Abstract
Background and purpose:
Adenosine suppresses immune responses through adenosine2A (A2A) receptors, by raising intracellular cAMP. Interleukin (IL)-18 up-regulates the expression of intercellular adhesion molecule (ICAM)-1 on monocytes, leading to production of pro-inflammatory cytokines such as IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-α by human peripheral blood mononuclear cells (PBMC). We have previously demonstrated that elevation of cAMP inhibits this IL-18-induced expression of adhesion molecules. In the present study, we examined the effect of adenosine on the IL-18-induced up-regulation of ICAM-1 on human monocytes and production of IL-12, IFN-γ and TNF-α by PBMC.
Experimental approach:
The expression of ICAM-1 was examined by flow cytometry. IL-12, IFN-γ and TNF-α were determined by ELISA assay.
Key results:
Adenosine inhibited the IL-18-induced up-regulation of ICAM-1 on human monocytes and it abolished the IL-18-enhanced production of IL-12, IFN-γ and TNF-α. While an A2A receptor antagonist reversed the action of adenosine, an A1 or A3 receptor antagonist enhanced them. An A2A receptor agonist, CGS21680, mimicked the effects of adenosine and its effects were abolished not only by the A2A receptor antagonist but also by A1 or A3 receptor agonists. Activation via A2A receptors resulted in elevation of cAMP in monocytes, whereas the stimulation of A1 or A3 receptors inhibited it, suggesting that intracellular signal transduction following ligation of A2A receptors might be blocked by activation of A1 or A3 receptors.
Conclusions and Implications:
Adenosine differentially regulates IL-18-induced adhesion molecule expression and cytokine production through several subtypes of its receptors.
Keywords: adenosine, interleukin-18, intercellular adhesion molecule-1, human peripheral blood mononuclear cells, cytokine
Introduction
Adenosine is an endogenous nucleoside that regulates many physiological processes through its four receptor subtypes: A1, A2A, A2B and A3. These adenosine receptors belong to the superfamily of seven transmembrane G-protein-coupled receptors. A1 and A3 subtypes inhibit adenylate cyclase via Gi protein, whereas A2A and A2B activate adenylate cyclase via Gs protein (Jacobson et al., 1992; Poulsen and Quinn, 1998). Stimulation via A2A receptors results in elevation of cAMP and the activation of protein kinase A protein kinase A (PKA) in human monocytes (Bshesh et al., 2002). The Gs-coupled high-affinity A2A receptor mediates many of the anti-inflammatory actions of adenosine in monocytes (Link et al., 2000) and is involved in the inhibitory activity of adenosine analogs on tumor necrosis factor (TNF)-α production by lipopolysaccharide-activated monocytes (Khoa et al., 2001; Zhang et al., 2005). Thus, the A2A receptor is likely to be important for the regulation of immune responses.
Interleukin (IL)-18 is a potent inflammatory cytokine secreted by antigen-presenting cells such as macrophages and dendritic cells in response to invading pathogens (Okamura et al., 1995; Janeway and Medzhitov, 2002). Serum levels of IL-18 were also found to be elevated during multiple sclerosis multiple sclerosis (MS) (Gomez and Sitkovsky, 2003) and rheumatoid arthritis (RA) (Cronstein et al., 1992; Marabito et al., 1998). Cell-to-cell interactions between monocytes and T cells mediated through intercellular adhesion molecule (ICAM)-1 engagement with their respective ligands play important roles in immune responses (Camacho et al., 2001). Recently, we found that IL-18 upregulated the expression of ICAM-1 on monocytes as well as influencing the production of IL-12, interferon (IFN)-γ and TNF-α in peripheral blood mononuclear cells (PBMC) (Takahashi et al., 2002, 2003). Blockade of the engagement of adhesion molecule by antibodies against ICAM-1 reduced cytokine production by IL-18-treated PBMC (Takahashi et al., 2002; 2003). This suggests that IL-18 induces cytokine production through upregulation of adhesion molecule expression on monocytes. However, little is known about the effect of adenosine on IL-18-initiated immune responses. In the present study, therefore, we investigated the effect of adenosine on the expression of adhesion molecules on monocytes and cytokine production by PBMC treated with IL-18 and the adenosine receptor subtypes what were involved therein.
Methods
Isolation of PBMC and monocytes
Normal human PBMC were obtained from ten healthy volunteers after IRB approval (Okayama University IRB No.106) (Table 1). PBMC were isolated from 20 to 50 ml of venous blood and they were treated with heparin. Monocytes were separated by counterflow centrifugal elutriation, as described previously (Takahashi et al., 2002; 2003). The PBMC and monocytes were then suspended at a final concentration of 1 × 106 cells ml−1 in the culture medium, RPMI 1640 medium (Nissui, Co. Ltd, Tokyo, Japan) supplemented with 10% (v/v) heat-inactivated fetal calf serum, 20 μg ml−1 kanamycin and 100 μg ml−1 streptomycin and penicillin (Sigma, St Louis, MO, USA), as described previously (Takahashi et al., 2002; 2003). The Figures 1, 2, 3, 4 and 6 relate to experiments with PBMC and Figure 5 relates to data from monocytes isolated from PBMC. The PBMC obtained from volunteers 1, 2, 3, 8 and 9 were used for Figures 1 and 2, and the PBMC obtained from volunteers 1, 2, 3, 4 and 5 were used for Figures 3 and 4. The monocytes obtained from volunteers 1, 3, 7, 8 and 9 were used for Figure 5, and the PBMC obtained from volunteers 1, 2, 3, 6 and 10 were used for Figure 6.
Table 1.
Blood donors
| Volunteers | Sex | Age (years) |
|---|---|---|
| 1 | Man | 47 |
| 2 | Man | 34 |
| 3 | Man | 33 |
| 4 | Man | 28 |
| 5 | Man | 35 |
| 6 | Man | 52 |
| 7 | Man | 30 |
| 8 | Woman | 37 |
| 9 | Woman | 66 |
| 10 | Woman | 28 |
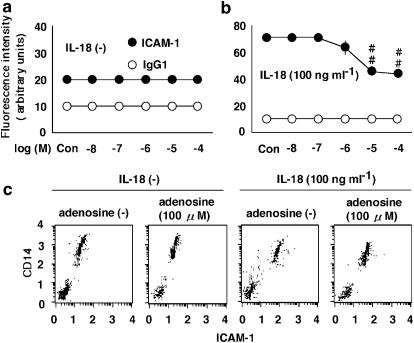
Figure 1.
The effects of adenosine on the expression of ICAM-1 on monocytes. (a and b) The effect of adenosine at concentrations ranging from 0.01 to 100 μM (Con, no adenosine) on the expression of ICAM-1 in the absence (a) or presence (b) of 100 ng ml−1 IL-18. PBMC were treated with adenosine or IL-18, and the expression of ICAM-1 on monocytes was examined by multi color flow cytometry using a combination of anti-CD14 antibody with anti-ICAM-1 antibody. The values shown as IgG1 represent the IgG1 isotype-matched control. Results are expressed as the means±s.e.m. of triplicate determinations from five donors. ##P< 0.01 compared with the value for IL-18 alone. When an error bar was within a symbol, the bar was omitted. (c) The dot plots of ICAM-1 are shown. The cells were treated with adenosine at 100 μM in the presence or absence of IL-18 at 100 ng ml−1 and then incubated for 24 h.
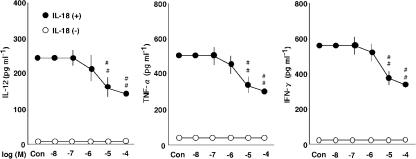
Figure 2.
The effects of adenosine on the production of IL-12, TNF-α and IFN-γ by PBMC. The effect of adenosine at concentrations ranging from 0.01 to 100 μM (Con, no adenosine) on the production of IL-12, TNF-α and IFN-γ in the presence (+) or absence (−) of 100 ng ml−1 IL-18. Results are expressed as the means±s.e.m. of triplicate determinations from five donors. ##P<0.01 compared with the value for IL-18 alone. When an error bar was within a symbol, the bar was omitted.
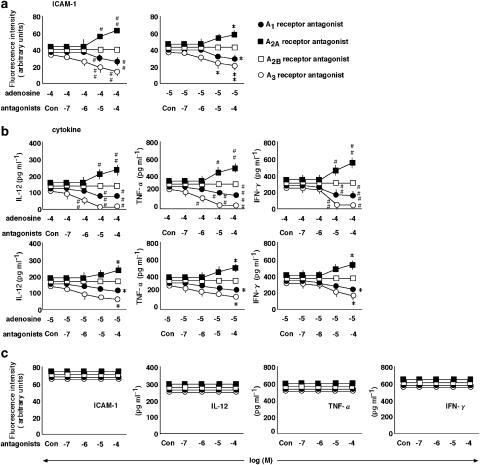
Figure 3.
The effects of A1, A2A, A2B and A3 receptor antagonists on the action of adenosine. (a) The effects of the selective A1, A2A, A2B and A3 receptor antagonists, DPCPX (•), ZM-241385 (▪), alloxazine (□) and MRS1220 (○), respectively, at concentrations ranging from 0.1 to 100 μM (Con, no antagonist) on the expression of ICAM-1 in the presence of IL-18 at 100 ng ml−1 and adenosine at 10 (right-hand graph) and 100 μM (left-hand graph). PBMC were treated with various stimulations and the expression of ICAM-1 on monocytes was examined by multi color flow cytometry using a combination of anti-CD14 antibody with anti-ICAM-1 antibody. (b) The effects of A1, A2A, A2B and A3 receptor antagonists (Con, no antagonist) on the production of IL-12, TNF-α and IFN-γ in the presence of IL-18 at 100 ng ml−1 and adenosine at 10 (lower graphs) and 100 μM (upper graphs). (c) The effects of antagonists at concentrations ranging from 0.1 to 100 μM (Con, no antagonist) on the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ by PBMC treated with IL-18 at 100 ng ml−1 in the absence of adenosine were determined. Results are expressed as the means±s.e.m. of triplicate determinations from five donors. #P<0.05, ##P<0.01 compared with the value for IL-18 and adenosine at 10 μM. *P<0.05, **P<0.01 compared with the value for IL-18 and adenosine at 100 μM. When an error bar was within a symbol, the bar was omitted.
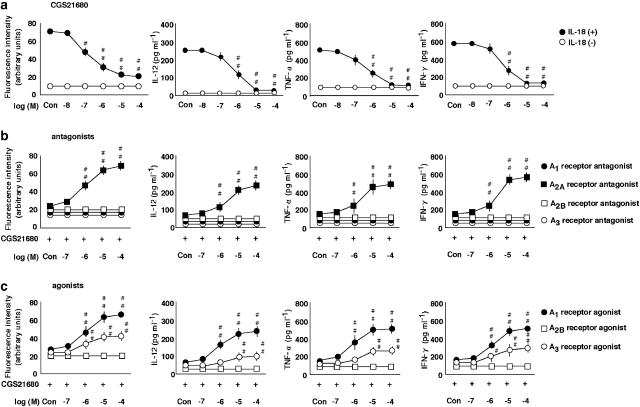
Figure 4.
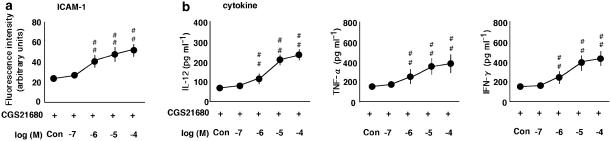
The effects of an A2A receptor agonist on the expression of ICAM-1 on monocytes and production of IL-12, TNF-α and IFN-γ by PBMC and their modulation. (a) The effect of a selective A2A receptor agonist, CGS-21680, at concentrations ranging from 0.1 to 100 μM (Con, no CGS 21680) on the expression of ICAM-1, and the production of IL-12, TNF-α and IFN-γ in the presence (+) or absence (−) of IL-18 at 100 ng ml−1. The expression of ICAM-1 on monocytes was examined by multi color flow cytometry using a combination of anti-CD14 antibody with anti-ICAM-1 The results are expressed as the means±s.e.m. of triplicate determinations from five donors. #P<0.05, ##P<0.01 compared with the value for IL-18 alone. (b) The effect of a selective A1, A2A, A2B and A3 receptor antagonists, DPCPX, ZM-241385, alloxazine and MRS1220, at concentrations ranging from 0.1 to 100 μM (Con, no antagonist) on the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ in the presence of IL-18 at 100 ng ml−1 and CGS-21680 at 100 μM. #P<0.05, ##P<0.01 compared with the value for IL-18 and CGS-21680. (c) The effect of a selective A1, A2B and A3 receptor agonists, CPA, NECA and IB-MECA, respectively, at concentrations ranging from 0.1 to 100 μM (Con, no agonist) on the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ in the presence of IL-18 at 100 ng ml−1 and CGS-21680 at 100 μM. #P<0.05, ##P<0.01 compared with the value for IL-18 and CGS-21680. When an error bar was within a symbol, the bar was omitted.
Figure 6.
The effect of H89 on CGS-21680-mediated inhibition of IL-18 induced ICAM-1 expression and the cytokine production. (a) The effect of a PKA inhibitor, H89, at concentrations ranging from 0.1 to 100 μM (Con, no H89) on the expression of ICAM-1 on monocytes in the presence of 100 ng ml−1 IL-18 and 100 μM of the selective A2A receptor agonist, CGS-21680, at 24 h. PBMC were treated as described and the expression of ICAM-1 on monocytes was examined by multi color flow cytometry using a combination of anti-CD14 antibody with anti-ICAM-1 antibody. (b) The effect of H89 (Con, no H89) on IL-12, TNF-α and IFN-γ production by PBMC in the presence of IL-18 and CGS-21680 after 24 h. Results are expressed as the means±s.e.m. of triplicate determinations from five donors. #P<0.05, ##P<0.01 compared with the value for IL-18 and CGS-21680. When an error bar was within a symbol, the bar was omitted.
Figure 5.
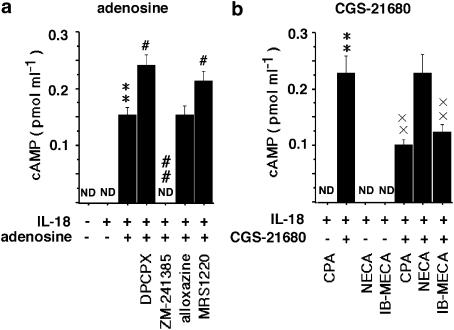
The effects of A2AR stimulation on the levels of cAMP in isolated monocytes treated with IL-18. (a) The effect of adenosine, the selective A1, A2A, A2B and A3 receptor antagonists, DPCPX, ZM-241385, alloxazine and MRS1220, respectively, on the production of cAMP in the presence of 100 ng ml−1 IL-18 at 30 min. (b) The selective A1, A2A, A2B and A3 receptor agonists, CPA, CGS-21680, NECA and IB-MECA, respectively, at 100 μM on the production of cAMP in the presence of IL-18. **P<0.01 compared with the value for IL-18 alone. #P<0.05, ##P<0.01 compared with the value for adenosine. × ×P<0.01 compared with the value for CGS-21680 and IL-18. The results are the means±s.e.m. of triplicate determinations from five donors. ND, not detected.
Flow cytometric analysis for adhesion molecule expression
Changes in the expression of human leukocyte antigen, ICAM-1, on monocytes were examined by multi color flow cytometry using a combination of anti-CD14 antibody with anti-ICAM-1 antibody. PBMC at 1 × 106 cells ml−1 were treated with adenosine, the selective A1 (DPCPX), A2A (ZM-241385), A2B (alloxazine) and A3 (MRS1220) receptor antagonists, the selective A1 (CPA), A2A (CGS-21680), A2B (NECA) and A3 (IB-MECA) receptor agonists (Table 2) or a PKA inhibitor, H89, each at concentrations ranging from 0.01 to 100 μM in the presence or absence of IL-18 at 100 ng ml−1. Treatment was carried out by incubation for 24 h. The cells were washed once with washing buffer (phosphate-buffered saline supplemented with 2.5% normal horse serum, 0.1% NaN3, and 0.01 M HEPES, pH 7.3). The cells were prepared for flow cytometric analysis and analyzed with a FACSCalibur (BD Biosciences, San Jose, CA, USA) as described previously (Takahashi et al., 2002; 2003). The data were processed using the CELL QUEST program. The data were expressed as the relative fluorescent intensities against isotype-matched control.
Table 2.
Adenosine receptor antagonists and agonists
| Antagonists | Agonists | |
|---|---|---|
| A1 | DPCPX | CPA |
| A2A | ZM-241385 | CGS-21680 |
| A2B | Alloxazine | NECA |
| A3 | MRS1220 | IB-MECA |
Enzyme-linked immunosorbent assay (ELISA) assay
PBMC at 1 × 106 cells ml−1 were used for analyzing IL-12, IFN-γ and TNF-α production. After culturing for 24 h at 37°C in a 5% CO2 and air mixture, cell-free supernatant were assayed for IL-12, IFN-γ and TNF-α protein by enzyme-linked immunosorbent assay (ELISA) employing the multiple antibody sandwich principle (IL-12 (p70), TNF-α and IFN-γ reagents were from R&D Systems, Minneapolis, MN, USA). The detection limits of the ELISA for IL-12, IFN-γ and TNF-α were 10 pg ml−1.
Measurement of cAMP production in monocytes
Monocytes at 1 × 106 cells ml−1 were incubated with the culture medium at 37°C in a 5% CO2 and air mixture. After 30 min, trichloroacetic acid at a final concentration of 5% and 3-isobutyl-1-methylxanthine, an inhibitor of phosphodiesterase, at 100 μM was added to cells at 2 × 105 cells. These were then frozen at −80°C until analyzed. Frozen samples were subsequently sonicated and assayed for cAMP using a cAMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions, for which no acetylation procedures were performed.
Statistical analysis
Statistical significances were evaluated using analysis of variance (ANOVA) followed by Dunnett's test. A probability value of less than 0.05 was considered to indicate statistical significance. The results were expressed as means±s.e.m. of triplicate findings from five donors. The IC50 is the concentration at which 50% of the maximum inhibition was achieved.
Reagents and drugs
Recombinant human IL-18 was purchased from MBL (Nagoya, Japan). Adenosine, CPA (2-chloro-N-cyclopentyladenosine), DPCPX (8-cyclopentyl-1,3-dipropylxanthine), CGS-21680 (2-(p-(2-carnonylethyl) phenylethylamino)-5-N-ethylcarboxamido adenosine), ZM-241385 (4-(2-(7-amino-2-(2-furyl)(1,2,4-triazolo(2,3-NECA (5′-N-ethylcarboxamido adenosine), alloxazine (benzo(g)pteridine-2,4 (1H,3H)-dione), IB-MECA (1-deoxy-1-(6-((3-iodophenyl) methyl)amino)-9H-purin-9-yl)-N-methyl-D-ribofuranuronamide), MRS1220 (N-(9-chloro-2-furan-2-yl-(1,2,4)triazolo (1,5-c)quinazolin-5-yl)-2-phenyl-acetamide) and H89 were purchased from Sigma Chemical. For flow cytometric analysis, fluorescein isothiocyanate (FITC)-conjugated mouse IgG1 monoclonal antibody (mAb) against ICAM-1 (6.5B5), which recognizes the D1 domain of ICAM-1 and phycoerythrin-conjugated anti-CD14 mAb, were purchased from DAKO (Glostrup, Denmark). An FITC-conjugated IgG1 isotype-matched control was obtained from Sigma Chemical.
Results
The effects of adenosine on the expression of ICAM-1 on monocytes and the production of IL-12, TNF-α and IFN-γ by PBMC
We investigated the effect of adenosine on the expression of ICAM-1 on monocytes (Figure 1) and its impact on the production of IL-12, TNF-α and IFN-γ by PBMC (Figure 2). Adenosine had no effect on the expression of ICAM-1 or cytokine production in the absence of IL-18. IL-18 induced the expression of ICAM-1 on monocytes and the production of IL-12, TNF-α and IFN-γ (Takahashi et al., 2002; 2003). Adenosine inhibited the IL-18-enhanced expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ (Figures 1 and 2). The IC50 values of adenosine for inhibition of the IL-18-enhanced ICAM-1 expression and IFN-γ production were both 20 μM, with the maximal effect being obtained at 100 μM.
The effect of a selective A1, A2A, A2B and A3 receptor antagonists on the action of adenosine
To investigate the involvement of four different subtype receptors in the action of adenosine, we examined the effects of the selective A1, A2A, A2B and A3 receptor antagonists, DPCPX, ZM-241385, alloxazine and MRS1220, respectively, on the adenosine-induced inhibition of ICAM-1 expression on monocytes and the production of IL-12, TNF-α and IFN-γ by PBMC in the presence of IL-18 (Figure 3a and b). The A2A receptor antagonist, ZM-241385, reversed both the adenosine-induced inhibition of expression of ICAM-1 and cytokine production, but the A2B receptor antagonist, alloxazine, had no effect. Conversely, the A1 and A3 receptor antagonists, DPCPX and MRS1220, enhanced the actions of adenosine. None of the antagonists had any effect in the absence of adenosine (Figure 3c).
The effects of an A2A receptor agonist on the IL-18-enhanced expression of ICAM-1 on monocytes and production of IL-12, TNF-α and IFN-γ by PBMC
The effects of the selective A1, A2A, A2B and A3 receptor agonists, CPA, CGS-21680, NECA and IB-MECA, respectively, on the expression of adhesion molecules and cytokine production in the presence or absence of IL-18 were determined (Figure 4). In the presence of IL-18, the A2A receptor agonist CGS-21680 inhibited the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ (Figure 4a). However, it had no effect on the cytokine production in the absence of IL-18 (data not shown). The A1, A2B and A3 receptor agonists had no effect in the presence or absence of IL-18 (data not shown). In addition, we investigated the effects of four different receptor antagonists on the A2A agonist-initiated inhibition of ICAM-1 expression and production of IL-12, TNF-α and IFN-γ in the presence of IL-18 (Figure 4b). The A2A receptor antagonist, ZM-241385, but not the other receptor antagonists reversed the action of the A2A receptor agonist, CGS-21680. Moreover, we examined the effect of the A1, A2B and A3 receptor agonists on the A2A receptor agonist-initiated inhibition of ICAM-1 expression on monocytes and production of IL-12, TNF-α and IFN-γ by PBMC in the presence of IL-18 (Figure 4c). The A1 and A3 receptor agonists, CPA and IB-MECA, but not the A2B receptor agonist, NECA, reversed the action of the A2A receptor agonist, CGS-21680.
The effects of A2A receptor-mediated stimulation on the levels of cAMP in monocytes treated with IL-18
We examined the involvement of the elevation of cAMP in the action of A2A receptor stimulation (Figure 5). IL-18 had no effect on the elevation of intracellular cAMP. Adenosine and the A2A receptor agonist, CGS-21680, but not the A1 and A3 receptor agonists, CPA and IB-MECA, induced the elevation of cAMP. The effect of adenosine was reduced by the A2A receptor antagonist, ZM-241385, and it was enhanced by the A1 and A3 receptor antagonists, DPCPX and MRS1220. The action of the A2A receptor agonist CGS-21680, was reduced by the A1 and A3 receptor agonists, CPA and IB-MECA. Neither the A2B receptor antagonist, alloxazine nor the corresponding agonist, NECA had any effect on A2A receptor stimulation by adenosine. A PKA inhibitor, H89, reversed the inhibitory effect of the A2A receptor agonist, CGS-21680, on the IL-18-elicited expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ (Figure 6).
Discussion and conclusions
We previously reported that blockade of the engagement of ICAM-1 and lymphocyte function-associated antigen (LFA)-1 by anti-ICAM-1 antibody and a statin-derived LFA-1 inhibitor, LFA703, reduced the IL-18-enhanced production of IL-12, TNF-α and IFN-γ by PBMC (Takahashi et al., 2002; 2003; 2005). This suggests that the engagement of ICAM-1 and LFA-1 might play an important role in IL-18-enhanced cytokine production. Here, as shown in Figures 1 and 2, we demonstrate that adenosine inhibited the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ in the presence of IL-18. This further suggests that adenosine-initiated inhibition of ICAM-1 expression might regulate cytokine production. Adenosine deaminase is known to convert adenosine to inosine by the removal of an amine group. We demonstrated that inosine at concentrations ranging from 0.01 to 100 μM had no effect on the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ in the presence of IL-18 (data not shown). Therefore, the actions of adenosine were independent of any conversion to inosine.
Zhang et al. (2005) reported the binding affinity profile of agonists and antagonists at human adenosine receptors. Adenosine inhibited monocyte function through activation of A2A, A2B and A3 receptors (Le Vraux et al., 1993; Bouma et al, 1994; Khoa et al., 2001; Zhang et al., 2005), and, thus, the involvement of adenosine receptor subtypes is controversial. In the present study, we found a complex interaction between adenosine receptor subtypes in mediating the overall effect of adenosine. The inhibitory effect of adenosine was blocked by the A2A receptor antagonist, ZM-241385, and, conversely, the A2A receptor agonist, CGS-21680, mimicked the effect of adenosine (Figures 3 and 4b). Unexpectedly, the inhibitory effect of adenosine was enhanced by the A1 and A3 receptor antagonists, DPCPX and MRS1220, respectively (Figure 3). In addition, we demonstrated that the A1 and A3 receptor agonists, CPA and IB-MECA, respectively, reduced the actions of the A2A receptor agonist (Figure 4). Thus, the inhibitory effect of adenosine was owing to the stimulation of A2A receptors and that effect was reduced by stimulation of A1 and A3 receptors.
Stimulation through the A2A receptor is known to result in the elevation of cAMP levels and PKA activation in human monocytes (Bshesh et al., 2002). As shown in Figure 5, stimulation via the A2A receptor increased cAMP levels in monocytes, which were again reduced by A1 and A3 receptor agonists. We have found that the activation of cAMP inhibited the IL-18-elicited expression of ICAM-1 and the production of IL-12, TNF-α and IFN-γ by PBMC (Takahashi et al., 2002; 2003). A PKA inhibitor, H89 reversed the effect of A2A receptor agonist on the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ (Figure 6). These results are consistent with the notion that cAMP might be involved in mediating the effects of A2A receptor agonists on the expression of ICAM-1 and production of IL-12, TNF-α and IFN-γ. Moreover, the inhibitory influence of A1 and A3 receptor agonists on the effects of A2A receptor agonist might depend on the inhibition of cAMP elevation induced by the latter. However, the expression of B7.2 and CD40 was not changed by adenosine or by the A2A receptor agonist, suggesting that the activation of cAMP was not sufficient for this outcome. Further study on the mechanism of the action of adenosine and A2A receptor agonist will be required to clarify this.
Ohta and Sitkovsky (2001) reported that the stimulation of A2A receptor signaling suppressed the inflammation and inhibited the tissue damage in mouse models of liver injury and endotoxin-induced septic shock. The A2A receptor-initiated inhibition of chemokine and cytokine production also reduced the inflammation and the tissue damage during reperfusion after hepatic ischemia (Harada et al., 2000; Day et al., 2004). Moreover, it has been suggested that adenosine may be useful for treating the chronic inflammatory diseases, including MS and RA (Cronstein et al., 1992; Marabito et al., 1998; Gomez and Sitkovsky, 2003). Adenosine induces bronchoconstriction in animal models and in patients with inflammatory airway diseases such as asthma and chronic obstructive pulmonary diseases (COPD), and adenosine receptors are present in many cell types involved in airway inflammation (Spicuzza et al., 2003). In the lung, A2A receptors activate a protective mechanism playing a critical role in the downregulation of inflammation and tissue damage in different models (Ohta and Sitkovsky, 2001; Thiel et al., 2005). The mechanism of adenosine's actions in these cases may involve IL-18 (Saha et al., 1999; Karni et al., 2002; Yamagata and Ichinose, 2006). The present study may explain, at least in part, the mechanism of action of adenosine on IL-18-initiated diseases, including MS, RA, asthma and COPD. In conclusion, we have demonstrated that adenosine regulates IL-18-enhanced expression of ICAM-1 and the production of IL-12, TNF-α and IFN-γ via the stimulation several subtypes of adenosine receptor.
Abbreviations
- COPD
chronic obstructive pulmonary diseases
- ICAM
intercellular adhesion molecule
- IFN
interferon
- IL
interleukin
- LFA
lymphocyte function-associated antigen
- PBMC
peripheral blood mononuclear cells
- PKA
protein kinase A
- TNF
tumor necrosis factor
- MS
multiple sclerosis
- RA
rheumatoid arthritis
Conflict of interest
The authors state no conflicts of interest.
References
- Bouma MG, Stad RK, van den Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- Bshesh K, Zhao B, Spight D, Biaggioni I, Feokistov I, Denenberg A, et al. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J Leukoc Biol. 2002;72:1027–1036. [PubMed] [Google Scholar]
- Camacho SA, Heath WR, Carbone FR, Sarvetnick N, LeBon A, Karlsson L, et al. A key role for ICAM-1 in generating effector cells mediating inflammatory responses. Nat Immunol. 2001;6:523–529. doi: 10.1038/88720. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Philips MR, Hirschhorm R, Abramson SB, Weissmannn G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- Gomez G, Sitkovsky MV. Targeting G protein-coupled A2a adenosine receptors to engineer inflammation in vivo. Int J Biochem Cell Biol. 2003;35:410–414. doi: 10.1016/s1357-2725(02)00177-2. [DOI] [PubMed] [Google Scholar]
- Harada N, Okajima K, Murakami K, Usune S, Sato C, Ohshima K, et al. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J Pharmacol Exp Ther. 2000;294:1034–1042. [PubMed] [Google Scholar]
- Jacobson KA, van Galen PJM, Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem. 1992;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Karni A, Koldzic DN, Bharanidharan P, Khoury SJ, Weiner HL. IL-18 is linked to raised IFN-gamma in multiple sclerosis and is induced by activated CD4(+) T cells via CD40-CD40 ligand interactions. J Neuroimmunol. 2002;125:134–140. doi: 10.1016/s0165-5728(02)00018-8. [DOI] [PubMed] [Google Scholar]
- Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monoElenkov, I.J. (2000) Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2001;164:436–442. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Le Vraux V, Chen YL, Masson I, De Sousa M, Giroud JP, Florentin I, Chauvelot-Moachon L. Inhibition of human monocyte TNF production by adenosine receptor agonists. Life Sci. 1993;52:1917–1924. doi: 10.1016/0024-3205(93)90632-d. [DOI] [PubMed] [Google Scholar]
- Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, Wilder RL, Elenkov IJ. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- Marabito L, Montesinos MC, Schreibman DM, Balter L, Thompson L, Resta R, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-59-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;2:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G protein-coupled adenosine receptors in down-regulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Poulsen SA, Quinn R. Adenosine receptors: new opportunities for future drugs. Bioorg Med Chem. 1998;6:619–641. doi: 10.1016/s0968-0896(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Saha N, Moldovan F, Tardif G, Pelletier JP, Cloutier JM, Martel-Pelletier J. Interleukin-1β-converting enzyme (caspase-1) in human osteoarthritic tissues. Localization and role in the maturation of interleukin-1β and interleukin-18. Arthritis Rheum. 1999;42:1577–1587. doi: 10.1002/1529-0131(199908)42:8<1577::AID-ANR3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Bonfiglio C, Polosa R. Research applications and implications of adenosine in diseased airways. Trends Pharmacol Sci. 2003;24:409–413. doi: 10.1016/S0165-6147(03)00193-7. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Iwagaki H, Tamura R, Xue D, Sano M, Mori S, et al. Unique regulation profile of prostaglandin E1 on adhesion molecule expression and cytokine production in human peripheral blood mononuclear cells. J Pharmacol Exp Ther. 2003;307:1188–1195. doi: 10.1124/jpet.103.056432. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Iwagaki H, Yoshino T, Mori S, Morichika T, Itoh H, et al. Prostaglandin E(2) inhibits IL-18-induced ICAM-1 and B7.2 expression through EP2/EP4 receptors in human peripheral blood mononuclear cells. J Immunol. 2002;168:4446–4454. doi: 10.4049/jimmunol.168.9.4446. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Mori S, Iwagaki H, Yoshino T, Tanaka N, Weitz-Schmidt G, et al. Differential effect of LFA703, pravastatin,and fluvastatin on production of IL-18 and expression of ICAM-1 and CD40 in human monocytes. J Leukoc Biol. 2005;77:400–407. doi: 10.1189/jlb.0904510. [DOI] [PubMed] [Google Scholar]
- Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PloS Biol. 2005;3:174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Ichinose M. Agents against cytokine synthesis or receptors. Eur J Pharmacol. 2006;533:289–301. doi: 10.1016/j.ejphar.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Hepburn L, Cruz G, Borman RA, Clark KL. The role of adenosine A2A and A2B receptors in the regulation of TNF-alpha production by human monocytes. Biochem Pharmacol. 2005;69:883–889. doi: 10.1016/j.bcp.2004.12.008. [DOI] [PubMed] [Google Scholar]