Abstract
Background and purpose:
Receptor subtypes involved in PGE2-induced nociception are still controversial. The present study investigated the prostanoid E receptor (EP) subtypes and the protein kinase (PK) pathways involved in the nociception induced by PGE2 injection in the mouse paw.
Experimental approach:
Paw-licking and mechanical allodynia were measured in vivo and protein kinase activation ex vivo by Western blots of extracts of paw skin.
Key results:
Intraplantar (i.pl.) injection of PGE2 into the mouse paw caused nociceptive behaviour of short duration with mean ED50 of 1.43 nmol. PGE2 produced a longer-lasting mechanical allodynia, with an ED50 of 0.05 nmol. Intraplantar injection of antagonists at EP3 or EP4, but not at EP1 or EP2 receptors inhibited PGE2-induced paw-licking. Paw-licking caused by PGE2 was blocked by an inhibitor of PKA but only partially decreased by inhibition of the extracellular-regulated kinase (ERK). Selective inhibitors of PKC, c-Jun N-terminal kinase (JNK) or p38, all failed to affect PGE2-induced paw-licking. An EP3 antagonist inhibited PGE2-induced mechanical allodynia. However, inhibitors of PKA, PKC or ERK, but not p38 or JNK, also partially inhibited PGE2-induced mechanical allodynia. Western blot analyses confirmed that i.pl. injection of PGE2 activated PKA, PKCα, and mitogen activated kinases (MAPKs) in the paw. Co-treatment with EP3 or EP4 receptor antagonists reduced PGE2-induced PKA and ERK, but not PKCα activation.
Conclusions and Implications:
The present results indicate that the nociceptive behaviour and mechanical allodynia caused by i.pl. PGE2 are mediated through activation of distinct EP receptors and PK-dependent mechanisms.
Keywords: prostaglandin E2, prostanoid E receptors, protein kinases, nociception, mitogen-activated protein kinases, mice
Introduction
Symptomatically, pain may be manifested spontaneously (stimulus-independent pain) or following challenge with noxious (hyperalgesia) or innocuous (allodynia) stimulation after damage to, or alterations in, sensory neurons (stimulus-evoked pain) (Woolf and Mannion, 1999). During an inflammatory event, pain generation is a consequence of a complex interaction between a number of inflammatory mediators, including prostaglandins, some of which (notably prostaglandin E2 (PGE2)) are known to exert a critical role in the generation and maintenance of the nociceptive response (see Samad et al. (2002) for review).
A great number of in vivo studies have shown that peripherally injected PGE2 produces hyperalgesia and allodynia both in experimental animals and in humans (Ferreira, 1972; Kuhn and Willis, 1973). This nociceptive effect seems to be related to the ability of PGE2 to sensitize peripheral terminals of small diameter, high threshold, primary afferent fibers to thermal, chemical and mechanical stimuli (Schaible and Schimdt, 1988; Kumazawa et al., 1993, 1996; Mizumura et al., 1993). Besides sensitization, a direct activation of nociceptors in vitro by high concentrations of PGE2 has been shown (Mense, 1981, Mizumura et al., 1987; Schaible and Schimdt, 1988), as well as the induction of spontaneous nociceptive behaviours following peripheral injection in vivo (Hong and Abbott, 1994).
The biological actions of PGE2 are attributed to its ability to interact with G-protein-coupled (prostanoid E receptor) EP receptors that have been classified into four subtypes (EP1–4) (see Kobayashi and Narumiya, 2002; Hata and Breyer, 2004 for review). EP receptors can be expressed in various tissues, including sensory neurons (Southall and Vasko, 2001). It has been suggested that EP2, EP3 and EP4 receptors could mediate the sensitizing effect of PGE2 in nociceptors and dorsal root ganglion (DRG) neurons (Kumazawa et al., 1993, 1996; Southall and Vasko, 2001). However, the receptor subtype(s) responsible for the stimulus-evoked and nociceptive behaviour in vivo produced by peripherally injected PGE2 still remains unknown.
The stimulation of EP receptors can result in activation of complex signal transduction pathways, depending on the receptor subtype stimulated and the cells being studied. Some studies have demonstrated that the mechanical hyperalgesia caused by peripheral PGE2 injection in rats is mediated by cAMP-protein kinase A (PKA) pathways (Ferreira and Nakamura, 1979; Taiwo and Levine, 1991; Aley and Levine, 1999). In contrast, thermal hyperalgesia produced by peripheral injection of PGE2 is only marginally reduced in mice with a targeted mutation of the type I regulatory subunit of PKA, suggesting that other intracellular pathways could also be involved in PGE2-induced nociceptive effects (Malmberg et al., 1997). In fact, the activation of EP receptors by PGE2 can stimulate other protein kinases (PK), including protein kinase C (PKC) and mitogen-activated protein kinases (MAPKs) – both of which exert a critical role in nociceptor excitation and sensitization (Burkey and Regan, 1995; Khasar et al., 1999; Aley et al., 2001; Fiebich et al., 2001).
In the present study we sought to investigate, by the use of pharmacological and Western blot procedures, the receptor subtype and also the signaling pathways involved in mechanical allodynia and nociceptive behaviour produced in vivo by intraplantar (i.pl.) injection of PGE2 in the mouse.
Methods
Animals
The experiments were conducted using male Swiss mice (25–35 g) kept in a 12 h light–dark cycle, with controlled humidity (60–80%) and temperature (21±1°C). Food and water were freely available. The animals were acclimatized to the laboratory for at least 2 h before testing and were used only once throughout the experiments. The studies reported in this manuscript were carried out in accordance with current guidelines for the care of laboratory animals and ethical guidelines for the investigation of experimental pain in conscious animals, according to Zimmermann (1983) and approved by the local University Committee (process number 262/CEUA). The number of animals and the intensity of noxious stimuli used here were the minimum necessary to demonstrate consistent effects of the drug treatments.
PGE2-induced paw licking
The procedure used was similar to that described previously (Ferreira et al., 2004). Twenty microliters of solutions of PGE2 (0.3–10 nmol per paw), 17-phenyl-trinor-PGE2 (3 nmol per paw) or a suspension of carrageenan (300 μg per paw) was injected subcutaneously under the plantar surface of the right hindpaw (i.pl. injection). The PGE2 analogue 17-phenyl-trinor-PGE2 is an agonist of mouse EP1 and EP3 receptors (Kiriyama et al., 1997). Separate groups of animals received an i.pl. injection of the appropriate vehicle (phosphate-buffered saline (PBS) or PBS plus ethanol 0.5%). The animals were placed individually in chambers (transparent glass cylinders, 20 cm in diameter) and were acclimatized for at least 20 min before algogen or vehicle injection. After challenge, the mice were observed individually for 15 min. The amount of time spent licking the injected paw was measured with a chronometer and was considered as indicative of nociceptive behaviour.
PGE2-induced mechanical allodynia
The mechanical threshold was measured using the up–down paradigm as described previously (Chaplan et al., 1994). Mice were first acclimatized (1–2 h) in individual clear Plexiglass boxes (9 × 7 × 11 cm) on an elevated wire mesh platform to allow the access to the plantar surface of the hind paws. Von Frey filaments of increasing stiffness (0.008–6.0 g) were applied to the same portion of the hind paw before and several times after i.pl. PGE2 (0.01–0.3 nmol per paw), 17-phenyl-trinor-PGE2 (0.1 nmol per paw) or carrageenan (300 μg per paw) injection. Mechanical allodynia was considered as a decrease in the threshold when compared with the same paws before injection with agonist. Each round of testing consisted of sets of six stimulations with different increasing filaments. When a filament induced paw withdrawals, a period of 1 min was allowed between stimulations. The mechanical threshold was expressed in milligrams (mg).
Effect of drug treatments
To assess the involvement of different EP receptor subtypes in the algogen responses induced by PGE2, the animals received an i.pl. injection of an EP1/2 receptor antagonist, AH-6809, (10–100 nmol per paw; Kiriyama et al., 1997), a selective EP3 receptor antagonist, L-826266 (0.1–30 nmol per paw; Schlemper et al., 2005) or a selective EP4 receptor antagonist, L-161982 (1–30 nmol per paw; Machwate et al., 2001) in association with PGE2, 17-phenyl-trinor-PGE2 or carrageenan. To assess the effect of post-treatment with antagonists on PGE2-induced nociceptive behaviour, another group of animals was injected with EP receptor antagonists 5 min (for nociceptive behaviour) or 15 min (for mechanical allodynia) after PGE2 injection.
To investigate some of the signalling pathways involved in PGE2-induced nociceptive response, we evaluated the effect of co-administration of selective kinase inhibitors – KT-5720 for PKA (0.1–3 nmol per paw), GF109203X for PKC (10 nmol per paw), SP600125 for c-Jun N-terminal kinase (JNK) (10 or 30 nmol per paw), PD98059 for mitogen-activated protein kinase kinase (MEK) (10 or 30 nmol per paw), or SB203580 for p38 (10 or 30 nmol per paw) – in association with PGE2. Another group of animals were injected with kinase inhibitors 5 min (for nociceptive behaviour) or 15 min (for mechanical allodynia) after PGE2 injection. The choice of doses for each drug was based on pilot experiments (not shown) or on data reported in the literature (Inoue et al., 2003; Ferreira et al., 2004, 2005; Claudino et al., 2006).
Western blot analysis
To confirm the possible activation of PKC, PKA or MAPKs after PGE2 injection into the mouse paw, western blot analysis was carried out as described previously (André et al., 2004) with minor modifications. The right paws of mice were taken from animals killed at different periods of time (1–60 min) after PGE2 treatment (3 nmol per paw). Paws were also collected from animals co-treated with L-826266 (10 nmol per paw) or L-161982 (10 nmol per paw) in association with PGE2 (3 nmol per paw). The skin and connective tissues of the plantar aspect of the hind paws were removed and homogenized in an ice-cold buffer containing protease and phosphatase inhibitors (100 mM Tris-HCl, pH 7.4; 2 mM EDTA; 2 μg/ml aprotinin, 0.1 mM phenylmethanesulphonyl fluoride, 200 mM NaF and 2 mM of sodium orthovanadate). The homogenate was first centrifuged at 1000 g for 10 min at 4°C; the pellet was discarded and the supernatant was further centrifuged at 35 000 g for 30 min at 4°C. The supernatant was collected as a cytoplasm-rich fraction. The resulting pellet was re-suspended and considered as a membrane-rich fraction. The protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA, USA). Equivalent amounts of proteins (10 μg for membrane and 40–70 μg for cytoplasm-rich fractions) were mixed in buffer (Tris 200 mM, glycerol 10%, sodium dodecyl sulphate 2%, β-mercaptoethanol 2.75 mM and bromophenol blue 0.04%) and boiled for 5 min. Proteins were resolved in 10% sodium dodecyl sulphate–polyacrylamide gel by electrophoresis and transferred on to polyvinylidene difluoride membranes. The membranes were blocked by incubation overnight with 10% non-fat dry milk solution and then incubated with anti-PKCα, anti-phosphorylated forms of protein kinase A regulatory subunit II (PKA RII) (phospho-PKA RII), extracellular-regulated kinase (ERK) (phospho-ERK1), JNK (phospho-JNK) or p38 (phospho-p38) antibodies. Following washing, the membranes were incubated with adjusted peroxidase-coupled secondary antibodies. The immunocomplexes were visualized using the enhanced chemiluminescence detection system (Amersham Biosciences, UK). Membranes were then incubated for 10 min in stripping buffer at room temperature and reincubated with anti-actin, which served as a loading control.
Statistical analysis
The results are presented as mean±s.e.m., except for the ED50 values (i.e. the dose of PGE2 necessary to produce 50% of the nociceptive response relative to the maximum effect), which are reported as geometric means accompanied by their respective 95% confidence limits. To obtain data that were derived purely by the treatments in algogen-induced nociception, the inhibition values were represented as the difference between the licking times of the vehicle-treated and algogen-treated animals. The statistical significance between groups was assessed by means of one-way analysis of variance (ANOVA) followed by Dunnett's or Student–Newman–Keuls' test, as appropriate. P-values <0.05 were considered as indicative of significance.
Drugs and reagents
AH-6809 and 17-phenyl-trinor-PGE2, were purchased from Cayman Chemicals (Ann Arbor, MI, USA); PGE2 was obtained from Sigma-Aldrich (St Louis, MO, USA); PD98059, GF 109203x, SB 203580, SP600125 and KT-5720 were purchased from Calbiochem (Cambridge, MA, USA); polyclonal antibodies anti-PKC-α, anti-phospho-JNK, anti-phospho-p38, anti-phospho-ERK1, anti-actin and anti-rabbit IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-phospho-PKA RII was purchased from Upstate Biotechnology (Lake Placid, NY, USA) and L-826266 and L-161982 were kindly supplied by Merck Frost (Kirkland, Québec, Canada).
Stock solutions of drugs (0.1–1 M) were prepared in absolute ethanol, except for AH-6809, which was prepared in 1% of NaHCO3 plus 0.9% NaCl and L-161982 that was directly dissolved in PBS. All drugs were stocked in siliconized plastic tubes and maintained at −18°C until use. The final concentration of ethanol did not exceed 0.5%, which alone had no effect on the nociceptive behaviour and on mechanical allodynia, respectively. KT-5720 was protected from light to avoid its photodegradation.
Results
Nociceptive behaviour and mechanical allodynia produced by i.pl. injection of PGE2
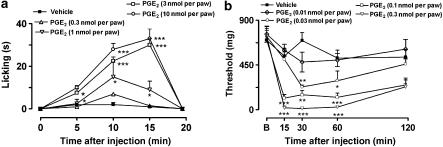
The i.pl. injection of PGE2 (0.3–10 nmol per paw) into the hindpaw of the mice produced dose-related paw licking when compared with vehicle-injected animals (Figure 1a). Paw licking induced by PGE2 had a short duration, lasting up to 15 min after its injection (Figure 1a). The calculated mean ED50 value (and the 95% confidence limits) for this effect was 1.43 (1.20–1.71) nmol per paw. The i.pl. injection of low doses of PGE2 (0.01–0.3 nmol per paw) produced dose–dependent mechanical allodynia (stimulus-evoked nociception reaction), assessed by the decrease in the mechanical withdrawal thresholds (Figure 1b). In contrast with PGE2-induced paw licking, PGE2-induced mechanical allodynia had a long duration, peaking within 15 min and lasting for up to 60 min after injection of PGE2 (Figure 1b). The calculated mean ED50 value (and 95% confidence limits) for mechanical allodynia was 0.05 (0.03–0.09) nmol per paw. Thus, at the ED50 level, PGE2 was about 28-fold more potent in inducing mechanical allodynia in comparison to PGE2-induced paw licking. Based on these initial experiments, we chose doses of 3 and 0.1 nmol per paw of PGE2 for subsequent experiments to evaluate mechanisms involved in paw licking and mechanical allodynia, respectively.
Figure 1.
Dose and time dependence for PGE2-induced paw licking (0.3–10 nmol per paw) (a) or mechanical allodynia (0.01–0.3 nmol per paw) (b) in mice. The paw licking is expressed as licking time (s) and the mechanical allodynia is expressed as threshold (mg). Each point on the curve represents the mean of 6–10 animals and vertical lines show the s.e.m. Asterisks denote the significance levels in comparison with the vehicle-treated group (one-way ANOVA followed by Dunnett's test). *P<0.05, **P<0.01 and ***P<0.001.
Effects of EP receptor antagonists on PGE2-, 17-phenyl-trinor-PGE2- or carrageenan-induced nociceptive responses
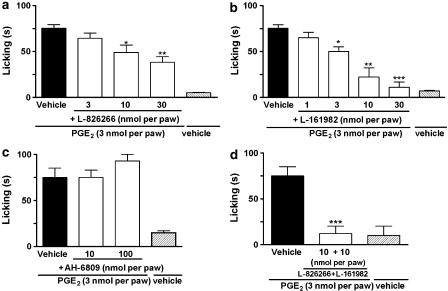
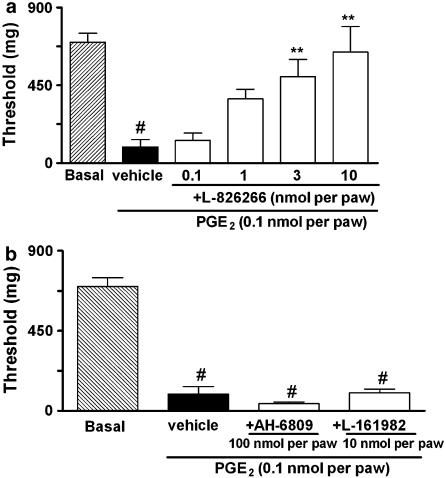
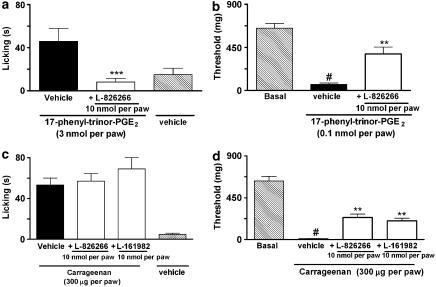
PGE2-induced paw licking was dose-dependently inhibited by the co-injection of the EP3 receptor antagonist, L-826266, (3–30 nmol per paw) or the EP4 receptor antagonist, L-161982, (1–30 nmol per paw) (Figure 2a and b, respectively). In contrast, the co-injection of the EP1/2 receptor antagonist, AH-6809 (10–100 nmol per paw) did not alter the paw licking induced by PGE2 (Figure 2c). The co-administration of EP3 receptor antagonist L-826266 (10 nmol per paw) plus the EP4 receptor antagonist L-161982 (10 nmol per paw) with PGE2 injection (3 nmol per paw) significantly inhibited PGE2-induced paw licking (Figure 2d). On the other hand, L-826266 (an EP3 receptor antagonist – 0.1–10 nmol per paw) inhibited PGE2-induced mechanical allodynia (Figure 3a). Local injection of L-161982 (10 nmol per paw) or AH-6809 (100 nmol per paw) was unable to alter the PGE2-induced mechanical allodynia in mice (Figure 3b). To gain further evidence about the role of the EP3 receptor in PGE2-induced mechanical allodynia and paw licking, we carried out some additional experiments using 17-phenyl-trinor-PGE2, a selective agonist at mouse EP1 and EP3 receptors (Figure 4a and b). Similar to PGE2, 17-phenyl-trinor-PGE2 (3 nmol per paw), produced significant paw licking and mechanical allodynia (0.1 nmol per paw) (Figure 4a and b). Co-injection of the EP3 receptor antagonist, L-826266 (10 nmol per paw), significantly inhibited both the paw licking and mechanical allodynia induced by 17-phenyl-trinor-PGE2. The inhibitions observed were 100 and 54±7%, respectively (Figure 4a and b). Thus, 17-phenyl-trinor-PGE2-induced paw licking and mechanical allodynia seem to be mediated, at least partly, through an activation of the EP3 receptor.
Figure 2.
Effect of i.pl. treatment with selective receptor antagonists for EP3 (L-826266, 3–30 nmol per paw (a)) EP4 (L-161982, 1–30 nmol per paw (b)) EP1/2 receptors (AH-6809, 10 and 100 nmol per paw (c)) or a combination of EP3 and EP4 receptor antagonists (L-826266 plus L-161982, each 10 nmol per paw) (d) on PGE2-induced paw licking in mice (PGE2, 3 nmol per paw). The drugs were co-injected with PGE2 and the effects of the drugs are expressed as licking time (s). Each column represents the mean of 7–8 animals and vertical lines show the s.e.m. Asterisks denote significant difference levels, *P<0.05, **P<0.01 and ***P<0.001, compared with the PGE2 plus vehicle-injected mice (black bar, one-way ANOVA followed by Dunnett's (a and b) or Student–Newman–Keuls' (c and d) test).
Figure 3.
Effect of i.pl. treatment with selective receptor antagonists for EP3 (L-826266, 0.1–10 nmol per paw (a)) EP1/2 (AH-6809, 100 nmol per paw) or EP4 receptors (L-161982, 10 nmol per paw (b)) on PGE2-induced mechanical allodynia (0.1 nmol per paw) in mice. The drugs were co-injected with PGE2 and the effects of the drugs are expressed as threshold (mg). Each column represents the mean of 6–12 animals and vertical lines show the s.e.m. #Denotes significant difference from basal group (P<0.01) (Student-Newman-Keuls' test). Asterisks denote the significance levels, **P<0.01, compared with the PGE2 plus vehicle-injected mice (black bar, one-way ANOVA followed by Dunnett's (a) or Student–Newman–Keuls' (b) test).
Figure 4.
Effect of i.pl. treatment with the selective EP3 receptor antagonist (L-826266, 10 nmol per paw) on 17-phenyl-trinor-PGE2-induced paw licking (3 nmol per paw (a)) or mechanical allodynia (0.1 nmol per paw (b)). In c and d, the effects of i.pl. treatment with the EP3 receptor antagonist (L-826266, 10 nmol per paw) or the EP4 receptor antagonist (L-161982, 10 nmol per paw) on paw-licking or mechanical allodynia induced by carrageenan (300 μg per paw) are shown. The effects of the drugs are expressed as licking time (s) or threshold (mg). Each column represents the mean of six animals and vertical lines show the s.e.m. #denotes significant difference from basal group (P<0.01) (Student–Newman–Keuls' test). Asterisks denote the significance levels, **P<0.01 and ***P<0.001, compared with the PGE2 plus vehicle-injected mice (black bar, one-way ANOVA followed by Student–Newman–Keuls' test).
To demonstrate that the EP antagonists are also effective against nociceptive responses induced by stimulating endogenous prostanoid synthesis, we tested the effect of EP3 and EP4 receptor antagonists in carrageenan-induced mechanical allodynia (Figure 4d). The i.pl. injection of carrageenan (300 μg per paw) caused a significant mechanical allodynia (thresholds of 630±50 and 11±2 mg before and 3 h after injection, respectively) (Figure 4d). The co-administration of the EP3 receptor antagonist (L-826266, 10 nmol per paw) or the EP4 receptor antagonist (L-161982, 10 nmol per paw) significantly inhibited the mechanical allodynia induced by carrageenan. The inhibitions observed were 34±6 and 38±6%, respectively (Figure 4d). Moreover, the i.pl. injection of carrageenan (300 μg per paw) also induced paw licking that was not significantly altered by the co-administration of the EP3 receptor antagonist (L-826266, 10 nmol per paw) or the EP4 receptor antagonist (L-161982, 10 nmol per paw) (Figure 4c).
Involvement of PKC, PKA and MAPKs in PGE2-induced nociception
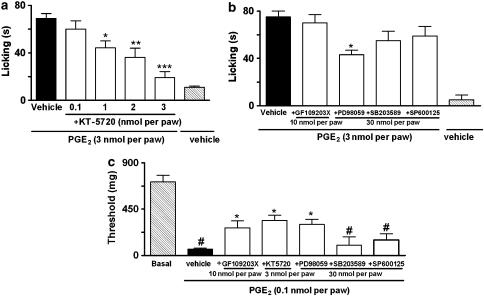
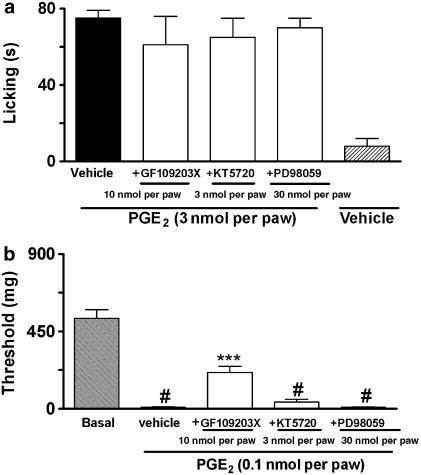
PGE2-induced paw licking and mechanical allodynia was significantly reduced by co-injection of the PKA inhibitor KT-5720 (0.1–3 nmol per paw) (Figure 5a and c). The inhibitions observed were 87±5 and 42±6% for paw licking and mechanical allodynia, respectively. However the post-treatment with KT-5720 (3 nmol per paw) did not alter PGE2-induced mechanical allodynia or paw licking (Figure 6a and b).
Figure 5.
Effect of i.pl. treatment with an inhibitor of PKA (KT-5720, 0.1–3 nmol per paw) (a), inhibitors of PKC (GF109203X, 10 nmol per paw), ERK (PD98059, 30 nmol per paw), p38 MAPK (SB203580, 30 nmol per paw) or JNK (SP600125, 30 nmol per paw) on PGE2-induced paw licking (3 nmol per paw) (b), inhibitors of PKC (GF109203X, 10 nmol per paw), PKA (KT-5720, 3 nmol per paw), ERK (PD98059, 30 nmol per paw), p38 MAPK (SB203580, 30 nmol per paw) or JNK (SP 600125, 30 nmol per paw) on mechanical allodynia induced by PGE2 (0.1 nmol per paw) (c). The effects of the drugs are expressed as licking time (s) or threshold (mg). Each column represents the mean of six animals and vertical lines show the s.e.m. #denotes significant difference from basal group, P<0.01 (Student–Newman–Keuls' test). Asterisks denote the significance levels, *P<0.05, **P<0.01 and ***P<0.001, compared with the PGE2 plus vehicle-injected mice (black bar, one-way ANOVA followed by Dunnett's (a) or Student–Newman–Keuls' (b and c) test).
Figure 6.
Effect of post-treatment with GF109203X (10 nmol per paw), KT5720 (3 nmol per paw) or PD98059 (30 nmol per paw) on PGE2-induced paw-licking (a) or mechanical allodynia (b) in mice. The effects of the drugs are expressed as licking time (s) or threshold (mg). Each column represents the mean of six animals and vertical lines show the s.e.m. #denotes significant difference from basal group, P<0.01 (Student–Newman–Keuls' test). Asterisks denote the significance levels, ***P<0.001, compared with the PGE2 plus vehicle-injected mice (black bar, one-way ANOVA followed by Student–Newman–Keuls' test).
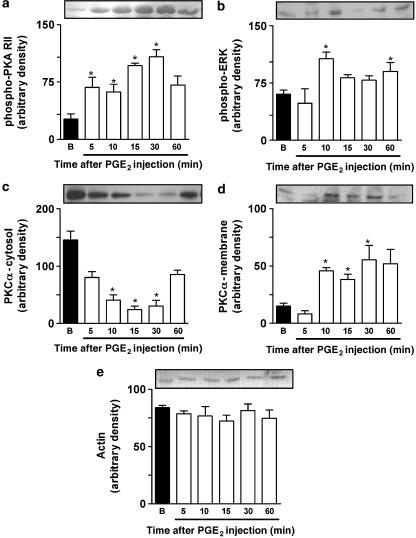
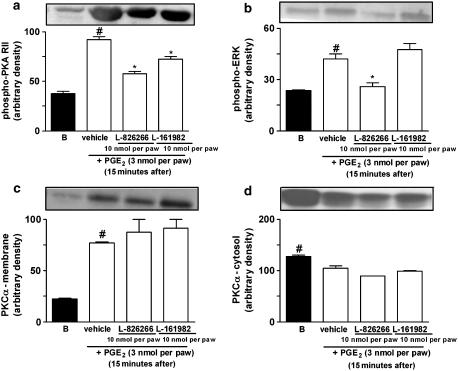
To further confirm the role played by PKA in PGE2-induced paw licking, we carried out western blot analysis on samples of the PGE2-injected paws (3 nmol per paw). It has been reported previously that autophosphorylation of the PKA regulatory subunit occurs when PKA is activated by cAMP (Tasken and Aandahl, 2004). Thus, the detection of phosphorylation of the PKA regulatory subunit is useful evidence for PKA activation. Our results showed that phosphorylation of the PKA regulatory subunit II (PKA RII) stimulated by PGE2 occurred within 5 min after PGE2 injection, an effect that peaked 15 min after PGE2 administration and lasted for up to 30 min (Figure 7a). Of note, the observed PKA activation had the same time course as the induction of paw licking following i.pl. injection of PGE2 (Figures 5a and 7a). The co-administration of the receptor antagonists for the EP3 receptor, L-826266 (10 nmol per paw), or the EP4 receptor, L-161982, (10 nmol per paw) significantly reduced PKA activation when assessed 15 min after PGE2 injection (3 nmol per paw) (Figure 8a).
Figure 7.
Representative images of western immunoblotting and densitometry analyses showing the time course of phosphorylation of PKA RII (phospho-PKA RII; (a)) ERK (phospho-ERK1, (b)) PKCα translocation from cytosol (c) to membrane (d) in response to i.pl. injection of PGE2 (3 nmol per paw) into mouse paw. Densities for actin are shown in (e). Mouse paw tissues were obtained from naive (basal, B) or PGE2-injected mice at the indicated times. Membrane levels of PKC-α and cytosolic levels of phospho-PKA RII, PKCα, phospho-ERK1 and actin were determined using specific antibody and indicated PK activation. Results were normalized by arbitrarily setting the densitometry of the basal group and are expressed as mean±s.e.m. (n=3). *P<0.05, as compared with basal values (one-way ANOVA followed by Dunnett's test).
Figure 8.
Representative images of western immunoblotting and densitometry analyses showing the effect of EP3 or EP4 receptor antagonists in PGE2-induced phosphorylation of PKA RII (phospho-PKA RII, (a)) of ERK1 (phospho-ERK1, (b)) or PKCα activation (PKCα membrane (c)) and PKCα cytosol (d)). Mouse paw tissues were obtained from naive (basal, B) or 15 min after PGE2 injection. Cytosolic levels of phospho-PKA RII were determined using a specific antibody. Results were normalized by arbitrarily setting the densitometry of the basal group and are expressed as mean±s.e.m. (n=3). #P<0.05, as compared with basal values (B), *P<0.05, as compared with vehicle group (one-way ANOVA followed by Student–Newman–Keuls' test).
We next investigated the possible role played by PKC in PGE2-induced nociceptive responses. The co-administration of the selective PKC inhibitor GF109203X (10 nmol per paw) significantly inhibited (32±5%) PGE2-induced mechanical allodynia, without altering PGE2-induced paw licking (Figure 5b and c). Post-treatment with GF 109203X (10 nmol per paw) also inhibited (32±4%) PGE2-induced mechanical allodynia, but not PGE2-induced paw licking (Figure 6a and b). Further, we assessed the time-course response for PKCα isoform activation following i.pl. injection of PGE2 (3 nmol per paw) as assessed by their translocation from cytosol to membrane fractions collected from skin samples of injected paws. We found that the i.pl. injection of PGE2 stimulated the translocation of PKCα isoform, an effect that peaked 15 min after injection and lasted for until 30 min after PGE2 injection (Figure 7c and d). Additionally, co-administration with EP3 (L-826266, 10 nmol per paw) or the EP4 (L-161982, 10 nmol per paw) receptor antagonists did not significantly reduce PKCα activation when assessed 15 min after PGE2 injection (3 nmol per paw) (Figure 8c and d).
Finally, we evaluated through the use of selective inhibitors and western blot analysis whether or not the different MAPKs are involved in the nociceptive responses produced by i.pl. injection of PGE2. The MEK (a kinase upstream to ERK) inhibitor PD98059 (30 nmol per paw) partially, but significantly reduced both the paw licking and the mechanical allodynia produced by i.pl. injection of PGE2. The observed inhibitions were 41±5 and 36±7%, respectively (Figure 5b and c). Post-treatment with PD98059 (30 nmol per paw) did not significantly inhibit PGE2-induced mechanical allodynia or paw licking (Figure 6a and b). Western blot analysis detected ERK activation at 10 and 60 min in PGE2-injected tissues (Figure 7b). Co-administration with EP3 (L-826266, 10 nmol per paw), but not EP4 (L-161982, 10 nmol per paw), receptor antagonists significantly reduced the ERK activation caused by PGE2 injection (3 nmol per paw) (Figure 8b). The co-injection of SP600125 (30 nmol per paw), a selective inhibitor of JNK, did not significantly affect PGE2-induced nociceptive responses (Figure 5b and c). The inhibitor of p38 MAK, SB203580 (30 nmol per paw) also failed to significantly affect both paw licking and mechanical allodynia induced by i.pl. injection of PGE2 (Figure 5b and c).
Discussion and conclusions
There is now a considerable amount of experimental evidence demonstrating the ability of PGE2 to induce stimulus-evoked nociception. Furthermore, it has been shown that the peripheral injection of PGE2 into humans or experimental animals induces both allodynia and hyperalgesia in response to mechanical stimulation (Ferreira, 1972; Taiwo and Levine, 1991). There is also evidence that high concentrations of PGE2 can directly excite peripheral terminals and also cell bodies of nociceptors in vitro (Mense, 1981; Mizumura et al., 1987; Schaible and Schimdt, 1988; Noda et al., 1997). For instance, it was reported that testicular nociceptors were excited by the addition of PGE2 at 1 μM, a concentration about 100 times higher than those necessary to sensitize these nociceptors to heat or to bradykinin (Mizumura et al., 1987, 1993). Confirming the previous in vitro experiments, our results show that i.pl. injection of PGE2 to mice caused dose-related paw licking. However, when compared with PGE2-induced mechanical allodynia, this prostanoid was about 28-fold less potent in causing paw licking. In agreement with our data, Hong and Abbott (1994) have shown that i.pl. injection of high doses of PGE2 into the rat paw produces paw licking.
To date, four distinct EP receptors have been cloned in different animal species and all of them are G-protein-coupled receptors (see Kobayashi and Narumiya, 2002 for review). EP2 and EP4 receptors stimulate adenylate cyclase via stimulatory G-protein (Gs) activation, whereas EP1 receptor activation normally stimulates phosphoinositide metabolism and mobilizes intracellular calcium through phospholipase C-linked G-protein protein stimulation (see Kobayashi and Narumiya, 2002 for review). On the other hand, EP3 receptor has several splice variants and its signalling pathways seem to involve a complex interaction with inhibitory G-protein, Gs and G13 activation (see Hatae et al., 2002; Hata and Breyer, 2004 for review). Furthermore, the restricted number of available selective agonists/antagonists for EP receptors has greatly limited the pharmacological strategies to elucidate the EP receptor subtypes involved in the nociceptive responses caused by PGE2 at a peripheral level.
The nociceptive effect of PGE2 could be mediated by a direct action on sensory neurons as the mRNA of all EP receptors was found in rat DRG (Southall and Vasko, 2001). Moreover, EP3 and EP4 receptors have been found both in DRG and in the peripheral nerve terminals of rats, whereas the EP4 receptors are colocalized with calcitonin gene-related peptide (Kopp et al., 2004). Studies carried out on mouse DRG have mRNA for the EP3 receptor, and to a lesser extent, for EP1 and EP4 receptors in small-sized neurons (Sugimoto et al., 1994; Oida et al., 1995).
L-826266 and L-161982 are highly potent (Ki values in nanomolar range) and selective receptor antagonists (affinity about 100–200 times greater on EP3 or EP4 receptors when compared with other prostanoid receptors) (Machwate et al., 2001; Michel Gallant, unpublished results). Through the use of these selective EP receptor antagonists, our present results support the participation of both EP3 and EP4, but not EP1 and EP2 receptors, in PGE2-induced paw licking. These results were not unexpected as PGE2, at least in mice, had been found to bind with higher affinity to both EP3 and EP4 receptors, when compared with its action at EP1 and EP2 receptors (Kiriyama et al., 1997). Notably, Southall and Vasko (2001) have shown that the combination of antisense RNAs directed to EP4 plus EP3 receptors, but not the individual antisense RNAs, abolished PGE2-induced cAMP production and neuropeptide release in cultured rat DRG. In addition, the EP3 receptor is also able to augment Gs-coupled EP4 receptor-stimulated adenylate cyclase activity (Hatae et al., 2002). Our data extend these previous results by demonstrating that PGE2-induced paw licking is consistently reduced by PKA inhibition. The role of PKA in the nociception caused by the EP1/3 receptor agonist 17-phenyl-trinor-PGE2 must be elucidated in further studies.
Another new finding of the present study is the demonstration, for the first time, that the activation of PKA, PKC and MAPKs could be detected in PGE2-injected paws. In addition, the antagonism of either EP3 receptors or EP4 receptors reduced PKA and ERK activation in PGE2-injected paws. Notably, the time course of activation of PKA and ERK following i.pl injection of PGE2 agreed well with its temporal ability to induce paw licking and mechanical allodynia. Burkey and Regan (1995) have reported that PGE2 is effective in activating ERK in COS-7 cells heterologously expressing EP3 receptors. Besides, the stimulation of EP3 receptors by PGE2 is known to be able to activate other MAPKs (Fiebich et al., 2001; Mendez and Lapointe, 2005). We have found that the peripheral injection of PGE2 causes activation of p38 and JNK. However, the activation of these kinases does not seem to be related to the production of nociceptive behaviours since selective inhibitors of p38 and JNK did not significantly alter paw licking or mechanical allodynia even in doses where they significantly reduced PGE2-induced paw oedema in mice (Claudino et al., 2006). Overall, our results have demonstrated, for the first time, that PKA stimulation largely accounts for PGE2-induced paw licking, whereas ERK has apparently only a minor role in this process.
The current knowledge concerning the EP receptor subtype and the intracellular signalling pathways involved in PGE2-induced sensitization of nociceptors and hyperalgesia are still controversial. Electrophysiological studies have suggested that both EP2 and EP3, but not EP1 receptor subtypes mediate PGE2-induced sensitization or activation of nociceptors (Kumazawa et al., 1993, 1996; Smith et al., 1998). Furthermore, the intradermal injection of PGE2 into rats produces mechanical hyperalgesia, an effect that is greatly reduced by EP1 receptor antagonism and by inhibitors of PKA, but not of PKC or ERK (Khasar et al., 1995, 1999; Aley and Levine, 1999; Cunha et al., 1999; Aley et al., 2001). Experiments using selective EP agonists have suggested the presence of more than one EP receptor subtype mediating PGE2-induced mechanical hyperalgesia in the rat paw (Khasar et al., 1995). The present study has extended these findings to mice by showing that EP3 receptors mediates the mechanical allodynia caused by PGE2, based on the data showing that the EP3 receptor antagonist L-826266 significantly reduced both PGE2 and 17-phenyl-trinor-PGE2-induced mechanical allodynia. However, a role for EP1 receptor in allodynia cannot be ruled out as 17-phenyl-trinor-PGE2-induced mechanical allodynia was not completely prevented by the EP3 receptor antagonist. In contrast with our pharmacological data, it has been reported that mechanical allodynia produced by PGE2 was not altered in EP3 receptor knockout mice (Reinold et al., 2005). The reason for such discrepant findings is presently unclear and requires additional study. However, we cannot exclude a role for EP3 receptor in allodynia, bearing in mind the physiological compensation that is often observed in gene-deleted mice (Mak et al., 2001).
Of note, it has been reported that PGE2 increases the phospholipase C activity as well as the translocation of PKCɛ in cultured DRG (Smith et al., 1998; Vellani et al., 2004). Moreover, evidence also suggests that PKC as well as PKA mediates the increase in sodium currents gated by tetrodotoxin-resistant sodium channels caused by PGE2, an effect that is essential to PGE2-induced mechanical hyperalgesia in rats (Gold et al., 1998; Khasar et al., 1998). To gain further insight into the signalling mechanisms involved in PGE2-induced mechanical allodynia, we have demonstrated by the use of both pharmacological tools and western blot analysis that PKA in conjunction with PKC and ERK largely accounts for the PGE2-induced mechanical allodynia in mice. The absence of effect of EP3 receptor antagonists in PKCα translocation suggests that other PKC subtypes, such as PKCɛ, are probably stimulated by the activation of EP3 receptors and can modulate the nociceptive response.
Finally, we have shown that EP3 or EP4 receptor antagonists were able to significantly reduce mechanical allodynia induced by carrageenan. It is now well-known that the levels of PGE2 are greatly increased in the paw tissue at 3 h but not 15 min after carrageenan injection (Herencia et al., 1998). This temporal profile could explain why EP receptor antagonists were not able to reduce paw licking that was observed at 30 min after carrageenan injection. The effect of EP receptor antagonists in models of pain related with earlier increases in peripheral PGE2 levels must be carried out to elucidate the role of this endogenous prostanoid in paw licking. In contrast to our data obtained with exogenous PGE2 injection, we found that the EP4 receptor antagonist was also capable of reducing mechanical allodynia when PGE2 was endogenously produced. This result indicates that the receptors and signalling pathways stimulated by PGE2 can be modulated by the presence of other inflammatory mediators.
On the basis of published data, both EP receptor antagonists and PK inhibitors, in the doses used here, seem to produce selective effects on their targets. It has been previously shown that AH-6809, L-826266 and L-161982 were about 235, 25 and 40 times, respectively, less potent than PGE2 in binding to EP receptors in cells expressing recombinant receptors (Abramovitz et al., 2000; Machwate et al., 2001; Michel Gallant, unpublished results). On the basis of dose ratios, the doses of EP receptor antagonists we used were maximally 1000 times (for AH-6809) or 100 times (for L-826266 and L-161982) higher than the PGE2 doses. In our opinion, the antinociceptive action of EP receptor antagonists would not reflect non-specific actions of these compounds as they were not capable of significantly altering the carrageenan-induced paw licking. On the other hand, the doses of AH6809 used in the present study seem to be pharmacologically effective as AH-6809, at 100 nmol per paw, can influence PGE2-induced paw oedema in mice (Claudino et al., 2006). The choice of dosage for kinase inhibitors was mainly based on the data reported in the literature, which previously have shown that these compounds produce pronounced antinociceptive actions not dependent on non-specific effects, when intraplantarly injected (Inoue et al., 2003; Ferreira et al., 2004, 2005; Claudino et al., 2006). Accordingly, KT-5720 was found effective to reduce PGE2-induced nociception in doses where it also decreased nocistatin-induced nociceptive responses, but did not alter PGE2-induced oedema (Inoue et al., 2003; Claudino et al., 2006). Similarly, PD-98059 inhibited PGE2- and phorbol ester-induced nociception in mice, in a dose where it failed to interfere with heat-induced paw withdrawal in rats (Zhuang et al., 2004; Ferreira et al., 2005). On the other hand, p38 and JNK inhibitors did not alter PGE2-induced allodynia and paw licking in doses where they markedly inhibit phorbol ester-induced nociceptive behaviour and PGE2-induced oedema (Ferreira et al., 2005; Claudino et al., 2006). Thus, based in the above evidence, we believe that the effects observed with the drugs we have used certainly reflect their actions on the selected targets.
In summary, our present results show that the i.pl injection of PGE2 into mice not only sensitizes nociceptors to produce mechanical allodynia, but also induces robust short-lasting paw licking. Although PGE2-induced paw licking is probably mediated through activation of EP3 and EP4 receptors, with the involvement of both PKA and ERK signalling pathways, PGE2-induced mechanical allodynia is most likely mediated through EP3 receptor, an action that is associated with activation of the PKA, PKC and ERK signalling mechanisms.
Acknowledgments
We acknowledge the financial support from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), PRONEX (Programa de Apoio aos Núcleos de Excelência), FAPESC (Fundação de Apoio a Ciência e Tecnologia do Estado de Santa Catarina) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil. Authors acknowledge the Merck Froost (Kirkland, Québec, Canada) that kindly supplied L-828266 and L-1616982. CALK is a PhD student in Pharmacology holding a grant from CNPq. JF was a holder of post-doctoral fellowship from CNPq and RFC is a MSc student in Pharmacology holding a grant from CAPES.
Abbreviations
- DRG
dorsal root ganglion
- EP receptor
prostanoid E receptor
- ERK
extracellular-regulated kinase
- Gs
stimulatory G-protein
- i.pl.
intraplantar
- JNK
c-Jun N-terminal kinase
- MAPKs
mitogen-activated protein kinases
- MEK
mitogen-activated protein kinase kinase
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- PK
protein kinase
- PKA
protein kinase A
- PKA RII
protein kinase A regulatory subunit II
- PKC
protein kinase C
Conflict of interest
The authors state no conflict of interest.
References
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E, Ferreira J, Malheiros A, Yunes RA, Calixto JB. Evidence for the involvement of vanilloid receptor in the antinociception produced by the dialdeydes unsaturated sesquiterpenes polygodial and drimanial in rats. Neuropharmacology. 2004;46:590–597. doi: 10.1016/j.neuropharm.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Burkey TH, Regan JW. Activation of mitogen-activated protein kinase by the human prostaglandin EP3A receptor. Biochem Biophys Res Commun. 1995;211:152–158. doi: 10.1006/bbrc.1995.1790. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Claudino RF, Kassuya CAL, Ferreira J, Calixto JB. Pharmacological and molecular characterization of the mechanisms involved in the prostaglandin E2 (PGE2)-induced edematogenic responses in mice. J Pharmacol Exp Ther. 2006;318:611–618. doi: 10.1124/jpet.106.102806. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Teixeira MM, Ferreira SH. Pharmacological modulation of secondary mediator systems-cyclic AMP and cyclic GMP – on inflammatory hyperalgesia. Br J Pharmacol. 1999;127:671–678. doi: 10.1038/sj.bjp.0702601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Triches KM, Medeiros R, Calixto JB. Mechanisms involved in the nociception produced by peripheral protein kinase Cactivation in mice. Pain. 2005;117:171–181. doi: 10.1016/j.pain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. Prostaglandin, aspirin-like drugs and analgesia. Nat New Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Nakamura M. I – Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979;18:179–190. doi: 10.1016/0090-6980(79)90103-5. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hull M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogenactivated protein kinase and protein kinase C. J Neurochem. 2001;79:950–958. doi: 10.1046/j.1471-4159.2001.00652.x. [DOI] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: advances in the study of EP3 receptor signaling. J Biochem. 2002;131:781–784. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- Herencia F, Ubeda A, Ferrandiz ML, Terencio MC, Alcaraz MJ, Garcia-Carrascosa M, et al. Anti-inflammatory activity in mice of extracts from Mediterranean marine invertebrates. Life Sci. 1998;62:PL115–PL120. doi: 10.1016/s0024-3205(97)01188-0. [DOI] [PubMed] [Google Scholar]
- Hong Y, Abbott FV. Behavioural effects of intraplantar injection of inflammatory mediators in the rat. Neuroscience. 1994;63:827–836. doi: 10.1016/0306-4522(94)90527-4. [DOI] [PubMed] [Google Scholar]
- Inoue M, Kawashima T, Allen RG, Ueda H. Nocistatin and prepro-nociceptin/orphanin FQ 160–187 cause nociception through activation of Gi/o in capsaicin-sensitive and of Gs in capsaicin-insensitive nociceptors, respectively. J Pharmacol Exp Ther. 2003;306:141–146. doi: 10.1124/jpet.103.049361. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Ouseph AK, Chou B, Ho T, Green PG, Levine JD. Is there more than one prostaglandin E receptor subtype mediating hyperalgesia in the rat hindpaw. Neuroscience. 1995;64:1161–1165. doi: 10.1016/0306-4522(94)00423-3. [DOI] [PubMed] [Google Scholar]
- Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid E receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;22:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Narumiya S. Prostanoids in health and disease; lessons from receptor-knockout mice. Adv Exp Med Biol. 2002;507:593–597. doi: 10.1007/978-1-4615-0193-0_90. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Nakamura K, Nusing RM, Smith LA, Hokfelt T. Activation of EP4 receptors contributes to prostaglandin E2-mediated stimulation of renal sensory nerves. Am J Physiol Renal Physiol. 2004;287:1269–1282. doi: 10.1152/ajprenal.00230.2004. [DOI] [PubMed] [Google Scholar]
- Kuhn DC, Willis AL. Prostaglandin E2, inflammation and pain threshold in rat paws. Br J Pharmacol. 1973;49:183–184. [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Koda H. Involvement of EP3 subtype of prostaglandin E receptors in PGE2-induced enhancement of the bradykinin response of nociceptors. Brain Res. 1993;632:321–324. doi: 10.1016/0006-8993(93)91169-s. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Koda H, Fukusako H. EP receptor subtypes implicated in the PGE2-induced sensitization of polymodal receptors in response to bradykinin and heat. J Neurophysiol. 1996;75:2361–2368. doi: 10.1152/jn.1996.75.6.2361. [DOI] [PubMed] [Google Scholar]
- Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, et al. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2) Mol Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- Mak TW, Penninger JM, Ohashi PS. Knockout mice: a paradigm shift in modern immunology. Nat Rev Immunol. 2001;1:11–19. doi: 10.1038/3509551. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Brandon EP, Idzerda RL, Liu H, Mcknight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, Lapointe MC. PGE2-induced hypertrophy of cardiac myocytes involves EP4 receptor-dependent activation of p42/44 MAPK and EGFR transactivation. Am J Physiol Heart Circ Physiol. 2005;288:H2111–H2117. doi: 10.1152/ajpheart.00838.2004. [DOI] [PubMed] [Google Scholar]
- Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E2. Brain Res. 1981;225:95–105. doi: 10.1016/0006-8993(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Mizumura K, Minagawa M, Tsujii Y, Kumazawa T. Prostaglandin E2-induced sensitization of the heat response of canine visceral polymodal receptors in vitro. Neurosci Lett. 1993;161:117–119. doi: 10.1016/0304-3940(93)90154-d. [DOI] [PubMed] [Google Scholar]
- Mizumura K, Sato J, Kumazawa T. Effects of prostaglandins and other putative chemical intermediaries on the activity of canine testicular polymodal receptors studied in vitro. Pflueg Arch. 1987;408:565–572. doi: 10.1007/BF00581157. [DOI] [PubMed] [Google Scholar]
- Noda K, Ueda Y, Suzuki K, Yoda K. Excitatory effects of algesic compounds on neuronal processes in murine dorsal root ganglion cell culture. Brain Res. 1997;751:348–351. doi: 10.1016/s0006-8993(97)00077-2. [DOI] [PubMed] [Google Scholar]
- Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, et al. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanism therapeutics targets. Trends Mol Med. 2002;8:390–396. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schimdt RF. Excitation and sensitization of fine articular afferents from cat's knee joint by prostaglandin E2. J Physiol. 1988;403:91–104. doi: 10.1113/jphysiol.1988.sp017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlemper V, Medeiros R, Ferreira J, Campos MM, Calixto JB. Mechanisms underlying the relaxation response induced by bradykinin in the epithelium-intact guinea-pig trachea in vitro. Br J Pharmacol. 2005;145:740–750. doi: 10.1038/sj.bjp.0706222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br J Pharmacol. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem. 2001;276:16083–16091. doi: 10.1074/jbc.M011408200. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Shigemoto R, Namba T, Negishi M, Mizuno N, Narumiya S, et al. Distribution of the messenger RNA for the prostaglandin E receptor subtype EP3 in the mouse nervous system. Neuroscience. 1994;62:919–928. doi: 10.1016/0306-4522(94)90483-9. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. J Physiol. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanism and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]