Abstract
Background and purpose:
The objective of this study was to investigate the possible interactions between the cannabinoid and cholinergic systems in memory and learning processes by using genetic and pharmacological approaches in two different behavioural models, the active avoidance and the object recognition test.
Experimental approach:
The effects induced by nicotine, physostigmine and scopolamine were studied in CB1 receptor knockout and wild-type mice in the active avoidance paradigm. In addition, the effects of pretreatment with the CB1 receptor antagonist rimonabant were evaluated on the responses induced by nicotine in the active avoidance and the object recognition tasks in wild-type mice.
Key results:
Nicotine (0.5 mgkg–1 s.c.) did not modify the performance of CB1 knockout and wild-type mice in this model, whereas scopolamine (0.5 mgkg–1 i.p.) impaired the performance in both genotypes. Physostigmine (0.1 mgkg–1 i.p.) increased the active avoidance performance in wild-type but not in CB1 knockout mice. Rimonabant (0.3, 1, 3, and 10 mgkg–1) did not modify the performance in the active avoidance test, given alone or co-administered with nicotine. In contrast, nicotine enhanced the performance in the object recognition task but this response was attenuated by rimonabant co-administration.
Conclusions and implications:
The present findings revealed that the cognitive effects of nicotine and physostigmine were attenuated in the absence of CB1 receptor activity. Scopolamine effects were independent from CB1 receptors.
Keywords: CB1 knockout mice, rimonabant, active avoidance, object recognition test, cannabinoid, cholinergic system, cognition, nicotine
Introduction
Cannabis sativa derivatives and tobacco remain two of the most widely abused drugs and represent worldwide public health problems. In the central nervous system (CNS), nicotine, the primary addictive substance in tobacco, activates the nicotinic acetylcoline receptors (nAChR), whereas Δ9-tetrahydrocannabinol (THC) the main psychoactive compound of Cannabis sativa, acts through the cannabinoid receptors: CB1 receptor mainly located in the CNS and CB2 receptor which is abundant in the immune cells (Munro et al., 1993). CB1 receptors are highly expressed in different brain areas that play an important role in the modulation of memory such as hippocampus, cortex and amygdala (Herkenham et al., 1990; Tsou et al., 1998). An overlapping distribution of nAChR and CB1 receptors has been reported in some of these structures (Picciotto et al., 2000), suggesting possible functional interactions between cannabinoid and cholinergic systems in cognitive control.
The involvement of nAChR in learning and memory processes has been recognized for several decades (Levin, 1992; Stolerman et al., 1995). Acetylcholine (ACh) activity seems essential to learn multiple tasks and plays an important role during the early stages of memory formation (Miranda et al., 2003). Nicotine agonists improve performance in several cognitive models in both rodents and humans (Levin et al., 2006) as well as acetylcholinesterase inhibitors, which enhance the availability of ACh in the synaptic cleft (Molchan et al., 1992; Degroot and Parent, 2001; Zarrindast et al., 2002), whereas anticholinergic drugs impair learning and memory in a variety of tasks (Fibiger, 1991; Gallagher and Colombo, 1995; Zarrindast et al., 2002). Thus, scopolamine, a muscarinic cholinergic receptor antagonist, induces a performance deficit that has been proposed as an animal model of dementia (Collerton, 1986; Jensen et al., 1987; Quartermain and Leo, 1988).
Although CB1 receptor activation impairs cognitive function (Schacter and Wagner 1999), the blockade of this receptor may increase learning and memory through an enhancement of ACh efflux in the brain. Thus, the CB1 antagonist rimonabant increases ACh efflux in the hippocampus and medial-prefrontal cortex (Gessa et al., 1998; Tzavara et al., 2003) and the genetic deletion of CB1 receptor improves cognitive processes in different behavioural paradigms (Maccarrone et al., 2002; Martin et al., 2002).
The behavioural and biochemical consequences of the interaction between the cannabinoid and cholinergic systems are poorly documented in spite of the current association of cannabis and tobacco in humans. In mice, nicotine facilitates hypothermia, antinociception, hypolocomotion and anxiolytic-like responses induced by THC (Valjent et al., 2002), whereas THC decreases somatic and motivational manifestations of nicotine withdrawal (Balerio et al., 2004). On the other hand, rimonabant abolishes nicotine-induced anxiolytic-like effects and increases the anxiogenic-like responses of nicotine (Balerio et al., 2006).
The aim of the present study was to investigate the possible interactions between the cannabinoid and cholinergic systems in cognitive processes by using pharmacological and genetic approaches in different behavioural paradigms. For this purpose, the effects induced by nicotine, physostigmine and scopolamine were studied in CB1 knockout and wild-type littermates mice in the active avoidance paradigm. In addition, the effects of the pretreatment with rimonabant were evaluated on the pharmacological responses induced by nicotine in the active avoidance and the object recognition tasks in wild-type mice.
Methods
Animals
Male CD1 mice (Charles River, France) weighing 22–28 g as well as male CB1 knockout mice and wild-type littermates weighing 30–35 g were used. The generation of mice lacking CB1 receptors was described previously (Ledent et al., 1999). In order to homogenize the genetic background of the mice, the first generation of heterozygotes were bred for 30 generations on a CD1 (Charles River, France) background, with selection for the mutant CB1 gene at each generation. Animals used in a given experiment originated from the same breeding series. All the animals were housed five per cage with food and water available ad libitum. They were acclimated to the laboratory conditions (12 h light–dark cycle, 21±1°C room temperature) and manipulated by the investigators during 1 week before the experiment. Behavioural tests and animal care were conducted in accordance with the standard ethical guidelines (NIH, publication no. 85–23, revised 1985; European Communities Directive 86/609/EEC) and approved by the local ethical committee (CEEA IMAS-UPF). All experiments were performed with the investigators being unaware of the treatment and/or genotype conditions.
Active avoidance procedure
Mice were trained to avoid an aversive unconditioned stimulus (US) associated with the presentation of a conditioned stimulus (CS) in a two-way shuttle box apparatus placed in a sound-attenuating box (Panlab SL, Barcelona, Spain) (Martin et al., 2002). The shuttle box apparatus consists of a box with two compartments (20 × 10 cm) connected by a 3 × 3-cm door. A light (10 W) switched on in the compartment in which the mouse was placed was used as a CS. The CS preceded by 5 s the onset of the US and overlapped it for 25 s. Using this procedure, the light was presented in the compartment for 30 s (5 s alone and 25 s together with the US). At the end of the 30 s period, both CS and US were automatically turned off. The US was an electric shock (0.2 mA) continuously applied to the grid of the floor. A conditioned response was recorded when the animal avoided the US by changing from the compartment where the animal received the CS into the opposite compartment within the 5 s after the onset of the CS. If animals failed to avoid the shock, they could escape it by crossing during the US (25 s). Between each trial session, there was an inter-trial interval of 30 s.
Animals were subjected to one daily 100-trial active avoidance session during 5 consecutive days. Each day the mice were placed in the shuttle box 10 min before starting the session to allow them to explore the box and to become familiar with the apparatus. Data from active avoidance paradigm was calculated as a ratio between conditioned changes and total changes. Data were also expressed as area under the curve (AUC) in order to facilitate the comparisons between groups by using a standard trapezoid method and the following equation, AUC=(0.5*A1*d+A2*d+ … +An-1*d+0.5*An*d), where the A1 to An are the ratio values and d is the time (days) elapsed between the consecutive measurements.
Object recognition test
Mice were placed in a Plexiglas open-field box (51 cm wide × 51 cm long × 58 cm high) with white vertical walls and a white floor divided into 25 equal squares, as reported previously (Meziane et al., 1998). The light intensity in the middle of the field was 30 lux. The objects to be discriminated were a marble (5.5 cm high, object A) and a plastic (4.5 cm high, object B) figure. First, mice were individually habituated to the open field for 50 min. The next day, they were submitted to a 10 min acquisition trial (first trial) during which they were placed in the open field in the presence of the object A. Locomotor activity (number of squares crossed), rearings and time that animal took to explore object A (animal's snout direct toward the object at a distance <1 cm) were recorded. A 10 min retention trial (second trial) occurred 24 h later. During this second trial, objects A and B were placed in the open field and locomotor activity, rearings and time that animal took to explore object A (tA) and object B (tB) were recorded. A recognition index was defined as (tB/(tA+tB)) × 100. Objects A and B were counterbalanced so that half of the animals in each experimental group were first exposed to the object A and then to the object B whereas the other half saw first as the object B and then the object A.
Statistical analysis
Data from the active avoidance test performed in CB1 knockouts and wild-type littermates were analysed using three-way analysis of variance (ANOVA) with repeated measures (genotype and treatment as between-subjects factors and day as within-subjects factor of variation). Subsequent two-way ANOVA followed by one-way and post hoc comparison (Dunnett's test) were used when required. AUC values were compared by using two-way ANOVA (genotype and treatment as a between-subjects factors), followed by one-way ANOVA and post hoc comparisons (Dunnett's test) when required. Data from active avoidance test performed using the pharmacological approach were analysed using three-way ANOVA with repeated measures (treatment and pretreatment as between-group factors and day as within-group factor of variation). In the object recognition task, recognition index values were compared using two-way ANOVA (treatment and pretreatment as between-subjects factor), followed by one-way ANOVA when required. In all the experiments, the level of significance was P<0.05. SPSS statistical package was used.
Drugs
The selective CB1 receptor antagonist rimonabant ((N-piperidin-1-yl)-5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxy amide), kindly provided by (Sanofi–Aventis, Paris, France) was dissolved in a solution of 1% of carboxymethylcellulose sodium salt (Merck, Madrid, Spain, Germany) and administered by intraperitoneal (i.p.) route. Nicotine hydrogen tartrate salt (Sigma, Madrid, Spain, France) was dissolved in physiological saline 0.9% and administered subcutaneously (s.c.). Physostigmine hemisulphate salt (Sigma) and scopolamine hydrochloride, (Sigma) were dissolved in physiological saline 0.9% and administered intraperitoneally. The injection volume was 20 ml kg−1 of body weight in the case for rimonabant and 10 ml kg−1 for the other drugs. Physostigmine (0.1 mg kg−1), scopolamine (0.5 mg kg−1) and nicotine (0.5 mg kg−1) were administered 30 min before the test. Rimonabant (0.03, 1 , 3 and 10 mg kg−1), was administered 35 min before testing.
Results
Genetic approach
Changes induced by scopolamine and physostigmine in the active avoidance paradigm in wild type and CB1 knockout mice
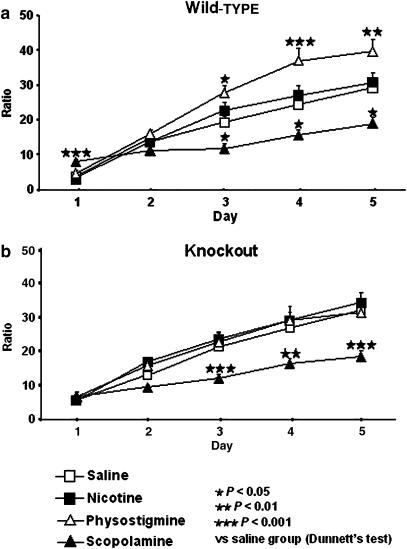
In the active avoidance paradigm, scopolamine decreased the performance in both, wild-type and CB1 knockout mice whereas physostigmine increased the performance in wild-type but not CB1 knockout mice (Figure 1). Nicotine did not produce any significant effect in this paradigm. Three-way ANOVA of ratio values revealed a significant effect of day (F(4,436)=372.72, P<0.001), treatment (F(3,109)=14.966, P<0.001), interaction between day and treatment (F(12,436)=13.175, P<0.001) and between day, genotype and treatment (F(12,436)=2.304, P<0.01). There is no significant effect of genotype, neither interaction between day and genotype nor between genotype and treatment.
Figure 1.
(a) CB1 wild-type mice treated with physostigmine (0.1 mg kg−1 i.p.) presented an enhancement in learning/memory evaluated in the active avoidance test (100-trial avoidance sessions per day for 5 days). Wild-type mice treated with scopolamine (0.5 mg kg−1 i.p.) showed a decrease in the learning performance. Data are expressed as a ratio between conditioned changes and total changes. Data represent mean±s.e.m. n=10–20 mice per experimental group. (b) CB1 knockout mice treated with scopolamine (0.5 mg kg−1 i.p.) showed a decrease in learning/memory evaluated during the active avoidance test (100-trial avoidance sessions per day for 5 days). Data are expressed as a ratio between conditioned changes and total changes. Data represent mean±s.e.m. n=10–20 mice per experimental group.
In wild-type animals, subsequent two-way ANOVA (day and treatment), revealed a significant effect of day (F(4,224)=167.12, P<0.001), treatment (F(3,56)=10.12, P<0.001) and interaction between both factors (F(12,224)=8.322, P<0.001). One-way ANOVA revealed in these animals a significant effect of treatment on day 1 (P<0.001), 3 (P<0.001), 4 (P<0.001) and 5 (P<0.001). Post hoc analysis showed a significant decrease in the performance of scopolamine-treated mice on days 3, 4 and 5. In contrast, a significant increase in learning performance of physostigmine-treated mice was observed on days 3, 4 and 5, when compared to saline-treated wild-type mice (Figure 1a).
In knockout animals, two-way ANOVA (day and treatment) revealed a significant effect of day (F(4,216)=205.69, P<0.001), treatment (F(3,54)=7.437, P<0.001) and interaction between these two factors (F(12,216)=5.705, P<0.001). One-way ANOVA showed a significant effect of treatment on day 2 (P<0.001), 3 (P<0.001), 4 (P<0.001) and 5 (P<0.001). Post hoc analysis indicated a significant decrease in the performance of scopolamine-treated mice on days 3, 4 and 5 when comparing with saline group (Figure 1b).
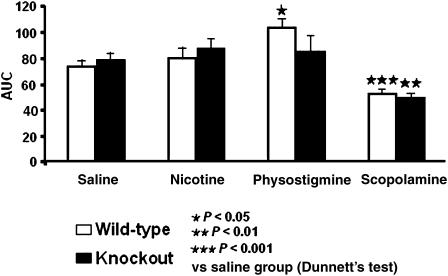
Data were also expressed as AUC in order to facilitate the comparison between groups (Figure 2). Two-way ANOVA (genotype and treatment) revealed significant effect of treatment (F(3,116)=16.707, P<0.001), but no effect of genotype, nor interaction between these two factors. One-way ANOVA, demonstrated a significant effect of treatment in both wild-type and knockout mice (F(3,116)=15.813, P<0.001; F(3,57)=7.559, P<0.001). In wild-type mice, subsequent post hoc analysis showed a significant decrease in the performance of scopolamine-treated animals and performance improvement in physostigmine-treated mice. In CB1 knockout animals, post hoc analysis only showed a decrease in the performance in scopolamine-treated mice (Figure 2).
Figure 2.
CB1 knockout and wild-type mice exhibited a decreased performance in the active avoidance test when treated with scopolamine (0.5 mg kg−1 i.p.), whereas only wild-type mice showed an enhanced performance when treated with physostigmine (0.1 mg kg−1 i.p.). Data represent mean±s.e.m. of n=10–20 mice per experimental group. Data are expressed as an AUC.
AUC was also expressed for number of conditioned changes. Knockout mice revealed an enhancement in the performance in the active avoidance compared to wild-type mice. Indeed, one-way ANOVA revealed a significant effect of genotype (F(1,38)=7.819, P<0.01) (Table 1).
Table 1.
AUC, obtained on the active avoidance paradigm (conditioned changes)
| Mean±s.e.m. | F-value | P-value | ||
|---|---|---|---|---|
| Genetical approach | Wild-type | 143.35±12.60 | ||
| 11.142 | <0.001 | |||
| CB1 knockout mice | Knockout | 198.58±14.60 | ||
| Vehicle | 132.66±12.57 | |||
| Pharmacological approach | 0.03 | 137.97±11.75 | ||
| 1 | 138.28±18.33 | 0.129 | NS | |
| Rimonabant (mg kg−1) | 3 | 131.97±10.94 | ||
| 10 | 125.56±17.61 |
Abbreviations: ANOVA, analysis of variance; AUC, area under the curve; NS, non significant.
A significant increase in the performance in the active avoidance paradigm was observed in CB1 knockouts compared to wild-type mice. The performance in this paradigm was not modified by rimonabant. One-way ANOVA (genotype for the genetical approach and treatment for the pharmacological approach).
Pharmacological approach
Lack of effects of rimonabant and nicotine in the active avoidance paradigm
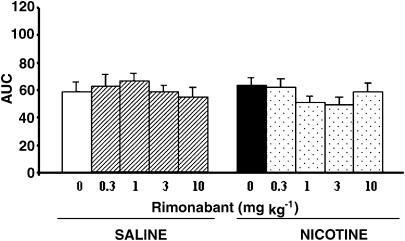
In the active avoidance paradigm, neither rimonabant nor nicotine have any effect in wild-type mice. Three-way ANOVA calculated for ratio values revealed a significant effect of day (F(4,628)=270.54, P<0.001) no significant effect of treatment (F(1,157)=1.164, ns) no effect of antagonist (F(4,157)=0.863, ns) and no interaction between either of these groups was observed (Figure 3a and b).
Figure 3.
(a) Rimonabant administration (0.03, 0.1, 1, 3 mg kg−1 i.p.) before the active avoidance test showed no effect in wild-type mice. Data are expressed as a ratio between conditioned changes and total changes and represent mean±s.em. n=16–19 mice per experimental group. (b) Nicotine administration (0.5 mg kg−1 s.c.) before the active avoidance test produced no effect in wild-type mice. There was no effect when animals were pretreated with rimonabant (0.03, 0.1, 1, 3 mg kg−1 i.p.) either. Data are expressed as a ratio between conditioned changes and total changes. Data represent mean±s.e.m. n=16–19 mice per experimental group.
Data were also expressed as AUC in order to facilitate the comparison between groups. Two-way ANOVA (pretreatment and treatment), revealed no effect of rimonabant (F(4,157)=655.53, ns) or nicotine (F(1,157)=1025.03, ns), nor interaction between these two factors (F(4,157)=661.12, ns) (Figure 4).
Figure 4.
Neither rimonabant (0.03, 0.1, 1, 3 mg kg−1 i.p.) nor nicotine (0.5 mg kg−1 s.c.) modified the performance in the active avoidance test in wild-type mice. Data are expressed as an AUC. Data represent mean±s.em. n=16–19 mice per experimental group.
AUC was also expressed for the number of conditioned changes. Rimonabant did not modify the performance in wild-type mice. Indeed, one-way ANOVA revealed no significant effect of the administration of the CB1 antagonist (Table 1).
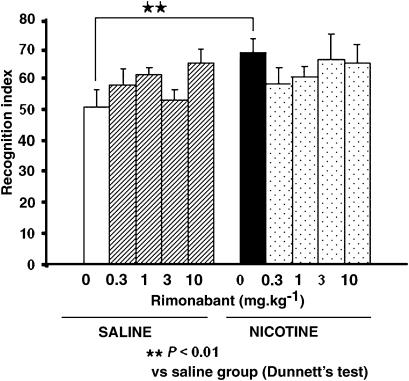
Nicotine increases the performance in the object recognition test
Two-way ANOVA revealed a significant effect of nicotine (F(1,118)=4.946,. P<0.05), but no effect of rimonabant nor interaction between these two factors. Subsequent one-way ANOVA only showed a significant increase in the recognition index values in nicotine-treated animals when compared with vehicle-treated animals (F(1,24)=10.608, P<0.01) (Figure 5).
Figure 5.
Recognition index measured at 24 h after the first trial, was increased in wild-type mice treated with nicotine (0.5 mg kg−1 s.c.), whereas animals that received rimonabant (0.03, 0.1, 1, 3 mg kg−1 i.p.) or both, rimonabant (0.03, 0.1, 1, 3 mg kg−1 i.p.) and nicotine (0.5 mg kg−1 s.c), exhibited a similar recognition index. Data represent mean±s.e.m. n=8–14 mice per experimental group.
Discussion
In this study, we investigated the possible interactions between the cannabinoid and cholinergic systems in memory and learning processes by using genetic and pharmacological approaches in two different behavioural models, the active avoidance and the object recognition test. Nicotine did not modify the performance of CB1 knockout and wild-type littermates in the active avoidance test. Our finding was in agreement with previous studies reporting that nicotine administration (0.35 mg kg−1) in NMRI mice did not improve the acquisition of the active avoidance test (Moragrega et al., 2005). Furthermore, nicotine (0.5, 1, 2 mg kg−1) had no effects on the performance in the active avoidance test, although it induced deficit in retrieval in C57BL/6J females and DBA/2J males and females, but not in C57BL/6J male mice (Gilliam and Schlesinger, 1985). In agreement, nicotine pretreatment affected active avoidance in a sexually dimorphic and dose–dependent manner (Yilmaz et al., 1997). Thus, in male Sprague–Dawley rats, nicotine was active at all the doses tested (0.2, 0.4, 0.6 mg kg−1) whereas in female rats, learning performance deteriorated only at the dose of 0.6 mg kg−1. In addition, prenatal administration of nicotine impaired active avoidance both in male and female Sprague–Dawley rats (Vaglenova et al., 2004), although an improved learning was revealed in similar experimental condition in females, but not in males (Genedani et al., 1983). Therefore, nicotine effects in the active avoidance test depend on a range of factors including strain, dose, time of administration, age and housing conditions. These discrepancies could be explained by differences in the emotional state. Thus, females show an enhanced stress response owing to the higher corticosterone level and faster onset compared to males (Carey et al., 1995; Carrasco and Van de Kar, 2003), and a mild decrease in anxiety has been reported in old mice compared to young animals (Maccarrone et al., 2002).
The effects of the cholinergic antagonist scopolamine on learning and memory were also evaluated in CB1 knockouts. Numerous pharmacological studies have demonstrated that scopolamine impairs learning in different tasks (Fibiger, 1991; Gallagher and Colombo, 1995; Zarrindast et al., 2002) and this impairment is directly related to a decrease in central cholinergic functions. In agreement with these reports, our study reveals an impairment in active avoidance performance after scopolamine administration in both wild-type and CB1 knockout mice, demonstrating that the amnesic effects of scopolamine are not mediated through the CB1 receptor. On the other hand, acetylcholinesterase inhibitors, such as physostigmine, that enhance the availability of ACh in the synaptic cleft, increase the performance in a variety of cognitive tasks (Molchan et al., 1992; Degroot and Parent, 2001; Zarrindast et al., 2002) and we found that physostigmine increased the active avoidance performance in wild-type mice. Interestingly, physostigmine did not modify the performance in CB1 knockout mice. An enhanced ACh release (Kathmann et al., 2001) and improved long-term potentiation in the hippocampus (Bohme et al., 2000) has been reported in mice lacking CB1 receptor, which are in part responsible for their improved memory function. Therefore, the responses mediated by physostigmine-induced enhancement of Ach activity could be impaired in the mutant mice that already show an enhanced Ach release as a precise concentration of this neurotransmitter seems to be required at the synaptic level to improve memory and learning processes. As reported previously (Martin et al., 2002), CB1 knockout mice showed an increase in the performance in the active avoidance task when compared to wild-type mice, as revealed by the modification of the number of conditioned changes. However, when the results are expressed as a ratio between conditioned and total changes, this difference did not reach statistical significance, showing that conditioned changes are more sensitive to these particular differences between genotypes. Endocannabinoids also modulate other pathways within the hippocampus, such as glutamatergic (Sullivan, 2000) and GABAergic activities (Hampson and Deadwyler, 2000; Wilson and Nicoll, 2001) that could also be involved in the altered response in CB1 knockout mice.
Using a pharmacological approach, we demonstrated that the CB1 antagonist rimonabant administered at a large range of doses (from 0.3 to 10 mg kg−1), and given alone or coadministered with nicotine did not modify the performance in the active avoidance test. Previous studies have reported controversial data on the effects of rimonabant on cognitive processes in rodents. Thus, rimonabant improved the performance of rats and mice in an olfactory recognition task (Terranova et al., 1996), facilitated memory acquisition and consolidation in the mouse elevated-Tmaze (Takahashi et al., 2005), enhanced spatial memory performance in the 8-arm radial maze (Lichtman, 2000) and improved memory in a delayed radial maze task (Wolff and Leander, 2003). However, rimonabant failed to enhance the performance in a variety of operant tasks in rats (Mansbach et al., 1996; Brodkin and Moerschbaecher, 1997; Mallet and Beninger, 1998), had no effect on a delayed nonmatch to sample task (Hampson and Deadwyler, 2000) and failed to modify the acquisition or consolidation of aversive memories (Marsicano et al., 2002). The apparent controversies could be explained because rimonabant seems to enhance memory consolidation rather than the acquisition or retrieval processes (Terranova et al., 1996). Thus, in most of the studies in which rimonabant improved the performance, this antagonist was administered after the original encounter with the cognitive paradigm (Terranova et al., 1996; Wolff and Leander, 2003). This hypothesis is in agreement with our findings revealing the absence of effect when rimonabant was administered before the exposure to the cognitive task. Another possible explanation for the lack of effect of rimonabant in these behavioural paradigms could be the particular biodistribution of this drug, which presents a preferential distribution to the peripheral tissues rather than to the CNS (Després et al., 2005). Another explanation for the difference between the results obtained with rimonabant and CB1 knockout mice could be the possibility of adaptive compensation in the genetic model.
In contrast with the active avoidance results, nicotine enhanced the performance in the two trial object recognition task. However, the results obtained in this behavioural paradigm showed a high variability that makes the interpretation difficult. Rimonabant enhanced the recognition index at all the doses tested, although significant differences were not revealed. Furthermore, nicotine did not significantly enhance the performance when combined with rimonabant, although the recognition index values were still high compared to saline. The particular enhancement in the availability of ACh that could result from the association of these two drugs could lead to an attenuation of the cognitive effects induced by nicotine alone. The active avoidance paradigm is a complex model in which other behavioural responses different from the cognitive processes, such as anxiety, play an important role in the trial performance. Several structures different from the hippocampus, that are involved in cognitive and emotional responses such as the prefrontal cortex and amygdala, also participate in the responses obtained after exposure to this paradigm (Holland and Bouton, 1999; LeDoux 2000). In contrast, object recognition test is considered a pure working memory task (Ennaceur and Delacour, 1988) in which the hippocampus plays a key role. Thus, cholinergic innervations of hippocampus by neurons in the medial septal area are critical for optimal memory performance in this model (Levin and Rezvani, 2002). Therefore, the different responses induced by nicotine in the active avoidance and object recognition task might be consequences of the distinct neurobiological substrate and cognitive responses evoked in these behavioural models.
In summary, the present findings demonstrate that the effects of nicotine and physostigmine are attenuated in the absence of CB1 receptor activity. However, scopolamine effects are independent of CB1 receptor activity. The cognitive responses induced by rimonabant in the active avoidance paradigm were different to those observed in CB1 knockout mice.
Acknowledgments
This study was supported by the National Institute on Drug Abuse-National Institutes of Health Grant 1R01DA016768, grants from Spanish Ministry of Science and Technology (SAF 2004/568, BFU2004-00920/BFI and GEN2003-20651-C06-04), and The European Commission (Newmood: LSHM-CT-2004-503474). SAB is a fellowship from Spanish MEC. AC was supported by a fellowship from generalitat de catalunya.
Abbreviations
- AUC
area under the curve
- CS
conditioned stimulus
- nAChR
nicotinic acetylcholine receptors
- THC
Δ9-tetrahydrocannabinol
- US
unconditioned stimulus
Conflict of interest
The authors state no conflict of interest.
References
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berlin) 2006;184:504–513. doi: 10.1007/s00213-005-0251-9. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. doi: 10.1016/s0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- Brodkin J, Moerschbaecher JM. SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther. 1997;282:1526–1532. [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. 1986;19:1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- Degroot A, Parent MB. Infusions of physostigmine into the hippocampus or the entorhinal cortex attenuate avoidance retention deficits produced by intra-septal infusions of the GABA agonist muscimol. Brain Res. 2001;920:10–18. doi: 10.1016/s0006-8993(01)02798-6. [DOI] [PubMed] [Google Scholar]
- Després JP, Galay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. Cholinergic mechanisms in learning, memory and dementia: a review of recent evidence. Trends Neurosci. 1991;14:220–223. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colombo PJ. Ageing: the cholinergic hypothesis of cognitive decline. Curr Opin Neurobiol. 1995;5:161–168. doi: 10.1016/0959-4388(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Genedani S, Bernardi M, Bertolini A. Sex-linked differences in avoidance learning in the offspring of rats treated with nicotine during pregnancy. Psychopharmacology (Berlin) 1983;80:93–95. doi: 10.1007/BF00427504. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Casu MA, Carta G, Mascia MS. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur J Pharmacol. 1998;355:119–124. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Schlesinger K. Nicotine-produced relearning deficit in C57BL/6J and DBA/2J mice. Psychopharmacology (Berlin) 1985;86:291–295. doi: 10.1007/BF00432216. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and contex in classical conditioning. Curr Opin Neuobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Jensen LH, Stephens DN, Sarter M, Petersen EN. Bidirectional effects of beta-carbolines and benzodiazepines on cognitive processes. Brain Res Bull. 1987;19:359–364. doi: 10.1016/0361-9230(87)90104-3. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of acetylcholine release in the brain of NMRI, CD-1 and C57BL/6J mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:50–56. doi: 10.1007/s002100000304. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Patitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB receptor knockout mice. Science. 1999;283:401–402. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic systems and cognitive function. Psychopharmacology (Berlin) 1992;108:417–431. doi: 10.1007/BF02247415. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology (Berlin) 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr Drug Targets CNS Neurol Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404:175–179. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Valverde O, Barbaccia ML, Castañe A, Maldonado R, Ledent C, et al. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berlin) 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Winston EN, Lowe JA., III Effects of the cannabinoid CB1 receptor antagonist SR141716A on the behavior of pigeons and rats. Psychopharmacology (Berlin) 1996;124:315–322. doi: 10.1007/BF02247436. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berlin) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, et al. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Role of cholinergic system on the construction of memories: taste memory encoding. Neurobiol Learn Mem. 2003;80:211–222. doi: 10.1016/s1074-7427(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Molchan SE, Martinez RA, Hill JL, Weingartner H.J, Thompson K, Vitiello B, et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Brain Res Rev. 1992;17:215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- Moragrega I, Carmen CM, Redolat R. Effects of housing and nicotine on shuttle-box avoidance in male NMRI mice. Behav Brain Res. 2005;164:178–187. doi: 10.1016/j.bbr.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Leo P. Alleviation of scopolamine amnesia by different retrieval enhancing treatments. Pharmacol Biochem Behav. 1988;30:1093–1096. doi: 10.1016/0091-3057(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Perspectives: neuroscience. Remembrance of things past. Science. 1999;285:1503–1504. doi: 10.1126/science.285.5433.1503. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Shoaib M. Nicotine psychopharmacology: addiction, cognition and neuroadaptation. Med Res Rev. 1995;15:47–72. doi: 10.1002/med.2610150105. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Fernandes MS. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett. 2005;380:270–275. doi: 10.1016/j.neulet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, et al. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berlin) 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J Neurosci. 2003;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol. 2003;477:213–217. doi: 10.1016/j.ejphar.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Kanit L, Okur BE, Pogun S. Effects of nicotine on active avoidance learning in rats: sex differences. Behav Pharmacol. 1997;8:253–260. [PubMed] [Google Scholar]
- Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. J Psychopharmacol. 2002;16:313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]