ca2+ sensitivity in both control (*P=0.001) and TNF-α+IL-1β-treated cells (#P=0.01, paired t-test).
Effects of Nalox on the Ca2+ transient and contraction of Alf-treated cells
Twenty-one control cells were equilibrated with 10 μM Nalox to inhibit the actions of Alf mediated via opioid receptors. Nalox alone had no effect on the amplitude of contraction (Figure 6a) or the Ca2+ transient (Figure 6b). In the presence of Nalox, Alf still led to an increase in contraction amplitude, an increase similar to that observed in the absence of Nalox. Similarly, Alf produced a small but significant increase in Ca2+ transient amplitude (Figure 6b). Figure 6c illustrates data for myofilament Ca2+ sensitivity for this group of cells under control conditions, with Nalox and following addition of Alf. These data show that myofilament Ca2+ sensitivity was unchanged by Nalox alone but enhanced by Alf in the presence of Nalox.
Figure 6.
Mean data (±s.e.m.) describing the effects of Nalox and Nalox+Alf on (a) contraction amplitude, on (b) Ca2+ transient amplitude and on (c) apparent myofilament Ca2+ sensitivity in control cells (n=21, from three animals). 10 μM Nalox alone had no effect on contraction amplitude, Ca2+ transient amplitude or myofilament Ca2+ sensitivity whereas Alf significantly increased all three parameters in the presence of Nalox (*P<0.05 vs control, repeated measures ANOVA).
Discussion and conclusions
The effects of TNF-α and IL-1β on contraction and Ca2+ transients of ventricular myocytes
Incubation of myocytes with TNF-α and IL-1β led to a time-dependent decrease in contractility accompanied by a decrease in the magnitude of the systolic Ca2+ transient, presumably as a result of a decrease in the Ca2+ content of the SR. Similar results have been observed recently in ventricular myocytes isolated from a caecal ligation and puncture model of sepsis (Zhu et al., 2005). In that study, it is thought that an enhanced leak of Ca2+ from the SR contributed to a decrease in SR Ca2+ content. However, others (Dettbarn et al., 1994) have implicated altered sphingomyelin metabolism in the cellular signalling cascade associated with cytokine exposure and postulate that increased levels of sphingosine inhibit Ca2+ transient magnitude by blocking the release of Ca2+ from the SR. In addition to altered Ca2+ regulation, a decrease in myofilament Ca2+ sensitivity has been reported following acute exposure to a high concentration of TNF-α (Goldhaber et al., 1996) and in an experimental model of endotoxaemia (Tavernier et al., 1998; Layland et al., 2005). We did not observe any difference in myofilament Ca2+-sensitivity between control and treated cells (Figure 6c). Whereas the technique used here (Spurgeon et al., 1992) is useful for assessing changes in myofilament Ca2+ sensitivity in any given cell on the addition and removal of a Ca2+ sensitizing agent, the variance between cells is quite large owing to the different inotropic state of cells and thus small differences in the gradient of the final phase of relaxation between cell populations may not be identified.
The effects of Alf on control and TNF-α and IL-1β-treated cells
Superfusion of control cells with Alf caused a positive inotropic response as has been demonstrated previously (Graham et al., 2004). The increase in contraction amplitude occurred secondarily to an increase in myofilament Ca2+ sensitivity and a significant but small increase in the systolic Ca2+ transient. Similar results were observed in treated cells with the important observation being that Alf increased contraction magnitude to a level not significantly different from control. This is of potential clinical utility, as Alf is routinely used for sedation of patients in intensive care units and, therefore, Alf may be the drug of choice in patients with hypotensive cardiac dysfunction.
Effects of Alf on Nalox-treated cells
Alf is an opioid anaesthetic and may well induce its effects via opioid receptor stimulation. Cardiac myocyte cell membranes express μ, κ and δ opioid receptors (Barron, 1999). The opioid-dependent effects of Alf would be expected to be blocked by a broad-spectrum competitive opioid antagonist such as Nalox, which is typically used to counter heroin and morphine overdose. Here, we used the highest dose of Nalox (10 μM) that did not affect contraction or Ca2+ transient amplitude in an attempt to block Alf-induced effects on all classes of opioid receptors. However, it should be noted that we did not carry out a comprehensive investigation of the dose dependency of Nalox antagonism at all classes of opioid receptors, which limits certain conclusions. These data illustrate that the positive inotropic effect of Alf was still present following pretreatment with 10 μM Nalox. This suggests that Alf may exert its positive inotropic effects through a route other than via opioid receptor stimulation; however the mechanisms involved remain to be elucidated.
In conclusion, the combined effects of low concentrations of TNF-α and IL-1β (which were ineffective individually at the concentrations used) caused a time-dependent negative inotropic response in ventricular myocytes supporting the concept that these two cytokines act synergistically. Contractile depression was accompanied by a decrease in the cytosolic Ca2+ transient, presumably as a consequence of a reduction in SR Ca2+ content. Administration of Alf ameliorated the negative inotropic effects of TNF-α and IL-1β, via a combination of an increase in myofilament sensitivity and a small enhancement of the Ca2+ transient. Therefore, the use of Alf in the critical care of septic patients may be beneficial by enhancing contractility and thus cardiac output.
Acknowledgments
We are grateful to The White Rose Consortium and The British Heart Foundation for financial support.
Abbreviations
- Alf
alfentanil
- Fr
fluorescence ratio
- IL-1β
interleukin-1β
- Nalox
naloxone
- NT
normal tyrode solution
- RCL
resting cell length
- SR
sarcoplasmic reticulum
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Amadou A, Nawrocki A, Best-Belpomme M, Pavoine C, Pecker F. Arachidonic acid mediates dual effect of TNF-alpha on Ca2+ transients and contraction of adult rat cardiomyocytes. Am J Physiol. 2002;282:C1339–C1347. doi: 10.1152/ajpcell.00471.2001. [DOI] [PubMed] [Google Scholar]
- Barron BA. Opioid peptides and the heart. Cardiovasc Res. 1999;43:13–16. doi: 10.1016/s0008-6363(99)00112-1. [DOI] [PubMed] [Google Scholar]
- Baumgarten G, Knuefermann P, Mann DL. Cytokines as emerging targets in the treatment of heart failure. Trends Cardiovasc Med. 2000;10:216–223. doi: 10.1016/s1050-1738(00)00063-3. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Lederer WJ. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986;406:536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Cao CM, Xia Q, Bruce IC, Shen YL, Ye ZG, Lin GH, et al. Influence of interleukin-2 on Ca2+ handling in rat ventricular myocytes. J Mol Cell Cardiol. 2003;35:1491–1503. doi: 10.1016/j.yjmcc.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- Dettbarn CA, Betto R, Salviati G, Palade P, Jenkins GM, Sabbadini RA. Modulation of cardiac sarcoplasmic reticulum ryanodine receptor by sphingosine. J Mol Cell Cardiol. 1994;26:229–242. doi: 10.1006/jmcc.1994.1026. [DOI] [PubMed] [Google Scholar]
- Edmunds NJ, Lal H, Woodward B. Effects of tumour necrosis factor-alpha on left ventricular function in the rat isolated perfused heart: possible mechanisms for a decline in cardiac function. Br J Pharmacol. 1999;126:189–196. doi: 10.1038/sj.bjp.0702294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MR, Naz JR, Graham MD, Bru-Mercier G, Harrison SM, Orchard CH. Decreased Ca2+ extrusion via Na+/Ca2+ exchange in epicardial left ventricular myocytes during compensated hypertrophy. Am J Physiol. 2005;288:H2431–H2438. doi: 10.1152/ajpheart.01069.2004. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI, Kim KH, Natterson PD, Lawrence T, Yang P, Weiss JN. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am J Physiol. 1996;271:H1449–H1455. doi: 10.1152/ajpheart.1996.271.4.H1449. [DOI] [PubMed] [Google Scholar]
- Graham MD, Hopkins PM, Harrison SM.The effects of alfentanil on cytosolic Ca2+ and contraction in rat ventricular myocytes Anesth Analg 2004981013–1016.table [DOI] [PubMed] [Google Scholar]
- Harrison SM, McCall E, Boyett MR. The relationship between contraction and intracellular sodium in rat and guinea-pig ventricular myocytes. J Physiol. 1992;449:517–550. doi: 10.1113/jphysiol.1992.sp019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 1999;276:R265–R276. doi: 10.1152/ajpregu.1999.276.1.R265. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, et al. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005;19:1137–1139. doi: 10.1096/fj.04-2519fje. [DOI] [PubMed] [Google Scholar]
- Li XQ, Zhao MG, Mei QB, Zhang YF, Guo W, Wang HF, et al. Effects of tumor necrosis factor-alpha on calcium movement in rat ventricular myocytes. Acta Pharmacol Sin. 2003;24:1224–1230. [PubMed] [Google Scholar]
- Murray DR, Freeman GL. Tumor necrosis factor-alpha induces a biphasic effect on myocardial contractility in conscious dogs. Circ Res. 1996;78:154–160. doi: 10.1161/01.res.78.1.154. [DOI] [PubMed] [Google Scholar]
- Negishi C, Kim JS, Lenhardt R, Sessler DI, Ozaki M, Vuong K, et al. Alfentanil reduces the febrile response to interleukin-2 in humans. Crit Care Med. 2000;28:1295–1300. doi: 10.1097/00003246-200005000-00006. [DOI] [PubMed] [Google Scholar]
- Negretti N, O'Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993;27:1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- Schreur KD, Liu S. Involvement of ceramide in inhibitory effect of IL-1 beta on L-type Ca2+ current in adult rat ventricular myocytes. Am J Physiol. 1997;272:H2591–H2598. doi: 10.1152/ajpheart.1997.272.6.H2591. [DOI] [PubMed] [Google Scholar]
- Spurgeon HA, duBell WH, Stern MD, Sollott SJ, Ziman BD, Silverman HS, et al. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol. 1992;447:83–102. doi: 10.1113/jphysiol.1992.sp018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGowan FX., Jr Rapid endotoxin-induced alterations in myocardial calcium handling: obligatory role of cardiac TNF-alpha. Anesthesiology. 2001;95:1396–1405. doi: 10.1097/00000542-200112000-00019. [DOI] [PubMed] [Google Scholar]
- Sugishita K, Kinugawa K, Shimizu T, Harada K, Matsui H, Takahashi T, et al. Cellular basis for the acute inhibitory effects of IL-6 and TNF-alpha on excitation–contraction coupling. J Mol Cell Cardiol. 1999;31:1457–1467. doi: 10.1006/jmcc.1999.0989. [DOI] [PubMed] [Google Scholar]
- Tavernier B, Garrigue D, Boulle C, Vallet B, Adnet P. Myofilament calcium sensitivity is decreased in skinned cardiac fibres of endotoxin-treated rabbits. Cardiovasc Res. 1998;38:472–479. doi: 10.1016/s0008-6363(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Tavernier B, Mebazaa A, Mateo P, Sys S, Ventura-Clapier R, Veksler V. Phosphorylation-dependent alteration in myofilament Ca2+ sensitivity but normal mitochondrial function in septic heart. Am J Respir Crit Care Med. 2001;163:362–367. doi: 10.1164/ajrccm.163.2.2002128. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303–2312. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bernecker OY, Manohar NS, Hajjar RJ, Hellman J, Ichinose F, et al. Increased leakage of sarcoplasmic reticulum Ca2+ contributes to abnormal myocyte Ca2+ handling and shortening in sepsis. Crit Care Med. 2005;33:598–604. doi: 10.1097/01.ccm.0000152223.27176.a6. [DOI] [PubMed] [Google Scholar]