Abstract
Background and purpose:
FTY720 is a potent immunomodulatory prodrug that is converted to its active phosphorylated form by a sphingosine kinase. Here we have studied whether FTY720 mimicked the action of sphingosine-1-phosphate (S1P) and exerted an anti-inflammatory potential in renal mesangial cells.
Experimental approach:
Prostaglandin E2 (PGE2) was quantified by an enzyme-linked immunosorbent-assay. Secretory phospholipase A2 (sPLA2) protein was detected by Western blot analyses. mRNA expression was determined by Northern blot analysis and sPLA2-promoter activity was measured by a luciferase-reporter-gene assay.
Key results:
Stimulation of cells for 24 h with interleukin-1β (IL-1β) is known to trigger increased PGE2 formation which coincides with an induction of the mRNA for group-IIA-sPLA2 and protein expression. FTY720 dose-dependently suppressed IL-1β-induced IIA-sPLA2 protein secretion and activity in the supernatant. This effect is due to a suppression of cytokine-induced sPLA2 mRNA expression which results from a reduced promoter activity. As a consequence of suppressed sPLA2 activity, PGE2 formation is also reduced by FTY720. Mechanistically, the FTY720-suppressed sPLA2 expression results from an activation of the TGFβ/Smad signalling cascade since inhibition of the TGFβ receptor type I by a specific kinase inhibitor reverses the FTY720-mediated decrease of sPLA2 protein expression and sPLA2 promoter activity.
Conclusions and implications:
In summary, our data show that FTY720 was able to mimic the anti-inflammatory activity of TGFβ and blocked cytokine-triggered sPLA2 expression and subsequent PGE2 formation. Thus, FTY720 may exert additional in vivo effects besides the well reported immunomodulation and its anti-inflammatory potential should be considered.
Keywords: FTY720, sphingosine-1-phosphate, TGFβ2, Smad, phospholipase A2, prostaglandins, mesangial cell
Introduction
Inflammation is an important cellular response triggered by various mechanical, chemical or immunological stress factors and is regulated by a delicate balance between local factors that finally determine the outcome of the disease process: progression or resolution. The inflammatory response is a complex and tightly regulated sequence of events that starts with an initial production of pro-inflammatory mediators that recruit professional inflammatory cells to clear the offending trigger. This is followed by an anti-inflammatory phase, in which resident cells may acquire the potential for protecting themselves from further activation and injury.
A hallmark of many forms of inflammatory kidney diseases, besides the production of a variety of inflammatory mediators, is the increased proliferation of mesangial cell and the enhanced synthesis of extracellular matrix components, which ultimately leads to the pathogenesis of glomerulosclerosis (Kashgarian and Sterzel, 1992; Pfeilschifter, 1989, 1994). The mechanisms underlying the proliferative response are still not completely understood although the involvement of mitogen-activated protein kinases (MAPKs) seems clear (Huwiler et al., 1995; Seger and Krebs, 1995).
Sphingosine 1-phosphate (S1P) is a sphingolipid molecule generated from ceramide by the sequential action of neutral ceramidase followed by sphingosine kinase (Olivera and Spiegel, 1993; Spiegel, 1999; Huwiler et al., 2000a, 2000b; Spiegel and Milstien, 2003). It has gained increasing attention owing to its mitogenic property for many cell types including mesangial cells (Zhang et al., 1991; Olivera and Spiegel, 1993; Katsuma et al., 2002). S1P mainly acts via cell surface receptors that were first identified as endothelial differentiation genes (edg) (Lee et al., 1998) and have now been renamed by the International Union of Pharmacological Societies (IUPHAR) as S1P receptors (Chun et al., 2002). These receptors are classical G-protein-coupled receptors and initiate a multitude of signaling cascades, including the MAPKs, the stress-activated protein kinase/N-terminal c-Jun kinase, the p38-MAPK, protein kinase B, but also the Smad signaling cascade (Xin et al., 2004).
Recently, a novel potent immunomodulatory agent, 2-amino-2-[2-(4-octyl-phenyl)ethyl]-1,3-propanediol hydrochloride (FTY720), was described that shows a different mode of action than the commonly used immunosuppressive drugs like cyclosporine A or FK506 that block the proliferation of lymphocytes by interfering with interleukin-2 production by T cells. FTY720 induces a characteristic depletion of circulating lymphocytes from peripheral blood by promoting lymphocyte homing into secondary lymphoid tissues (Chiba et al., 1998).
FTY720 shows structural similarities with the sphingolipid, sphingosine and can be phosphorylated by the sphingosine kinases (Brinkmann et al., 2002; Mandala et al., 2002). This phosphorylation not only occurs in vitro, but can also be detected in vivo in the blood of mice and rats after administration of FTY720 (Brinkmann et al., 2002; Mandala et al., 2002). The phosphorylated form of FTY720 closely resembles S1P in structure and consequently is able to bind to the same S1P receptors. Receptor binding studies revealed differential binding affinities. Thus, the S1P4-receptor displays a 20-fold higher affinity for the FTY720-phosphate than for the natural ligand S1P (Mandala et al., 2002), whereas the S1P2-receptor does not bind FTY720-phosphate, but only S1P (Mandala et al., 2002). On the other side, the non-phosphorylated FTY720 showed a KD value of 300nM at the S1P1 receptor, but does not bind to the other S1P receptors (Mandala et al., 2002). Additionally, it was reported that FTY720 acts as a competitive inhibitor of S1P-induced chemotaxis in T cells (Graeler and Goetzl, 2002).
In this study, we show that in cultures of renal mesangial cells, FTY720 exerts an anti-inflammatory capacity and is able to reduce cytokine-triggered secretory phospholipase A2 (sPLA2) mRNA and protein expression and subsequent Prostaglandin E2 (PGE2) formation. Mechanistically, this effect is due to an activation of the transforming growth factor (TGF)-β/Smad signaling cascade which has an inhibitory action on the sPLA2 promoter activity.
Methods
Cell culturing
Rat renal mesangial cells were cultivated and characterized as described previously (Pfeilschifter et al., 1986). Passages 7–22 were used for the experiments in this study.
Cell stimulation and Western blot analysis
Confluent mesangial cells in 60-mm-diameter dishes were stimulated with the indicated substances in Dulbecco's modified Eagle medium (DMEM) containing 0.1 mg ml−1 of fatty acid-free bovine serum albumin (BSA). Thereafter, the medium was withdrawn and the cells washed once with ice-cold phosphate-buffered saline solution. Cells were scraped into ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 2 mM EGTA, 40 mM β-glycerophosphate, 50 mM sodiumfluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 1 μM pepstatin A, 1 mM phenylmethyl sulfonyl fluoride) and homogenized by 10 passes through a 26G-needle fitted to a 1 ml syringe. Samples were centrifuged for 10 min at 14000 g and the supernatant was taken for protein determination. Cell extracts containing 50 μg of protein were prepared and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred onto nitrocellulose paper as described (Huwiler et al., 2000a, 2000b). After the transfer, immunostaining was performed as described in detail previously (Huwiler et al., 2000a, 2000b). Antibodies were diluted in blocking buffer as indicated in the legends of the figures. Bands were detected by the enhanced chemiluminescence (ECL) method as recommended by the manufacturer.
Northern blot analysis
Total RNA was isolated using guanidinium isothiocyanate solution. Twenty micrograms of RNA was separated by electrophoresis on 1% agarose-formaldehyde gels. RNA was transferred to a nylon membrane and crosslinked by UV light. Blots were hybridized with a 416 bp RT-PCR product of rat IIA-sPLA2 (forward primer: GGTCCTCCTGTTGCTAG CAG; reverse primer: CTTTGCAAAACTTGTTGGGG). All probes were labeled with [α-32P]-dCTP using the Multiprime DNA Labeling system (Amersham Pharmacia Biotech, Freiburg, Germany). Hybridization was carried out at 42°C for 20 h, and the membranes were exposed on an Imaging system. To correct for variations in RNA amounts, blots were finally rehybridized with a 32P-labeled GAPDH cDNA probe.
sPLA2 activity assay
Equal volumes of supernatants were taken for an in vitro assay using 14C-oleic acid-labeled Escherichia coli as a substrate (Märki and Franson, 1986) in a total volume of 0.2 ml including 20 mM Tris-HCl, pH 8.5 and 10 mM CaCl2. Samples were incubated for 60 min at 37°C and stopped by addition of 2.5 ml Dole reagent. Liberated 14C-labeled fatty acids were extracted by adding 1.5 ml heptane and 1 ml water followed by vigorous vortexing. The heptane phase was loaded onto a silica gel column and 14C-labeled free fatty acids were eluted with diethylether and counted in a β-counter.
Determination of arachidonic acid release
Mesangial cells in 24-well plates were labeled for 24 h with 1 μCi ml−1 of [3H]arachidonic acid in DMEM including 0.1 mg ml−1 of BSA. Thereafter, cells were washed three times to remove all non-incorporated radioactivity and stimulated as indicated in the figure legends in DMEM containing 1 mg ml−1 of BSA as a trap for the released [3H]arachidonic acid. Supernatants were collected and centrifuged. Cells were dissolved in 0.5 M NaOH, and radioactivity in supernatants and cell extracts were counted in a β-counter. The percentage of [3H]arachidonic acid released from total incorporated radioactivity was calculated.
PGE2 determination
Equal volumes of supernatants were subjected to a PGE2-ELISA according to the manufacturer's instructions.
Cell transfections and promoter studies
A 2.67 kb promoter fragment of rat IIA-sPLA2 was cloned from rat genomic DNA by PCR using the following primers: forward: GCGCCGACGCGTGAAAATCCCTGACTTGATTC; reverse: GCGCCGCTCGAGGTTTTTCCTGTACTCCCAATG according to a previous report (Scholz-Pedretti et al., 2002) and fused into the luciferase reporter gene-containing vector pGL3. Mesangial cells were cultured in 6-well plates and were transfected with 400 ng of plasmid DNA plus 40 ng Renilla luciferase DNA per well by use of the Effectene transfection reagent following the manufacturer's recommendations. Values for sPLA2-IIA promotor activity were calculated from the ratio of firefly/renilla luciferase activities and expressed as relative luciferase activity.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by a Bonferroni's post hoc test for multiple comparisons (GraphPad InStat version 3.00 for Windows NT, GraphPad Software, San Diego, CA, USA).
Chemicals
FTY720 was obtained from Cayman Chemicals, (Ann Arbor, MI, USA); FTY720-phosphate was kindly provided by Dr V Brinkmann, (Novartis Pharma Ltd, Basel, Switzerland); interleukin-1β (IL-1β) was obtained from Cell Concept, (Umkirch, Germany); TGFβ2 was from R&D systems, (Wiesbaden, Germany); the TGFβ receptor type I inhibitor was from Merck Biosciences, (Schwalbach, Germany), Rediprime II random prime labelling system, Nick columns, the PGE2 enzyme immunoassay, [α-32P] dCTP, hyperfilms MP and horseradish-coupled secondary antibodies were from Amersham Pharmacia Biotech, (Freiburg, Germany); Effectene Transfection Reagent was from Qiagen, (Hilden, Germany); Trizol and all cell culture nutrients were from Invitrogen, (Karlsruhe, Germany).
Results
FTY720 is expected to become active via phosphorylation to FTY720-phosphate and thereby turns into a S1P mimetic (Brinkmann et al., 2002; Mandala et al., 2002). As we have shown previously that TGFβ and S1P have an anti-inflammatory capacity and reduce the expression of pro-inflammatory enzymes including the sPLA2 –IIA (Pfeilschifter et al., 1990; Schalkwijk et al., 1992; Xin et al., 2004), we investigated whether FTY720 may have an effect on sPLA2-IIA expression similar to that seen with TGFβ and S1P.
Stimulation of mesangial cells for 24 h with the pro-inflammatory cytokine IL-1β caused a marked induction of sPLA2 -IIA protein secretion into the cell culture supernatant (Figure 1a) as reported previously (Pfeilschifter et al., 1989; Schalkwijk et al., 1991; Xin et al., 2004). This induction of sPLA2 protein was dose-dependently reduced in the presence of FTY720 (Figure 1a) and a significant suppression of sPLA2-IIA protein secretion was observed even at low concentrations (10–100 nM). At 1–3 μM, a marked inhibition was obtained, although basal levels were not reached. In parallel, IL-1β-triggered sPLA2 activity in the medium was also decreased, although only partially (Figure 1b). This may be explained by the fact that in mesangial cells, IL-1β also induces another subtype of PLA2, the group V-sPLA2 (van der Helm et al., 2000), which may account for the remaining sPLA2 activity and is not affected by FTY720.
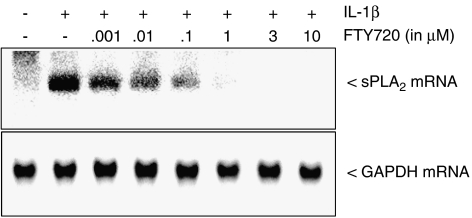
Figure 1.
Effect of FTY720 on IL-1β-induced protein secretion and activity of sPLA2 in mesangial cells. Quiescent mesangial cells were stimulated for 24 h with either vehicle (−) or interleukin-1β (IL-1β, 1 nM; +) in the absence or presence of the indicated concentrations of FTY720 or FTY-phosphate (pFTY; both in μM). Thereafter, supernatants were taken for protein precipitation followed by protein separation by SDS-PAGE, transfer to nitrocellulose and Western blot analysis using a specific monoclonal antibody against rat IIA-sPLA2 antibody at a dilution of 1 : 60 (a), or sPLA2 activity was measured in the supernatants as described in the Methods section (b). Data in (a) show one representative blot out of at least three independent experiments. Data in (b) are expressed as % of maximal IL-1β-induced activity and are means±s.d. (n=3), **P<0.01, ***P<0.001 considered statistically significant when compared to the IL-1β-stimulated values.
The reduction of sPLA2-IIA protein was owing to an inhibition of IL-1β-induced mRNA expression, which also occurred in a concentration-dependent manner (Figure 2, upper panel). In contrast, the mRNA expression of the housekeeping enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) did not change upon cell stimulation (Figure 2, lower panel).
Figure 2.
Effect of FTY720 on IL-1β-induced mRNA expression sPLA2 in mesangial cells. Quiescent mesangial cells were stimulated for 24 h with either vehicle (−) or interleukin-1β (IL-1β, 1 nM; +) in the absence or presence of the indicated concentrations of FTY720 (in μM). Thereafter, mRNA was extracted and subjected to a Northern blot analysis using a probe for either rat IIA-sPLA2 (upper panel) or GAPDH (lower panel). Data are representative of at least four independent experiments giving similar results.
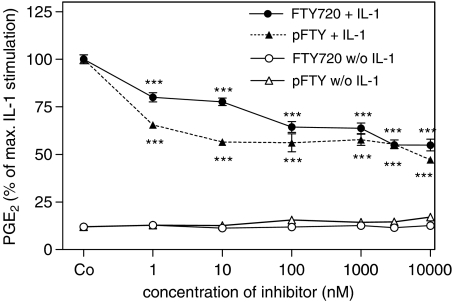
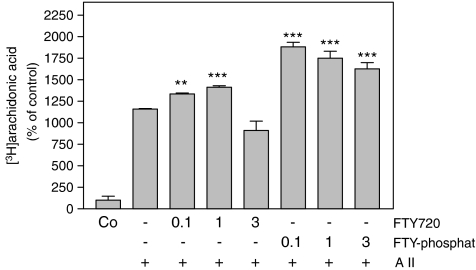
As PLA2 cleaves arachidonic acid from phospholipids and thus catalyses the rate-limiting step in prostaglandin synthesis, we next examined PGE2 formation as a functional read-out in mesangial cells. As seen in Figure 3, IL-1β-induced PGE2 formation was reduced in the presence of FTY720, whereas in the absence of IL-1β FTY720 did not alter PGE2 formation significantly (Figure 3). Remarkably, even at higher concentrations of FTY720 only a partial reduction of cytokine-triggered PGE2 formation was seen. This is most likely to be owing to the fact that cytokine-induced PGE2 formation is not only regulated by the action of the group IIA sPLA2 but also by the group V sPLA2 as well as the cPLA2 (Pfeilschifter et al., 1993; Schalkwijk et al., 1993; van der Helm et al., 2000). However, the cPLA2 activity, as measured by specific [3H]arachidonic acid released from angiotensin II-stimulated cells, was not reduced, but rather slightly enhanced by FTY720 (Figure 4). FTY-phosphate exerted a more pronounced increase of angiotensin II-stimulated arachidonic acid release (Figure 4), consistent with our previous findings that FTY720 and FTY-phosphate are able to activate the MAPK/ERK cascade (Xin et al., 2006), which is critically involved in cPLA2 phosphorylation and activation.
Figure 3.
Effect of FTY720 and FTY-phosphate on IL-1β-induced prostaglandin E2 formation in mesangial cells. Quiescent mesangial cells were stimulated for 24 h with either vehicle (Co) or the indicated concentrations (in nM) of FTY720 and FTY-phosphate (pFTY) in the absence (w/o IL-1) or presence (+IL-1) of interleukin-1β (1 nM). Thereafter, supernatants were taken for determination of PGE2 using an ELISA as described in the Methods Section. Data are expressed as % of IL-1β stimulation and are means±s.d. (n=3–4). ***P<0.001 considered statistically significant when compared to IL-1β-stimulated value.
Figure 4.
Effect of FTY720 and FTY-phosphate on cPLA2 activity in renal mesangial cells. [3H]arachidonic acid-labeled mesangial cells were stimulated for 1 h with either vehicle (Co) or angiotensin II (A II; 300 nM; +) in the absence (−) or presence of the indicated concentrations (in μM) of FTY720 or FTY-phosphate (both pretreated for 30 min before stimulation). Thereafter, liberated [3H]arachidonic acid was determined as described in the Methods Section. Data are expressed as % of control stimulations and are means±s.d. (n=3). **P<0.01, ***P<0.001 considered statistically significant when compared to the angiotensin II-stimulated values.
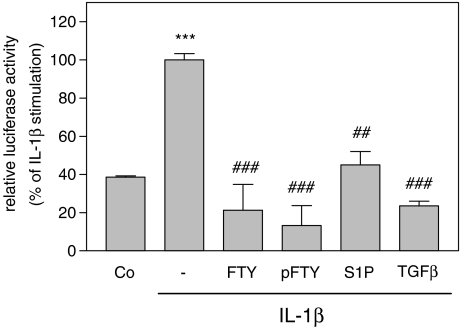
To elucidate further the mechanism by which FTY720 blocked sPLA2 mRNA expression, sPLA2 promoter activation was measured. To this end, a 2.67 kb fragment of the rat sPLA2 promoter was cloned and fused to a luciferase-containing vector construct according to a previous report (Scholz-Pedretti et al., 2002). Transfection of mesangial cells with this sPLA2 promoter fragment followed by stimulation for 24 h with IL-1β induced an increased promoter activity (Figure 5), thus confirming previous reports (Scholz-Pedretti et al., 2002; Petry et al., 2005). The cytokine-induced promoter activation was blocked in the presence of FTY720 or FTY-phosphate (Figure 5). A similar inhibition of sPLA2 promoter activity was also obtained with TGFβ2 and S1P (Figure 5). Importantly, the stability of sPLA2 mRNA, which may also contribute to increased mRNA steady-state levels, was not affected by FTY720 (data not shown).
Figure 5.
Effect of FTY720 and FTY-phosphate on IL-1β-stimulated sPLA2 promoter activity in mesangial cells. Subconfluent mesangial cells were cotransfected with the rat IIA-sPLA2 promoter DNA plus the plasmid coding for Renilla luciferase (pRL-CMV). At 24 h after transfection, cells were rendered serum-free and stimulated for 24 h with either vehicle (Co), or interleukin-1β (IL-1β, 1 nM) in the absence (−) or presence of FTY720 (FTY; 3 μM), FTY-phosphate (pFTY; 3 μM), S1P (10 μM) and TGFβ2 (10ng ml−1). The ratio between beetle luciferase activity and Renilla luciferase activity was calculated and depicted as % of the IL-1β-stimulated values. Data are means±s.d. (n=3), ***P<0.001 considered statistically significant compared to the vehicle-stimulated control value. ##P<0.01, ###P<0.001 compared to the IL-1β-stimulated values.
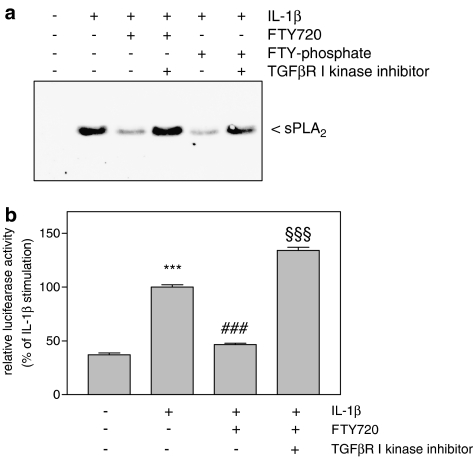
As TGFβ2 is well known to act via the Smad signaling cascade, and also S1P and FTY720 have been shown to crossactivate the TGFβ/Smad signaling cascade (Xin et al., 2004, 2006), it was possible that this mechanism was also responsible for the FTY720-mediated inhibition of sPLA2 mRNA expression. To address this point, cells were pretreated with a specific kinase inhibitor of the TGFβ receptor type I (Inman et al., 2002). As seen in Figure 6a, the FTY720- and also the FTY720-phosphate-mediated decrease of sPLA2-IIA protein expression was completely reversed by the TGFβ receptor type I inhibitor. Moreover, the inhibition by FTY720 of IL-1β-triggered sPLA2 promoter activation was also abolished by the TGFβ receptor inhibitor (Figure 6b).
Figure 6.
Effect of TGFβ receptor inhibition on FTY720-mediated reduction of sPLA2 protein expression and promoter activity in mesangial cells. (a) Mesangial cells were pretreated for 30 min with the TGFβR I kinase inhibitor (at 10 μM) before stimulation with vehicle (−) or IL-1β in the absence or presence of FTY720 (FTY; 3 μM) and FTY-phosphate (pFTY; 3 μM) as indicated. Thereafter, supernatants were taken for protein precipitation followed by SDS-PAGE, transfer to nitrocellulose and Western blot analysis using a specific monoclonal antibody against rat IIA-sPLA2 antibody at a dilution of 1 : 60. Data are representative of five independent experiments giving similar results. (b) Cells cotransfected for 24 h with the rat IIA-sPLA2 promoter DNA plus the plasmid coding for Renilla luciferase (pRL-CMV) were rendered serum-free and stimulated for 24 h with either vehicle (−), interleukin-1β (IL-1β, 1 nM), IL-1β plus FTY720 (3 μM), IL-1β plus FTY720 plus TGFβR I kinase inhibitor (10 μM; pretreated for 30 min before stimulation). The ratio between beetle luciferase activity and Renilla luciferase activity was calculated and depicted as relative luciferase activity. Data are means±s.d. (n=3), ***P<0.001 is considered statistically significant compared to the vehicle-stimulated values; ###P<0.001 compared to the IL-1β-stimulated values; §§§P<0.001 compared to the IL-1β plus FTY720-stimulated values.
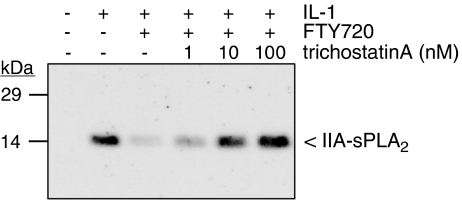
Additionally, the involvement of histone deacetylation was tested, as this process is often involved in regulation of gene transcription (Verdone et al., 2005). To this end, the histone deacetylase inhibitor, trichostatin A (Yoshida et al., 1990) was used. As seen in Figure 7, the FTY720-triggered reduction of sPLA2-IIA protein expression was dose-dependently reversed by trichostatin A, showing significant effects already at 1 nM. As various promoters are known to be regulated by methylation, the involvement of this process in the activation of the sPLA2-IIA promoter was assessed by using the demethylase inhibitor 5-azocytidine (Helm, 2006). However, in the presence of 5-azocytidine neither the cytokine-mediated nor the FTY720-mediated effect on sPLA2 mRNA expression was altered (data not shown). All these data further confirm that FTY720 acts on the transcriptional level and that histone deacetylation takes place, but that methylation is not an important regulatory step in transcription of the sPLA2 gene.
Figure 7.
Effect of the histone deacetylase inhibitor trichostatin A on the FTY720-mediated inhibition of sPLA2 expression in mesangial cells. Mesangial cells were stimulated for 24 h with either vehicle (−), interleukin-1β (IL-1β, 1 nM), or IL-1β plus FTY720 (3 μM) in the presence of the indicated concentrations of trichostatin A (in nM; pretreated for 30 min before stimulation) as indicated. Thereafter, supernatants were taken for protein precipitation followed by protein separation by SDS-PAGE, transfer to nitrocellulose and Western blot analysis using a specific monoclonal antibody against rat IIA-sPLA2 antibody at a dilution of 1 : 60. Data are representative of three independent experiments giving similar results.
Discussion
FTY720 has been introduced as a potent immunomodulatory agent that promotes lymphocyte homing into the lymph nodes. However, the detailed mechanism of action of FTY720 is only partially resolved. FTY720 is converted to FTY-phosphate by a sphingosine kinase activity and thereby is turned into a S1P receptor agonist. However, Matloubian et al. (2004) showed that the critical event in the immunomodulatory action of FTY720 is not the activation of the S1P1 receptor per se, but rather the subsequent selective depletion of S1P1 receptor subtype from lymphocytes. The S1P1 receptor knockout mice showed the same phenotype as FTY720-treated mice, that is retention and accumulation of activated T cells in lymphoid organs (Matloubian et al., 2004). Whether FTY720 is able to downregulate the S1P1 receptor also in other cell types has not yet been systematically tested. Preliminary data in mesangial cells, however, show that FTY720 does not reduce S1P1 receptor mRNA nor protein expression (data not shown), thus suggesting a cell type-specific action of FTY720.
The finding that FTY720 is a potent immunosuppressive agent that promotes migration and homing of T cells (Lee et al., 1998) strongly resembles the homing of T cells and immunosuppression mediated by TGFβ (Wahl et al., 2004) and suggests that similar signaling mechanisms are involved, which may converge at the Smad protein level. In this context, it is worth noting that S1P, which shares identical receptors with FTY-phosphate, with the exception of S1P2 also activates the Smad protein signaling cascade and mimics TGFβ-induced cell responses in mesangial cells (Xin et al., 2004), keratinocytes (Sauer et al., 2004) and murine Langerhans cells (Radeke et al., 2005). In the latter study, a role for S1P as an endogenous immunosuppressive mediator affecting migration and development of tolerogenic dendritic cells was proposed (Radeke et al., 2005). Furthermore, the S1P-induced Smad activation was blocked by the putative S1P3-receptor antagonist suramin (Xin et al., 2004), leading to the suggestion that this specific S1P-receptor subtype could be involved in the Smad activation pathway. However, only the use of highly selective receptor subtype antagonists will prove which receptor subtype is involved.
It is now becoming more and more clear that FTY720 exerts additional cellular effects besides immunosuppression. In this study, we show for the first time that FTY720 is able to exert an anti-inflammatory effect on mesangial cells. Evidence for this is provided by the decreased expression of the sPLA2-IIA, which is considered a pro-inflammatory enzyme and represents the rate-limiting step in the generation of inflammatory prostaglandins. Nevertheless, as the sPLA2-IIA is not the only enzyme induced under inflammatory conditions, but also the sPLA2-V (van der Helm et al., 2000) and the cPLA2 (Pfeilschifter et al., 1993; Schalkwijk et al., 1993), this multiplicity of PLA2 enzymes may explain why there was only partial inhibition by FTY720 of PGE2 generation (Figure 3).
Mechanistically, this suppression of sPLA2 gene transcription was owing to the activation of the TGFβ/Smad signaling cascade. Evidence for this was given by the findings that the specific TGFβ receptor type I kinase inhibitor was able to abolish the reducing effect of FTY720 and FTY-phosphate on sPLA2 protein expression (Figure 6a) and also sPLA2 promoter activity (Figure 6b). In this regard, it is noteworthy that FTY720 and FTY-phosphate are indeed able to trigger phosphorylation and activation of Smad proteins (Xin et al., 2006), which are classical members of the TGFβ signaling pathway (Massague, 1998; ten Dijke and Hill, 2004). As already reported for S1P (Xin et al., 2004), the FTY720-triggered Smad activation is also blocked by suramin, which acts as an antagonist at the S1P3 receptors (Xin et al., 2006).
Besides its immunosuppressive effect, TGFβ also possesses an anti-inflammatory potential (Ling and Robinson, 2002). Thus, various pro-inflammatory mechanisms are decreased by TGFβ in cell culture studies including cytokine-triggered inducible NO synthase expression (Pfeilschifter and Vosbeck, 1991; Kunz et al., 1997) and sPLA2 expression (Pfeilschifter et al., 1990; Mühl et al., 1992; Schalkwijk et al., 1992). Moreover, TGFβ reduces lipopolysaccharide-induced toll-like receptor 4 signaling by facilitating proteasomal degradation of MyD88 (Naiki et al., 2005). The anti-inflammatory effect of TGFβ is also seen in vivo because transgenic mice overexpressing TGFβ are protected against renal inflammation in mouse obstructive and diabetic models (Wang et al., 2005). Conversely, TGFβ knockout mice die from massive systemic inflammatory reactions (Shull et al., 1992).
The mechanism by which Smad complexes are able to reduce cytokine-induced sPLA2 promoter activity and gene transcription is still unclear. Whereas Smad complexes can positively affect gene transcription of various matrix-related genes by binding to and activating Smad-binding elements (SBE) on promoter sequences, how Smad complexes lead to repression of genes remains obscure, as negative SBE elements on DNA have not been characterized. However, because the affinity of Smad proteins for DNA is generally low, accessory proteins exist that facilitate Smad/DNA binding (for review, see: Massague, 1998). Thus, Smads may either interact with co-activators such as p300, AP-1, p53, C/EBP, p68 RNA helicase or SP-1, or with co-repressors including the proto-oncogenes Ski/Sno, DACH-1, EID-2 or others. The induction or activation of one of these co-repressors by TGFβ and FTY720 in mesangial cells is a tempting hypothesis, but clearly, further studies will be needed to unravel the mechanism of repression of sPLA2 gene transcription.
FTY720 has been positively tested in human clinical trials for prevention of graft rejection after kidney transplantation (Brinkmann et al., 2001). Furthermore, it is suggested to have beneficial effects in the treatment of multiple sclerosis (Gonsette, 2004), an immune-mediated disease characterized by inflammatory demyelination in the central nervous system. Thus, in a rat model of experimental autoimmune encephalomyelitis (EAE), FTY720 was reported to have an ameliorating effect (Fujino et al., 2003). FTY720 is presently tested in clinical trials for multiple sclerosis. Phase II results showed a beneficial effect, at a low daily dose of 1.25 mg.
Furthermore, a favorable effect of FTY720 on the cardiovascular system was proposed, as FTY720 induces vasodilatation of mouse aortae (Tolle et al., 2005). This vasodilating effect was abolished not only in mice deficient in endothelial NO synthase (eNOS) but also in S1P3 receptor deficient mice, suggesting a role for FTY720 in NO formation and the action of FTY720 through the S1P3 receptor (Tolle et al., 2005). Indeed, the authors could show that FTY720 rapidly stimulated Akt and eNOS phosphorylation in human endothelial cell cultures, leading to increased NO release (Tolle et al., 2005). Also, FTY720 had beneficial effects in a rat model of anti-Thy1-induced chronic progressive glomerulonephritis (Peters et al., 2004). This was attributed to depletion of lymphocytes and the cessation of the inflammatory reaction resulting in a slowing of disease progression towards chronic tubulointerstitial fibrosis and renal insufficiency. Very recently, Payne et al. (2006) reported that FTY720 directly inhibits the cPLA2 activity in mast cells and thereby reduces antigen-induced secretion of PGD2 without affecting mast cell degranulation. As FTY720, in our cell system, has no inhibiting effect on cPLA2 activity, measured by [3H]arachidonic acid release (Figure 4), we can exclude the possibility that the inhibition by FTY720 of PGE2 formation in our system was owing to inhibition of cPLA2.
However, not much is known about potential side effects that may arise upon long-term FTY720 treatment. So far, only the occurrence of a transient bradycardia has been described which was proposed to be owing to an agonistic effect of the active compound FTY720-phosphate on the S1P3 receptor on atrial sinus node myocytes which in turn leads to a negative chronotropic response (Budde et al., 2002; Sanna et al., 2004). Furthermore, in rats a significant reduction of sodium excretion was observed at repeated high doses of FTY720 (Tawadrous et al., 2002) pointing towards an effect on the renal tubular system. Moreover, our previous data obtained from mesangial cell cultures indicate that FTY720 is able to upregulate various profibrotic factors such as the connective tissue growth factor (CTGF), tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) and collagen type IV (Xin et al., 2006). Also these cell responses exerted by FTY720 are shared by both TGFβ and S1P. It is relevant here to note that when an acute inflammatory response is not appropriately terminated, it may turn into a chronic inflammatory reaction associated with a profibrotic response, causing organ fibrosis and organ failure.
In summary, we have shown that FTY720 exerts an anti-inflammatory potential in renal mesangial cells, very similar to that shown by TGFβ by suppressing cytokine-induced sPLA2 promoter activity, mRNA expression and protein secretion and subsequent PGE2 formation. Thus, FTY720 may represent a lead compound for novel therapeutic strategies to cope with inflammatory and/or immune diseases.
Acknowledgments
This work was supported by the Swiss National Foundation (3100A0-111806), the German Research Community (FOG784, GRK757/2, EB 257/2-2), the Wilhelm Sander-Stiftung, the Novartis Foundation and the European Community (FP6: LSHM-CT-2004-005033).
Abbreviations
- BSA
bovine serum albumin
- cPLA2
cytosolic phospholipase A2
- CTGF
connective tissue growth factor
- DMEM
Dulbecco's modified Eagle medium
- ECL
enhanced chemiluminescence
- Edg
endothelial differentiation gene
- eNOS
endothelial NO synthase
- FTY720
2-amino-2-[2-(4-octyl-phenyl)ethyl]-1, 3-propanediol hydrochloride
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- MAPK
mitogen-activated protein kinase
- pFTY
FTY-phosphate
- PGE2
prostaglandin E2
- S1P
sphingosine-1-phosphate
- SBE
Smad-binding element
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- sPLA2
secretory phospholipase A2
- TGFβ
transforming growth factor-β
- TIMP-1
tissue inhibitor of metalloproteinase-1
Conflict of interest
The authors state no conflict of interest.
References
- Brinkmann V, Davies MD, Heise CE, Albert R, Cottens S, Hof R. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: a novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol Sci. 2001;21:49–52. doi: 10.1016/s0165-6147(99)01419-4. [DOI] [PubMed] [Google Scholar]
- Budde K, Schmouder RL, Brunkhorst R, Nashan B, Lucker PW, Mayer T. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol. 2002;13:1073–1083. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]
- Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- Gonsette RE. New immunosuppressants with potential implication in multiple sclerosis. J Neurol Sci. 2004;223:87–93. doi: 10.1016/j.jns.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine-1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- Helm H. Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res. 2006;34:721–733. doi: 10.1093/nar/gkj471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000a;1485:63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Stabel S, Fabbro D, Pfeilschifter J. Platelet-derived growth factor and angiotensin II stimulate the mitogen-activated protein kinase cascade in renal mesangial cells: comparison of hypertrophic and hyperplastic agonists. Biochem J. 1995;305:777–784. doi: 10.1042/bj3050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Wartmann M, van den Bosch H, Pfeilschifter J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br J Pharmacol. 2000b;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Kashgarian M, Sterzel RB. The pathobiology of the mesangium. Kidney Int. 1992;41:524–529. doi: 10.1038/ki.1992.74. [DOI] [PubMed] [Google Scholar]
- Katsuma S, Hada Y, Ueda T, Shiojima S, Hirasawa A, Tanoue A. Signalling mechanisms in sphingosine 1-phosphate-promoted mesangial cell proliferation. Genes Cells. 2002;7:1217–1230. doi: 10.1046/j.1365-2443.2002.00594.x. [DOI] [PubMed] [Google Scholar]
- Kunz D, Walker G, Pfeilschifter J. Transforming growth factor-β2 inhibits interleukin 1β-induced expression of inducible nitric oxide synthase in rat renal mesangial cells. Inflammation Res. 1997;46:327–331. doi: 10.1007/s000110050196. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Ling E, Robinson DS. Transforming growth factor-β1: its anti-inflammatory and pro-fibrotic effects. Clin Exp Allergy. 2002;32:175–178. doi: 10.1046/j.1365-2222.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Märki F, Franson R. Endogenous suppression of neutral-active and calcium-dependent phospholipase A2 in human polymorphonuclear leukocytes. Biochim Biophys Acta. 1986;879:49–156. doi: 10.1016/0005-2760(86)90097-4. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mühl H, Geiger T, Pignat W, Märki F, van den Bosch H, Cerletti N. Transforming growth factors type-β and dexamethasone attenuate group II phospholipase A2 gene expression by interleukin-1 and forskolin in rat mesangial cells. FEBS Lett. 1992;301:190–194. doi: 10.1016/0014-5793(92)81245-h. [DOI] [PubMed] [Google Scholar]
- Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-β differentially inhibits MyD88-dependent, but not TRAM-and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J Biol Chem. 2005;280:5491–5495. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Payne SG, Oskeritzian CA, Griffiths R, Subramanian P, Barbour SE, Chalfant CE. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood. 2006;109:1077–1085. doi: 10.1182/blood-2006-03-011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Martini S, Wang Y, Shimizu F, Kawachi H, Kramer S. Selective lymphocyte inhibition by FTY720 slows the progressive course of chronic anti-thy 1 glomerulosclerosis. Kidney Int. 2004;66:1434–1443. doi: 10.1111/j.1523-1755.2004.00906.x. [DOI] [PubMed] [Google Scholar]
- Petry C, Huwiler A, Eberhardt W, Kaszkin M, Pfeilschifter J. Hypoxia increases group IIA phospholipase A(2) expression under inflammatory conditions in rat renal mesangial cells. J Am Soc Nephrol. 2005;16:2897–2905. doi: 10.1681/ASN.2004121051. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Cross-talk between transmembrane signalling systems: a prerequisite for the delicate regulation of glomerular haemodynamics by mesangial cells. Eur J Clin Invest. 1989;19:347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Mesangial cells orchestrate inflammation in the renal glomerulus. News Physiol Sci. 1994;9:271–276. [Google Scholar]
- Pfeilschifter J, Vosbeck K. Transforming growth factor-β2 inhibits interleukin 1β- and tumour necrosis factor-α induction of nitric oxide synthase in rat renal mesangial cells. Biochem Biophys Res Commun. 1991;175:372–379. doi: 10.1016/0006-291x(91)91574-v. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Kurtz A, Bauer C. The role of phospholipase C and protein kinase C in vasoconstrictor-induced prostaglandin synthesis in cultured rat renal mesangial cells. Biochem J. 1986;234:125–130. doi: 10.1042/bj2340125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Pignat W, Leighton J, Märki F, Vosbeck K, Alkan S. Transforming growth factor-β2 differentially modulates interleukin-1β - and tumour necrosis-factor-α-stimulated phospholipase A2 and prostaglandin E2 synthesis in rat renal mesangial cells. Biochem J. 1990;270:269–271. doi: 10.1042/bj2700269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Pignat W, Vosbeck K, Märki F. Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. Biochem Biophys Res Commun. 1989;159:385–394. doi: 10.1016/0006-291x(89)90003-x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Schalkwijk C, Briner VA, van den Bosch H. Cytokine-stimulated secretion of group II phospholipase A2 by rat mesangial cells. Its contribution to arachidonic acid release and prostaglandin synthesis by cultured rat glomerular cells. J Clin Invest. 1993;92:2516–2523. doi: 10.1172/JCI116860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke HH, von Wenckstern H, Stoidtner K, Sauer B, Hammer S, Kleuser B. Overlapping signaling pathways of sphingosine-1-phosphate and TGF-β in the murine Langerhans cell line XS52. J Immunol. 2005;174:2778–2786. doi: 10.4049/jimmunol.174.5.2778. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS. Sphingosine-1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, Glick AB. Involvement of Smad signalling in sphingosine-1-phosphate-mediated biological responses of keratinocytes. J Biol Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- Schalkwijk C, Pfeilschifter J, Märki F, van den Bosch H. Interleukin-1β, tumor necrosis factor and forskolin stimulate the synthesis and secretion of group II phospholipase A2 in rat mesangial cells. Biochem Biophys Res Commun. 1991;174:268–275. doi: 10.1016/0006-291x(91)90515-9. [DOI] [PubMed] [Google Scholar]
- Schalkwijk C, Pfeilschifter J, Märki F, van den Bosch H. Interleukin-1β- and forskolin-induced synthesis and secretion of group II phospholipase A2 and prostaglandin E2 in rat mesangial cells is prevented by transforming growth factor-β. J Biol Chem. 1992;267:8846–8851. [PubMed] [Google Scholar]
- Schalkwijk CG, Vervoordeldonk M, Pfeilschifter J, van den Bosch H. Interleukin-1β-induced cytosolic phospholipase A2 activity and protein synthesis is blocked by dexamethasone in rat mesangial cells. FEBS Lett. 1993;333:339–343. doi: 10.1016/0014-5793(93)80683-l. [DOI] [PubMed] [Google Scholar]
- Scholz-Pedretti K, Gans A, Beck K-F, Pfeilschifter J, Kaszkin M. Potentiation of TNF-α-stimulated group IIA phospholipase A2 expression by peroxisome proliferator-activated receptor α activators in rat mesangial cells. J Am Soc Nephrol. 2002;13:611–620. doi: 10.1681/ASN.V133611. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S. Sphingosine 1-phosphate: a prototype of a new class of second messengers. J Leukoc Biol. 1999;65:341–344. doi: 10.1002/jlb.65.3.341. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Tawadrous MN, Mabuchi A, Zimmermann A, Wheatley AM. Effects of immunosuppressant FTY720 on renal and hepatic hemodynamics in the rat. Transplantation. 2002;74:602–610. doi: 10.1097/00007890-200209150-00004. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Tolle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schonfelder G. Immunomodulator FTY720 Induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res. 2005;96:913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- van der Helm HA, Aarsman AJ, Janssen MJ, Neys FW, van den Bosch H. Regulation of the expression of group IIA and group V secretory phospholipases A(2) in rat mesangial cells. Biochim Biophys Acta. 2000;1484:215–224. doi: 10.1016/s1388-1981(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Cell Biol. 2005;83:344–353. doi: 10.1139/o05-041. [DOI] [PubMed] [Google Scholar]
- Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-β: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- Wang W, Koka V, Lan HY. Transforming growth factor-β and Smad signalling in kidney diseases. Nephrology. 2005;10:48–56. doi: 10.1111/j.1440-1797.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- Xin C, Ren S, Eberhardt W, Pfeilschifter J, Huwiler A. The immunomodulator FTY720 and its phosphorylated derivative activate the Smad signalling cascade and upregulate connective tissue growth factor and collagen type IV expression in renal mesangial cells. Br J Pharmacol. 2006;147:164–174. doi: 10.1038/sj.bjp.0706452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H. Sphingosine-1-phosphate cross-activates the Smad signaling cascade and mimics TGFβ-induced cell responses. J Biol Chem. 2004;279:35255–35280. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]