Abstract
Background and purpose:
A traditional Japanese herbal medicine, hochu-ekki-to, has been used for the symptomatic treatment of the common cold and to reduce the frequency of colds in patients with chronic obstructive pulmonary disease. However, the inhibitory effects of hochu-ekki-to on infection by rhinovirus (RV), the major cause of common colds, have not been studied.
Experimental approach:
Human tracheal epithelial cells in culture were infected with a major group rhinovirus-RV14. Virus output and viral RNA were measured along with interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α), mRNA for intercellular adhesion molecule (ICAM)-1 and acidic endosomes in cells.
Key results:
RV14 infection increased virus titers, the content of cytokines in supernatants and RV14 RNA in the cells. Hochu-ekki-to decreased virus output, RV14 RNA in the cells, susceptibility to RV infection and supernatant cytokine concentrations after RV14 infection. Hochu-ekki-to reduced mRNA for ICAM-1, the receptor for RV14, the concentration of the soluble form of ICAM-1 and the number and fluorescence intensity of acidic endosomes in the cells, from which RV RNA enters into the cytoplasm, at RV14 infection. Glycyrrhizin, one of the chemical constituents of hochu-ekki-to, reduced supernatant virus titers dose-dependently.
Conclusion and implications:
Hochu-ekki-to inhibited RV14 infection by decreasing ICAM-1 and by blocking entry of viral RNA into the cytoplasm from the endosomes, in airway epithelial cells. Glycyrrhizin may be partly responsible for inhibition of RV infection by hochu-ekki-to. Hochu-ekki-to could modulate airway inflammation by reducing production of cytokines in RV infections.
Keywords: Hochu-ekki-to, common cold, intercellular adhesion molecule, endosome, rhinovirus
Introduction
Rhinoviruses (RVs) are the major cause of the common cold and the most common acute infective illnesses in humans (Couch, 2001). They are also associated with acute exacerbations of bronchial asthma (Nicholson et al., 1993; Johnston et al., 1995) and chronic obstructive pulmonary disease (COPD) (Seemungal et al., 2001; Sethi, 2004). Several mechanisms have been proposed and the manifestations of RV-induced pathogenesis are suggested to be the result of virus-induced mediators of inflammation (Zhu et al., 1996; Sethi, 2004; Johnston, 2005).
Hochu-ekki-to is a traditional Japanese herbal medicine, which originated in China and is composed of 10 species of medicinal plants. It has been used for the treatment of complaints of general fatigue caused by common colds and of severe weakness. Sugiyama and Kitamura (1997) administered hochu-ekki-to to nine patients with COPD for 4 or 5 months, and found that the frequency of common cold in patients treated with hochu-ekki-to for 3 months was significantly lower than that in 17 patients without treatment of hochu-ekki-to. On the basis of these results, they concluded that hochu-ekki-to reduces the frequency of common cold in patients with COPD. Hochu-ekki-to has various immunoactive effects, including increased immunity in elderly persons (Kuroiwa et al., 2004), mitogenic activity of lymphocytes (Iwama et al., 1986) and an augmentation of natural killer activity (Utsuyama et al., 2001). However, the inhibitory effects of hochu-ekki-to on RV infection, the major cause of COPD exacerbations (Seemungal et al., 2001; Sethi, 2004), are still uncertain.
Recent reports revealed that the major group of RVs enters the cytoplasm of infected cells after binding to its receptor, the intercellular adhesion molecule-1 (ICAM-1) (Greve et al., 1989; Casasnovas and Springer, 1994). The entry of RNA of a major group rhinovirus-RV14 into the cytoplasm of infected cells is said to be mediated by destabilization from receptor binding and by endosomal acidification (Casasnovas and Springer, 1994). The macrolide antibiotics, bafilomycin (Pérez and Carrasco, 1993; Suzuki et al., 2001) and erythromycin (Suzuki et al., 2002), inhibit infection by the major group of RVs, via a reduction of ICAM-1 expression and an increase in endosomal pH. Glucocorticoids also inhibit RV14 infection via the reduction of ICAM-1 (Suzuki et al., 2000). Hochu-ekki-to protects against murine cytomegalovirus infection (Hossain et al., 1999) and Listeria monocytogenes infection (Yamaoka et al., 1998). However, the effects of hochu-ekki-to on infection by RV have not been studied.
RV infection induces the production of cytokines including interleukin (IL)-1, IL-6 and IL-8 (Subauste et al., 1995; Zhu et al., 1996; Terajima et al., 1997). These cytokines have proinflammatory effects (Akira et al., 1990) and may be related to the pathogenesis of RV infections (Couch, 2001; Johnston, 2005). Hochu-ekki-to increases production of interferon (IFN)-γ in a murine model (Ishimitsu et al., 2001). However, the effects of hochu-ekki-to on cytokine production in airway epithelial cells following RV infection have not been studied.
Here, we have studied, in cultures of human airway epithelial cells infected with RV-14, the effects of hochu-ekki-to on output of virus and the associated levels of viral RNA, as measures of infection. We also examined the effects of hochu-ekki-to on the production of ICAM-1 and of cytokines and on endosomal pH to clarify the mechanisms underlying its inhibition of infection by RV.
Methods
Human tracheal epithelial cell culture
Isolation and culture of the human tracheal surface epithelial cells were performed as described previously (Terajima et al., 1997; Suzuki et al., 2001, 2002). The human tracheal surface epithelial cells were plated at 5 × 105 viable cells ml−1 in plastic tubes with round bottoms (16 mm diameter and 125 mm length, Becton Dickinson, Franklin Lakes, NJ, USA) coated with human placental collagen. Cells were covered with 1 ml of a mixture of DMEM and Ham's F-12 (DF-12) medium (50:50, v/v) containing 2% ultroser G (USG) (BioSepra, Cergy-Saint-Christophe, France) and antibiotics (Terajima et al., 1997; Suzuki et al., 2001, 2002). The tubes were loosely covered with a screw cap and were laid down with a slant of ∼5° and kept stationary in a humid incubator (Terajima et al., 1997; Suzuki et al., 2001, 2002). Because of the position of the plastic tubes, the cells were attached and proliferated mainly on the inner surface of the lateral wall of the tubes, and the surface area of culture vessels of the plastic tubes covered by the cells was 11.4±0.1 cm2 (n=3). Cells were cultured at 37°C in 5% CO2−95% air.
Tracheas for cell cultures were obtained after death from 25 patients (age, 72±3 years; 9 female, 16 male) without complications with bronchial asthma or COPD. The causes of death included malignant tumor other than lung cancer (n=9), acute myocardial infarction (n=4), renal failure (n=3), congestive heart failure (n=2), cerebral bleeding (n=2), rupture of an aortic aneurysm (n=1), cerebral infarction (n=1), sepsis (n=1), mitral stenosis (n=1) and malignant lymphoma (n=1). Of 25 patients, seven patients were ex-smokers and 18 patients were nonsmokers. This study was approved by the Tohoku University Ethics Committee.
Preparations and biochemical composition of hochu-ekki-to
Hocchu-ekki-to is composed of 10 species of medicinal plants, including Astragali Radix, Atractylodis Lanceae Rhizoma, Ginseng Radix, Angelicae Radix, Bupleuri Radix, Zizyphi Fructus, Aurantii Nobilis Pericarpium, Glycyrrhizae Radix, Cimicifugae Rhizoma and Zingiberis Rhizoma. Analysis of the biochemical composition of hochu-ekki-to with 3D-high–performance liquid chromatography (HPLC) revealed that the major components are hesperidin, glycyrrhizin and saikosaponin b2 (data from Tsumura Co., Tokyo, Japan), as described below. Other components include astragaloside IV, hinesol, ginsenoside and Z-ligustilide (data from Tsumura), although their contents were not measured.
Preparation of culture medium containing hochu-ekki-to
To prepare the culture medium containing hochu-ekki-to, the powder form of hochu-ekki-to (obtained from Tsumura Co.) was dissolved in dimethylsulfoxide (DMSO) at a concentration of 10 mg ml−1 by vortexing for 1 min at room temperature. The mixture was centrifuged at 1000 r.p.m. for 5 min to remove any insoluble ingredients. The concentration of hesperidin, one of the major components of hochu-ekki-to, has been used as an indicator of the concentration of hochu-ekki-to in the DMSO solution. Analysis of the biochemical composition of hochu-ekki-to with 3D-HPLC revealed that 1 g of hochu-ekki-to powder contains 5.7 mg of hesperidin, 5.0 mg of glycyrrhizin and 0.1 mg of saikosaponin b2 (data from Tsumura Co.). The powder form of G. Radix, liquorice roots (1 g), contains 25 mg of glycyrrhizin. The peak plasma concentration of glycyrrhizin was 228±106 ng ml−1 (n=12), 13 h after ingestion of 0.5 g of the powder form of liquorice roots (data from Tsumura Co.). After ingestion of 2.5 g hochu-ekki-to, a usual oral dose, the peak plasma concentration of glycyrrhizin was assumed to be 228 ng ml−1. Furthermore, the plasma concentration of glycyrrhizin was maintained at 100 ng ml−1, 24 h after ingestion of 0.5 g of liquorice roots (data from Tsumura Co.). On the basis of these data, the plasma concentration of hesperidin and glycyrrhizin was assumed to be 100 ng ml−1. In the present study, the concentration of glycyrrhizin in the solution of hochu-ekki-to in DMSO (10 mg ml−1) was 43±1 μg ml−1 (n=3) and the concentration of hesperidin in the same solution was 56±1 μg ml−1 (n=3). We have used as our standard treatment, solutions of hochu-ekki-to diluted to yield final concentrations of 100 ng ml−1 of hesperidin. In the experiments shown in Figure 2, the dilutions of hochu-ekki-to were made in terms of the glycrrhizin content, to match those of the pure glycrrhizin.
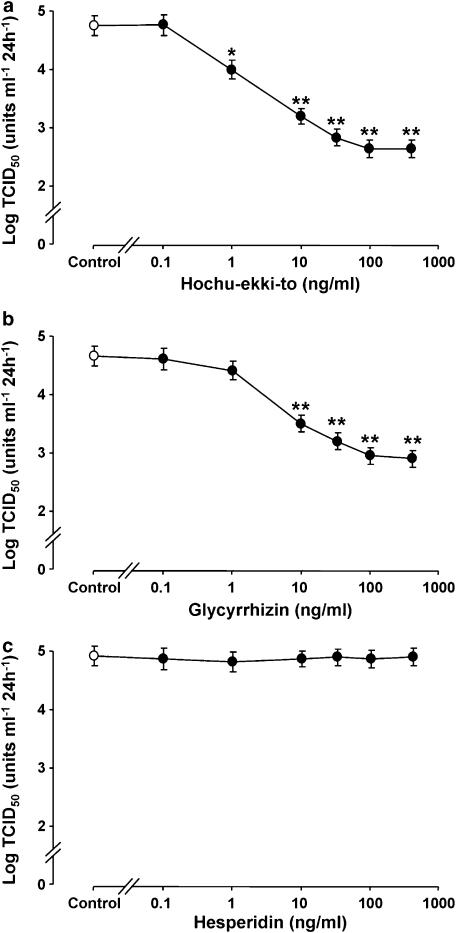
Figure 2.
Concentration–response effects of hochu-ekki-to (a) glycyrrhizin (b) and hesperidin (c) on viral release in supernatants collected during 24–72 h after infection. The cells were treated with hochu-ekki-to, glycyrrhizin, hesperidin or vehicle (control; DMSO, 0.2%) from 3 days before RV14 infection until the end of the experiments after RV14 infection. The rates of change in RV14 concentration in the supernatant are expressed as TCID50 units ml−1 24 h−1. Results are means±s.e.m. from five different tracheae. Significant differences from vehicle (control) are indicated by *P<0.05 and **P<0.01. Note that in these experiments the concentrations of hochu-ekki-to have been adjusted to provide the same concentrations of glycyrrhizin as shown in (b); a solution of 1 g ml−1 of hochu-ekki-to contains 5 mg ml−1 of glycyrrhizin.
The effects of pure hesperidin or glycyrrhizin on RV14 infection in human tracheal epithelial cells were also studied, and the concentrations used were matched to those of hochu-ekki-to, based on these data. The solutions of hesperidin (Sigma, St Louis, MO, USA) or glycyrrhizin (Wako, Osaka, Japan) in DMSO (10 mg ml−1) were diluted in double-distilled water and in DF-12 medium containing 2% USG and antibiotics.
Effects of hochu-ekki-to on viral infection
To examine the effects of hochu-ekki-to on the viral titers, on the cytokine contents in supernatants, and on the expression of ICAM-1 and RV14 RNA in the cells, the cultured human tracheal epithelial cells were treated with 100 ng ml−1 of hochu-ekki-to or vehicle (DMSO, 0.2%), from 3 days before RV14 infection until the end of the experiments after RV14 infection (Suzuki et al., 2001). The cells were then exposed to RV14 (105 TCID50 units ml−1) or vehicle (Eagle's minimum essential medium) for 60 min. The opening of tubes was sealed with rubber plugs and cells were cultured at 33°C with rolling in an incubator (HDR-6-T, Hirasawa, Tokyo, Japan) as described previously (Terajima et al., 1997; Suzuki et al., 2001, 2002).
To measure the time course of viral release during the first 24 h, we used three separate cultures from the same trachea. We collected the culture supernatants at either 1, 12 or 24 h after RV14 infection. To measure the viral titer during 1–3 days after RV14 infection, we used one culture from each trachea after collecting supernatants at 1 day (24 h) after RV14 infection. After collecting supernatants at 1 day after infection, the cells were rinsed with phosphate-bufferd saline (PBS) and 1 ml of DF-12 medium containing 2% USG was replaced. Supernatants were also collected at 3 days after infection. To measure the viral titer during 3–5 days after RV14 infection, after collecting supernatants at 3 days after infection, the cells were rinsed with PBS and 1 ml of the fresh medium was replaced. Supernatants were also collected at 5 days after RV14 infection. The cells were then rinsed with PBS and 1 ml of the fresh medium was replaced. Supernatants were also collected at 7 days after infection to measure the viral titer during 5–7 days after RV14 infection.
Viral stocks and detection and titration of viruses
RV14 stocks were prepared from patients with common colds by infecting human embryonic fibroblast cells as described (Terajima et al., 1997; Suzuki et al., 2001, 2002). Detection and titration of RV14 were performed by observing the cytopathic effects of viruses on fibroblast cells as described previously (Terajima et al., 1997; Suzuki et al., 2001, 2002). The fibroblasts were exposed to collected supernatants and the amount of sample required to infect 50% of the fibroblasts (tissue culture infective dose (TCID50)) was determined. To demonstrate the time course of viral release, we measured the rates of change in RV14 concentration in the supernatant. The rates were obtained by dividing the value of RV14 titer (TCID50 units per ml) in supernatants by incubation time and are expressed as TCID50units ml−1 24 h−1.
Qantification of RV RNA
To quantify the RV RNA and GAPDH mRNA expression in the cells after RV14 infection, real-time quantitative polymerase chain reaction with reverse transcription (RT-PCR) using the Taqman technique (Roche Molecular Diagnostic Systems, Mannheim, Germany) was performed as described previously (Martell et al., 1999; Suzuki et al., 2002). Taqman technology exploits the 5'-3' nucleolytic activity of AmpliTaq DNA polymerase (Holland et al., 1991; Heid et al., 1996; Martell et al., 1999). We used the program PrimerExpress (Applied Biosystems, Foster City, CA, USA) to design the probe and primers according to the guidelines for the best performance of the PCR. The standard curve was obtained between the fluorescence emission signals and Cτ by means of 10-fold dilutions of the total RNA, extracted from 105 TCID50 units ml−1 of RV14 in the supernatants of the human embryonic fibroblasts 7 days after infection with RV14 (104 TCID50 units ml−1). Real-time quantitative RT-PCR for GAPDH was also performed using the same PCR products. The expression of RV RNA was normalized to the constitutive expression of GAPDH mRNA.
Study protocol
To examine the concentration-dependent effects of hochu-ekki-to on RV14 infection, cells were treated with hochu-ekki-to at concentrations ranging from 0.1 to 500 ng ml−1.
The effects of hochu-ekki-to on the susceptibility to RV14 infection were evaluated as described previously (Subauste et al., 1995; Suzuki et al., 2002) using epithelial cells pretreated with hochu-ekki-to (100 ng ml−1, 3 days) or vehicle (DMSO, 0.2%, 3 days). The epithelial cells were then exposed to serial 10-fold dilutions of RV14 or vehicle of RV14 (Eagle's minimum essential medium) for 1 h at 33°C. The presence of RV in the supernatants collected for 1–3 days after infection was determined with the human embryonic fibroblast cell assay described above to assess whether infection occurred at each dose of RV used.
Measurement of ICAM-1 expression
The mRNA of ICAM-1 was examined with real-time RT-PCR analysis as described previously (Suzuki et al., 2002). Furthermore, concentrations of a soluble form of ICAM-1 (sICAM-1) in culture supernatants were measured with enzyme-linked immunoassay (EIA).
Measurement of cytokine production
We measured IL-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α in culture supernatants by specific enzyme-linked immunosorbant assays (ELISAs) (Terajima et al., 1997; Suzuki et al., 2002). To examine the effects of hochu-ekki-to, supernatants were collected before and after RV14 infection. Cells were pretreated with hochu-ekki-to for 3 days before RV14 infection. Twenty-four hours before RV14 infection, cells were rinsed with PBS, and fresh DF-12 medium containing 2% USG was replaced. Supernatants were collected just before RV14 infection and these supernatants were used to provide baseline values of cytokine release. After RV14 infection, supernatants were collected at 1, 3 and 5 days after RV14 infection. Cells were rinsed with PBS and fresh DF-12 medium containing 2% USG was replaced. Then supernatants were also collected at 7 days after RV14 infection. These supernatants were used as the samples for 1, 3, 5 and 7 days after RV14 infection for the measurement of cytokine release. To demonstrate the time course of cytokine release, we expressed the rates of change in cytokine concentration in the supernatant. The rates were obtained by dividing the value of cytokines concentration in supernatants by incubation time and are expressed as pg ml−1 24 h−1.
Measurement of changes in acidic endosomes distribution
The fluorescence intensity of acidic endosomes in the cells was measured as described previously with a dye, LysoSensor DND-189 (Molecular Probes, Eugene, OR, USA) (Suzuki et al., 2001, 2002). The effects of hochu-ekki-to on the distribution of acidic endosomes were examined from 100 s before to 300 s after the treatment with hochu-ekki-to (100 ng ml−1) or vehicle (DMSO, 0.2%). Fluorescence intensity of acidic endosomes was measured in 100 human tracheal epithelial cells and the mean value of fluorescence intensity after treatment with hochu-ekki-to was expressed as the percentage of control value (the fluorescence intensity of the cells treated with vehicle).
Measurement of LDH concentration
The amount of lactate dehydrogenase (LDH) in the culture supernatants was measured as described by Amador et al. (1963).
Statistical analysis
Results are expressed as means±s.e.m. Statistical analysis was performed using two-way repeated measures of analysis of variance (ANOVA). Subsequent post hoc analysis was performed using Bonferroni's method. For all analyses, values of P<0.05 were assumed to be significant; n refers to the number of donors (tracheae) from which cultured epithelial cells were used.
Results
Effects of hochu-ekki-to on RV infection in human tracheal epithelial cells
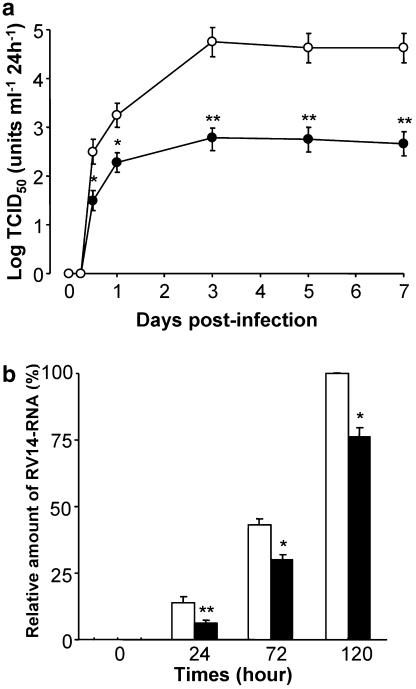
Exposing confluent human tracheal epithelial cell monolayers to RV14 (105 TCID50 units ml−1) consistently led to infection. No detectable virus was revealed at 1 h after infection but the viral content of the culture medium progressively increased between 1 and 12 h after infection (Figure 1a). Evidence of continuous viral production was obtained by demonstrating that each of the viral titers of supernatants collected during either 12–24 h, 1–3, 3–5 or 5–7 days after infection contained significant levels of RV14 (Figure 1a). The viral titer levels in supernatants increased significantly with time for the first 3 days. Treatment of the cells with hochu-ekki-to (100 ng ml−1) significantly decreased the viral titers of RV14 in supernatants from 12 h after infection (Figure 1a). RV14 titer levels in culture supernatants of the cells from seven ex-smokers did not differ from those in 18 nonsmokers (data not shown). No virus was detected in supernatants after infection of UV-inactivated RV14 (Terajima et al., 1997).
Figure 1.
(a) The time course of viral release in supernatants of human tracheal epithelial cells obtained at different times after exposure to 105 TCID50 units per ml of RV14 in the presence of hochu-ekki-to (100 ng ml−1; RV+hochu-ekki-to) or vehicle (DMSO, 0.2%; RV). The rates of change in RV14 concentration in the supernatant are expressed as TCID50 units ml−1 24 h−1. Results are means±s.e.m. from five different tracheae. Significant differences from viral infection with vehicle are indicated by *P<0.05 and **P<0.01. (b) Time course of replication of rhinovirus RNA from human tracheal epithelial cells after infections of RV14 in the presence of hochu-ekki-to (100 ng ml−1) or vehicle (DMSO, 0.2%; control) as detected by real-time quantitative RT-PCR. Results are expressed as relative amounts of RNA expression (%) compared with those of maximal RV14 RNA at day 5 (120 h), and reported as means + s.e.m. from five samples. Significant differences from control at each time are indicated by *P<0.05 and **P<0.01.
Further evidence of the inhibitory effects of hochu-ekki-to on RV14 replication in human tracheal epithelial cells was provided by real-time RT-PCR analysis. The amount of RV14 RNA in the cells increased with time until 120 h after RV14 infection (Figure 1b). Hochu-ekki-to (100 ng ml−1) also reduced the amount of RV14 RNA in the cells (Figure 1b). No RV14 RNA was detected in the cells after infection of ultraviolet (UV)-inactivated RV14.
Hochu-ekki-to inhibited RV14 infection concentration dependently, and the maximum effect was obtained between 100 and 500 ng ml−1 (Figure 2a).
To examine the effects of the major chemical components of hochu-ekki-to on RV14 infection, cells were pretreated with either glycyrrhizin or hesperidin with concentrations ranging from 0.1 to 500 ng ml−1. Glycyrrhizin reduced RV14 titers of supernatants collected during 1–3 days concentration dependently, and the maximum effect was between 100 and 500 ng ml−1 (Figure 2b). The inhibitory effects of glycyrrhizin were consistent and reproducible even when the solution of glycyrrhizin was stored in a refrigerator for 48 h (data not shown). Titers of RV14 from the cells treated with 1 ng ml−1 of hochu-ekki-to were significantly lower than those in the cells treated with vehicle (DMSO, 0.2%) (Figure 2a). In contrast, RV14 titers in the cells treated with 1 ng ml−1 of glycyrrhizin did not differ from those in the cells treated with vehicle (DMSO, 0.2%) (Figure 2b). Hesperidin at concentrations up to 500 ng ml−1 did not reduce RV14 titers of supernatants collected during 1–3 days (Figure 2c).
To examine whether RV14 infection or hochu-ekki-to-induced cytotoxic effects on the cultured cells, and caused cell detachment from the tubes after the cells made a confluent sheet, we counted the cell numbers after RV14 infection and after the treatment with hochu-ekki-to. The cell numbers were constant in the confluent epithelial cells in the control medium, and the coefficient of variation was small (6.8%; n=15). Neither RV14 infection (105 TCID50 units ml−1; 5 days) nor hochu-ekki-to treatment (100 ng ml−1; 5 days) had any effect on the cell numbers (data not shown). Cell viability, assessed by the exclusion of Trypan blue (Terajima et al., 1997), was consistently >96% in the hochu-ekki-to-treated culture. RV14 infection and hochu-ekki-to treatment (100 ng ml−1) did not alter the concentrations of LDH in the supernatants: LDH in the supernatants was 31±2 IU l−1 before RV14 infection, 33±3 IU l−1 3 days after RV14 infection (n=5) and 32±2 IU l−1 after hochu-ekki-to treatment (100 ng ml−1; 5 days; n=5).
Likewise, glycyrrhizin (100 ng ml−1) did not have an effect on the cell numbers (data not shown). Cell viability was consistently >96% in the glycyrrhizin-treated culture. The amount of LDH in the supernatants after glycyrrhizin treatment (100 ng ml−1; 5 days) (32±2 IU l−1, n=5) did not differ from that before the treatment (31±2 IU l−1, n=5).
Effects of hochu-ekki-to on susceptibility to type 14 RV infection
Treatment of the cells with hochu-ekki-to decreased the susceptibility of the cells to infection by RV14. The minimum dose of RV14 necessary to cause infection in the cells treated with hochu-ekki-to (100 ng ml−1, 3 days) (3.1±0.2 log TCID50 units ml−1, n=5) was significantly higher than that in cells treated with vehicle (2.1±0.2 log TCID50 units ml−1; n=5).
Effects of hochu-ekki-to on the expression of ICAM-1
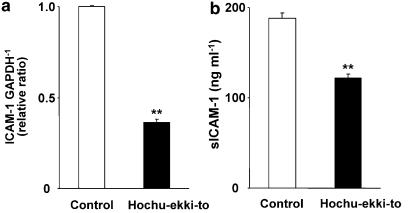
To examine the effects of hochu-ekki-to on the expression of ICAM-1, the human tracheal epithelial cells were treated with hochu-ekki-to (100 ng ml−1) or vehicle (DMSO, 0.2%) for 3 days and the mRNA was extracted and supernatants were collected. Hochu-ekki-to inhibited the baseline ICAM-1 mRNA expression in the cells before RV14 infection (Figure 3a) by more than 50% compared with that of the cells treated with vehicle (Figure 3a). Furthermore, concentrations of sICAM-1 protein in supernatants of the cells treated with hochu-ekki-to (100 ng ml−1) was significantly lower than those in the cells treated with vehicle (Figure 3b).
Figure 3.
(a) The expression of ICAM-1 mRNA in human tracheal epithelial cells 3 days after treatment with hochu-ekki-to (100 ng ml−1) or vehicle (0.2% DMSO, control) detected by real-time quantitative RT-PCR. ICAM-1 mRNA was normalized to the constitutive expression of GAPDH mRNA. Results are means±s.e.m. from five different tracheae. Significant differences from control values are indicated by **P<0.01. (b) The sICAM-1 concentrations in supernatants of human tracheal epithelial cells 3 days after treatment with hochu-ekki-to (100 ng ml−1) or vehicle (0.2% DMSO, control). The cells were treated with hochu-ekki-to or vehicle for 3 days before RV14 infection. At 1 day before RV14 infection, cells were rinsed with PBS and fresh DF-12 medium containing 2% USG was replaced. Then, supernatants were collected just before RV14 infection and the sICAM-1 concentrations in supernatants were measured. Results are means±s.e.m. from five different tracheae. Significant differences from control values are indicated by **P<0.01.
Effects of hochu-ekki-to on cytokine production
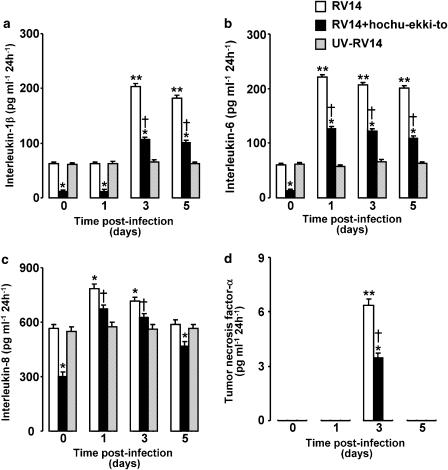
Hochu-ekki-to (100 ng ml−1) reduced the baseline secretion (shown as day 0) of IL-1β, IL-6 and IL-8 for 24 h before RV14 infection compared with that in the cells treated with vehicle (Figure 4). Secretion of IL-6 and IL-8 increased 24 h after RV14 infection and the secretion of IL-1β increased 3 days after RV14 infection. Hochu-ekki-to (100 ng ml−1) also reduced the RV14 infection-induced secretion of IL-1β, IL-6 and IL-8 compared with that in the cells treated with vehicle (Figure 4).
Figure 4.
Time course of release of cytokines (a, IL-1β; b, IL-6; c, IL-8; d, TNF-α) into supernatants of human tracheal epithelial cells after infection of RV14 in the presence of hochu-ekki-to (100 ng ml−1) or vehicle (DMSO, 0.2%; RV14) or after UV-inactivated RV14 (UV-RV14). The rates of change in cytokines concentration in the supernatant are expressed as pg ml−1 24 h−1. Results are means±s.e.m. from five different tracheae. Significant differences from values before RV14 infection (time 0) in the presence of vehicle (DMSO, 0.2%) are indicated by *P<0.05 and **P<0.01. Significant differences from corresponding values of RV14 plus vehicle (RV14) are indicated by +P<0.05.
TNF-α was not detectable in supernatants for 24 h before, or for 24 h after, RV14 infection but increased markedly 3 days after RV14 infection (Figure 4d). This increase was reduced by hochu-ekki-to compared with that in the cells treated with vehicle (Figure 4d). UV-inactivated RV14 did not increase the secretion of IL-1β, IL-6, IL-8 and TNF-α (Figure 4). Furthermore, secretion of IL-1β, IL-6, IL-8 and TNF-α in culture supernatants of the cells from seven ex-smokers did not differ from those of 18 nonsmokers (data not shown).
Effects of hochu-ekki-to on the acidification of endosomes
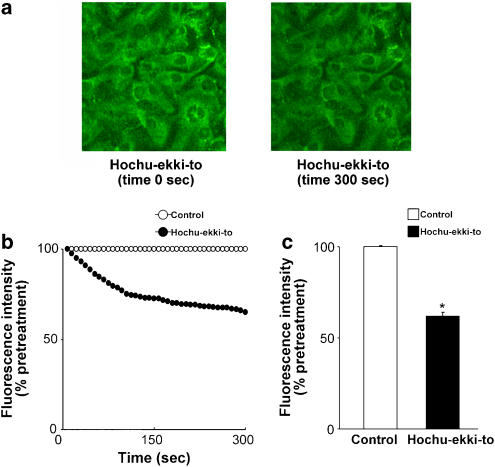
The effects of hochu-ekki-to on the changes in the distribution and the fluorescence intensity of acidic endosomes were examined from 100 s before until 300 s after the treatment with hochu-ekki-to (100 ng ml−1) or vehicle (DMSO, 0.2%). Acidic endosomes in human tracheal epithelial cells were stained green with LysoSensor DND-189 (Figure 5a). Green fluorescence from acidic endosomes was observed in a granular pattern in the cytoplasm (Figure 5a). Hochu-ekki-to (100 ng ml−1) decreased the number and the fluorescence intensity of acidic endosomes with green fluorescence in the cells with time (Figure 5).
Figure 5.
(a) Changes in the distribution of acidic endosomes with green fluorescence in the human tracheal epithelial cells before (time 0 s) and 300 s (time 300 s) after treatment with hochu-ekki-to (100 ng ml−1). Data are representative of three different experiments. (b) Time-course changes in the intensity of green fluorescence from acidic endosomes in human tracheal epithelial cells after treatment with either hochu-ekki-to (100 ng ml−1) or vehicle (0.2% DMSO). Inhibitors were added at time=0. (c) The fluorescence intensity of acidic endosomes 300 s after the addition of hochu-ekki-to (100 ng ml−1) or vehicle (0.2% DMSO, control). Results are means±s.e.m. from five different tracheae. Significant differences from control values are indicated by *P<0.05.
Discussion
In the present study, we have shown that a traditional Japanese herbal medicine, hochu-ekki-to, reduced viral titers in the supernatants and viral RNA of a major group rhinovirus-RV14, in cultured human tracheal epithelial cells. Pretreatment with hochu-ekki-to inhibited the expression of mRNA and protein of ICAM-1, the receptor for the major group of RVs (Greve et al., 1989), before RV14 infection. The magnitude of inhibitory effects of hochu-ekki-to on ICAM-1 mRNA expression was similar to that of dexamethasone and erythromycin (Suzuki et al., 2000, 2002). Because the minimum dose of RV14 necessary to cause infection in the cells treated with hochu-ekki-to was significantly higher than that in the cells treated with vehicle, hochu-ekki-to may inhibit RV14 infection at least partly by reducing the production of its receptor, ICAM-1, as observed in human tracheal epithelial cells treated with dexamethasone (Suzuki et al., 2000) and erythromycin (Suzuki et al., 2002). Furthermore, hochu-ekki-to reduced the fluorescence intensity of acidic endosomes, from which RV RNA enters the cytoplasm of the epithelial cells. The magnitude of inhibitory effects of hochu-ekki-to on the fluorescence intensity of acidic endosomes was similar to that of bafilomycin A1 (Suzuki et al., 2001) and erythromycin (Suzuki et al., 2002). Hochu-ekki-to may thus also act by inhibiting RV14 RNA entry across acidic endosomes as demonstrated in HeLa cells and human tracheal epithelial cells treated with bafilomycin A1 (Pérez and Carrasco, 1993; Suzuki et al., 2001) and erythromycin (Suzuki et al., 2002).
Glycyrrhizin, one of the major chemical components of hochu-ekki-to, reduced RV14 titers in the supernatants concentration dependently, whereas another component hesperidin, did not reduce RV14 titers. On the other hand, the magnitude of the inhibitory effect of glycyrrhizin was smaller than that of hochu-ekki-to. These findings suggestthat glycyrrhizin could be one of the constituents of hocchu-ekki-to that contributes to the overall inhibition of RV infection.
RVs are the major cause of the common cold and the most common acute infective illnesses in humans (Couch, 2001). Furthermore, various viruses have been reported in exacerbations in patients with COPD and bronchial asthma, including RV, influenza virus and respiratory syncytial virus (Nicholson et al., 1993; Johnston et al., 1995; Seemungal et al., 2001; Sethi, 2004). Seemungal et al. reported that 64% of COPD exacerbations were associated with a previous cold (Seemungal et al., 2001). Seventy-seven viruses were detected in 39% of COPD exacerbations and 39 (58%) viruses were RV. Infections by RVs were also associated with the acute exacerbations of bronchial asthma (Nicholson et al., 1993; Johnston et al., 1995). Thus, RV infections are likely to be a major cause of acute exacerbations of COPD and bronchial asthma.
Various mechanisms have been reported in the pathogenesis of exacerbations of COPD and bronchial asthma, including acute airway inflammation such as airway edema and eosinophil infiltration, acute airway hyper-reactivity and mucus hypersecretion (Wedzicha and Donaldson 2003; Sethi, 2004; Johnston, 2005). ICAM-1 interacts physiologically with leukocyte function-associated antigen-1, expressed on leukocytes, and thus plays a vital role in the recruitment and migration of immune effector cells to sites of local inflammation observed in patients with bronchial asthma and COPD (Riise et al., 1994; Grunberg and Sterk, 1999). Therefore, reduced ICAM-1 expression by hochu-ekki-to, in the present study, might also suggest the modulation of airway inflammation by hochu-ekki-to after RV infection.
Furthermore, neutrophilic and eosinophilc inflammation in the exacerbations of bronchial asthma and COPD are associated with a variety of mediators including IL-6 and IL-8, the production and secretion of which are stimulated by RV14 in airway epithelial cells as shown in the present and previous studies (Subauste et al., 1995; Zhu et al., 1996; Terajima et al., 1997). Hochu-ekki-to reduces eosinophilia in ovoalbumin-sensitized mice through the reduced production of IL-4 (Ishimitsu et al., 2001), suggesting that hochu-ekki-to may also modulate eosinophil-related inflammation in bronchial asthma. In the present study, RV14 infection increased the production of IL-1β, IL-6, IL-8 and TNF-α and hochu-ekki-to also reduced this increased production of IL-1β, IL-6, IL-8 and TNF-α. Because hochu-ekki-to reduced viral titer of RV14 in cell supernatants, this inhibition of RV14 infection could be the cause of the reduced production of the proinflammatory cytokines in the cells.
Hochu-ekki-to also reduced the baseline production of cytokines, including IL-1β, IL-6 and IL-8 before RV14 infection. The role of the baseline production of these cytokines is uncertain. However, as we have demonstrated previously, endogenously produced IL-1β is associated with the expression of ICAM-1 after RV infection in human tracheal epithelial cells (Terajima et al., 1997). In the present study, hochu-ekki-to reduced the RV titer levels and IL-1β secretion in culture supernatants of the cells. Pretreatment with hochu-ekki-to also inhibited the expression of mRNA and protein of ICAM-1. These findings suggest that production of ICAM-1 might be reduced by hochu-ekki-to partly through the reduction of IL-1β and hochu-ekki-to might inhibit RV14 infection at least partly by reducing the production of its receptor, ICAM-1. However, the antiviral and anti-inflammatory consequences of baseline reductions in IL-6 and IL-8 are still not clear.
Endosomal pH is probably regulated by vacuolar H+-ATPases (Mellman et al., 1986), and ion transport across the Na+ H+−1 antiporters (Marshansky and Vinay, 1996). Inhibitors of Na+ H+−1 antiporters, such as 5-(N-ethyl-N-isopropyl)amiloride (EIPA) and N′′-(3-(hydroxymethyl)-5-(1H-pyrrol-1-yl)benzoyl)guanidine methonesulfonate (FR168888) as well as a vacuolar H+-ATPase inhibitor, bafilomycin, increased endosomal pH and inhibit RV14 infection in cultured human tracheal epithelial cells (Suzuki et al., 2001). Although we have no direct data from the present study, a similarly mediated increase in endosomal pH by hochu-ekki-to may explain its inhibitory effect on RV infection.
Recent reports revealed that the major group of RVs enters the cytoplasm of infected cells after binding to its receptor ICAM-1 (Greve et al., 1989). The entry of RNA of RV14 into the cytoplasm of infected cells is suggested to be mediated by the destabilization from receptor binding and by endosomal acidification (Casasnovas and Springer, 1994). The inhibitory effects of hochu-ekki-to on infection by RV14 and its effects on the endosomal pH in the present study are consistent with those of bafilomycin and erythromycin in previous studies (Pérez and Carrasco, 1993; Suzuki et al., 2002). Furthermore, the inhibitory effects of hochu-ekki-to on ICAM-1 expression in airway epithelial cells could also be associated with inhibition of RV14 infection, as reported previously for the inhibitory effects of dexamethasone, bafilomycin and erythromycin (Suzuki et al., 2000, 2001, 2002).
Hochu-ekki-to (100 ng ml−1) reduced RV14 titer levels by about 1.5 logs of TCID50 but it reduced the amount of RV14 RNA in the cells by only 25–50%, showing that inhibition of RV14-RNA replication was less that that of RV14 release into supernatants. The reason for this discrepancy is uncertain but may reflect inhibition of one or more of the many steps between RV14-RNA replication and the release of virus particles in the culture medium.
Likewise, hochu-ekki-to inhibited ICAM-1 mRNA expression by 60%, sICAM-1 concentrations in supernatants by 45% and fluorescent from acidic endosomes by 40% on RV14 infection. In contrast, hochu-ekki-to inhibited RV14-RNA replication by 32% at 72 h and by 25% at 120 h, although RV14-RNA replication was inhibited by by 55% at 24 h after RV14 infection. These findings suggest that all viral binding, internalization, entry into the cytoplasm and replication steps might be equally inhibited by hochu-ekki-to, on and during the first 24 h after RV14 infection. However, the effects of hochu-ekki-to on RV replication or on viral binding, internalization and release into the cytoplasm during the later stages of infection (72–120 h) are not clear.
The RV14 titer levels in culture supernatants are low, but are consistent with the levels in throat swabs in patients with the symptoms of common cold as reported previously (Numazaki et al., 1987). Zhu et al. (1996) demonstrated the increased symptom score of common cold after challenge of RV-strain H with 800 TCID50 and RV39 with 2500 TCID50. RV-titer levels in their study were similar to those of RV14 in supernatants in this study, suggesting that minimal levels of RV infection and virus release may cause the symptoms of the common cold. On the other hand, the rate of release of virus is constant from days 3 to 7. We have no data on viral release after 7 days and no data to show when it starts to fall. Because the time course of viral release after 7 days is also important in the pathogenesis of RV infection, further studies over a longer period are needed.
In summary, this is the first report that a traditional Japanese herbal medicine, hochu-ekki-to, inhibits infection by RV14 and decreases the susceptibility of cultured human tracheal epithelial cells to RV14 infection, probably through the inhibition of ICAM-1 expression and endosomal acidification. Hochu-ekki-to reduced baseline and RV infection-induced release of proinflammatory cytokines in supernatants including IL-1β, IL-6 and IL-8 and RV infection-induced release of TNF-α. Hochu-ekki-to may inhibit infection by the major group of RVs and modulate the inflammatory responses in airway epithelial cells after RV infection.
Acknowledgments
We thank Mr Grant Crittenden for reading the manuscript. This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of the Japanese government to MY (16590732), by Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases from the Ministry of Health, Labour and Welfare of the Japanese government to MY (17243601) and supported from the Ministry of Health, Labour and Welfare of the Japanese government to KN (17170801).
Abbreviations
- COPD
chronic obstructive pulmonary disease
- DMSO
dimethylsulfoxide
- EIA
enzyme-linked immunoassay
- ELISA
enzyme-linked immunosorbent assay
- ICAM-1
intercellular adhesion molecule-1
- LDH
lactate dehydrogenase
- IFN
interferon
- IL
interleukin
- RV
rhinovirus
- sICAM-1
soluble form of ICAM-1
- TCID
tissue culture infective dose
- TNF
tumor necrosis factor
- USG
ultroser G
Conflict of interest
The authors state no conflict of interest.
References
- Akira S, Hirano S, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- Amador E, Dorfman LE, Wacker EC. Serum lactic dehydrogenase activity: an analytical assessment of current assays. Clin Chem. 1963;9:391–399. [PubMed] [Google Scholar]
- Casasnovas JM, Springer TA. Pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): an intermediate in which ICAM-1 is bound and RNA is released. J Virol. 1994;68:5882–5889. doi: 10.1128/jvi.68.9.5882-5889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB.Rhinoviruses Fields Virology 2001Lippincott Williams and Wilkins: Philadelphia, PA; 777–797.In: Knipe DM, Howley PM, (eds)4th edn [Google Scholar]
- Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Grunberg K, Sterk PJ. Rhinovirus infections: induction and modulation of airways inflammation in asthma. Clin Exp Allergy. 1999;29:65–73. doi: 10.1046/j.1365-2222.1999.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA. 1991;88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Takimoto H, Hamano S, Yoshida H, Ninomiya T, Minamishima Y, et al. Protective effects of hochu-ekki-to, a Chinese traditional herbal medicine against murine cytomegalovirus infection. Immunopharmacology. 1999;41:169–181. doi: 10.1016/s0162-3109(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Ishimitsu R, Nishimura H, Kawauchi H, Kawakita T, Yoshikai Y. Dichotomous effect of a traditional Japanese medicine, bu-zhong-yi-qi-tang on allergic asthma in mice. Int Immunopharmacol. 2001;1:857–865. doi: 10.1016/s1567-5769(01)00022-4. [DOI] [PubMed] [Google Scholar]
- Iwama H, Amagaya S, Ogihara Y. Effects of five kampohozais on the mitogenic activity of lipopolysaccharide, concanavalin A, phorbol myristate acetate and phytohemagglutin in vivo. J Ethnopharmacol. 1986;18:193–204. doi: 10.1016/0378-8741(86)90031-0. [DOI] [PubMed] [Google Scholar]
- Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc. 2005;2:150–156. doi: 10.1513/pats.200502-018AW. [DOI] [PubMed] [Google Scholar]
- Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. Br Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa A, Liu S, Yan H, Eshita A, Naitoh S, Nagayama A. Effect of a traditional Japanese herbal medicine, Hochu-ekki-to (Bu-Zhong-Yi-Qi Tang), on immunity in elderly persons. Int Immunopharmacol. 2004;4:317–324. doi: 10.1016/j.intimp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Vinay P. Proton gradient formation in early endosomes from proximal tubes. Biochem Biophys Acta. 1996;1284:171–180. doi: 10.1016/s0005-2736(96)00123-x. [DOI] [PubMed] [Google Scholar]
- Martell M, Gomez J, Esteban JI, Sauleda S, Quer J, Cabot B, et al. High-throughput real-time reverse transcription-PCR quantitation of hepatitis C virus RNA. J Clin Microbiol. 1999;37:327–332. doi: 10.1128/jcm.37.2.327-332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Ann Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki Y, Oshima T, Ohmi A, Tanaka A, Oizumi Y, Komatsu S, et al. A microplate methods for isolation of viruses from infants and children with acute respiratory infections. Microbiol Immunol. 1987;31:1085–1095. doi: 10.1111/j.1348-0421.1987.tb01340.x. [DOI] [PubMed] [Google Scholar]
- Pérez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riise GC, Larsson S, Lofdahl CG, Andersson BA. Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur Respir J. 1994;7:1673–1677. doi: 10.1183/09031936.94.07091673. [DOI] [PubMed] [Google Scholar]
- Seemungal T, Harper-Owen R, Brownmik A, Moric I, Sanderson G, Message S, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- Sethi S. New developments in the pathogenesis of acute exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis. 2004;17:113–119. doi: 10.1097/00001432-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Kitamura S. The effects of Hochu-ekki-to on the patients with chronic obstructive pulmonary disease. Nippon Kyobu Rinsho. 1997;56:105–109. [Google Scholar]
- Suzuki T, Yamaya M, Sekizawa K, Hosoda K, Yamada N, Ishizuka S, et al. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. Am J Respir Crit Care Med. 2002;165:1113–1118. doi: 10.1164/ajrccm.165.8.2103094. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamaya M, Sekizawa K, Hosoda M, Yamada N, Ishizuka S, et al. Bafilomycin A1 inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am J Physiol. 2001;280:L1115–L1127. doi: 10.1152/ajprenal.2001.280.6.F1115. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamaya M, Sekizawa K, Yamada N, Nakayama K, Ishizuka S, et al. Effects of dexamethasone on rhinovirus infection in cultured human tracheal epithelial cells. Am J Physiol. 2000;278:L560–L571. doi: 10.1152/ajplung.2000.278.3.L560. [DOI] [PubMed] [Google Scholar]
- Terajima M, Yamaya M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, et al. Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1β. Am J Physiol. 1997;273:L749–L759. doi: 10.1152/ajplung.1997.273.4.L749. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Seidler H, Kitagawa M, Hirokawa K. Immunological restoration and anti-tumor effect by Japanese herbal medicine in aged mice. Mech Ageing Dev. 2001;122:341–352. doi: 10.1016/s0047-6374(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48:1204–1213. [PubMed] [Google Scholar]
- Yamaoka Y, Kawakita T, Kishihara K, Nemoto K. Effect of traditional Chinese medicine, Bu-zhong-yi-gi-tang, on the protection against an oral infection with Listeria monocytogenes. Immunopharmacology. 1998;39:215–223. doi: 10.1016/s0162-3109(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Tang W, Ray A, Wu Y, Einarsson O, Landry ML, et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]