Abstract
Background and purpose:
Topiramate is a novel anticonvulsant known to modulate the activity of several ligand- and voltage-gated ion channels in neurons. The mechanism of action of topiramate, at a molecular level, is still unclear, but the phosphorylation state of the channel/receptor seems to be a factor that is able to influence its activity. We investigated the consequences of phosphorylation of the sodium channel on the effect of topiramate on tetrodotoxin (TTX)-sensitive transient Na+ current (INaT).
Experimental approach:
INaT was recorded in dissociated neurons of rat sensorimotor cortex using whole-cell patch-clamp configuration.
Key results:
We found that topiramate (100 μM) significantly shifted the steady-state INaT inactivation curve in a hyperpolarized direction. In neurons pre-treated with a PKC-activator, 1-oleoyl-2-acetyl-sn-glycerol (OAG; 2 μM), the net effect of topiramate on steady-state INaT inactivation was significantly decreased. In addition, OAG also slightly shifted the INaT activation curve in a hyperpolarized direction, while perfusion with topiramate had no effect on the parameters of INaT activation.
Conclusions and Implications:
These data show that PKC-activation can modulate the effect of topiramate on INaT. This suggests that channel phosphorylation in physiological or pathological conditions (such as epiliepsy), can alter the action of topiramate on sodium currents.
Keywords: transient Na+ current, topiramate, PKC, sodium channel, antiepileptic drug
Introduction
It has been demonstrated that a number of traditional and new anti-epileptic drugs significantly inhibit the transient Na+ current (INaT; Ragsdale and Avoli, 1998), an effect that can play a primary role in preventing the occurrence or spread of epileptic ictal events. However, the functional properties of voltage-gated Na+ channels in the brain are subject to modulation by the activation of second messenger systems (Cantrell and Catterall, 2001) by either physiological or pathological (epileptic) events. In particular, protein kinase C (PKC)-mediated channel phosphorylation modifies the properties of INaT by favoring Na+ channel inactivation at depolarized membrane potentials (Godoy and Cukierman, 1994; O'Reilly et al., 1997; Franceschetti et al., 2000). This effect is comparable to that of a number of anti-epileptic drugs that principally act by stabilizing the inactivated state of the channel and therefore the concomitant action of PKC activation and anti-epileptic drugs could lead to a cumulative inhibitory effect on INaT.
Topiramate (2,3:4,5-bis-O-(1-methyl-ethylidene)-∃-D-fructopyranose sulfamate) is a structurally novel anticonvulsant, currently used for treating several seizure disorders (Bourgeois, 1998; Sander, 1998; Reife et al., 2000; Biton et al., 2005). Electrophysiological studies have revealed several effects of topiramate on ion channels, including negative modulation of voltage-gated Na+ (Zona et al., 1997; Taverna et al., 1999; Curia et al., 2004) and Ca2+ channels (Zhang et al., 2000), blockade of kainate-evoked inward current (Gibbs et al., 2000; Skradski and White, 2000) and modulation of GABAergic current (White et al., 1997; Herrero et al., 2002). In addition, topiramate is able to provoke depression of sustained repetitive firing that is mediated by Na+ currents (DeLorenzo et al., 2000; McLean et al., 2000).
Although the exact mechanism of action of topiramate at a molecular level is not completely understood, results from previous studies suggest that the phosphorylated state of target receptors/channels can affect the activity of topiramate (Gibbs et al., 2000; Angehagen et al., 2004; Curia et al., 2004).
In the present study, we confirmed the previously reported effect of topiramate (Taverna et al., 1999) and an activator of protein kinase C, 1-oleoyl-2-acetyl-sn-glycerol (OAG; Franceschetti et al., 2000) on the transient component of the TTX-sensitive sodium current (INaT) and we investigated whether the phosphorylation state of sodium channels could influence the effect of topiramate.
Methods
Cell preparation
All experimental procedures were carried out in compliance with the European 86/609/UE law and the guidelines of the Ethics Committee of ‘C Besta' Institute.
Sprague–Dawley rats aged 10–17 days (Charles River, Italy) were anesthetized with ether and decapitated. Their brains were removed and placed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF), containing (in mM): 124 NaCl, 3.5 KCl, 2 CaCl2, 2 MgCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10 glucose (pH 7.35 with NaOH). Coronal slices (300 μm thick) were cut from the sensorimotor cortex using a vibratome, treated with 1 mg ml−1 of Protease XIV (Sigma, Italy) to digest the extracellular matrix, and then stored in enzyme-free ACSF. Immediately before the recordings, single neurons were dissociated using fire-polished Pasteur pipettes and plated in a Petri dish (Falcon, Becton Dickinson and Company, Franklin Lakes, NJ, USA) coated with Concanavalin A (50 μg ml−1) to allow cell adhesion.
Electrophysiology
Whole-cell patch clamp recordings were performed at room temperature using a 200B amplifier (Molecular Devices, Palo Alto, CA, USA). All data were digitized using a Digidata 1200 interface (sampling frequency: 10 kHz) and pClamp 8.0 (Molecular Devices) and analyzed with Origin 6.0 software (OriginLab, Northampton, MA, USA).
Borosilicate glass electrodes (3–4 MΩ) were filled with a solution containing (in mM): 120 CsF, 2 MgCl2, 10 HEPES, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N',-tetraacetic acid-CsOH, 2 Na2ATP, 10 phosphocreatine-diTris, 0.3 Na-GTP, and 20 U ml−1 creatine phosphokinase, pH 7.2. As external bath solution, a modified ACSF was used, in which 105 mM choline-Cl partially substituted NaCl, in order to reduce the voltage error owing to the large Na+ current observed in pyramidal neurons. CdCl2, NiCl2 and TEA-Cl in the external solution (respectively 0.4, 0.3 and 20 mM), and Cs in the intracellular one, were added to block calcium and potassium currents.
OAG (Sigma, Italy) 2 μM and topiramate (RAW Johnson Pharmaceutical Research Institute, Rarity, NJ, USA) 100 μM, were dissolved in ACSF and applied using a micromanifold, whose tip was positioned no more than 100 μm from the soma of the selected neuron. Previous studies show that topiramate acts on sodium channels in a dose-dependent manner (DeLorenzo et al., 2000; McLean et al., 2000) with an IC50 value of 48.9 μM and a saturating concentration of 500 μM (Zona et al., 1997). For our experiments, we chose the concentration of 100 μM of topiramate in order to compare our data with the ones previously collected from several laboratories (Zona et al., 1997; Taverna et al., 1999; Franceschetti et al., 2000; Curia et al., 2004). In addition, this concentration corresponds to 34 μg ml−1, a value that is just over the upper limit of clinically relevant plasma levels in epileptic patients (Contin et al., 2002).
Voltage-clamp recordings were performed on 32 neurons dissociated from somatosensory cortex of Sprague–Dawley rats. Principal cells were identified from interneurons by the larger size and the pyramidal-like shape of the soma, which is preserved after the dissociation process. All the traces presented in the figures represent the transient component of the TTX-sensitive sodium current (INaT), obtained from off-line digital subtraction of recordings before and after application of TTX.
In the episodic stimulus protocol, used to evaluate the voltage dependence of steady-state inactivation, eight preconditioning pulses, from −90 to −20 mV and lasted 300 ms, preceded the test pulse at −15 mV, which lasted 50 ms. Steady-state inactivation curves were obtained by fitting the data points with a Boltzmann relationship in the form: I/IMAX=1/{1+exp[(V1/2−V)/k]}, where I/IMAX is the relative current, V1/2 the voltage at which half-maximal inactivation is reached and k the slope factor.
The episodic stimulus protocol used to evaluate the voltage dependence of steady-state activation, was composed of nine test pulses (from −60 to −20 mV) lasted 200 ms. The activation curves were obtained by fitting the data points with a Boltzmann equation in the form: G/GMAX=1/{1+exp[(V1/2−V)/K]}, where GMAX is maximal peak conductance, G peak conductance at each test voltage, V1/2 the voltage at which half-maximal activation is reached and k the slope factor. Reversal potential for sodium current was measured experimentally from each neuron.
Data analysis
The data are presented as mean values±standard error of the mean (s.e.m.) and were statistically analyzed using analysis of variance or Wilcoxon's tests. Values of P<0.05 were taken as showing a significant difference between means.
Results
Voltage dependence of steady-state inactivation
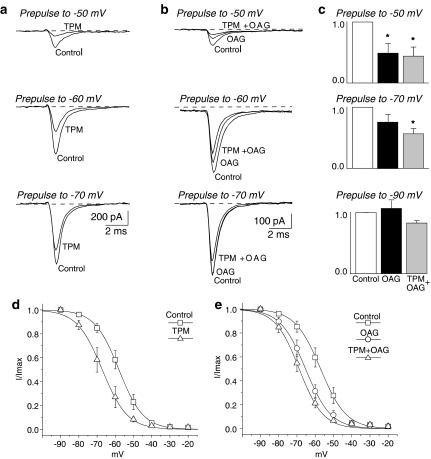
Topiramate (100 μM) inhibited the peaks evoked by more depolarized conditioning pulses (cf. −50 versus −70 mV conditioning pulse in Figure 1a), thus leading to a significant hyperpolarizing shift (9.3±1.2 mV) in the steady-state INaT inactivation curve (Figure 1d, Table 1).
Figure 1.
Effects of topiramate (TPM) and OAG on steady-state INaT inactivation. (a and b) TTX-subtracted current traces evoked by 50 ms step depolarization to −15 mV after a 300 ms prepulse at different membrane potentials. (a) Na+ currents recorded in a neuron exposed to topiramate 100 μM. (b) Na+ current recorded in a neuron exposed to OAG 2 μM, and then to OAG plus topiramate. (c) The normalized peak amplitude of INaT evoked after conditioning pulses to −90, −70 and −50 mV during perfusion with OAG (black bar) or OAG + topiramate (grey bar) compared with the normalized value of the current peak measured under control conditions (white bar; *=P<0.05; n=8). (d and e) Steady-state inactivation curve obtained by plotting the current peaks (normalized to maximal values) against the prepulse potential. The curves show the fit obtained using a Boltzmann function applied to the data points calculated under control conditions and in the presence of topiramate (d), and under control conditions, in the presence of OAG and OAG plus topiramate (e).
Table 1.
INaT steady-state inactivation parameters (mean values±s.e.m.)
|
Steady-state inactivation |
Activation |
|||||
|---|---|---|---|---|---|---|
| n | V1/2 (mV) | k | n | V1/2 (mV) | K | |
| Controls | 6 | −57.9±2.1 | 5.9±0.3 | 6 | −35.7±0.7 | 3.1±0.4 |
| TPM | −67.3±2.7* | 5.9±0.3 | −36.9±0.7 | 3.3±0.3 | ||
| Controls | 8 | −56.1±1.6 | 6.2±0.4 | 7 | −34.7±0.7 | 2.9±0.2 |
| OAG | −66.0±1.6** | 5.9±0.3 | −38.5±1.0* | 3.6±0.2* | ||
| TPM+OAG | −70.5±1.7*,• | 5.7±0.2 | −39.4±1.0* | 3.9±0.1* | ||
Abbreviations: OAG, 1-oleoyl-2-acetyl-sn-glycerol; TPM, topiramate.
=P<0.05
=P<0.01, in comparison with the values measured under control conditions.
=P<0.05, in comparison with the values measured in the presence of OAG.
Pretreatment with OAG (2 μM) had a progressively increasing inhibitory effect on the peak current evoked after depolarizing prepulses positive to −70 mV (Figure 1b–c). Between 5 and 8 min after the start of OAG perfusion, the average value of the steady-state inactivation midpoint shifted in a negative direction by 10.2±0.9 mV compared to control conditions (Table 1). Neurons exposed to OAG were subsequently perfused with OAG together with topiramate (n=5). Alternatively, the medium containing OAG was immediately replaced with a medium containing topiramate alone (n=3). Under both conditions, the steady-state INaT inactivation curve further shifted in a hyperpolarizing direction, and the midpoint became 4.2±0.7 mV more negative than the one measured in the presence of OAG alone (n=8; Figure 1e, Table 1). We obtained similar results in five more neurons preincubated for 20–30 min with OAG. In these cells, addition of topiramate to OAG shifted the midpoint of the steady-state INaT inactivation curve from −62.4±2.6 (k=6.4±0.1) to −69.5±2.7 mV (k=6.5±0.2) (P<0.05).
On the basis of these results, we can conclude that topiramate was still capable of inducing a small hyperpolarizing shift of INaT inactivation in phosphorylated channels (average: −4.9±0.6 mV), but that this effect is significantly smaller than in non-phosphorylated ones (−9.3±1.2 mV; P<0.05) (Table 1).
INaT activation
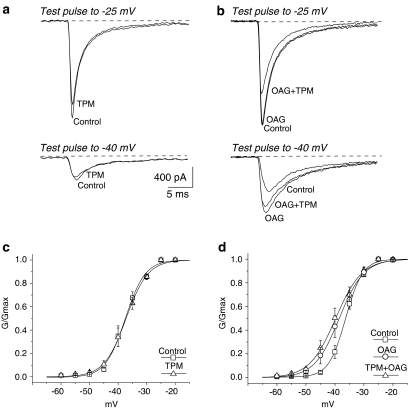
Addition of topiramate to neurons incubated in standard ACSF (n=6) inhibited INaT by 20.6±7. 7% (P<0.05), without affecting either the voltage-dependence or slope of the activation curve (Figure 2a, c; Table 1).
Figure 2.
Effects of topiramate (TPM) and OAG on INaT activation. (a and b) TTX-subtracted current traces evoked from holding potential (−70 mV) to respectively −40 and −25 mV. (a) Na+ currents recorded in a neuron exposed to topiramate 100 μM. (b) Na+ current recorded in a neuron exposed to OAG 2 μM, and then to OAG plus topiramate. In (b), note that the increased amplitude of the current evoked by a depolarizing step to −40 mV in a neuron perfused with OAG is only partially counteracted during perfusion with both OAG and topiramate. (c and d) Voltage-dependent Na+ conductance; the curves show the Boltzmann function fit applied to the data points calculated under control conditions and in the presence of topiramate (c), and under control conditions, in the presence of OAG and OAG plus topiramate (d).
In the presence of OAG, the maximal peak INaT amplitude did not change significantly when evoked by step potentials to −25 mV, but was increased when evoked by step potentials to −40 mV (Figure 2b). The activation curve was consistently shifted in a hyperpolarizing direction (3.8±0.7 mV; P<0.05; n=7) and concurrently became less steep (Figure 2d; Table 1). Addition of topiramate to the perfusion containing OAG inhibited the maximal INaT amplitude by 19.2±6.1% (n=7, P<0.05), without further changes in the INaT activation curve (Figure 2d; Table 1). The extent of this inhibitory effect on INaT was similar to that found in the neurons perfused with topiramate alone.
Discussion and conclusions
Our results indicate that the inhibitory effect of topiramate resulting from a hyperpolarizing shift of the steady-state INaT inactivation curve (Zona et al., 1997; Taverna et al., 1999) is partially prevented by pretreatment with the PKC activator OAG. Moreover, topiramate was unable to counteract the OAG-induced hyperpolarizing shift on INaT activation.
Both OAG and topiramate individually induced a comparable hyperpolarizing shift on INaT steady-state inactivation, thus confirming that both experimental manipulations are capable of reducing Na+-dependent membrane excitability by favouring channel inactivation. PKC-dependent phosphorylation may therefore act as an endogenous system that reduces cell excitability following a sufficiently large depolarization.
However, at the same time, this change seems to limit the inhibitory effect of topiramate, which acts on Na+ channels by shifting the INaT inactivation curve toward more hyperpolarized potentials. Chemically different anti-epileptic drugs acting on Na+ channels seem to bind preferentially to extracellular channel sites, which became available only in the case of conformational changes leading to inactivation (Kuo, 1998). Our findings suggest that PKC-dependent phosphorylation may act by partially preventing topiramate binding to inactivated channels or by lessening its stabilizing effect on channel inactivation. Shank et al. (2000) hypothesized that topiramate binds selectively to protein kinase A-mediated phosphorylation site only when the channels are in the dephosphorylated state. After binding the site, topiramate could exert either a positive or negative allosteric modulation. The variable activity of topiramate could be a result of the variable state of phosphorylation of the channels. In particular Shank et al. (2000) suggested an inverse relation between the level of the topiramate effect on the channel conductance and the degree of channel phosphorylation.
A direct excitatory mechanism owing to PKC activation may come from the small but consistent hyperpolarizing shift in the voltage dependence of INaT activation owing to OAG perfusion (Franceschetti et al., 2000). This effect enhances membrane excitability by increasing the availability of Na+ channels prone to open at negative membrane potentials that are very close to resting levels. Topiramate did not change the activation parameters in the neurons perfused with OAG, or in those perfused with standard ACSF. However, it exerted its inhibitory effect by decreasing the current amplitude. This result is similar to that found by evaluating the persistent fraction of sodium currents, for which activation was also shifted in a hyperpolarizing direction in the presence of OAG (Astman et al., 1998; Franceschetti et al., 2000), but was left unchanged by topiramate perfusion (Curia et al., 2004).
The inhibitory role played by topiramate on INaT includes a reduction of the peak amplitude that is bigger in comparison with that observed for INaP (Curia et al., 2004). A further inhibitory effect on INaT is the hyperpolarizing shift of the steady-state inactivation curve. However, because INaP plays a crucial role in boosting near-threshold depolarizations (Curia et al., 2004), even small reductions of its amplitude can significantly modify the excitable properties of neurons. Thus, both INaP and INaT can be important targets for TMP action on neuronal hyperexcitability. The concomitant effects on both current fractions suggest that topiramate can act on different functional states of the same channel.
It has been estimated that complete seizure control is achievable in 54–82% of patients with primary generalized epilepsy syndromes. In fact, a substantial group of patients with untreatable epilepsy is present (Faught, 2004). The interaction of PKC activation with the effects of topiramate may be an important mechanism leading to the uneven efficacy of topiramate on Na+ channels in different patients or at different times during the natural course of an epileptic disorder. Our data suggest that activation of the PKC second messenger pathway, that has been shown to occur in a number of experimental models of epilepsy (Chen et al., 1992; Osonoe et al., 1994; Akiyama et al., 1995), may actually change Na+-dependent excitability in epileptic patients, thereby altering the sensitivity of Na+ channels to topiramate.
Acknowledgments
We thank Dr B Rosati for critically reading the paper. This work was supported by Italian Ministry of Health and by Mariani Foundation for Pediatric Neurology.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- INaT
transient Na+ current
- OAG
1-oleoyl-2-acetyl-sn-glycerol
Conflict of interest
The authors state no conflict of interest.
References
- Akiyama K, Ono M, Kohira I, Daigen A, Ishihara T, Kuroda S. Long-lasting increase in protein kinase C activity in the hippocampus of amygdala-kindled rat. Brain Res. 1995;679:212–230. doi: 10.1016/0006-8993(95)00221-b. [DOI] [PubMed] [Google Scholar]
- Angehagen M, Ben-Menachem E, Shank R, Rönnbäck L, Hansson E. Topiramate modulation of kainate-induced calcium currents is inversely related to channel phosphorylation level. J Neurochem. 2004;88:320–325. doi: 10.1046/j.1471-4159.2003.02186.x. [DOI] [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ currents in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. doi: 10.1152/jn.1998.80.3.1547. [DOI] [PubMed] [Google Scholar]
- Biton V, Bourgeois BF, YTC/YTCE Study Investigators Topiramate in patients with juvenile myoclonic epilepsy. Arch Neurol. 2005;62:1705–1708. doi: 10.1001/archneur.62.11.1705. [DOI] [PubMed] [Google Scholar]
- Bourgeois BFD. New antiepileptic drugs. Arch Neurol. 1998;55:1181–1183. doi: 10.1001/archneur.55.9.1181. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Desai MA, Klann E, Winder DG, Sweatt JD, Conn PJ. Amygdala kindling alters protein kinase C activity in dentate gyrus. J Neurochem. 1992;59:1761–1769. doi: 10.1111/j.1471-4159.1992.tb11008.x. [DOI] [PubMed] [Google Scholar]
- Contin M, Riva R, Albani F, Avoni P, Baruzzi A. Topiramate therapeutic monitoring in patients with epilepsy: effect of concomitant antiepileptic drugs. Ther Drug Monit. 2002;24:332–337. doi: 10.1097/00007691-200206000-00002. [DOI] [PubMed] [Google Scholar]
- Curia G, Aracri P, Sancini G, Mantegazza M, Avanzini G, Franceschetti S. Protein-kinase C-dependent phosphorylation inhibits the effect of the antiepileptic drug topiramate on the persistent fraction of sodium currents. Neuroscience. 2004;127:63–68. doi: 10.1016/j.neuroscience.2004.04.040. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sombati S, Coulter DA. Effects of topiramate on sustained repetitive firing and spontaneous recurrent seizure discharges in cultured hippocampal neurons. Epilepsia. 2000;41 s1:S40–S44. doi: 10.1111/j.1528-1157.2000.tb06048.x. [DOI] [PubMed] [Google Scholar]
- Faught E. Treatment of refractory primary generalized epilepsy. Rev Neurol Dis. 2004;1 s1:S34–S43. [PubMed] [Google Scholar]
- Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones. J Physiol. 2000;528:291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JW, III, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultures hippocampal neurons. Epilepsia. 2000;41 s1:S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Godoy MG, Cukierman S. Diacylglycerol-induced activation of protein kinase C attenuates Na+ currents by enhancing inactivation from the closed state. Eur J Physiol. 1994;429:245–252. doi: 10.1007/BF00374319. [DOI] [PubMed] [Google Scholar]
- Herrero AI, Del Olmo N, Gonzalez-Escalada JR, Solis JM. Two new actions of topiramate: inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology. 2002;42:210–220. doi: 10.1016/s0028-3908(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Kuo CC. A common anticonvulsant binding site for phenytoin carbamazepine and lamotrigine in neuronal Na+ channels. Mol Pharmacol. 1998;54:713–723. [PubMed] [Google Scholar]
- McLean MJ, Bukhari AA, Wamil AW. Effects of topiramate on sodium-dependent action-potential firing by mouse spinal cord nourons in cell culture. Epilepsia. 2000;41 s1:S21–S24. [PubMed] [Google Scholar]
- O'Reilly JP, Cummins TR, Haddad GG. Oxygen deprivation inhibits Na+ currents in rat hippocampal neurons via protein-kinase C. J Physiol. 1997;503:479–488. doi: 10.1111/j.1469-7793.1997.479bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osonoe K, Ogata S, Iwata Y, Mori N. Kindled amygdaloid seizures in rats cause immediate and transient increase in protein kinase activity followed by transient suppression of the activity. Epilepsia. 1994;35:850–854. doi: 10.1111/j.1528-1157.1994.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, Avoli M. Sodium channels as molecular targets for antiepileptic drugs. Brain Res Rev. 1998;26:16–28. doi: 10.1016/s0165-0173(97)00054-4. [DOI] [PubMed] [Google Scholar]
- Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia. 2000;41 s1:S66–S71. doi: 10.1111/j.1528-1157.2000.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Sander JW. Using topiramate in patients with epilepsy: practical aspects. Can J Neurol Sci. 1998;25:S16–S18. doi: 10.1017/s0317167100034867. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics and mechanism of action. Epilepsia. 2000;41 s1:S3–S9. [PubMed] [Google Scholar]
- Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultures neurons. Epilepsia. 2000;41 s1:S45–S47. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Taverna S, Sancini G, Mantegazza M, Franceschetti S, Avanzini G. Inhibition of transient and persistent Na+ current fractions by the new anticonvulsant topiramate. J Pharmacol Exper Ther. 1999;288:960–968. [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of high-voltage-activated calcium channels in dentate gyrus cells by topiramate. Epilepsia. 2000;41 s1:S52–S60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neurosci Lett. 1997;231:123–126. doi: 10.1016/s0304-3940(97)00543-0. [DOI] [PubMed] [Google Scholar]