Abstract
Background and purpose:
Atrial fibrillation (AF) is the most common electrical cardiac disorder in clinical practice. The major trigger for AF is focal ectopic activity of unknown origin in sleeves of cardiac muscle that extend into the pulmonary veins. We examined the role of noradrenaline in the genesis of ectopic activity in the pulmonary vein.
Experimental approach:
Mechanical activity of strips of pulmonary vein isolated from male Wistar rats was recorded via an isometric tension meter. Twitch contractions of cardiac myocytes were evoked by electrical field stimulation in a tissue bath through which flowed Krebs-Heinseleit solution warmed to 36-37°C and gassed with 95% O2 5% CO2.
Key results:
The superfusion of noradrenaline induced ectopic contractions in 71 of 76 different isolated pulmonary veins. Ectopic contractions in the pulmonary vein were not associated with electrically evoked twitch contractions. The effect of noradrenaline on the pulmonary vein could be replicated by the simultaneous, but not separate, application of the α adrenoceptor agonist phenylephrine and the β adrenoceptor agonist isoprenaline. The use of selective agonists and antagonists for adrenoceptor subtypes showed that ectopic activity in the pulmonary vein arose from the simultaneous stimulation of α1 and β1 adrenoceptors. The application of noradrenaline to isolated strips of left atrium did not induce ectopic contractions (n=10).
Conclusions:
These findings suggest an origin for ectopic activity in the pulmonary vein that requires activation of both α and β adrenoceptors. They also open new perspectives towards our understanding of the triggering of AF.
Keywords: Atrial fibrillation, pulmonary vein, α adrenoceptors, β adrenoceptors, automatism, ectopic activity
Introduction
Atrial fibrillation (AF) is a commonly occurring arrhythmia and a major contributor to cardiovascular morbidity. Pacemaker activity from cells within the myocardial sleeves that extend into the pulmonary veins is thought to result in the formation of ectopic beats that initiate and sustain AF (Haissaguerre et al., 1998; Chen et al., 1999; Hocini et al., 2000).
Despite many investigations, the mechanism generating focal activity in the pulmonary veins remains unknown (see Nattel, 2002; Page and Roden, 2005 for reviews). Most studies have concentrated upon pathological alterations to the electrophysiology of cardiac muscle cells in the pulmonary vein, including the possibility of delays in action potential conduction (Chen et al., 2000; Verheule et al., 2002), alterations of action potential duration and effective refractory period (Chen et al., 2001; Jalife et al., 2002; Scherlag et al., 2002), high-frequency firing and cellular Ca2+ overload causing early after depolarizations (Schauerte et al., 2001; Miyauchi et al., 2004; Patterson et al., 2005), alterations in ion channels that might cause abnormal automaticity (Bosch and Nattel, 2002; Khan, 2004; Melnyk et al., 2005), and finally, automatism resulting from the activity of nodal myocytes in the pulmonary vein (Masani, 1986; Chen and Yeh, 2003; Perez-Lugones et al., 2003).
The contractile function of the pulmonary vein has been the object of little attention. Lal et al. (1999) used isolated rings of large pulmonary veins and studied the effect of several contractile and relaxant agents and the role of endothelium on vascular smooth muscle cells. MacLeod and Hunter (1967) studied the functional responses of the cardiac myocytes in electrically simulated pulmonary veins. They obtained positive inotropic responses with different adrenoceptor agonists. While we were investigating the contractile properties of isolated pulmonary veins from the rat, we observed that noradrenaline (NA) could induce ectopic contractions. This agrees with a previous report that NA provoked spontaneous action potentials in pulmonary veins of the guinea pig (Cheung, 1980). In this study, we present evidence that ectopic activity in the pulmonary vein arises from simultaneous stimulation of α1 and β1 adrenoceptors. We also show that this particular response to adrenoceptor stimulation is specific to the cardiac myocytes of the pulmonary vein compared to those of the left atrium.
Methods
Animals
Experiments were performed on male Wistar rats purchased from CER Janvier (Le Genest, St Isle, France) following national guidelines on animal care.
Preparation of the pulmonary vein
Rats were anesthetized by intraperitoneal injection of pentobarbital (60 mg kg−1), followed 20 min later by intravenous injection of heparin (500 IU kg−1). The heart and lungs were rapidly removed from the animal as one block and placed in a dissecting dish that contained Krebs–Henseleit solution composed of 119 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4, 1.36 mM CaCl2 and 5.5 mM glucose. A strip of tissue was dissected from the right superior pulmonary vein. This was mounted in a 3 ml organ bath continuously perfused at a flow rate of 5 ml min−1 with Krebs–Henseleit solution aerated with 95% O2 and 5% CO2 and maintained between 36 and 37°C. Electrical field stimulation (FS: 0.1 Hz, 2 ms duration, two times threshold voltage) was applied via three platinum electrodes attached to the walls of the organ bath. Tension was continuously recorded with a force–displacement transducer connected to a Powerlab system and a PC computer running Chart 4.0 software (ADInstruments, Castle Hill, NSW, Australia). The baseline tension was adjusted to obtain 90% of the maximal contraction amplitude evoked by FS under basal conditions and if necessary readjusted during the experiment. Experiments began after a stabilization period of at least 1 h. Compounds were diluted in the perfusion medium.
The same procedure was followed to record tension from strips of cardiac muscle obtained from the superior wall of the left atrium.
Data analysis
Analysis of evoked and ectopic contractions was performed with the Peak Parameters Module of Chart software. Data are reported as mean±s.e.m. values obtained from n different preparations.
Chemicals
Standard chemicals were of reagent grade and obtained from Merck KG (Darmstadt, Germany). Agonists and antagonists of adrenoceptors were prepared as stock solutions in either distilled water, ethanol or dimethyl sulphoxide and kept frozen (−20°C) until just before use. Their final dilution in physiological solution took into account their respective pD2, pA2 or Ki values, which were obtained from either the IUPHAR database (http://www.iuphar-db.org) or published data (Leblais et al., 2004; Baker, 2005). (−)-NA, S(−)-atenolol, prazosin hydrochloride, R(−)-denopamine and UK 14304 were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France). Cirazoline hydrochloride, yohimbine hydrochloride, formoterol hemifumarate, ICI 118551, BRL 37344, and SR 59230A were obtained from Tocris Cookson (Avonmouth, UK).
Results
NA evokes spontaneous ectopic contractions in the pulmonary vein
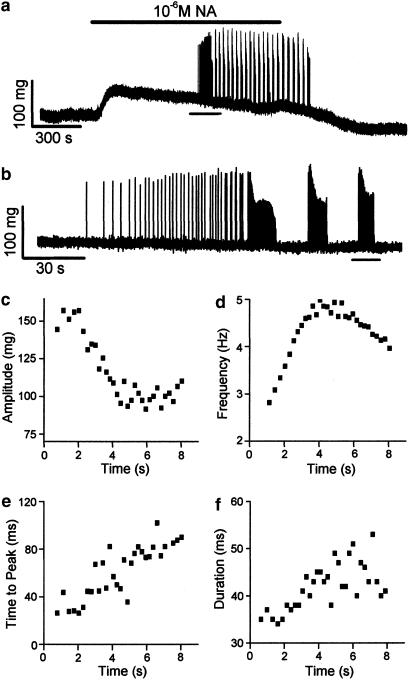
The superfusion of an isolated segment of a rat pulmonary vein with NA evoked spontaneous ectopic contractions as illustrated by a typical trace in Figure 1a and reproduced in nine different preparations. The onset of the application of NA produced an increase in basal tension, which was essentially sustained and declined upon the washout of the agonist. Ectopic contractions appeared with a latency of 12.9±1.9 min after the onset of the superfusion of between 10−6 and 10−5 M NA. Initially, these ectopic contractions consisted of isolated individual contractions that then formed clearly defined bursts (Figure 1b). The bursts of ectopic contractions had a period of 49±4 s (range 28–65 s) and duration of 18±2 s (range 9–30 s). Ectopic contractions within a burst were not uniform (Figure 1c-f). The amplitude of ectopic contractions declined (Figure 1c) and their frequency increased first and then declined during the burst (Figure 1d). Both the time to peak (Figure 1e) and the duration (Figure 1f) of ectopic contractions show a tendency to increase during the burst.
Figure 1.
NA-induced ectopic contractions in the pulmonary vein. (a) A continuous recording of tension in an isolated pulmonary vein. NA was superfused across the preparation for the period indicated by the bar above the trace. The horizontal line under the recording indicates the region that is shown upon an expanded timescale in (b). (c–f) Analysis of the ectopic contractions, which were recorded during the burst indicated by the line under the trace in (b). (c) Contraction amplitude. (d) Contraction frequency. (e) Time to peak contraction. (f) Contraction duration measured as the width at 50% of the amplitude.
Notwithstanding their variation, these characteristics make it likely that ectopic contractions arise from the cardiac myocytes present in the pulmonary vein as vascular smooth muscle cells contract upon a much longer timescale, which probably corresponds to the action of NA upon baseline tension shown in Figure 1a. To confirm the cardiac myocyte origin of ectopic contractions, electrical FS was used to evoke twitch contractions in pulmonary veins (n=17) and as a control, in isolated strips of the left atrium (n=10). The time to peak of FS – evoked contractions in pulmonary vein ranged between 35 and 44 ms and in atrial strips ranged between 36 and 41 ms. These values are similar to those recorded for ectopic contractions at the onset of a burst (Figure 1e). The duration of FS-evoked contractions in pulmonary vein ranged between 25 and 39 ms, and in atrial strips ranged between 28 and 31 ms. These are comparable with the range of values shown by ectopic contractions (Figure 1f).
The concentration of NA required to induce ectopic activity in different isolated pulmonary veins is shown in Figure 2. A minimal concentration of 10−6 M NA was necessary to evoke ectopic contractions. In total, during this study, the superfusion of between 10−6 and 10−5 M NA induced ectopic contractions in 71 of 76 different isolated pulmonary veins (93%). In isolated strips of the left atrium, the superfusion of NA at concentrations that ranged between 10−8 and 10−4 M did not induce ectopic contractions (n=10).
Figure 2.
Dose-dependent induction of ectopic activity by NA. The numbers in parentheses represent the number of individual preparations.
Ectopic contractions require simultaneous activation of α1 and β1 adrenoceptors
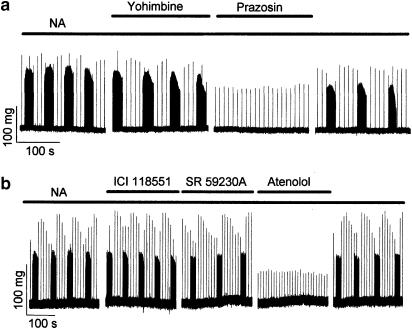
Ectopic activity induced by NA was inhibited by the application of either the α1-adrenoceptor antagonist prazosin (Figure 3a) or the β1-adrenoceptor antagonist atenolol (Figure 3b). Ectopic activity was not inhibited by either the α2-adrenoceptor antagonist yohimbine (Figure 3a), the β2-adrenoceptor antagonist ICI 118551 (Figure 3b), or the β3-adrenoceptor antagonist SR 59230A (Figure 3b).
Figure 3.
The effect of α- and β-adrenoceptor antagonists on ectopic contractions induced by NA. Traces represent segments of otherwise continuous recordings of tension obtained from isolated pulmonary veins. Ectopic contractions were induced by superfusion of 10−5 M NA. (a) Antagonists of α-adrenoceptors. For the periods indicated by the bars above the traces, the solution superfusing the preparation also contained either the α2-receptor antagonist yohimbine (10−7 M) or the α1-receptor antagonist prazosin (5 × 10−6 M). Similar results were obtained in six different preparations. (b) Antagonists of β-adrenoceptors. For the periods indicated by the bars, the solution also contained either the β1-receptor antagonist atenolol (5 × 10−6 M), the β2-receptor antagonist ICI118551 (10−7 M) or the β3-receptor antagonist SR59230A (5 × 10−7 M). Similar results were obtained in five different preparations.
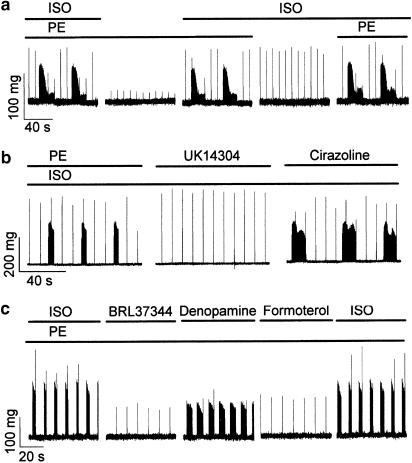
Ectopic activity could not be induced by the application of either the α-adrenoceptor agonist phenylephrine (PE) or the β-adrenoceptor agonist isoprenaline (ISO), but it could be induced by their mixture (Figure 4a). Agonists of subtypes of the adrenoceptors were tested by inducing ectopic activity with a mixture of PE and ISO and then either replacing PE with different α-adrenoceptor agonists or replacing ISO with different β-adrenoceptor agonists. Ectopic activity was supported by the α1-adrenoceptor agonist cirazoline (Figure 4b) and the β1-adrenoceptor agonist denopamine (Figure 4c). Ectopic activity disappeared when PE was replaced by the α2-adrenoceptor agonist UK 14304 (Figure 4b) and when ISO was replaced by either the β2-adrenoceptor agonist formoterol (Figure 4c) or the β3-adrenoceptor agonist BRL 37344 (Figure 4c).
Figure 4.
Ectopic contractions in the pulmonary vein require activation of both α1- and β1-adrenoceptors. Traces represent segments of otherwise continuous recordings of tension from different isolated pulmonary veins. Ectopic contractions were induced by the superfusion of the preparations with solutions that contained PE (5 × 10−6 M) and ISO (10−7 M). (a) Ectopic contractions require the simultaneous application of PE and ISO. The periods for which the preparation was exposed to the different agonists are indicated by bars above the trace. Similar results were obtained in six different preparations. (b) Agonists of α-adrenoceptors. For the periods indicated by the bars, PE was replaced first by the α2-receptor agonist UK14304 (5 × 10−6 M) and then by the α1-receptor agonist cirazoline (10−6 M). Similar results were obtained in five different preparations for UK14304 and six different preparations for cirazoline. (c) Agonists of β-adrenoceptors. For the periods indicated by the bars, ISO was replaced by either the β3-receptor agonist BRL37344 (10−7 M), the β1-receptor agonist denopamine (5 × 10−7 M), or the β2-receptor agonist formoterol (10−8 M). Similar results were obtained in six different preparations for denopamine and five each for BRL37344 and formoterol.
Ectopic activity depends upon a balance between α- and β-adrenoceptor activation
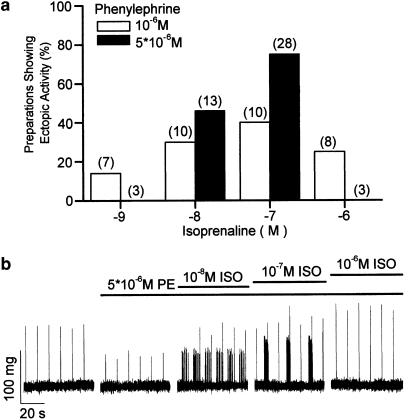
The ability of mixtures of PE and ISO to induce ectopic activity in pulmonary veins was variable. There was no single mixture of given concentrations of these agonists or a single ratio between concentrations of PE and ISO that could induce ectopic activity in every preparation. Thus, we were able to induce ectopic activity with the application of a mixture of PE and ISO in only 40 of 52 different preparations (77%), whereas in each of these preparations NA had evoked ectopic contractions. Figure 5a illustrates the incidence of ectopic activity in pulmonary veins exposed to different combinations of concentrations of PE and ISO. Although there was a tendency for the incidence of ectopic activity to increase with increasing concentrations of both PE and ISO, the application of the highest concentration of ISO (10−6 M) was less effective. This observation was examined in detail in experiments such as that shown in Figure 5b where the addition of 10−8 M ISO to 5 × 10−6 M PE-induced low-frequency ectopic contractions. Increasing the concentration of ISO to 10−7 M increased the frequency of ectopic contractions, which formed distinct bursts. Further increasing the concentration of ISO to 10−6 M resulted in the disappearance of ectopic activity. The inhibition of ectopic activity by an excess of ISO was observed in nine preparations.
Figure 5.
Effects of agonist concentration upon ectopic contractions in the pulmonary vein. (a) The effects of the concentration of ISO upon the incidence of ectopic activity recorded in the presence of either 10−6 (open columns) or 5 × 10−6 M (filled columns) PE. The numbers in parentheses represent n different preparations. (b) Induction and suppression of ectopic activity by ISO. These traces represent segments of an otherwise continuous recording of tension in an isolated pulmonary vein. The periods for which the preparation was exposed to PE and different concentrations of ISO are indicated by the bars above the traces.
Different reactions of pulmonary vein and left atrium to adrenoceptor stimulation
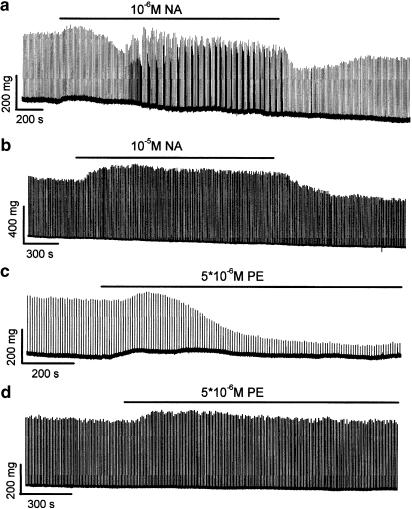
As reported above, pulmonary vein cardiac myocytes generate ectopic contractions in response to the application of NA whereas no ectopic activity is recorded in atrial cardiac myocytes. As shown by the typical trace in Figure 6a and reproduced in 17 experiments, NA induced a biphasic inotropic response from FS-evoked twitch contractions in the pulmonary vein. An initial increase in twitch amplitude (+22.7±3.7% after 5.6±0.5 min) was followed by a decline to less than the basal level (−26.8±5.3% after 14.6±1.3 min in 12 of 17 experiments). Isolated ectopic contractions appeared after 15.8±1.2 min and then formed distinct bursts. In isolated strips of the left atrium, NA induced a sustained positive increase in FS-evoked twitch contraction amplitude and it did not induce ectopic contractions (a typical trace is shown in Figure 6b, n=10). The responses of pulmonary vein and atrial cardiac myocytes to PE are also different. A slowly developing but important reduction in the amplitude of FS-evoked contractions (−69.8±2.8% after 16.1±0.6 min, n=11) is recorded in pulmonary vein preparations (Figure 6c), whereas a slight and mostly sustained increase in the amplitude of FS-evoked contractions is observed in atrial preparations (Figure 6d, n=8). Both pulmonary vein and atrial preparations showed positive inotropic responses to the superfusion of ISO (not shown).
Figure 6.
A comparison of the responses of the pulmonary vein (a and c) and the left atrium (b and d) to adrenoceptor stimulation. Each preparation was subjected to 0.1 Hz FS. The periods for which preparations were superfused with solutions, which contained either NA (a and b) or PE (c and d) are indicated by the bars above the traces.
Discussion
This study shows that nonselective adrenoceptor stimulation induces ectopic contractions in the pulmonary vein of the rat, which are entirely independent of electrically induced activity. This phenomenon is not observed in the left atrium.
Induction of ectopic contractions in isolated pulmonary veins of the rat requires a critical balance between the activation of α1- and β1-adrenoceptors. NA readily achieved this balance but it was more difficult to replicate with mixtures of PE and ISO in vitro. These results suggest that at some point from the receptors to the effectors there are functional or intermolecular interactions that give rise to the generation of ectopic contractions. These interactions could concern elements of the traditional α1-adrenoceptor signalling pathway (inositol (1,4,5)-triphosphate and diacylglycerol), the mobilization of intracellular Ca2+ and the activation of protein kinase C (PKC) and the classical signalling cascade of the β1-adrenoceptors (cAMP and protein kinase A (PKA)-dependent protein phosphorylation). It may involve novel time-dependent dynamic signalling mechanisms and G-protein-independent signalling pathways that have been recently reported for cardiac adrenoceptors (see Xiao et al., 2006 for review). For example, the time-dependent switch of the β1-adrenoceptor signalling pathway from PKA to Ca2+/calmodulin-dependent protein kinase II (Wang et al., 2004) is not incompatible with the delay between the onset of application of NA and the first appearance of ectopic contractions. Whatever the mechanism involved, it remains to be seen whether these complex interactions act on one or more effectors to induce ectopic contraction. It could also be suggested that interactions between different effectors result in ectopic contractions.
This study identifies an automatism that is particular to cardiac myocytes in the pulmonary vein. The requirement for simultaneous α1- and β1-adrenoceptor stimulation and the loss of ectopic activity upon exposure to an excess of ISO clearly distinguish it from classical nodal cell electrophysiology with its dependence upon β-adrenoceptor stimulation (Biel et al., 2002; Zorn-Pauly et al., 2004). Yamamoto et al. (2006) have shown that HCN4 that underlies the current If is not expressed in rat pulmonary veins. Our results are also clearly different from the recent proposal that PKA-mediated phosphorylation of multiple intracellular targets is responsible for sinoatrial node cell rhythmicity (Vinogradova et al., 2006). The presence of specialized conduction myocytes in pulmonary veins is disputed (Masani, 1986; Perez-Lugones et al., 2003; Anderson and Ho, 2004; Yamamoto et al., 2006) though if present these cells could give rise to spontaneous activity. Spontaneous action potentials have been reported in the cardiac myocytes of isolated pulmonary veins of different species including guinea-pig (Cheung, 1980), dog (Chen et al., 2000) and rabbit (Chen et al., 2006) but not in the rat (Miyauchi et al., 2005). The results in rabbit and dog have been questioned (Honjo et al., 2003; Wang et al., 2003, 2005). It is worth noting that we never observed spontaneous contractions in our pulmonary vein preparations in the absence of adrenoceptor stimulation.
Our study excludes an exclusive role for pathological alterations of cardiac cell action potentials in the generation of ectopic foci in the pulmonary vein of the rat as it was not necessary to stimulate the tissue electrically to develop ectopic contractions. This is not to say that the application of β-adrenoceptor agonists combined with rapid electrical stimulation cannot result in the development of extra systoles via the development of early- or delayed-after depolarizations (Schauerte et al., 2001; Miyauchi et al., 2004; Patterson et al., 2005). It does not deny that the application of acetylcholine and/or vagal stimulation can reduce action potential duration, effective refractory period and thus provide a substrate for the establishment of micro-re-entry circuits (Chen et al., 2001; Jalife et al., 2002; Scherlag et al., 2002).
A number of results in this study show that there are functional differences between the cardiac myocytes in the pulmonary vein and the left atrium, notwithstanding the direct physical and electrophysiological connection between them (de Bakker et al., 2002; Yeh, 2004). The most obvious difference is the ability of NA to induce ectopic contractions in the pulmonary vein but not in the left atrium. The inotropic responses of the pulmonary vein and left atrium to NA and PE also differ. They provoke a biphasic slight positive inotropy followed by an intense negative inotropic effect in the pulmonary vein, whereas a monophasic positive inotropic response is recorded in the left atrium. This may reflect a differential repartition of α1-adrenoceptor subtypes as α1A- and α1B-adrenoceptors elicit opposing effects on contractility in isolated ventricular myocytes of adult rat (Gambassi et al., 1998). More recently, Wang et al. (2006) showed separate negative and positive inotropic effects of α1-adrenoceptor stimulation in, respectively, the left and right ventricle of the mouse. They excluded α1-receptor subtypes from being responsible and suggested instead the different embryological origins of the left and right ventricles. Separate embryological origins for the cardiac myocytes in the pulmonary vein and the left atrium have been proposed by Kruithof et al. (2003) and Anderson et al. (2006).
In conclusion, although this study has been confined to the behaviour of an isolated pulmonary vein of the rat in response to stimulation with NA, this does not limit the interest of this work to rodent physiology or to adrenoceptor stimulation. It can be postulated that combinations of hormones and/or neurotransmitters that coactivate separate signalling pathways could provoke ectopic activity in the pulmonary veins of different species including man. However, the relationship between ectopic activity in a pulmonary vein and the induction and the maintenance of AF will depend upon a number of elements. For example, how such ectopic activity is conducted into the left atrium will depend upon the anatomy of the extension of cardiac myocytes in the pulmonary veins. In man, cardiac myocyte sleeves extend for 1–2 cm into the pulmonary veins (Hassink et al., 2003), whereas in the rat they run along the full length of the veins and even extend into the pulmonary parenchyma (Hashizume et al., 1998). Also, the pulmonary veins in the rat drain into a common sinus before joining the left atrium, whereas they independently join the roof of the left atrium in man. Therefore, the direct extrapolation from our results to the triggering of AF by ectopic foci in human pulmonary veins and to a pharmacological intervention against AF would not be realistic at this stage. Nevertheless, the specificity of the pulmonary vein compared with the left atrium holds out the hope of a selective pharmacological intervention.
Abbreviations
- AF
atrial fibrillation
- FS
field stimulation
- IP3
inositol 1,4,5-triphosphate
- PKA
protein kinase A
- PKC
protein kinase C
References
- Anderson RH, Brown NA, Moorman AFM. Development and structures of the venous pole of the heart. Dev Dyn. 2006;235:2–9. doi: 10.1002/dvdy.20578. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Ho SY. “Specialised” conducting cells in the pulmonary vein. J Cardiovasc Electrophysiol. 2004;15:121. doi: 10.1046/j.1540-8167.2004.03525.x. [DOI] [PubMed] [Google Scholar]
- Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Schneider A, Wahl C. Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med. 2002;12:206–213. doi: 10.1016/s1050-1738(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Bosch RF, Nattel S. Cellular electrophysiology of atrial fibrillation. Cardiovasc Res. 2002;54:259–269. doi: 10.1016/s0008-6363(01)00529-6. [DOI] [PubMed] [Google Scholar]
- Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- Chen SA, Yeh HI. Specialised conduction cells in human pulmonary veins: fact and controversy. J Cardiovasc Electrophysiol. 2003;14:810–811. doi: 10.1046/j.1540-8167.2003.03259.x. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, et al. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implications in initiation of atrial fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen YC, Tai CT, Yeh HI, Lin CI, Chen SA. Angiotensin II and angiotensin II receptor blocker modulate the arrhythmogenic activity of pulmonary veins. Br J Pharmacol. 2006;147:12–22. doi: 10.1038/sj.bjp.0706445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung DW. Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J Physiol (Lond) 1980;314:445–456. doi: 10.1113/jphysiol.1981.sp013718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker JMT, Ho SY, Hocini M. Basic and clinical electrophysiology of pulmonary vein ectopy. Cardiovasc Res. 2002;54:287–294. doi: 10.1016/s0008-6363(01)00532-6. [DOI] [PubMed] [Google Scholar]
- Gambassi G, Spurgeon HA, Ziman BD, Lakatta EG, Capogrossi MC. Opposing effect of α1-adrenergic receptor subtypes on Ca2+ and pH homeostasis in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 1998;274:H1152–H1162. doi: 10.1152/ajpheart.1998.274.4.H1152. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Tango M, Ushiki T. Three-dimensional cytoarchitecture of rat pulmonary venous walls: a light and scanning electron microscope study. Anat Embryol. 1998;198:473–480. doi: 10.1007/s004290050197. [DOI] [PubMed] [Google Scholar]
- Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins. A postmortum analysis in patients with and without atrial fibrillation. J Am Coll Cardiol. 2003;42:1108–1114. doi: 10.1016/s0735-1097(03)00918-5. [DOI] [PubMed] [Google Scholar]
- Hocini M, Haissaguerre M, Shah D, Jais P, Peng JT, Yamane T, et al. Multiple sources initiating atrial fibrillation from a single pulmonary vein identified by a circumferential catheter. Pacing Clin Electrophysiol. 2000;23:1828–1831. doi: 10.1111/j.1540-8159.2000.tb07030.x. [DOI] [PubMed] [Google Scholar]
- Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, et al. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation. 2003;107:1937–1943. doi: 10.1161/01.CIR.0000062645.38670.BD. [DOI] [PubMed] [Google Scholar]
- Jalife J, Berebfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–216. doi: 10.1016/s0008-6363(02)00223-7. [DOI] [PubMed] [Google Scholar]
- Khan R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc Res. 2004;64:387–394. doi: 10.1016/j.cardiores.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Kruithof BPT, Van den Hoff MJB, Tensink-Taekema S, Moorman AFM. Recruitment of intra- and extracardiac cells into the myocardial lineage during mouse development. Anat Rec A. 2003;271:303–314. doi: 10.1002/ar.a.10033. [DOI] [PubMed] [Google Scholar]
- Lal H, Williams KI, Woodward B. Chronic hypoxia differentially alters the responses of pulmonary arteries and veins to endothelin-1 and other agents. Eur J Pharmacol. 1999;371:11–21. doi: 10.1016/s0014-2999(99)00174-0. [DOI] [PubMed] [Google Scholar]
- Leblais V, Pourageaud F, Ivorra MD, Guibert C, Marthan R, Muller B. Role of α-adrenergic receptors in the effect of the β-adrenergic receptor ligand, CGP 12177, bupranolol, and SR 592230A, on the contractions of rat intrapulmonary artery. J Pharmacol Exp Ther. 2004;309:137–145. doi: 10.1124/jpet.103.061192. [DOI] [PubMed] [Google Scholar]
- MacLeod DP, Hunter EG. The pharmacology of the cardiac muscle of the great veins of the rat. Can J Physiol Pharmacol. 1967;45:463–473. doi: 10.1139/y67-055. [DOI] [PubMed] [Google Scholar]
- Masani F. Node-like cells in the myocardial layer of the pulmonary vein of rats: an ultrastructural study. J Anat. 1986;145:133–142. [PMC free article] [PubMed] [Google Scholar]
- Melnyk P, Ehrlich JR, Pourrier M, Villneuve L, Cha TJ, Nattel S. Comparison of ion channel distribution and expression in cardiomyocytes of canine pulmonary veins versus left atrium. Cardiovasc Res. 2005;65:104–116. doi: 10.1016/j.cardiores.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Fishbein MC, Karagueuzian HS. Electrical current-induced atrial and pulmonary vein action potential shortening and repetitive activity. Am J Physiol Heart Circ Physiol. 2004;287:H178–H186. doi: 10.1152/ajpheart.00085.2004. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Hayashi H, Miyauchi M, Okuyama Y, Mandel WJ, Chen PS, et al. Heterogenous pulmonary vein myocardial cell repolarisation, implications for re-entry and triggered activity. Heart Rhythm. 2005;2:1339–1345. doi: 10.1016/j.hrthm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature (Lond) 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- Page RL, Roden DM. Drug therapy for atrial fibrillation: where do we go from here. Nat Rev Drug Discovery. 2005;4:899–910. doi: 10.1038/nrd1876. [DOI] [PubMed] [Google Scholar]
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Perez-Lugones A, McMahon JT, Ratliff NB, Saliba WI, Schweikert RA, Marrouche NF, et al. Evidence of specialised conduction cells in human pulmonary veins of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:803–809. doi: 10.1046/j.1540-8167.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- Schauerte P, Scherlag BT, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, et al. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol. 2001;12:592–599. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Scherlag BJ, Yamanashi WS, Schauerte P, Scherlag M, Sun YX, Hou Y, et al. Endovascular stimulation within the left pulmonary vein to induce slowing of heart rate and paroxysmal atrial fibrillation. Cardiovasc Res. 2002;54:470–475. doi: 10.1016/s0008-6363(02)00239-0. [DOI] [PubMed] [Google Scholar]
- Verheule S, Wilson EE, Arora R, Engle SK, Scott LR, Olgin JE. Tissue structure and connexin expression of canine pulmonary veins. Cardiovasc Res. 2002;55:727–738. doi: 10.1016/s0008-6363(02)00490-x. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to α1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H2013–H2017. doi: 10.1152/ajpheart.00167.2006. [DOI] [PubMed] [Google Scholar]
- Wang TM, Chiang CE, Sheu JR, Tsou CH, Chang HM, Luk HN. Homogenous distribution of fast response action potentials in canine pulmonary vein sleeves: a contradictory report. Int J Cardiol. 2003;89:187–195. doi: 10.1016/s0167-5273(02)00474-6. [DOI] [PubMed] [Google Scholar]
- Wang TM, Luk HN, Sheu JR, Wu HP, Chiang CE. Inducibility of abnormal automaticity and triggered activity in myocardial sleeves of canine pulmonary veins. Int J Cardiol. 2005;104:59–66. doi: 10.1016/j.ijcard.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhu W, Wang S, Yang D, Crow WT, Xiao RP, et al. Sustained β1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signalling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, et al. Subtype-specific α1- and β-adrenoceptor signalling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Dobrzynski H, Tellez J, Niwa R, Billeter R, Honjo H, et al. Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc Res. 2006;72:271–281. doi: 10.1016/j.cardiores.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Yeh HI.Immunohistology of the thoracic veins Thoracic Vein Arrhythmias: Mechanisms and Treatment 2004Blackwell Futura Press: MA, USA; 99–110.In: Chen SA, Hassaguerre M, Zipes DP (eds). [Google Scholar]
- Zorn-Pauly K, Schaffer P, Pelkmann B, Lang P, Machler H, Rigler B, et al. If in left human atrium: a potential contributor to atrial ectopy. Cardiovasc Res. 2004;64:250–259. doi: 10.1016/j.cardiores.2004.07.001. [DOI] [PubMed] [Google Scholar]