Abstract
Background and purpose:
A strategy to treat Alzheimer's disease (AD) is to increase the soluble form of amyloid precursor protein (sAPPα), a promnesic protein, in the brain. Because strong evidence supports beneficial effects of 5-hydroxytryptamine 5-HT4 receptor agonists in memory and learning, we investigated the role of 5-HT4 receptors on APP processing in 8 weeks-old male C57BL/6j mice.
Experimental approach:
Mice were given, subcutaneously, prucalopride or ML 10302 (s.c.), two highly selective 5-HT4 receptor agonists and, up to 240 min later, the hippocampus and cortex were analysed by Western blot for sAPPα determination.
Key results:
Prucalopride (5 or 10 mg kg-1) significantly increased sAPPα levels in the hippocampus and cortex, but did not modify the expression level of APP mRNA as detected by quantitative RT-PCR. A selective 5-HT4 receptor antagonist, GR125487 (1 mg kg-1, s.c.) inhibited prucalopride induced- increase in sAPPα levels. In addition, levels of sAPPα were increased by ML10302 only at 20 mg kg-1 and was limited to the cortex. Also, prucalopride increased sAPPα levels in the cortex of a transgenic mouse model of AD, expressing the London mutation of APP. Furthermore, the combined injection of a selective acetylcholinesterase inhibitor, donepezil and prucalopride induced a synergic increase in sAPPα levels in the cortex and hippocampus.
Conclusions and implications:
Our results demonstrate that the 5-HT4 receptor plays a key role in the non-amyloidogenic pathway of APP metabolism in vivo and give support to the beneficial use of 5-HT4 agonists for AD treatment.
Keywords: 5-HT4 receptor agonist, amyloid precursor protein, Alzheimer's disease, acetylcholinesterase inhibitor
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by the appearance of senile plaques mainly composed of amyloid β-protein (Aβ), and the development of neurofibrillary tangles in patients' brains (Dickson, 1997). AD patients also have cognitive deficits, impaired long-term potentiation (LTP), learning and memory deficits (Walsh and Selkoe, 2004) and a consistent deficit in cholinergic neurotransmission. Several acetylcholine esterase inhibitors such as donepezil are available in the market for the treatment of patients with mild-to-moderate AD. However, beneficial effects of this symptomatic treatment can only be maintained for up to 36 months (Racchi et al., 2004).
As the hippocampus plays an important role in spatial memory and LTP, it is important to study this brain region to understand the development of the disease. Recently, by injecting a 5-hydroxytryptamine (5-HT4) receptor agonist to rats and implanting a recording electrode in the hippocampal CA1 region, Kemp and Manahan-Vaughan (2005) showed, in novelty exploration experiments, that 5-HT4 receptors play a key role in the regulation of synaptic plasticity and the determination of particular properties of stored synaptic information. Moreover, 5-hydroxytryptamine (5-HT4) receptor agonists are known to improve memory in different behavioural experiments in rodents (Marchetti-Gauthier et al., 1997; Galeotti et al., 1998; Lamirault and Simon, 2001; Moser et al., 2002; Lelong et al., 2003).
The 5-HT4 receptor is one of the seven subtypes of 5-HT receptors. It is a 7-transmembrane domain protein coupled to a G-protein positively linked to the activation of adenylate cyclase (Bockaert et al., 1990). Autoradiographic studies using the 5-HT4 receptor antagonists [125I]SB207710 and [3H]GR113808 in rat, mouse, guinea pig or post-mortem human brain showed that the 5-HT4 receptor is present at a high density in the limbic system including the hippocampus and frontal cortex (Waeber et al., 1994; Varnas et al., 2003), suggesting a role of this subtype of 5-HT receptor in memory and cognition.
Whereas drugs are currently available that may slightly ameliorate late-stage symptoms such as cognitive deficits, no drugs are in the market that specifically target the cellular mechanisms of AD, namely the generation of the neurotoxic Aβ from the amyloid precursor protein (APP). APP is a transmembrane glycoprotein that can be cleaved by β- and γ-secretases generating Aβ (Kerr and Small, 2005). In contrast, cleavage of APP by α-secretase occurs in the Aβ sequence and releases a large soluble N-terminal ectodomain, named non-amyloidogenic soluble form of amyloid precursor protein (sAPPα), into the extracellular space. Interestingly, sAPPα exerts proliferative effects as well as neurotrophic and neuroprotective effects in a variety of cell types (Turner et al., 2003). In addition, intracerebroventricular administration of sAPPα can improve spatial memory in mice, thus confirming the hypothesis of an action of this protein on early memory processes (Bour et al., 2004). Considerable emphasis is being placed on the pharmacological modulation of APP processing, aiming to enhance cleavage of APP by α-secretase and reduce Aβ formation. Recent in vitro studies have shown that activation of 5-HT4 receptors stimulates the secretion of the sAPPα (Robert et al., 2001; Lezoualc'h et al., 2003). However, in vivo information on the effect of 5-HT4 receptor ligands on APP metabolism is still lacking.
We studied changes in sAPPα levels in the hippocampus and cortex following treatment with either 5-HT4 receptor ligands or a combination of a 5-HT4 receptor agonist with an acetylcholinesterase inhibitor, donepezil. These experiments were performed in adult male C57BL/6j mice and also in a transgenic mouse line overexpressing the ‘London' mutant of human APP (APP/V717I) (Moechars et al., 1999). These transgenic mice are a good animal model of AD as they display several features of the disease such as increased levels of intracellular and extracellular amyloid peptides and cognitive deficits early in life (4–9 weeks) (Moechars et al., 1999). In addition, two 5-HT4 receptor agonists, prucalopride and ML10302, and one 5-HT4 receptor antagonist, GR125487 were used in this study. Prucalopride is a highly selective partial agonist (pEC50=7.48) at 5-HT4 receptors (Briejer et al., 2001a). It belongs to the newer benzamide family and does not display any antagonistic activity at 5-HT3 receptors. It has been used in electrophysiology and gastroenterology studies (Briejer et al., 2001b; Qi et al., 2003; Spencer et al., 2004). ML10302, an ester of 4-amino-5-chloro-2-methoxybenzoic acid, is a partial agonist at 5-HT4 receptors in vitro (Mialet et al., 2000a, 2000b). GR125487 is a 5-HT4 receptor antagonist with an ester linkage, which increases its bioavailability in rats and mice (Porras et al., 2002; Lelong et al., 2003). Moreover, it has greater potency (pKb=10) than another 5-HT4 receptor antagonist, GR113808 (pKb=9) (Eglen et al., 1995).
Methods
Animals
Adult male C57BL/6j wild-type mice (8-weeks old, weighing 23–27 g) from Janvier (Le Genest-Saint-Isle, France) were used in this study. Mice were housed in a 12-h light–dark cycle with food and water ad libitum. Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national and international legislation (Council Directive # 87–848, 19 October 1987, ‘Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale', permissions # 92–196 to AMG).
Adult APP/London transgenic mice (5–8-months old) used in this study were of the FVB/N genetic background and expressed human APP(V717I) under control of the mouse thy1 gene promoter (Moechars et al., 1999).
Treatment
The control group of mice received a subcutaneous (s.c.) injection of 0.9% NaCl. The treated groups (10 mice per group) received a single s.c. injection either of prucalopride (1, 5 or 10 mg kg−1 in a volume of 5 ml kg−1) or ML10302 (5, 10 or 20 mg kg−1 in a volume of 5 ml kg−1) dissolved in 0.9% NaCl.
For the dose–response relationship of 5-HT4 receptor agonists, mice were killed 90 min after the drug injection by cervical dislocation, the brains were quickly removed and the hippocampus and cortex were dissected at −20°C as described previously (Gardier et al., 2003). The hippocampus and cortex were immediately put in dry ice and weighed before being stored at −80°C until Western blotting was performed.
For the time-course study, each mouse received a single s.c. injection of prucalopride (5 mg kg−1) or saline. Then, they were killed 0, 30, 90 or 240 min after the injection by cervical dislocation. Mice brains were then treated as described above.
To assess the acute selective effect of prucalopride, we studied the blockade of its effects by the 5-HT4 receptor antagonist GR125487. Four groups of mice received either saline+saline, saline+prucalopride, GR125487+saline or GR125487+prucalopride. GR125487 (1 mg kg−1) or saline was administered s.c. 30 min before prucalopride (5 mg kg−1, s.c.) or saline. The mice were killed 90 min after the prucalopride injection and their brains were then treated as described above.
To study the effects of the combined treatment of prucalopride with the acetylcholine esterase inhibitor, donepezil, four groups of mice received either saline+saline, prucalopride+saline, saline+donepezil or prucalopride+donepezil. Donepezil (0.75 mg kg−1, s.c.) or saline was administered 30 min after prucalopride (5 mg kg−1, s.c.) or saline. The mice were killed 90 min after the prucalopride injection and their brains were then treated as described above.
Antibodies
R1736 (antibody kindly provided by Dr Dennis Selkoe, Harvard Medical School, Boston, MA, USA) is a rabbit polyclonal antiserum raised to a synthetic peptide of amino acids 595–611 of APP (Haass et al., 1992; Robert et al., 2001). WO-2 mouse antibody (the Genetics Company, Schlieren, Switzerland) recognizes the N terminus of human amyloid β-peptide. The β-actin mouse antibody was purchased from Abcam (UK).
Sample preparation
Hippocampus and cortex were homogenized by sonication in 150 and 300 μl of 50 mM Tris, pH 7.4, 150 mM NaCl and proteinase inhibitors (Sigma, France), respectively, and cleared by centrifugation (100 000 g, 45 min, 4°C). Only the resulting supernatant was used for experiments.
Measurement of sAPPα and β-actin by Western blot
To investigate the role of an acute treatment of 5-HT4 receptor agonists on sAPPα levels, we measured the expression of this protein in homogenates from the hippocampus and cortex of treated and untreated mice by Western-blot analysis (polyclonal antiserum R1736, 1:4000 dilution; goat anti-rabbit immunoglobulin antibody, 1:15 000; Amersham Pharmacia Biotech, (Orsay, France) or WO-2 1:1000 with anti-mouse immunoglobulin antibody for transgenic mice). Immunoreactive bands were then visualized by the ECL plus detection kit (Amersham Pharmacia Biotech, Orsay, France) on Kodak ML lights films. The membrane was stripped and blocked again before being probed by β-actin antibody (monoclonal anti-β-actin, 1:5000 dilution; Abcam, Cambridge, UK), used as internal control. After 1-h incubation with a horseradish peroxidase-linked goat anti-mouse immunoglobulin antibody (Cell Signaling, Ozyme, Saint-Quentin-en-Yvelines, France) at 1:2000 dilution, immunoreactive bands were visualized by the ECL detection kit (Amersham Pharmacia Biotech, Orsay, France) on Kodak ML lights films. After digitization, densitometric values were evaluated by using an image analysis.
Real-time reverse transcriptase-polymerase chain reaction
Total RNA was extracted from frozen hippocampus and cortex of saline and treated mice using a conventional guanidinium-thiocyanate acid-phenol-chloroform procedure with the Extract-All reagent according to the manufacturer's description (Eurobio, Les Ulis, France). Integrity and concentration of total RNA were directly analysed on an RNA 6000 Nano LabChip (Agilent, Wilmington, DE, USA) following the manufacturer's instruction. Total RNA (5 μg) of each sample was reversely transcribed using 0.5 μg of oligo dT and 200 U Superscript II reverse transcriptase (Invitrogen, Cergy Pontoise, France). Quantitative RT-PCR was conducted with a LightCycler system (RocheAQ) using a LightCycler-FastStart DNA Master SYBR Green I kit (Roche) and specific primer pair for APP695 (5′-GATGGCGGTGAAGACAAAGT-3′; 5′-CTTTGGCTTTCTGGAAATGG-3′) or β-actin (5′-AGAGGGAAATCGTGCGTGAC-3′; 5′-CAATAGTGATGACCTGGCCGT-3′).
For each pair of primers, PCR assays performed upon RT negative controls (i.e. no-template or no-reverse transcriptase controls) produced no amplification signal, suggesting that the formation of primer dimer and the contamination by genomic DNA were negligible. To normalize APP (695 isoform) mRNA levels, β-actin gene transcripts were quantified in each cDNA to control sample-to-sample differences in RNA concentration and quality or in reverse transcription efficiency. APP transcript levels were normalized to the content of β-actin, leading to APP values expressed in arbitrary units.
Statistical analysis
For data comparison, optical density ratios were calculated as described in Deplanque et al. (2003). Statistical analyses were performed using the computer software StatView 4.02 (Abacus Concepts Inc., Berkeley, CA, USA). Data are means±s.e.m. of sAPPα levels expressed as percentage of control values (mice which received a saline injection). Values were compared between the different groups by using a one-way analysis of variance (ANOVA) followed by least significant difference tests (PLSD). The significance level was set at P<0.05.
Drugs and reagents
Prucalopride, donepezil and GR125487 were a generous gift from Dr X Langlois (Janssen Pharmaceutics, Beerse, Belgium), Eisai Co., Ltd. (Tokyo, Japan) and GlaxoSmithKline (Middlesex, UK), respectively. The synthesis of ML10302 (2-piperidinoethyl 4-amino-5-chloro-2-methoxybenzoate) was performed in Biocis UMR 8076 (Châtenay-Malabry, France).
Results
Effects of 5 HT4 receptor agonists on sAPPα levels in mice
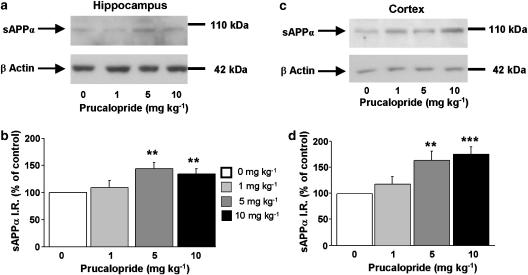
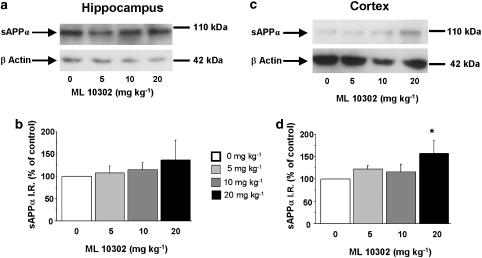
To analyse brain sAPPα levels, adult male C57BL/6j wild-type mice were injected with a single dose of prucalopride (1, 5 or 10 mg kg−1, s.c.), ML10302 (5, 10 or 20 mg kg−1, s.c.) or saline (5 ml kg−1). After 90 min treatment with the 5-HT4 agonist, sAPPα was determined by Western blot in hippocampus and cortex extracts. As shown in Figure 1, prucalopride significantly increased sAPPα levels in a dose-dependent manner in the hippocampus (Figure 1a and b) and in the cortex (Figure 1c and d) to 5 and 10 mg kg−1. Although sAPPα level was significantly increased in the cortex of mice treated with 20 mg kg−1 ML 10302 as compared to saline (Figure 2c and d), we did not observe any effect of this partial agonist on sAPPα levels in the hippocampal region (Figure 2a and b).
Figure 1.
Dose–response relationship of systemic prucalopride administration on sAPPα levels in the hippocampus (a and b) and cortex (c and d) of 8-weeks-old male C57BL/6j mice. The mice received a range of prucalopride doses (0, 1, 5 and 10 mg kg−1, s.c.) and Western-blot analysis was performed on sAPPα levels in hippocampal and cortical homogenates, 90 min after the single drug injection. In (a and c) representative immunoblots illustrating the effects of increasing doses of prucalopride on sAPPα levels are shown. In (b and d), the mean densitometric values (±s.e.m.) of sAPPα levels (shown here and in all similar figures as immunoreactive sAPPα=sAPPα IR), relative to β-actin (used as internal control) are expressed as percentages of values in untreated control mice. One-way ANOVA for hippocampus, F(3,35)=5.45; for cortex, F(3,33)=7.85. **P<0.01 and ***P<0.001 compared with saline-treated mice (clear bar); least significant difference test; n=8 per group.
Figure 2.
Dose–response relationship of systemic ML 10302 administration on sAPPα levels in the hippocampus (a and b) and cortex (c and d) of 8-week-old male C57BL/6j mice. The mice received a range of ML 10302 doses (0, 5, 10 and 20 mg kg−1, s.c.) and Western-blot analysis was performed on sAPPα levels from hippocampal and cortical homogenates 90 min after the single drug injection. In (a and c) representative immunoblots illustrating the effects of increasing doses of ML10302 on sAPPα levels are shown. In (b and d), the mean densitometric values (±s.e.m.) of sAPPα levels, relative to β-actin (used as internal control) are expressed as percentages of values in untreated control mice. One-way ANOVA for hippocampus, F(2,16)=1; for cortex, F(2,19)=4.51. *P<0.05 compared with saline-treated mice (clear bar); least significant difference test; n=5 mice per group.
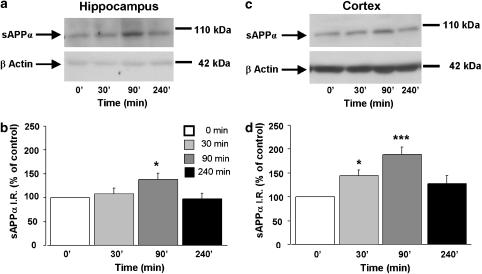
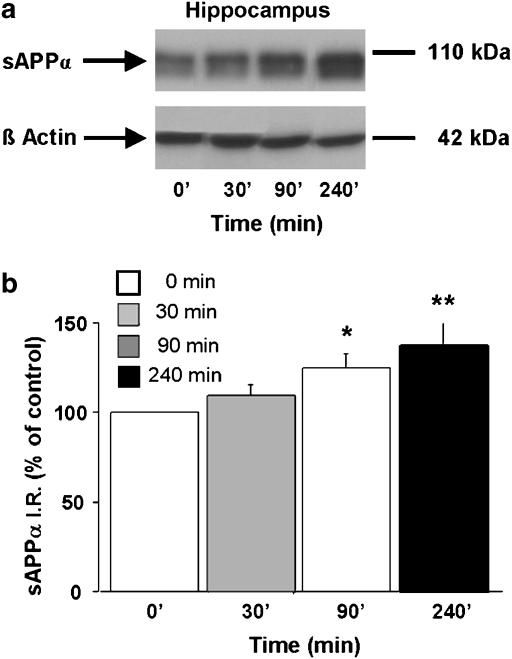
Next, to determine the time course of prucalopride-induced increase in sAPPα levels, a second set of mice was injected with a single dose of either prucalopride (5 mg kg−1, s.c.) or saline and sAPPα levels were measured in hippocampal and cortical homogenates 30, 90 and 240 min after the injections. Figure 3 showed that prucalopride-induced increase in sAPPα levels reached a maximum 90 min after its injection in the hippocampus and the cortex.
Figure 3.
Time-course study of the effect of prucalopride on sAPPα level in the hippocampus (a and b) and cortex (c and d) in 8-week-old male C57BL/6j mice. The mice were treated with a single dose of prucalopride (5 mg kg−1, s.c.) or saline (5 ml kg−1). sAPPα and β-actin levels (used as internal control) were measured in brain homogenates 30, 90 and 240 min after the injection. In (a and c) representative immunoblots illustrating the effects of increasing time on sAPPα levels are shown. In (b and d), the mean densitometric values (±s.e.m.) of sAPPα levels, relative to β-actin (used as internal control) are expressed as percentages of values in untreated control mice. One-way ANOVA for hippocampus, F(3,26)=3.36; for cortex, F(3,22)=7.53. *P<0.05 and ***P<0.001 compared with saline-treated mice (t=0; clear bar); least significant difference test: n=7 mice per group.
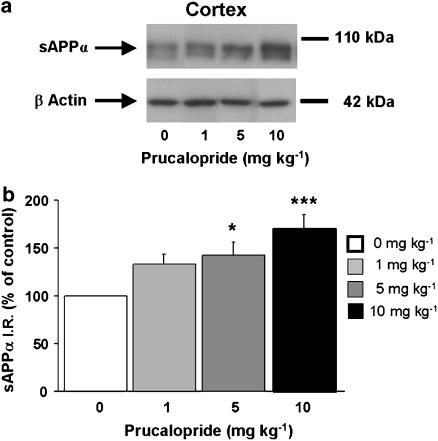
Finally, we tested whether prucalopride may influence sAPPα levels in a transgenic mouse model of AD expressing the human APP/London mutation. Injection (s.c.) of 5 or 10 mg kg−1 prucalopride significantly increased sAPPα levels in the cortex of transgenic mice (Figure 4). An increase in sAPPα level in the hippocampus was also observed at 90 and 240 min (Figure 5). We found in these experiments that sAPPα migrates as a doublet of bands, a major band and a less intense and lower migrating band (Figures 4 and 5). These two bands may correspond to different glycosylation states of sAPPα.
Figure 4.
Dose–response relationship of systemic prucalopride administration on sAPPα levels in the cortex of adult APP/V717I transgenic mice. The mice received prucalopride in a range of doses (0, 1, 5 and 10 mg kg−1, s.c.). Western-blot analysis was performed to measure brain sAPPα levels at 90 min after the injection. In (a) a representative immunoblot illustrating the effects of increasing doses of prucalopride on sAPPα levels is shown. In (b), the mean densitometric values (±s.e.m.) of sAPPα levels, relative to β-actin (used as internal control) are expressed as percentages of values in untreated control mice. One-way ANOVA, F(3,14)=7.746. *P<0.05 and ***P<0.001 compared with saline-treated mice; least significant difference test; n=4 mice per group.
Figure 5.
Time-course study of sAPPα secretion in the hippocampus by prucalopride in adult APP/V717I transgenic mice. The mice were treated with a single dose of prucalopride (5 mg kg−1, s.c.) or saline (5 ml kg−1) (clear bar). Brain sAPPα and β-actin levels (used as internal control) were measured 30, 90 and 240 min after the injection. In (a) a representative immunoblot illustrating the effects of time on sAPPα levels is shown. In (b), the mean densitometric values (±s.e.m.) of sAPPα levels, relative to β-actin (used as internal control), are expressed as percentages of values in untreated control mice. One-way ANOVA, F(3,11)=5.414. *P<0.05 and **P<0.01 compared with saline-treated mice (t=0); least significant difference test; n=4 mice per group.
Effects of the selective blockade of 5-HT4 receptors by GR125487 on prucalopride-induced increase in brain sAPPα levels
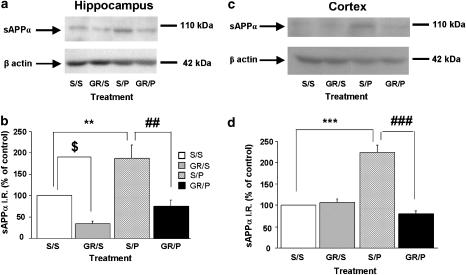
Next, we examined whether a highly and potent selective 5-HT4 antagonist, GR125487, blocked the prucalopride-induced increase in sAPPα (Figure 6). Pretreatment with a low dose of the GR125487 (1 mg kg−1 s.c., 30 min before prucalopride administration) completely prevented the increase in sAPPα induced by prucalopride (5 mg kg−1, s.c.) in the hippocampus (Figure 6a and b) and cortex of wild-type mice (Figure 6c and d). This result indicates that the effect of prucalopride on sAPPα is specifically mediated by the 5-HT4 receptor. Interestingly, GR125487 reduced significantly the basal level of sAPPα in the hippocampus (Figure 6a and b). In contrast, injection of GR125487 did not modify the relative basal level of sAPPα in the cortex (Figure 6c and d).
Figure 6.
Effects of the selective blockade of 5-HT4 receptors by GR125487 on prucalopride-induced increases in sAPPα levels in the hippocampus (a and b) and cortex (c and d) of mice. A low dose of the selective 5-HT4 receptor antagonist, GR125487 (1 mg kg−1, s.c.), was administered 30 min before prucalopride, and it completely blocked increases in sAPPα levels induced by prucalopride (5 mg kg−1, s.c.) in the hippocampus and cortex. The hippocampus and cortex were removed 90 min after prucalopride injection and Western-blot analysis was performed on sAPPα levels. In (a and c) representative immunoblots illustrating the effects of different treatments (S=saline; P=prucalopride, 5 mg kg−1; GR=GR125487, 1 mg kg−1) on sAPPα levels are shown. In (b and d), the mean densitometric values (±s.e.m.) of sAPPα levels, relative to β-actin (used as internal control), are expressed as percentages of values in untreated control mice. One-way ANOVA, for P vs GR+P, in hippocampus, F(3,16)=6.659; in cortex, F(3,14)=40.835; for GR vs saline in hippocampus, F(3,16)=6.659. $P<0.05, **P<0.01 and ***P<0.0001 compared with saline-treated mice (clear bar); ##P<0.001 and ###P<0.0001 compared with prucalopride-treated mice; least significant difference test; n=4–8 mice per group.
Effects of co-treatment with prucalopride and donepezil on brain sAPPα levels in mice
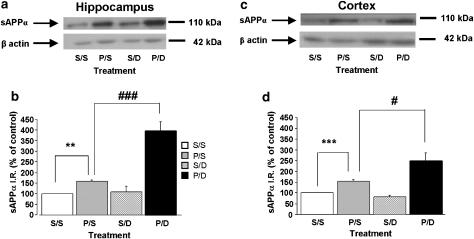
The possible interaction between prucalopride and the cholinesterase inhibitor donepezil was examined using a dose of donepezil (0.75 mg kg−1, s.c.), which, given alone, had no significant effect on sAPPα level in the cortex and hippocampus of wild-type mice (Figure 7). Injection of this dose of donepezil, 30 min after prucalopride (5 mg kg−1, s.c.), markedly increased sAPPα levels compared with prucalopride alone, in the hippocampus (Figure 7a and b) and the cortex (Figure 7c and d).
Figure 7.
Synergic effect of prucalopride with donepezil on sAPPα secretion in the hippocampus (a and b) and cortex (c and d) of wild-type mice. An inactive dose of the selective acetylcholine esterase inhibitor, donepezil (0.75 mg kg−1, s.c.), was administered 30 min after prucalopride. This combination induced a synergic increase in sAPPα levels in the hippocampus and cortex. The hippocampus and cortex were removed 90 min after prucalopride injection and Western-blot analysis was performed to determine sAPPα levels. In (a and c) representative immunoblots illustrating the effects of different treatments (S=saline, P=prucalopride, 5 mg kg−1, D=donepezil, 0.75 mg kg−1) on sAPPα levels are shown. In (b and d), the mean densitometric values (±s.e.m.) of sAPPα levels, relative to β-actin (used as internal control), are expressed as percentages of values in untreated control mice (clear bar). One-way ANOVA, for P only vs P+D, in hippocampus, F(3,9)=27.28; in cortex, F(3,14)=10.03. **P<0.01 and ***P<0.001 compared with saline-treated mice. #P<0.01 and ###P<0.001 compared with prucalopride-treated mice; least significant difference test; n=4–6 mice per group.
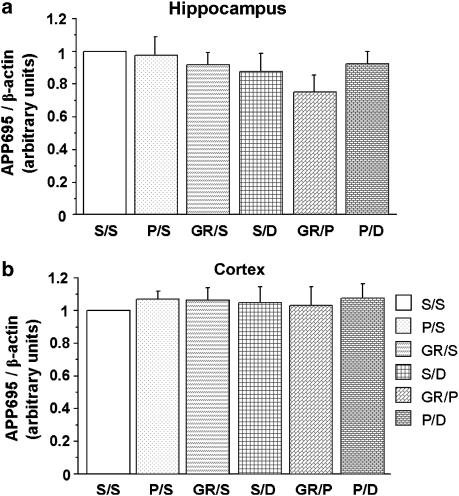
Finally, to exclude any effect of the different compounds on APP gene expression, we measured APP695mRNA transcripts in the hippocampus and the cortex of wild-type mice upon injection of prucalopride (5 mg kg−1), GR125487 (1 mg kg−1), donepezil (0.75 mg kg−1), prucalopride (5 mg kg−1) and GR125487 (1 mg kg−1), or prucalopride (5 mg kg−1), and donepezil (0.75 mg kg−1). APP695 is known to be the major APP isoform in neurons (Tanaka et al., 1989). Whatever the treatment administered to the mice, no significant variation of APP695 mRNA was observed in the hippocampus (Figure 8a) or cortex (Figure 8b).
Figure 8.
Drug treatments do not modify APP695 mRNA expression levels in the hippocampus (a) and cortex (b) of wild-type mice. Different treatments (S=saline, P=prucalopride, 5 mg kg−1, GR=GR125487 1 mg kg−1, D=donepezil, 0.75 mg kg−1) were administered to the mice, and the hippocampus and cortex were removed 90 min after prucalopride injection. Quantitative RT-PCR was then performed to analyse APP695 mRNA expression. The values shown are mean±s.e.m. of APP695 mRNA, normalized to β-actin mRNA, and are expressed relative to the ratio in untreated control mice (clear bar=1.00). Data from 4 to 5 mice per group.
Discussion
The results reported here suggest that 5-HT4 receptor agonists such as prucalopride increased the level of sAPPα in adult mice hippocampus and cortex. We showed by Western-blot analysis that prucalopride induced sAPPα production in these two brain regions in a transient and dose-dependent manner. This effect was also found in the cortex, but not in the hippocampus, with ML10302, a weak 5-HT4 receptor agonist. In addition, prucalopride-induced increase in sAPPα level in the hippocampus and cortex was blocked by GR125487, a highly selective 5-HT4 receptor antagonist. These data thus suggest that the effects of a single administration of a 5-HT4 receptor agonist on sAPPα levels in these two brain regions result from interactions between 5-HT neurotransmission and APP processing. Indeed, endogenous 5-HT may enhance intracellular events leading to the cleavage of APP by α-secretase within the Aβ domain to generate sAPPα. The present study provides the first in vivo selective link between these two components, the 5-HT4 receptor and APP, in C57BL/6j mice and in transgenic mice with the APP London mutation. In addition, the co-administration of prucalopride and donepezil, an acetylcholinesterase inhibitor, had a synergistic effect on hippocampal and cortical increases in sAPPα levels.
Ligands for the 5-HT4 receptor can be of interest in central nervous system research (Bockaert et al., 2004). As prucalopride has a high potency (pEC50 of 7.8) and affinity (pKi of 8.6) at the 5-HT4receptor, it can be considered as one of the best ligands to use in pharmacological experiments. ML 10302 has a pEC50 of 8.6 and it is also highly selective for 5-HT4 receptors.
The present data are consistent with our previous in vitro studies showing that 5-HT4 receptor agonists such as prucalopride increased the extracellular levels of sAPPα production in primary neurons and cell lines (Robert et al., 2001, 2005; Lezoualc'h and Robert, 2003; Maillet et al., 2003). We found here that ML10302 was less potent than prucalopride to induce sAPPα production in the cortex. This is probably due to the fact that ML10302 is an ester and consequently is easily hydrolysed in vivo and does not efficiently cross the blood–brain barrier (Langlois and Fischmeister, 2003). These characteristics could explain the high dose of ML10302 required here (20 mg kg−1, s.c.) to induce an effect in the cortex.
Although the density of 5-HT4 receptors is higher in the hippocampus (117 fmol mg−1 protein) than the cortex (83 fmol mg−1) (Waeber et al., 1994), we found that the effect of prucalopride on sAPPα levels was more potent in the cortex than the hippocampus, and ML10302 did not influence sAPPα production in the hippocampus. This is particularly interesting as there is evidence for a loss of 5-HT4 receptors in AD in the cortex and the hippocampus (−23 and −66%, respectively (Bockaert et al., 2004)). To explain these differences between these two brain regions, one could speculate that the effect of 5-HT4 receptor ligands on sAPPα production in the hippocampus are mediated by specific 5-HT4 receptor isoforms which display pharmacological properties distinct from those expressed in the cortex. Indeed, it is known that structural differences in the C-terminal tails of 5-HT4 receptor isoforms are known to influence and contribute to the specificity of their functional pharmacological profile to a given 5-HT4 receptor ligand (Mialet et al., 2000a, 2000b; Maillet et al., 2004). These functional differences may explain the brain region-dependent specificities observed with GR125487 and 5-HT4 receptor agonists on sAPPα production. Similar observations have been reported for 5-HT4 receptor ligands at the periphery (Langlois and Fischmeister, 2003). For instance, ML 10302 described as a potent 5-HT4 receptor agonist in the gastrointestinal tract us was a weak and partial agonist at this receptor subtype in cardiac myocytes (Langlois and Fischmeister, 2003).
The fact that prucalopride increased brain sAPPα levels in a transgenic mouse model of AD expressing the London mutation of APP under the control of promoter indicates that the 5-HT4 receptor may influence APP metabolism rather than APP gene expression (Figures 4 and 5). In addition, we found that acute injection of prucalopride in non-transgenic mice failed to influence the expression levels of APP695mRNA as determined by quantitative PCR (Figure 8). These data argue for a direct stimulating effect of 5-HT4 receptors on α-secretase activity. Accordingly, we showed recently that secretion of sAPPα induced by the 5-HT4e receptor isoform was not due to a general stimulation of the constitutive secretory pathway, but rather to its specific effect on α-secretase activity in different cell lines (Robert et al., 2005). Indeed, zinc metalloprotease inhibitors such as batimastat, marimastat and TAPI were effective in blocking sAPPα release induced by activation of 5-HT4 receptors in human neuroblastoma IMR32 and CHO cell lines (Robert et al., 2005). Moreover, we found that an inactive form of an α-secretase candidate, ADAM10, inhibited 5-HT-induced sAPPα secretion (Robert et al., 2005). Altogether, these data suggest the involvement of either one or more α-secretases in the regulation of APP ectodomain shedding induced by the 5-HT4 receptor.
In addition, Cho and Hu (2007) demonstrated recently that activation of 5-HT4 receptors inhibited the secretion of β-amyloid peptides in primary cultures of cortical neurons in Tg2576 transgenic mice expressing the Swedish mutation of APP. A neuroprotective effect was also observed. As sAPPα has neuroprotective properties, this in vitro study is consistent with our results. It reinforces our hypothesis that 5-HT4 receptor agonists may act in the brain on the secretase activity, but not on APP levels. However, an increase in sAPPα does not necessarily imply a decrease in sAPPβ or Aβ. Thus, the measurements of the effects of prucalopride on other parameters, such as full-length APP (APPFL) and β-secretase processing must be further investigated. In a preliminary set of experiments, we failed to find prucalopride effects on cortical APPFL, the C-terminal membrane-associated fragment (CTF)α and CTFβ levels in 5–8-month-old huAPP transgenic mice.
The maximal enhancement of sAPPα by prucalopride occurred within 90 min after s.c. administration, in the hippocampus and within 30 min in the cortex, and 90 min after ML 10302 in the cortex. These results are also consistent with the rapid response observed in vitro (Robert et al., 2001). However, in contrast to in vitro experiments, increases in hippocampal and cortical sAPPα levels returned to control values after 240 min after treatment suggesting a rapid exocytosis of sAPPα. This clearance could be explained by binding of sAPPα to ApoE as it was shown (with Ca2+ imaging) in primary cultures of rat hippocampal neurons that ApoE interacts directly with sAPPα (Barger and Mattson, 1997). In addition, Lelong et al. (2003) showed that a highly selective partial agonist, RS 67333 (1 mg kg−1, intraperitoneal (i.p.)), and a less selective full agonist, BIMU 1 (10 mg kg−1, i.p.), at 5-HT4 receptors, reversed scopolamine-induced deficit in working memory tests in mice, when given only 30 min before testing. These effects were selectively blocked by GR125487. RS 67333 was also used in old or young rats (at 1 and 10 mg kg−1, i.p., respectively) to test for spatial memory. The drug was injected twice, 30 min before the acquisition phase and 15 min after. This treatment enhanced place and object recognition (Lamirault and Simon, 2001; Lelong et al., 2003). These experiments showed that 5-HT4 receptor agonists act rapidly in memory processes. Given the promnesic properties of sAPPα, memory amelioration observed in different behavioural tests with 5-HT4 receptor agonists could be explained by a rapid production of sAPPα. This would be consistent with the results of the present time-course study of prucalopride performed in 8-week-old C57BL/6j mice.
Acetylcholinesterase inhibitors are currently administered to AD patients. However, beneficial effects cannot be maintained for more than 36 months (Racchi et al., 2004). A polytherapy might thus be of interest in the treatment of the cognitive deficits related to ‘normal' or pathological aging. The absence of any effect of donepezil on APPmRNA accumulation is consistent with the literature (Racchi et al., 2004). Our demonstration that prucalopride can act synergistically with donepezil on sAPPα levels is consistent with previous behavioural experiments performed in young rats showing that a co-administration of galanthaminium bromide (0.3 mg kg−1, i.p.), an acetylcholinesterase inhibitor and RS 67333 (0.1 mg kg−1, i.p.) improved memory in the place recognition test (Lamirault et al., 2003). The synergistic effect of donepezil and prucalopride on sAPPα could be explained by the combined activation of mutiple mechanisms and signalling pathways, which enhance α-secretase activity. Indeed, donepezil is known to exert its effects not only by enhancing the metabolism of membrane APP towards the non-pathogenic pathway through acetylcholine receptors and protein kinase C (Pakaski and Kasa, 2003), but also by promoting trafficking of the α-secretase candidate, ADAM 10, to the membranes, thus further enhancing α-secretase activity (Zimmermann et al., 2004). On the other hand, the enhancing effect of the 5-HT4 receptor on α-secretase activity involves cAMP-regulated guanine nucleotide exchange factor, Epac and the small GTPase Rac (Maillet et al., 2003; Robert et al., 2005).
In conclusion, the present in vivo experiments underlined the link between 5-HT4 receptors, a receptor involved in synaptic plasticity in the hippocampus, and sAPPα, a neuroprotective protein. Moreover, the synergistic effect of a 5-HT4 receptor agonist with an acetylcholine esterase inhibitor on APP metabolism suggests that 5-HT4 receptors can be an interesting pharmacological target in the treatment of AD. Such a combined therapy may allow the use of lower doses of each of these compounds, thereby attenuating the adverse effects of an individual drug.
Acknowledgments
This study was supported by the French ‘Ministère de l'Enseignement Supérieur et de la Recherche (MESR). M Cachard-Chastel was recipient of a fellowship from the MESR (France) during the performance of this work. We are grateful to pharmaceutical companies (Dr Xavier Langlois at Johnson-Johnson and Eisai Co., Ltd.) for generous gifts of prucalopride and donepezil, respectively. We thank Dr Dennis Selkoe for providing R1736 antiserum (Harvard Medical School, Boston, MA). We also thank Christelle Reperant for her technical assistance.
Abbreviations
- Aβ
amyloid β-protein
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- LTP
long-term potentiation
- RT-PCR
reverse transcriptase–polymerase chain reaction
- sAPPα
non-amyloidogenic soluble form of amyloid precursor protein
Conflict of interest
The authors state no conflict of interest.
References
- Barger SW, Mattson MP. Isoform-specific modulation by apolipoprotein E of the activities of secreted beta-amyloid precursor protein. J Neurochem. 1997;69:60–67. doi: 10.1046/j.1471-4159.1997.69010060.x. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Sebben M, Dumuis A. Pharmacological characterization of 5-hydroxytryptamine4(5-HT4) receptors positively coupled to adenylate cyclase in adult guinea pig hippocampal membranes: effect of substituted benzamide derivatives. Mol Pharmacol. 1990;37:408–411. [PubMed] [Google Scholar]
- Bour A, Little S, Dodart JC, Kelche C, Mathis C. A secreted form of the beta-amyloid precursor protein (sAPP695) improves spatial recognition memory in OF1 mice. Neurobiol Learn Mem. 2004;81:27–38. doi: 10.1016/s1074-7427(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Briejer MR, Bosmans JP, Van Daele P, Jurzak M, Heylen L, Leysen JE, et al. The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound. Eur J Pharmacol. 2001a;423:71–83. doi: 10.1016/s0014-2999(01)01087-1. [DOI] [PubMed] [Google Scholar]
- Briejer MR, Prins NH, Schuurkes JA. Effects of the enterokinetic prucalopride (R093877) on colonic motility in fasted dogs. Neurogastroenterol Motil. 2001b;13:465–472. doi: 10.1046/j.1365-2982.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- Cho S, Hu Y. Activation of 5-HT4 receptors inhibits secretion of beta-amyloid peptides and increases neuronal survival. Exp Neurol. 2007;203:274–278. doi: 10.1016/j.expneurol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Wong EH, Dumuis A, Bockaert J. Central 5-HT4 receptors. Trends Pharmacol Sci. 1995;16:391–398. doi: 10.1016/s0165-6147(00)89081-1. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Bartolini A. Role of 5-HT4 receptors in the mouse passive avoidance test. J Pharmacol Exp Ther. 1998;286:1115–1121. [PubMed] [Google Scholar]
- Gardier AM, David DJ, Jego G, Przybylski C, Jacquot C, Durier S, et al. Effects of chronic paroxetine treatment on dialysate serotonin in 5-HT1B receptor knockout mice. J Neurochem. 2003;86:13–24. doi: 10.1046/j.1471-4159.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmachers MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewskio BL, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb Cortex. 2005;15:1037–1043. doi: 10.1093/cercor/bhh204. [DOI] [PubMed] [Google Scholar]
- Kerr ML, Small DH. Cytoplasmic domain of the beta-amyloid protein precursor of Alzheimer's disease: function, regulation of proteolysis, and implications for drug development. J Neurosci Res. 2005;80:151–159. doi: 10.1002/jnr.20408. [DOI] [PubMed] [Google Scholar]
- Lamirault L, Guillou C, Thal C, Simon H. Combined treatment with galanthaminium bromide, a new cholinesterase inhibitor, and RS 67333, a partial agonist of 5-HT4 receptors, enhances place and object recognition in young adult and old rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:185–195. doi: 10.1016/S0278-5846(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Lamirault L, Simon H. Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5-HT4 receptors. Neuropharmacology. 2001;41:844–853. doi: 10.1016/s0028-3908(01)00123-x. [DOI] [PubMed] [Google Scholar]
- Langlois M, Fischmeister R. 5-HT4 receptor ligands: applications and new prospects. J Med Chem. 2003;46:319–344. doi: 10.1021/jm020099f. [DOI] [PubMed] [Google Scholar]
- Lelong V, Lhonneur L, Dauphin F, Boulouard M. BIMU 1 and RS 67333, two 5-HT4 receptor agonists, modulate spontaneous alternation deficits induced by scopolamine in the mouse. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:621–628. doi: 10.1007/s00210-003-0743-2. [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F, Robert SJ. The serotonin 5-HT4 receptor and the amyloid precursor protein processing. Exp Gerontol. 2003;38:159–166. doi: 10.1016/s0531-5565(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, et al. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Lezoualc'h F. New insights into serotonin 5-HT4 receptors: a novel therapeutic target for Alzheimer's disease. Curr Alzheimer Res. 2004;1:79–85. doi: 10.2174/1567205043332252. [DOI] [PubMed] [Google Scholar]
- Marchetti-Gauthier E, Roman FS, Dumuis A, Bockaert J, Soumireu-Mourat B. BIMU1 increases associative memory in rats by activating 5-HT4 receptors. Neuropharmacology. 1997;36:697–706. doi: 10.1016/s0028-3908(97)00058-0. [DOI] [PubMed] [Google Scholar]
- Mialet J, Berque-Bestel I, Sicsic S, Langlois M, Fischmeister R, Lezoualc'h F. Pharmacological characterization of the human 5-HT(4(d)) receptor splice variant stably expressed in Chinese hamster ovary cells. Br J Pharmacol. 2000a;131:827–835. doi: 10.1038/sj.bjp.0703641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mialet J, Dahmoune Y, Lezoualc'h F, Berque-Bestel I, Eftekhari P, Hoebeke J, et al. Exploration of the ligand binding site of the human 5-HT(4) receptor by site-directed mutagenesis and molecular modeling. Br J Pharmacol. 2000b;130:527–538. doi: 10.1038/sj.bjp.0703356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Moser PC, Bergis OE, Jegham S, Lochead A, Duconseille E, Terranova JP, et al. SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J Pharmacol Exp Ther. 2002;302:731–741. doi: 10.1124/jpet.102.034249. [DOI] [PubMed] [Google Scholar]
- Pakaski M, Kasa P. Role of acetylcholinesterase inhibitors in the metabolism of amyloid precursor protein. Curr Drug Targets CNS Neurol Disord. 2003;2:163–171. doi: 10.2174/1568007033482869. [DOI] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, De Deurwaerdere P, Esposito E, Spampinato U. Central serotonin4 receptors selectively regulate the impulse-dependent exocytosis of dopamine in the rat striatum: in vivo studies with morphine, amphetamine and cocaine. Neuropharmacology. 2002;43:1099–1109. doi: 10.1016/s0028-3908(02)00212-5. [DOI] [PubMed] [Google Scholar]
- Qi HB, Luo JY, Liu X. Effect of enterokinetic prucalopride on intestinal motility in fast rats. World J Gastroenterol. 2003;9:2065–2067. doi: 10.3748/wjg.v9.i9.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racchi M, Mazzucchelli M, Porrello E, Lanni C, Govoni S. Acetylcholinesterase inhibitors: novel activities of old molecules. Pharmacol Res. 2004;50:441–451. doi: 10.1016/j.phrs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Robert S, Maillet M, Morel E, Launay JM, Fischmeister R, Mercken L, et al. Regulation of the amyloid precursor protein ectodomain shedding by the 5-HT4 receptor and Epac1. FEBS Lett. 2005;579:1136–1142. doi: 10.1016/j.febslet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Robert SJ, Zugaza JL, Fischmeister R, Gardier AM, Lezoualc'h F. The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J Biol Chem. 2001;276:44881–44888. doi: 10.1074/jbc.M109008200. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Brown JT, Richardson JC, Medhurst AD, Sehmi SS, Calver AR, et al. Modulation of hippocampal excitability by 5-HT4 receptor agonists persists in a transgenic model of Alzheimer's disease. Neuroscience. 2004;129:49–54. doi: 10.1016/j.neuroscience.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Shiojiri S, Takahashi Y, Kitaguchi N, Ito H, Kameyama M, et al. Tissue-specific expression of three types of beta-protein precursor mRNA: enhancement of protease inhibitor-harboring types in Alzheimer's disease brain. Biochem Biophys Res Commun. 1989;165:1406–1414. doi: 10.1016/0006-291x(89)92760-5. [DOI] [PubMed] [Google Scholar]
- Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Pike VW, Hall H. Distribution of 5-HT4 receptors in the postmortem human brain – an autoradiographic study using [125I]SB 207710. Eur Neuropsychopharmacol. 2003;13:228–234. doi: 10.1016/s0924-977x(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology. 1994;33:527–541. doi: 10.1016/0028-3908(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Gardoni F, Marcello E, Colciaghi F, Borroni B, Padovani A, et al. Acetylcholinesterase inhibitors increase ADAM10 activity by promoting its trafficking in neuroblastoma cell lines. J Neurochem. 2004;90:1489–1499. doi: 10.1111/j.1471-4159.2004.02680.x. [DOI] [PubMed] [Google Scholar]