Abstract
Background and purpose:
GPRC6A is a novel member of family C of G protein-coupled receptors with so far unknown function. We have recently described both human and mouse GPRC6A as receptors for L-α-amino acids. To date, functional characterization of wild-type GPRC6A has been impaired by the lack of activity in quantitative functional assays. The aim of this study was thus to develop such an assay and extend the pharmacological characterization of GPRC6A.
Experimental approach:
We have engineered a novel cell-based inositol phosphate turnover assay for wild-type mouse GPRC6A based on transient co-expression with the promiscuous GαqG66D protein, known to increase receptor signalling sensitivity. This assay allowed for measurements of L-α-amino acid potencies. Furthermore, in combination with an assay measuring inward currents at Ca2+-activated chloride channels in Xenopus oocytes, the divalent cation-sensing ability of the receptor was examined.
Key results:
Using our novel assay, we demonstrate that the basic L-α-amino acids ornithine, lysine, and arginine are the most potent agonists at wild-type mouse GPRC6A. Using two different assay systems, we show that divalent cations do not activate the Gq signalling pathway of mouse GPRC6A per se but positively modulate the amino-acid response.
Conclusions and Implications:
This is the first reported assay for a wild-type GPRC6A successfully applied for quantitative pharmacological characterization of amino acid and divalent cation responses at mouse GPRC6A. The assay enables further search for GPRC6A ligands such as allosteric modulators, which may provide essential information about the physiological function of GPRC6A.
Keywords: GPRC6A, G protein-coupled receptor, promiscuous G proteins, L-α-amino acids, divalent cations, positive modulation
Introduction
The G protein-coupled receptor, family C, group 6, subtype A (GPRC6A) belongs to a large family of G protein-coupled receptors (GPCRs), which includes the calcium-sensing receptor (CaR) (Brown et al., 1993), the metabotropic glutamate (mGlu) receptors (Pin and Duvoisin, 1995), the GABAB receptors (Kaupmann et al., 1997), and the T1R taste receptors (Hoon et al., 1999). All members share the same structural characteristics: a large extracellular amino-terminal domain containing the endogenous ligand binding region and a seven-transmembrane domain responsible for the interaction with the G protein (Pin et al., 2003).
We recently cloned human GPRC6A (hGPRC6A) and showed it to be a promiscuous L-α-amino-acid receptor with preference for basic amino acids (Wellendorph and Bräuner-Osborne, 2004; Wellendorph et al., 2005). Wild-type hGPRC6A is not surface expressed in mammalian cell lines, which complicated the characterization of the receptor. However, a chimeric receptor comprised of the agonist-binding domain of hGPRC6A fused to the seven-transmembrane domain of the closely related goldfish receptor 5.24 (termed h6A/5.24) was expressed on the cell surface and was functional through the Gαq pathway (Wellendorph et al., 2005). In contrast to hGPRC6A, mouse GPRC6A (mGPRC6A) is surface expressed in heterologous expression systems and stimulates Ca2+-activated chloride channels in Xenopus oocytes upon activation. Expression in Xenopus oocytes has previously allowed qualitative pharmacological analysis which showed that mGPRC6A, like hGPRC6A, is a promiscuous L-α-amino acid receptor. However, agonist potencies could not be established using the Xenopus oocyte expression system, and attempts to establish a quantitative pharmacological assay for mGPRC6A have so far failed (Kuang et al., 2005; Wellendorph et al., 2005).
The use of chimeric Gα proteins in basic research and in characterization of GPCRs has been very successful (Kostenis et al., 2005b). In this regard, the carboxy terminus of the G protein α subunit (Conklin et al., 1993, 1996) and a conserved glycine within the linker I region of the Gαq subunit (G66) (Heydorn et al., 2004; Kostenis et al., 2005a) have been shown to be key determinants for the specificity of the interaction between the receptor and the G protein. In this study, we explore the ability of a mutated Gαq protein to enhance the signalling sensitivity of wild-type mGPRC6A.
Several members of family C GPCRs are capable of sensing divalent cations. CaR is directly activated by Ca2+ and Mg2+ (Brown et al., 1993), and both the GABAB receptor (Wise et al., 1999; Galvez et al., 2000) and the mGlu subtype 1 receptor (Saunders et al., 1998; Francesconi and Duvoisin, 2004) have been reported to be positively modulated by Ca2+. Among human family C receptors, GPRC6A is phylogenetically most closely related to CaR (34% overall amino-acid identity) (Wellendorph and Bräuner-Osborne, 2004) but, interestingly, phylogenetic analysis of the amino acids lining the predicted orthosteric ligand binding pocket shows that this segment of GPRC6A is most closely related to the mGlu receptors (Silve et al., 2005). From the similarity to both CaR and mGlu receptors, it is tempting to speculate that GPRC6A may also be either activated or positively modulated by divalent cations. Previous experiments in the Xenopus oocyte expression system have shown that GPRC6A is not activated by 4–5 mM Ca2+ (Kuang et al., 2005; Wellendorph et al., 2005), which are concentrations sufficient to fully activate CaR (Brown et al., 1993). However, it has been demonstrated that the response to some L-amino acids is potentiated by Ca2+ (Kuang et al., 2005). In contrast to these findings, it was recently reported that Ca2+ and Mg2+ directly activates mGPRC6A heterologously expressed in mammalian cells when measuring extracellular signal-regulated kinase phosphorylation, serum-response-element promotor-luciferase reporter activity and increase in intracellular calcium in Gαqi5 co-transfected cells (Pi et al., 2005), although high concentrations of cation (⩾20 mM) were needed for obtaining robust responses in the majority of the assays. The conflicting findings pertaining to the effects of Ca2+ and Mg2+ at GPRC6A encouraged us to expand the studies of mGPRC6A sensitivity to divalent cations.
We report the development of a novel quantitative assay system, which allowed us to determine the potencies of various L-α-amino acids at wild-type mGPRC6A. Furthermore, we used the Xenopus oocyte expression system and the novel assay to demonstrate that mGPRC6A is not directly activated, but was positively modulated by the divalent cations Ca2+ and Mg2+.
Methods
Cell culture and transfections
tsA201 cells (a transformed HEK293 cell line) (Chahine et al., 1994) were cultured in GlutaMAX-I Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% dialyzed fetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 μg ml−1) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Constructs encoding mGPRC6A and the indicated Gα protein constructs were transiently co-transfected into cells at a 1:1 ratio using PolyFect according to the manufacturer's protocol (Qiagen, West Sussex, UK). PolyFect was similarly used for transfections of constructs encoding the human glucagon-like peptide-1 (GLP-1) receptor, the rat GABAB(1b) and GABAB(2) receptors, the rat CaR and the human 5-HT2C receptor.
Inositol phosphate (IP) turnover assay
The day after transfection, tsA201 cells were split into poly-D-lysine-coated 96-well tissue culture plates in inositol-free DMEM, supplemented with 10% dialyzed fetal bovine serum, penicillin (100 U ml−1), streptomycin (100 μg ml−1) and 0.15 MBq ml−1 myo-[2-3H]inositol (GE Healthcare, Buckinghamshire, UK). Two days after transfection, cells were washed with assay buffer 1 (Hanks' Balanced Salt Solution (HBSS) containing 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2 and 1 mg ml−1 BSA, pH 7.4), and preincubated in 100 μl assay buffer 1 for 2 × 2 h at 37°C, as previously described (Kuang et al., 2003; Wellendorph et al., 2005). The cells were then washed and subsequently incubated in 50 μl assay buffer 2 (HBSS containing 1 mM CaCl2, 1 mM MgCl2 and 20 mM LiCl) for 30 min at 37°C. Following this incubation, the cells were stimulated with 50 μl of the indicated agonists in assay buffer 2 for 30 min at 37°C. When experiments were conducted in the presence of varying concentrations of Mg2+, the cells were stimulated with 50 μl of the indicated agonist in HBSS containing 1 mM CaCl2, 20 mM LiCl, and the indicated concentrations of MgCl2.
The reactions were stopped by exchanging the buffer with 50 μl 10 mM ice-cold formic acid and incubating the cells at 4°C for at least 30 min. Yttrium silicate scintillation proximity assay beads (GE Healthcare, Buckinghamshire, UK) were used for measuring radioactivity from generated [3H]IP, essentially as previously described (Brandish et al., 2003). In brief, 20 μl of the formic acid cell extracts were transferred to white 96-well plates and 1 mg yttrium silicate scintillation proximity assay beads suspended in 80 μl water added to each well. The plates were sealed, shaken vigorously for 1 h and centrifuged at 1500 r.p.m. for 5 min. Radioactivity was quantified in a Packard TopCount microplate scintillation counter and responses read as counts per minute (CPM). All experiments were performed in triplicate and repeated in at least three independent experiments.
cAMP assay
The day after transfection, tsA201 cells were split into poly-D-lysine-coated 96-well tissue culture plates in GlutaMAX-I DMEM, supplemented with 10% dialyzed fetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 μg ml−1). Two days after transfection, cells were washed with assay buffer 1 as described above and incubated in assay buffer 1 for 2 × 2 h. The cells were then washed with HBSS and incubated in 50 μl assay buffer 3 (HBSS containing 1 mM CaCl2, 1 mM MgCl2 and 1 mM 3-isobutyl-1-methylxanthine) for 20 min at 37°C. Following this incubation, the cells were stimulated with 50 μl of the indicated ligand in assay buffer 3 for 10 min at 37°C in the absence or presence of 10 μM forskolin. The agonist responses were determined as the increase or decrease in formation of cAMP, which was measured by use of the cAMP-[125I] Direct Biotrak Assay kit, according to the manufacturer's protocol (GE Healthcare, Buckinghamshire, UK).
Electrophysiology
Preparation of oocytes from Xenopus laevis and injection with in vitro-transcribed cRNA encoding mGPRC6A were carried out as previously described (Wellendorph et al., 2005). Whole-cell currents were recorded on oocytes 4–5 days after injection using two-electrode voltage clamp at −80 mV in divalent cation-free Ringer's solution. Unless otherwise noted, the divalent cation-free Ringer's solution contained 115 mM NaCl, 2.5 mM KCl and 10 mM HEPES (pH 7.6). Recordings were performed at ambient temperatures (20–22°C) using the OC-725C Oocyte Clamp amplifier (Warner Instruments, Hamden, CT, USA) with a Digidata 1322A interface (Molecular Devices, Sunnyvale, CA, USA). The pClamp9 suite of programs (Molecular Devices, Sunnyvale, CA, USA) was used to control stimulation parameters and data acquisition. Currents were digitized at 100 Hz. The microelectrodes were fabricated from borosilicate glass capillaries (GC150TF-10, Harvard Apparatus, Holliston, MA, USA) and pulled on a PC-10 puller (Narishige Instruments, Tokyo, Japan). Microelectrodes were filled with 3 M KCl and had 0.5–2.5 MΩ resistance. The ligands were dissolved in Ringer's solution and applied to the oocytes by gravity-driven perfusion using a Valvebank 8 (Automate Scientific, San Francisco, CA, USA). Absence of divalent cations in the Ringer's solution resulted in drift of the recorded baseline current of voltage-clamped oocytes corresponding to a 150–300 nA increase in the inward current per minute. The drift was approximately linear and was ameliorated when 1.8 mM CaCl2 or 1.8 mM MgCl2 was included in the Ringer's solution. Representative traces of recordings in divalent cation-free Ringer's solution were corrected for the drift of baseline current using the Clampfit program included in the pClamp9 suite of programs (Molecular Devices, Sunnyvale, CA, USA).
Data analysis
Concentration–response curves were analyzed using Prism 4.0b (GraphPad Software, San Diego, CA, USA). The curves were fitted by non-linear regression using the Equation Y=Ymin+(Ymax−Ymin)/(1+10^((log EC50−X)*nH)), where X is the logarithm of the agonist concentration, Y is the response, Ymax is the maximal response and Ymin the minimal response, EC50 is the agonist concentration that produces half-maximum response and nH denotes the Hill slope. The maximal concentration used for generation of concentration–response curves was 10 mM. Owing to the low potency of L-Ser, this compound was tested at concentrations eliciting submaximal effects. Using the assumption that L-Ser is a full agonist at the receptor, the concentration–response curves were fitted to the maximal response to L-Arg obtained at 10 mM, which was always included as a control. Inactive amino acids were tested at 1 mM for antagonism of the mGPRC6A response mediated by 100 μM L-ornithine (L-Orn).
Statistical procedures
Statistical analysis of the results was performed where appropriate. An unpaired, one-tailed t-test was performed to determine whether each of the tested Gα proteins upon cotransfection with mGPRC6A gave rise to a significant increase in the observed response to 1 mM L-Orn. Significant differences between potencies of agonists were calculated using one-way analysis of variance (ANOVA) followed by Tukey's test. One-way ANOVA followed by Dunnett's test, was used to compare all measurements in a given experiment to the control. Specific details are indicated in the figure and table legends. Statistical significance was determined at the following levels: *P<0.05, **P<0.01, ***P<0.001.
Materials
GlutaMAX-I DMEM, dialyzed fetal bovine serum, penicillin, streptomycin, HBSS and BSA were all from Invitrogen (Paisley, UK). Inositol-free DMEM was homemade from compounds purchased from Sigma-Aldrich (St Louis, MO, USA). All buffer reagents and tested compounds were from Sigma-Aldrich (St Louis, MO, USA).
Results
Development of a robust functional assay for mGPRC6A using promiscuous Gα proteins
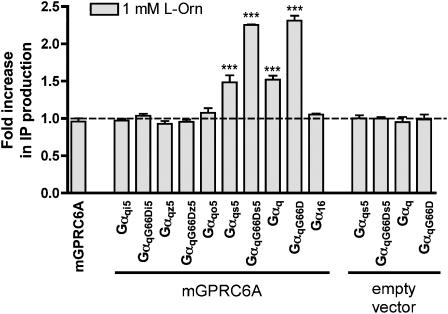
It has been demonstrated that mGPRC6A signals through the Gq pathway (Kuang et al., 2005; Wellendorph et al., 2005). In our efforts to develop a functional assay for wild-type mGPRC6A, we therefore applied an IP turnover assay to tsA cells transiently expressing mGPRC6A. However, we were unable to observe an increase in the IP production in response to 1 mM of the known agonist L-Orn (Figure 1).
Figure 1.
Ability of mGPRC6A to signal through various Gα proteins. tsA cells co-transfected with either mGPRC6A or empty vector, and the indicated Gα proteins were stimulated with 1 mM L-Orn for 30 min at 37°C. The formation of IP was determined as described under Methods. Results are shown as fold increase in IP production upon ligand stimulation, normalized to non-stimulated cells. Data are means±s.d. of triplicate determinations of a single representative experiment. Two additional experiments gave similar results. An unpaired, one-tailed t-test was performed to determine whether each observed response was significantly larger than the corresponding control: ***P<0.001.
In an attempt to increase the signalling sensitivity of mGPRC6A, we co-transfected mGPRC6A with a number of different chimeric and mutated Gα proteins and measured the increase in IP production upon stimulation with 1 mM L-Orn. No ligand-dependent increase in IP production could be detected in cells co-transfected with mGPRC6A and Gαq chimeras containing the carboxy–terminus of Gαi, Gαz and Gαo. However, cotransfection of mGPRC6A and Gαqs5, followed by ligand stimulation resulted in a measurable increase in IP formation (∼1.5-fold) relative to non-stimulated cells, and the ligand-stimulated increase in IP formation was even more pronounced in cells co-transfected with mGPRC6A and the mutated GαqG66Ds5 protein (∼2.2-fold). Ligand stimulation of cells co-transfected with mGPRC6A and Gαq or GαqG66D resulted in increased IP production of the same magnitudes as those observed with cell co-transfected with mGPRC6A and Gαqs5 or GαqG66Ds5, respectively. No increase in IP production could be detected in cells co-transfected with mGPRC6A and Gα16 (Figure 1). As the observed increase in IP production could be related to the coupling of the G proteins to an endogenous receptor, we co-transfected the cells with empty vector and each of the pertinent G proteins. Cotransfection with Gαqs5, GαqG66Ds5, Gαq, or GαqG66D did not give rise to increased IP formation upon application of L-Orn (Figure 1).
Mammalian cell culture media contain high levels of L-α-amino acids, which are agonists at mGPRC6A and therefore likely to cause desensitization of the receptor. In the developed IP turnover assay protocol, an additional incubation in assay buffer for 2 × 2 h before measurement of responses was therefore included and proven to enhance the signal-to-noise ratio (data not shown). The usefulness of such preincubation steps has also previously been demonstrated in generating functional assays for GPRC6A and the closely related goldfish receptor 5.24 (Kuang et al., 2003; Wellendorph et al., 2005). All experiments in mammalian cells were thus performed with this preincubation step.
The GαqG66D protein is applicable for functional characterization of mGPRC6A
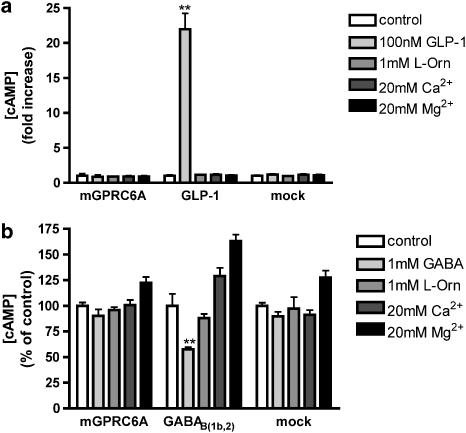
The Gαqs5 protein has been reported to allow Gαs-coupled receptors to couple to the Gq pathway (Conklin et al., 1993, 1996). Contrary to the previous reports of coupling of mGPRC6A to Gαq, the results from cotransfection of mGPRC6A and Gαqs5 indicate that mGPRC6A may also signal through Gαs. In order to clarify the significance of coupling of mGPRC6A to the Gs pathway, we measured the ligand-dependent formation of cAMP in a functional assay. Application of agonist did not evoke a response in cells transiently expressing mGPRC6A, whereas the GLP-1 receptor, used as positive control, gave rise to a 21±2-fold (mean±s.e.m.) increase in cAMP formation in response to application of 100 nM GLP-1 (Figure 2a). These results indicate that the indirectly observed Gαs coupling of mGPRC6A is not sufficient for direct coupling to the Gs pathway. Although we did not see any activation of the Gαi pathway using the chimeric/mutated Gα proteins (Figure 1), we also tested whether activation of mGPRC6A led to a decrease in cAMP in forskolin-stimulated cells. Application of agonist did not evoke a response in cells transiently expressing mGPRC6A, whereas the GABAB(1b,2) receptor, used as positive control, gave rise to a significant decrease in cAMP formation in response to application of 1 mM GABA (Figure 2b). As cotransfection of mGPRC6A with either GαqG66D or GαqG66Ds5 gave a similar increase in IP formation, we therefore decided that the GαqG66D protein, which theoretically explores the previously reported Gαq pathway, was appropriate for further characterization of mGPRC6A.
Figure 2.
Ability of mGPRC6A to (a) activate or (b) inhibit cAMP formation. tsA cells transfected with mGPRC6A, GLP-1, GABAB(1b,2) or empty vector were stimulated with the indicated ligands for 10 min at 37°C in either the absence (a) or presence (b) of 10 μM forskolin. The formation of cAMP was determined as described under Methods. Results are shown (a) as fold increase in cAMP production upon ligand stimulation, normalized to non-stimulated cells and (b) as percent cAMP concentration compared with non-stimulated cells. Data are means±s.d. of triplicate determinations of a single representative experiment. Two additional experiments gave similar results. Asterisks indicate significant stimulation (a) or inhibition (b) compared with the control: **P<0.01 (ANOVA followed by Dunnett's test).
Quantitative pharmacological characterization of amino acids at mGPRC6A reveals a preference for basic L-α-amino acids
Next, we used the IP turnover assay to determine the activity of L-Orn, L-citrulline (L-Cit), and the 20 proteinogenic amino acids, some of which have been shown to activate both hGPRC6A and mGPRC6A in the Xenopus oocyte expression system (Wellendorph et al., 2005). Basic amino acids were found to be the most potent agonists at mGPRC6A with the following rank-order of potency: L-Orn ⩾L-Lys>L-Arg. Also, L-Cys, L-Ala, Gly and L-Ser were found to be agonists (Table 1). Other tested L-α-amino acids, as well as all corresponding D-α-amino acids, were inactive when tested in a concentration of 1 mM. All active amino acids were found to be full agonists at mGPRC6A except Gly, which displayed partial agonism with a maximal response of 49±9% (mean±s.e.m.) relative to the maximal activation of mGPRC6A obtained with 10 mM L-Arg. In agreement with the observation of partial agonism, 10 mM Gly was able to inhibit a response mediated by 500 μM L-Orn by 33±2% (mean±s.e.m.). Furthermore, L-Ser was tested at concentrations eliciting submaximal effects and the potency estimated using the assumption of full agonism as described under data analysis.
Table 1.
Agonist potencies of L-α-amino acids at mGPRC6A transiently co-expressed with GαqG66D in tsA cells
| L-α-amino acid | EC50 (μM) | pEC50±s.e.m. | N | Concentration of L-α-amino acid (mean±s.d.) in mouse plasmaa (μM) |
|---|---|---|---|---|
| L-Orn | 63.6 | 4.20±0.001 | 3 | 86±20 |
| L-Lys | 135 | 3.97±0.18 | 4 | 366±55 |
| L-Arg | 284 | 3.58±0.08b | 6 | 137±22 |
| L-Cys | 356c | 3.46±0.09b | 3 | Not determined |
| L-Ala | 486 | 3.41±0.18b,d | 4 | 431±85 |
| Gly | 538e | 3.30±0.09b,f | 4 | 340±54 |
| L-Ser | 1160g | 2.94±0.03b,d,f | 3 | 181±25 |
The IP turnover assay was used to determine the EC50 values of various amino acids at mGPRC6A. The pEC50 values of all the listed amino acids were compared to determine statistically significant differences (P<0.05; ANOVA followed by Tukey's test).
Taken from Komarov and Reddy (1998).
Significantly different from L-Orn.
L-Cys displayed a small but significant effect on mock-transfected cells at high concentrations (⩾1 mM). The EC50 value might thus be overestimated.
Significantly different from L-Lys.
Gly displayed partial agonism with a maximal response of 49±9% (mean±s.e.m.) relative to the maximal activation of mGPRC6A obtained by stimulation with 10 mM L-Arg.
Significantly different from L-Arg.
Owing to low potency, L-Ser was tested at concentrations eliciting sub-maximal effect. Assuming full agonism, concentration–response curves for this compound were fitted using the maximal response to L-Arg (10 mM), which was always included as control.
None of the inactive L-α-amino acids or the D-α-amino acids were antagonists when tested against 100 μM L-Orn (Ki>1 mM). None of the active amino acids showed any effect on cells transfected with GαqG66D alone or mock-transfected cells when tested in 10 mM concentrations. An exception was L-Cys, which in the IP turnover assay elicited a small response in mock-transfected cells when applied in high concentrations (⩾1 mM). The measured potency of this compound may therefore be underestimated, as higher concentrations of L-Cys are needed in order to reach maximal responses (Table 1).
mGPRC6A is not activated but rather positively modulated by the divalent cations Ca2+ and Mg2+
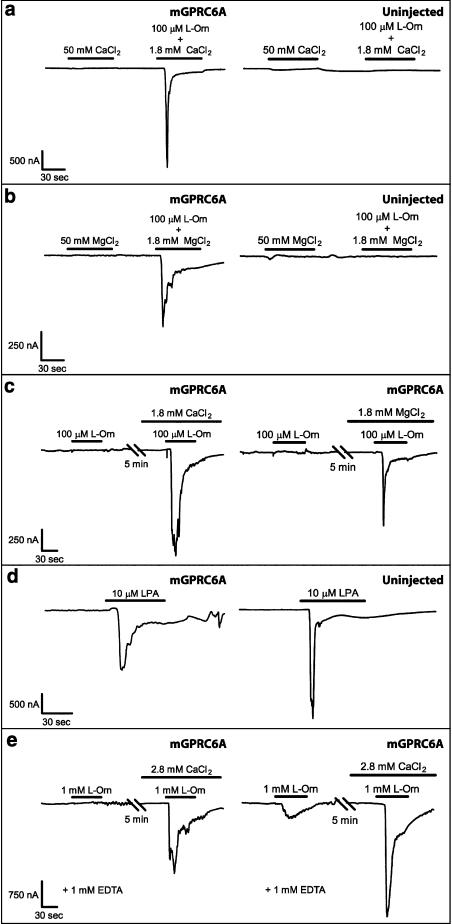
In order to study the cation-sensing ability of mGPRC6A, we initially used the Xenopus oocyte expression system to examine the response to Ca2+ and Mg2+ in oocytes injected with cRNA encoding mGPRC6A. No responses could be detected upon application of 50 mM Ca2+ or Mg2+ in mGPRC6A-expressing Xenopus oocytes, whereas subsequent addition of 100 μM L-Orn and either 1.8 mM Ca2+ or Mg2+ elicited robust responses. No responses were observed for uninjected oocytes when 50 mM Ca2+, 50 mM Mg2+, or 100 μM L-Orn (including either 1.8 mM Ca2+ or Mg2+) were applied (Figure 3a and b). These results indicate that the divalent cations Ca2+ and Mg2+ do not activate mGPRC6A directly.
Figure 3.
Representative traces obtained from two-electrode voltage clamp recordings on Xenopus oocytes that are either uninjected or injected with cRNA encoding mGPRC6A. (a) No responses could be detected at Ca2+-activated chloride channels upon application of 50 mM CaCl2 alone in either mGPRC6A-expressing (N=9) or uninjected Xenopus oocytes (N=6). The divalent cation-free Ringer's solution was supplemented with 1.8 mM MgCl2. (b) No responses could be detected at Ca2+-activated chloride channels upon application of 50 mM MgCl2 alone in either mGPRC6A-expressing (N=5) or uninjected Xenopus oocytes (N=4). The divalent cation-free Ringer's solution was supplemented with 1.8 mM CaCl2. (c) L-Orn (100 μM) did not activate mGPRC6A in the absence of divalent cations. Co-application of 100 μM L-Orn and either 1.8 mM CaCl2 (N=7) or 1.8 mM MgCl2 (N=8) restored mGPRC6A-mediated responses. Recordings were first made in divalent cation-free Ringer's solution, and then the oocytes were allowed to equilibrate for 5 min in divalent cation-free Ringer's solution supplemented with either 1.8 mM CaCl2 or 1.8 mM MgCl2. The recordings shown were from two different oocytes. The part of the traces recorded in divalent cation-free Ringer's solution without CaCl2 or MgCl2 was corrected for drift of baseline current, as described in the Methods section. No mGPRC6A-mediated responses were observed upon application of either 1.8 mM CaCl2 alone or 1.8 mM MgCl2 alone to mGPRC6A-expressing oocytes voltage clamped in divalent cation-free Ringer's solution. (d) In both mGPRC6A-expressing (N=3) and uninjected (N=3) oocytes, 10 μM LPA was able to evoke responses at Ca2+-activated chloride channels mediated by endogenously expressed GPCRs in the absence of divalent cations. The recordings shown were from two different oocytes. The traces were recorded in divalent cation-free Ringer's solution and corrected for drift of baseline current as described in the Methods section. (e) Recordings were made in a total of 12 mGPRC6A-expressing oocytes. Of these, six oocytes did not respond to application of 1 mM L-Orn in divalent cation-free Ringer's solution supplemented with 1 mM EDTA (N=6). However, six oocytes showed small responses to 1 mM L-Orn under the same conditions (N=6). Recordings were first made in divalent cation-free Ringer's solution supplemented with 1 mM EDTA, and the oocytes were then allowed to equilibrate for 5 min in divalent cation-free Ringer's solution supplemented with 1 mM EDTA and 2.8 mM CaCl2. The recordings shown were from two different oocytes. The part of the traces recorded in divalent cation-free Ringer's solution without CaCl2 was corrected for drift of baseline current, as described in the Methods section.
Next, we examined activation of GPRC6A in the absence of divalent cations. Under these conditions, 100 μM L-Orn was unable to activate mGPRC6A. However, subsequent co-application of 100 μM L-Orn and either 1.8 mM Ca2+ or Mg2+ could restore the mGPRC6A-mediated response to L-Orn (Figure 3c). To demonstrate that the Ca2+-activated chloride channels of the oocytes were not compromised by the complete absence of divalent cations in the buffer, lysophosphatidic acid (LPA), which activates endogenously expressed GPCRs (Guo et al., 1996), was applied to the oocytes. In both mGPRC6A-expressing and uninjected oocytes, 10 μM LPA evoked robust responses at Ca2+-activated chloride channels in the absence of divalent cations (Figure 3d).
These results could either indicate that the presence of both an amino acid and either of the divalent cations Ca2+ and Mg2+ are necessary to activate mGPRC6A or that the response to amino acids is potentiated by the divalent cations Ca2+ and Mg2+. To examine this in further detail, we used ethylenediaminetetraacetic acid (EDTA) to remove putative trace amounts of extracellular divalent cations and examined the response to a higher concentration of L-Orn (1 mM). Even though half of the oocytes still showed no response to L-Orn, the other half were activated by L-Orn and showed a weak response. Subsequent addition of Ca2+ gave rise to responses upon application of 1 mM L-Orn, which demonstrates that the oocytes were not compromised by the use of EDTA (Figure 3e).
Examination of mGPRC6A expressed in mammalian cells confirms that Mg2+ is not an agonist at the receptor
Next, we examined the effects of Ca2+ and Mg2+ in tsA cells co-transfected with mGPRC6A and GαqG66D in the IP turnover assay. Ca2+ showed significant effect on mock-transfected cells (data not shown), which limited the studies to Mg2+. However, to ensure proper cell adhesion, 1 mM Ca2+, which is close to the physiologically relevant concentration, was present in all experiments.
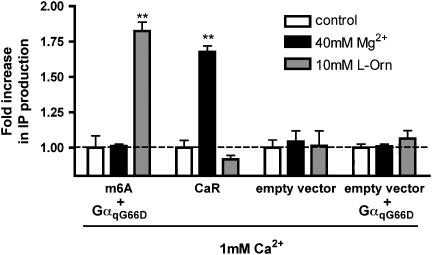
Initially, we compared the effect of 40 mM Mg2+ with the effect of 10 mM L-Orn at mGPRC6A. Rat CaR was used as a reference. The results show that L-Orn, but not Mg2+, activates mGPRC6A, whereas the reverse is shown for rat CaR. No response was observed in mock-transfected cells or cells co-transfected with empty vector and GαqG66D when either Mg2+ or L-Orn was applied (Figure 4).
Figure 4.
mGPRC6A is not activated by Mg2+ per se. tsA cells co-transfected with mGPRC6A and GαqG66D or rat CaR were incubated with either 40 mM Mg2+ or 10 mM L-Orn for 30 min at 37°C. Mock-transfected cells and cells co-transfected with empty vector and GαqG66D were used as control. All experiments were performed in the presence of 1 mM Ca2+. The formation of IP was determined as described under Methods. Results are shown as fold increase in IP production, normalized to the control. Data are means±s.d. of triplicate determinations of a single representative experiment. Two additional experiments gave similar results. Asterisks indicate significant differences compared with the control: **P<0.01 (ANOVA followed by Dunnett's test).
Mg2+ is a positive modulator of mGPRC6A and affects the efficacy of the L-Orn-generated response
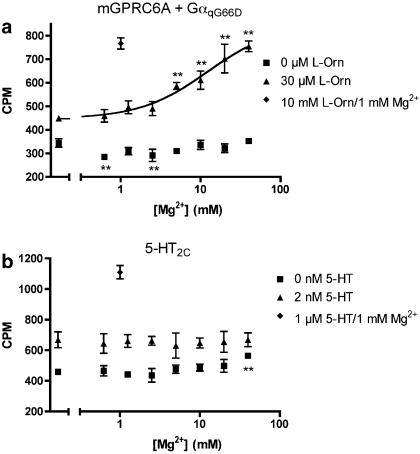
The results from the oocyte experiments indicate that Ca2+ and Mg2+ potentiate the response to L-Orn at mGPRC6A. We wished to study this in further detail using the IP turnover assay with cells co-transfected with mGPRC6A and GαqG66D. The results show that in the presence of 30 μM L-Orn (∼EC25), extracellular Mg2+ increased the magnitude of L-Orn-induced IP formation in a concentration-dependent manner. Again, no response was observed when Mg2+ was applied separately to the cells (Figure 5a). Furthermore, no increase in IP production was observed when 30 μM L-Orn and 40 mM Mg2+ were co-applied to cells co-transfected with empty vector and GαqG66D (data not shown). As the effects of Mg2+ could be either receptor-, assay- or cell-specific, we performed a corresponding experiment in cells transiently expressing the 5-HT2C receptor (Saltzman et al., 1991). When increasing concentrations of Mg2+ was applied in the absence or presence of 2 nM 5-HT (∼EC25), no increase in IP formation was observed (Figure 5b). These results demonstrate that potentiation of responses to L-Orn by Mg2+ is specific for mGPRC6A.
Figure 5.
Measurement of IP production as a function of extracellular Mg2+ concentration at mGPRC6A and 5-HT2C. (a) Response to Mg2+ measured in cells transiently co-expressing mGPRC6A and GαqG66D in the absence or presence of 30 μM L-Orn (∼EC25). The response to maximal concentration (10 mM) of L-Orn in the presence of 1 mM Mg2+ was used as control. (b) Response to Mg2+ measured in cells transiently expressing the 5-HT2C receptor, in the absence or presence of 2 nM 5-HT (∼EC25). The response to maximal concentration (1 μM) of 5-HT in the presence of 1 mM Mg2+ was used as control. All experiments were performed in the presence of 1 mM Ca2+. The formation of IP was determined as described under Methods. Results are expressed as CPM and are means±s.d. of triplicate determinations of a single representative experiment. Two additional experiments gave similar results. Asterisks indicate significant differences from the measurements where only Ca2+ was present: **P<0.01 (ANOVA followed by Dunnett's test).
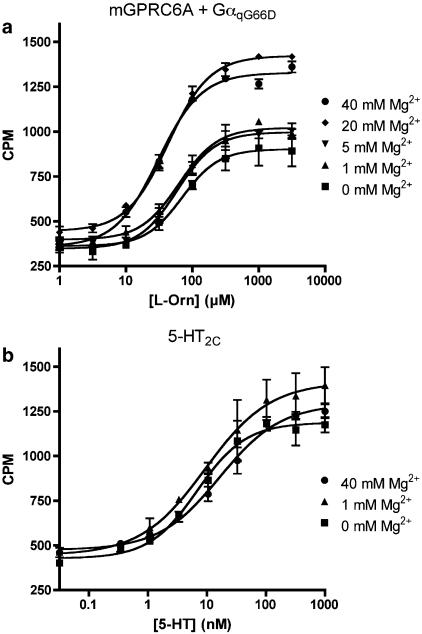
Next, we generated concentration–response curves of L-Orn in the presence of varying concentrations of Mg2+. The results show that the maximal response to L-Orn is markedly enhanced by increasing concentrations of Mg2+ with a maximal response of 192±19% at 40 mM Mg2+ relative to the response measured in the presence of 1 mM Mg2+. Furthermore, the EC50 value is significantly decreased upon addition of 40 mM Mg2+, relative to the EC50 value obtained in the presence of 1 mM Mg2+ (P<0.05). All experiments were performed in the presence of 1 mM Ca2+ (Figure 6a and Table 2). No increase in IP production was observed upon co-application of the maximal used concentration of L-Orn and Mg2+ at cells co-transfected with empty vector and GαqG66D (data not shown).
Figure 6.
Concentration–response curves of ligands at mGPRC6A and 5-HT2C, respectively, in the presence of varying concentrations of extracellular Mg2+. (a) Concentration–response curves of L-Orn with 0, 1, 5, 20, and 40 mM Mg2+ present generated from stimulation of IP formation in tsA cells transiently co-expressing mGPRC6A and GαqG66D. (b) Concentration–response curves of 5-HT with 0, 1, and 40 mM Mg2+ present generated from stimulation of IP formation in tsA cells transiently expressing the 5-HT2C receptor. All experiments were performed in the presence of 1 mM Ca2+. The formation of IP was determined as described under Methods. Results are expressed as CPM and are means±s.d. of triplicate determinations of a single representative experiment. Two additional experiments gave similar results.
Table 2.
Effect of Mg2+ on the potency and maximal activation of L-Orn at mGPRC6A
| EC50 (μM) | pEC50±s.e.m. | Max±s.e.m.a (%) | n | |
|---|---|---|---|---|
| L-Orn/0 mM Mg2+ | 63.2 | 4.21±0.07 | 103±5 | 3 |
| L-Orn/1 mM Mg2+ | 63.6 | 4.20±0.001 | 100 | 3 |
| L-Orn/5 mM Mg2+ | 61.2 | 4.23±0.10 | 122±6 | 3 |
| L-Orn/20 mM Mg2+ | 39.5 | 4.41±0.05 | 184±18 | 3 |
| L-Orn/40 mM Mg2+ | 34.5 | 4.46±0.03* | 192±19 | 3 |
The IP turnover assay was used to determine the EC50 value of L-Orn in the presence of various concentrations of Mg2+. Significant difference from L-Orn/1 mM Mg2+ (pEC50 value) was determined:
P<0.05 (ANOVA followed by Dunnett's test).
All experiments were performed in the presence of 1 mM Ca2+.
The maximal responses are normalized to the maximal response to L-Orn at 1 mM Mg2+.
Corresponding experiments were performed at 5-HT2C to ensure that the observed modulation of the response was not an assay artefact. In contrast to the effect of Mg2+ on mGPRC6A, the results showed a non-significant increase in the EC50 value of 5-HT at 5-HT2C when the extracellular concentration of Mg2+ was increased from 1 to 40 mM. Furthermore, 40 mM Mg2+ had no marked effect on the maximal response of 5-HT at 5-HT2C (Figure 6b and Table 3).
Table 3.
Effect of Mg2+ on the potency and maximal activation of 5-HT at the 5-HT2C receptor
| EC50 (nM) | pEC50±s.e.m. | Max±s.e.m.a (%) | n | |
|---|---|---|---|---|
| 5-HT/0 mM Mg2+ | 5.51 | 8.28±0.07 | 88±3 | 4 |
| 5-HT/1 mM Mg2+ | 10.8 | 7.98±0.07 | 100 | 3 |
| 5-HT/40 mM Mg2+ | 17.4 | 7.81±0.11 | 90±4 | 4 |
The IP turnover assay was used to determine the EC50 value of 5-HT in the presence of various concentration of Mg2+. Significant difference from 5-HT/1 mM Mg2+ (pEC50 value) was examined: P>0.05 (ANOVA followed by Dunnett's test).
All experiments were performed in the presence of 1 mM Ca2+.
The maximal responses are normalized to the maximal response to 5-HT at 1 mM Mg2+.
Discussion
In this study, we examined the ability of various promiscuous and chimeric Gα proteins to enhance the signalling sensitivity of mGPRC6A. The Gα proteins were chosen for their previously reported ability to couple receptors from various coupling classes to the Gq pathway. Co-expression of wild-type mGPRC6A with Gαqs5, GαqG66Ds5, Gαq or GαqG66D gave rise to measurable responses upon application of the agonist L-Orn, indicating that mGPRC6A can couple to two different signalling pathways, Gq and Gs. We and others have previously shown in Xenopus oocytes that GPRC6A couples to the Gq pathway (Kuang et al., 2005; Wellendorph et al., 2005). In addition, it has been shown that a chimeric receptor containing the large amino-terminal domain of the goldfish receptor 5.24 and the seven-transmembrane domain from mGPRC6A couples to the Gq pathway and elevates intracellular Ca2+ concentrations when activated (Kuang et al., 2005). The coupling to the Gs pathway, as well as a newly reported coupling to the Gi pathway (Pi et al., 2005) are indirectly demonstrated by the coupling of mGPRC6A to Gαqs5 and Gαqi5, respectively. We therefore evaluated the coupling to Gαs and Gαi by examining activation of mGPRC6A in a cAMP assay. However, we were unable to observe agonist-induced responses in the cAMP assay by L-Orn, Ca2+, or Mg2+ and therefore cannot provide any direct indication for the coupling of mGPRC6A to Gαs or Gαi. It is known that the coupling of receptors to multiple G proteins depends on the expression level of both receptor and G proteins (Hermans, 2003; Kukkonen, 2004). The coupling of mGPRC6A to Gαs observed indirectly in the IP turnover assay might therefore be explained by overexpression of the Gαqs5 protein under the experimental conditions. This coupling to Gαs may or may not be physiologically relevant and further examination of the non-Gq signalling pathways in general is required to clarify the separate significance of these.
We have previously shown that mGPRC6A responds to L-Orn, L-Arg, L-Lys, L-Ser and L-Ala in the Xenopus oocyte expression system (Wellendorph et al., 2005). A similar system has been used to show responses at mGPRC6A for the same amino acids as well as Gly, L-His and L-Cys, albeit the two latter compounds were only active when extracellular Ca2+ was elevated from 1.8 to 5 mM (Kuang et al., 2005). The use of the IP turnover assay for cells co-expressing mGPRC6A and GαqG66D allowed determination of the potencies of the endogenous L-α-amino acids. Except for L-His, all the above-mentioned amino acids were agonists at mGPRC6A. As also shown for hGPRC6A (Wellendorph et al., 2005), the basic amino acids are the most potent agonists at mGPRC6A, and most of the amino acids which are agonists at hGPRC6A, are also active at mGPRC6A.
Prominent members of the family C GPCRs are capable of sensing both amino acids and cations. The GABAB receptor is positively modulated by Ca2+ (Wise et al., 1999; Galvez et al., 2000) and the more generally accepted view is that the same is true for the mGlu subtype 1 receptor (Saunders et al., 1998; Francesconi and Duvoisin, 2004). However, some have reported that Ca2+ acts as an agonist at the mGlu subtype 1 receptor (Kubo et al., 1998) and others claim that Ca2+ has no direct effect (Nash et al., 2001). CaR is activated by Ca2+ and Mg2+ (Brown et al., 1993) and the response is reported to be allosterically modulated by a range of L-α-amino acids (Conigrave et al., 2000; Mun et al., 2004). GPRC6A is activated by L-α-amino acids (Kuang et al., 2005; Wellendorph et al., 2005), and we examined the cation-sensing ability of the receptor in the Xenopus oocyte expression system. We were unable to demonstrate activation of mGPRC6A in response to high concentration of either Ca2+ or Mg2+. However, robust responses were observed in response to 100 μM L-Orn in the presence of these divalent cations, and the fact that a response to L-Orn, although weak, was also observed in the absence of divalent cations demonstrates that divalent cations are not a prerequisite for activation of mGPRC6A. Collectively, these data therefore indicated that the response to L-Orn at mGPRC6A was potentiated by both Ca2+ and Mg2+.
Owing to a marked effect of Ca2+ on mock-transfected cells, we were unable to examine the effect of Ca2+ in the IP turnover assay. However, we found that Mg2+ did not independently activate mGPRC6A and that Mg2+ acted as a positive modulator of L-Orn responses on mGPRC6A at relatively high concentrations (⩾5 mM). The results pertaining to the potentiating effect of Mg2+ on L-Orn responses obtained using the IP turnover assay were therefore in accordance with the results obtained at mGPR6A expressed in Xenopus oocytes.
The cation-sensing ability of mGPRC6A corresponds well with the recently reported conservation of the Ca2+-binding pocket of CaR in GPRC6A as well as other family C GPCRs (Silve et al., 2005), and our results question the recently reported direct activation of GPRC6A by Ca2+ and Mg2+ (Pi et al., 2005). However, the observation by Pi et al. (2005) might correlate with the notion that Mg2+ acts as an apparent agonist in the presence of L-amino acids. Accordingly, small amounts of amino acids in the applied assay buffers may explain the reported agonism of Ca2+ and other cations. An interesting alternative explanation is that most of the responses measured by Pi et al. (2005) were mediated through non-Gq pathways. It is thus possible that L-α-amino acids and cations activate different signalling pathways, as has recently been shown to be the case for CaR (Rey et al., 2005) and mGlu subtype 1 receptor (Tateyama and Kubo, 2006). Furthermore, the reported discrepancies in the sensitivity of mGPRC6A to divalent cations could also be explained by differences in the cell lines used.
It remains to be shown whether the physiologically more relevant ligands are L-α-amino acids or divalent cations. A comparison of the EC50 values of the amino acids at mGPRC6A with the plasma concentrations of the amino acids (Table 1) indicate that sensing of these compounds by mGPRC6A is possible at physiologically relevant concentrations. However, the measured EC50 values cannot necessarily be extrapolated to the in vivo situation, as they are generated in a recombinant system notably with overexpression of an artificial promiscuous Gα protein. Regarding the action of Mg2+ and Ca2+ (plasma concentrations of ∼0.45 and ∼1.25 mM, respectively; Fanestil et al., 1999), it seems evident that the response to L-Orn is greatly enhanced by relatively low concentrations of these cations. Furthermore, 1 mM Ca2+, which is close to the physiologically relevant concentration, sufficed to cause a response to L-Orn when measured in the IP turnover assay, a response that was unaffected by the absence or presence of 1 mM Mg2+. Under normal physiological conditions, the activity of GPRC6A may therefore be governed by changes in the level of amino acids rather than minor changes in the level of divalent cations. However, as discussed above, the divalent cations might have more pronounced effects on non-Gq signalling pathways. Further studies are therefore needed to establish the physiologically relevant signalling pathway(s). Given the broad tissue expression of GPRC6A (Wellendorph and Bräuner-Osborne, 2004; Kuang et al., 2005; Pi et al., 2005), it might very well turn out that different ligands and signalling pathways are employed in different tissues.
In conclusion, we have shown that mGPRC6A, like the human and goldfish orthologues (Speca et al., 1999; Wellendorph et al., 2005), is a promiscuous L-α-amino-acid receptor with preference for basic amino acids. In addition, we have shown that mGPRC6A is positively modulated by Ca2+ and Mg2+. Finally, we describe an assay for characterization of ligands at wild-type mGPRC6A, which enables screening and future identification of potent and selective allosteric modulators. Such compounds have proven essential for delineation of the physiological function of many other family C receptors (Mombereau et al., 2004; Xu et al., 2004; Niswender et al., 2005), and will hopefully reveal more information about the physiological function of GPRC6A as well.
Acknowledgments
Anders A Jensen, Anders Balsgaard and Mie Kusk are greatly acknowledged for previous attempts at developing an assay for mGPRC6A in mammalian cells. Dr Evi Kostenis (7TM Pharma A/S, Hørsholm, Denmark) is acknowledged for the generous gift of chimeric and promiscuous Gα proteins and Drs Janet Clark, Alan Saltzman and Stuart Sealfon are acknowledged for the receptor cDNAs. We thank Jan Egebjerg for fruitful discussions and Mark Aplin, Maria Brinck Hansen and Kasper Lind Hansen for technical assistance. This work was supported by the Danish Medical Research Council, Apotekerfonden af 1991, Savværksejer Jeppe Juhl og hustru Ovita Juhls Mindelegat, EU grant HPAW-CT-2002-80057 (PW) and the Novo Scholarship Programme in Biotechnology and Pharmaceutical Sciences (BC).
Abbreviations
- CaR
calcium-sensing receptor
- Cit
citrulline
- CPM
counts per minute
- DMEM
Dulbecco's Modified Eagle's Medium
- GLP-1
glucagon-like peptide-1
- GPCR
G protein-coupled receptor
- GPRC6A
G protein-coupled receptor, family C, group 6, subtype A
- HBSS
Hanks' Balanced Salt Solution
- hGPRC6A
human GPRC6A
- IP
inositol phosphate
- LPA
lysophosphatidic acid
- mGlu
metabotropic glutamate
- mGPRC6A
mouse GPRC6A
- Orn
ornithine
Conflict of interest
The authors state no conflict of interest.
References
- Brandish PE, Hill LA, Zheng W, Scolnick EM. Scintillation proximity assay of inositol phosphates in cell extracts: high-throughput measurement of G-protein-coupled receptor activation. Anal Biochem. 2003;313:311–318. doi: 10.1016/s0003-2697(02)00630-9. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Chahine M, Bennett PB, George AL, Jr, Horn R. Functional expression and properties of the human skeletal muscle sodium channel. Pflügers Arch. 1994;427:136–142. doi: 10.1007/BF00585952. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Herzmark P, Ishida S, Voyno-Yasenetskaya TA, Sun Y, Farfel Z, Bourne HR. Carboxyl-terminal mutations of Gqα and Gsα that alter the fidelity of receptor activation. Mol Pharmacol. 1996;50:885–890. [PubMed] [Google Scholar]
- Fanestil DD, Hyde RH, Blakely P, Vaughn DA. Dietary magnesium, not calcium, regulates renal thiazide receptor. J Am Soc Nephrol. 1999;10:458–563. doi: 10.1681/ASN.V103458. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Divalent cations modulate the activity of metabotropic glutamate receptors. J Neurosci Res. 2004;75:472–479. doi: 10.1002/jnr.10853. [DOI] [PubMed] [Google Scholar]
- Galvez T, Urwyler S, Prézeau L, Mosbacher J, Joly C, Malitschek B, et al. Ca2+ requirement for high-affinity γ-aminobutyric acid (GABA) binding at GABAB receptors: involvement of serine 269 of the GABABR1 subunit. Mol Pharmacol. 2000;57:419–426. doi: 10.1124/mol.57.3.419. [DOI] [PubMed] [Google Scholar]
- Guo Z, Liliom K, Fischer DJ, Bathurst IC, Tomei LD, Kiefer MC, et al. Molecular cloning of a high-affinity receptor for the growth factor-like lipid mediator lysophosphatidic acid from Xenopus oocytes. Proc Natl Acad Sci USA. 1996;93:14367–14372. doi: 10.1073/pnas.93.25.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Ward RJ, Jorgensen R, Rosenkilde MM, Frimurer TM, Milligan G, et al. Identification of a novel site within G protein α subunits important for specificity of receptor-G protein interaction. Mol Pharmacol. 2004;66:250–259. doi: 10.1124/mol.66.2.250. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Komarov AM, Reddy MN. Effect of septic shock on nitrate, free amino acids, and urea in murine plasma and urine. Clin Biochem. 1998;31:107–111. doi: 10.1016/s0009-9120(97)00168-9. [DOI] [PubMed] [Google Scholar]
- Kostenis E, Martini L, Ellis J, Waldhoer M, Heydorn A, Rosenkilde MM, et al. A highly conserved glycine within linker I and the extreme C terminus of G protein α subunits interact cooperatively in switching G protein-coupled receptor-to-effector specificity. J Pharmacol Exp Ther. 2005a;313:78–87. doi: 10.1124/jpet.104.080424. [DOI] [PubMed] [Google Scholar]
- Kostenis E, Waelbroeck M, Milligan G. Techniques: promiscuous Gα proteins in basic research and drug discovery. Trends Pharmacol Sci. 2005b;26:595–602. doi: 10.1016/j.tips.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem. 2005;93:383–391. doi: 10.1111/j.1471-4159.2005.03025.x. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Wang M, Pattabiraman N, Kotra LP, Hampson DR. Molecular similarities in the ligand binding pockets of an odorant receptor and the metabotropic glutamate receptors. J Biol Chem. 2003;278:42551–42559. doi: 10.1074/jbc.M307120200. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP. Regulation of receptor-coupling to (multiple) G proteins. A challenge for basic research and drug discovery. Receptors Channels. 2004;10:167–183. doi: 10.3109/10606820490926151. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Mun HC, Franks AH, Culverston EL, Krapcho K, Nemeth EF, Conigrave AD. The Venus Fly Trap domain of the extracellular Ca2+-sensing receptor is required for L-amino acid sensing. J Biol Chem. 2004;279:51739–51744. doi: 10.1074/jbc.M406164/200. [DOI] [PubMed] [Google Scholar]
- Nash MS, Saunders R, Young KW, Challiss RA, Nahorski SR. Reassessment of the Ca2+ sensing property of a type I metabotropic glutamate receptor by simultaneous measurement of inositol 1,4,5-trisphosphate and Ca2+ in single cells. J Biol Chem. 2001;276:19286–19293. doi: 10.1074/jbc.M007600200. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Jones CK, Conn PJ. New therapeutic frontiers for metabotropic glutamate receptors. Curr Top Med Chem. 2005;5:847–857. doi: 10.2174/1568026054750254. [DOI] [PubMed] [Google Scholar]
- Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- Saltzman AG, Morse B, Whitman MM, Ivanshchenko Y, Jaye M, Felder S. Cloning of the human serotonin 5-HT2 and 5-HT1C receptor subtypes. Biochem Biophys Res Commun. 1991;181:1469–1478. doi: 10.1016/0006-291x(91)92105-s. [DOI] [PubMed] [Google Scholar]
- Saunders R, Nahorski SR, Challiss RA. A modulatory effect of extracellular Ca2+ on type 1α metabotropic glutamate receptor-mediated signalling. Neuropharmacology. 1998;37:273–276. doi: 10.1016/s0028-3908(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Silve C, Petrel C, Leroy C, Bruel H, Mallet E, Rognan D, et al. Delineating a Ca2+ binding pocket within the venus flytrap module of the human calcium-sensing receptor. J Biol Chem. 2005;280:37917–37923. doi: 10.1074/jbc.M506263200. [DOI] [PubMed] [Google Scholar]
- Speca DJ, Lin DM, Sorensen PW, Isacoff EY, Ngai J, Dittman AH. Functional identification of a goldfish odorant receptor. Neuron. 1999;23:487–498. doi: 10.1016/s0896-6273(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. Dual signaling is differentially activated by different active states of the metabotropic glutamate receptor 1α. Proc Natl Acad Sci USA. 2006;103:1124–1128. doi: 10.1073/pnas.0505925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P, Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Braüner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- Wise A, Green A, Main MJ, Wilson R, Fraser N, Marshall FH. Calcium sensing properties of the GABAB receptor. Neuropharmacology. 1999;38:1647–1656. doi: 10.1016/s0028-3908(99)00119-7. [DOI] [PubMed] [Google Scholar]
- Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]