Abstract
Myocardial ischaemia/reperfusion injury leading to myocardial infarction is one of the most frequent causes of debilitation and death in man. Considerable research has been undertaken to investigate the possibility of reducing myocardial infarction and increasing cell survival by activating certain endogenous prosurvival signaling pathways. Thus, it has been established that the activation of the PI3K (Phosphoinositide-3 kinase)/Akt (Protein kinase B, PKB) signaling pathway is essential for protection against ischaemia/reperfusion injury. This pathway has been shown to be activated by mechanical procedures (e.g. pre and post conditioning) as well as by a number of pharmacological agents. Although the activation of this prosurvival signaling pathway induces the phosphorylation of a large number of substrates implicated in increased cell survival, when activated over a prolonged period this pathway can have detrimental consequences by facilitating unwanted growth and malignancies. Importantly PTEN (phosphatase and tensin homolog deleted on chromosome ten), is the main phosphatase which negatively regulates the PI3K/Akt pathway. In this review we discuss: a) the significance and the limitations of inhibiting PTEN in myocardial ischaemia/reperfusion injury; b) PTEN and its relationship to ischaemic preconditioning, c) the role of PTEN in the development of tolerance to chronic administration of drugs known to limit infarction by activating PI3K/Akt pathway when given acutely, and d) the possible role of PTEN in the ischaemic/reperfused diabetic heart. The experimental evidence discussed in this review illustrates the importance of PTEN inhibition in the protection of the heart against ischaemia/reperfusion injury.
Keywords: myocardium, ischaemia, reperfusion, infarction, Akt, PTEN
Introduction
The normal cell has the necessary enzymatic equipment for controlling both its death and its survival (Jin and El-Deiry, 2005), these two processes being finely balanced (Horbinski and Chu, 2005). The main prosurvival pathway is the PI3K/Akt signaling cascade. Akt, when activated, has the ability to phosphorylate two categories of downstream substrates implicated in the life/death balance: (i) the antiapoptotic substrates, which, when phosphorylated, are activated and contribute to survival and (ii) the proapoptotic substrates, which, when phosphorylated, become inactive (Franke et al., 2003). PI3K/Akt pathway has been demonstrated to play an important role in protecting the myocardium against ischaemia–reperfusion injury in all species, both in vitro and in vivo (Hausenloy and Yellon, 2004). The main downregulator of this prosurvival pathway is phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a dual protein–lipid phosphatase which dephosphorylates the secondary messenger produced by PI3K and interrupts the downstream activation of Akt (Hlobilkova et al., 2003). In addition, it is worth mentioning that PTEN may play a significant role in pathological conditions associated with the ischaemic heart disease, such as diabetes and obesity (Sasaoka et al., 2006). Therefore, blocking PTEN may prove important, particularly in increasing myocardial survival following an ischaemic episode (Oudit et al., 2004).
PI3K/Akt: the main prosurvival pathway in the ischaemic/reperfused myocardium
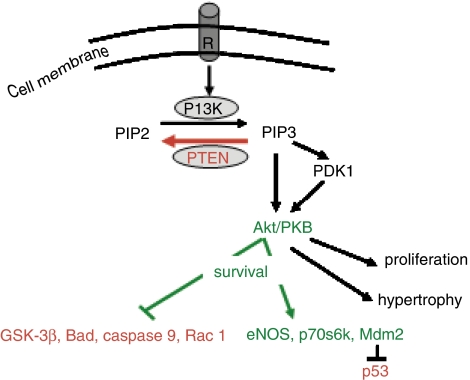
PI3K/Akt is an intracellular signaling pathway, which plays significant roles in a variety of biological processes involving cell survival, growth and migration (Wetzker and Rommel, 2004). It has been demonstrated in the myocardium that the activation of this pathway by procedures such as ischaemic pre- or postconditioning or by the administration of pharmacological agents is crucial for the salvage of the ischaemic/reperfused myocardium. Ischaemic preconditioning (which consists of short, sublethal ischaemic episodes interspersed with reperfusion, before a sustained ischaemic insult) is considered to be the most powerful endogenous mechanism of protection against ischaemic injury (Murry et al., 1986). Preconditioning protects the heart by reducing myocardial infarction and this protection is due, in principal, to the activation of the PI3K/Akt pathway either before the lethal ischaemic insult (Tong et al., 2000; Mocanu et al., 2002) or at reperfusion following a sustained ischaemic period (Hausenloy et al., 2005). Postconditioning, which consists of short ischaemic episodes at the commencement of reperfusion (Zhao et al., 2003), has also been demonstrated to achieve significant protection via Akt upregulation (Zhu et al., 2006). As expected, a large number of pharmacological agents, which are known to activate PI3K/Akt signaling pathway, have also been shown to protect against myocardial infarction. In this regard, insulin (Jonassen et al., 2001), urocortin (Brar et al., 2002), atorvastatin (Bell and Yellon, 2003a), bradykinin (Bell and Yellon, 2003b), erythropoietin (Parsa et al., 2003; Bullard et al., 2005) and glucagon-like peptide 1 (Bose et al., 2005) have all been shown to reduce the extent of necrotic tissue developed within the myocardium at risk following a lethal ischaemic insult. The protection observed is, in part, achieved via PI3K/Akt activation, supporting the hypothesis that pharmacological manipulation and upregulation of this pro-survival kinase is essential for protecting the myocardium from lethal ischaemia/reperfusion-induced cell death (Hausenloy and Yellon, 2004). Briefly, Akt once activated, may induce its antiapoptotic effects via the phosphorylation of two types of substrate (Figure 1): (a) the proapoptotic substrates such as glycogen synthase kinase-3-beta (Nishihara et al., 2006) or Bad (Jonassen et al., 2001), which, after phosphorylation exhibit an increased affinity for the cytosolic 14-3-3 proteins and become inactive by binding to them or (b) the antiapoptotic substrates such as p70s6 kinase (Jonassen et al., 2001), eNOS (endothelial nitric oxide synthase) (Bell and Yellon, 2003b) or MDM2 (mouse double minute) (Mocanu and Yellon, 2003), which, after phosphorylation become activated and stimulate cellular processes essential for an increased survival. However, it must also be noted that the chronic activation of this pathway may lead to hypertrophy and malignancy. As such there appears to be a fine balance between the potentially beneficial effects of activating this signaling pathway acutely and the potentially harmful effects of sustained activation of this pathway (Franke et al., 2003). The principal factor protecting against the long-term activation of the PI3K/Akt pathway in normal cells is PTEN, a unique dual protein–lipid phosphatase (Leslie and Downes, 2004).
Figure 1.
PTEN acts as a lipid phosphatase reversing the reaction catalyzed by the PI3K, that is dephosphorylating the second-messenger PtdIns(3,4,5)P3 (PIP3) to the precursor PtdIns(4,5)P2 (PIP2). The role of PIP3 is to recruit Akt and PDK1 at the membrane level. PDK1 phosphorylates Akt which thereafter acts upon numerous targets, activating the antiapoptotic substrates and inhibiting the proapoptotic substrates (sharp arrows, activation; blunt arrows, inhibition). R, membrane receptor.
PTEN: a ubiquitous phosphatase which protects the cell against hypertrophy and malignancy
PTEN – also called MMAC1 (mutated in multiple advanced cancers) or TEP-1 (TGF-β regulated and epithelial cell-enriched phosphatase), was discovered relatively recently (Li et al., 1997; Steck et al., 1997). It is a highly conserved dual (protein and lipid) phosphatase, responsible for negatively regulating PI3 kinase activation (Hlobilkova et al., 2003). The mechanisms involved in the negative regulation of the PI3K/Akt pathway by PTEN are presented in Figure 1. In summary, the result of PI3 kinase activity is the phosphorylation of phosphatidylinositol (4,5)-bisphosphate (or PtdIns(4,5)P2), abreviated as PIP2, into the secondary messenger phosphatidylinositol (3,4,5)-trisphosphate (or PtdIns(3,4,5)P3), commonly abbreviated as PIP3. This metabolite is an intracellular second messenger, which mediates downstream signaling by recruiting and activating 3-phosphoinositide-dependent kinase 1 (PDK-1) followed by the activation of PKB/Akt. PTEN has the ability to dephosphorylate PIP3 to its precursor, PIP2, thereby blocking the cascade of events generated as a consequence of the accumulation of the secondary messenger in the plasmalemma. PTEN is present ubiquitously in cells and its activity is reflected by its cellular level, which can be modulated by transcription. However, this activity can also be downregulated by phosphorylation or oxidation as discussed in the next section. Interestingly, there is no redundancy in this inhibitory mechanism of PI3-kinase/Akt activation, making PTEN an important ‘switch' in maintaining cellular homeostasis and normal development. It is known that homozygous PTEN knockout mice are not viable whereas the heterozygous animals develop numerous tumors. In addition, in humans, many tumor types are characterized by deficient PTEN expression (Ghebranious and Donehower, 1998).
PTEN regulation
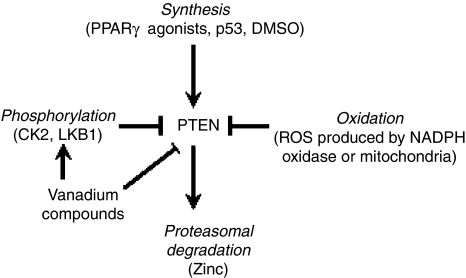
As outlined above, PTEN is ubiquitously present in normal cells, its degree of activity depends upon its cellular level, its localisation and its interactions with other proteins or lipids (Gericke et al., 2006). Among the metabolites which induce PTEN transcription are the peroxisome proliferator-activated receptor γ-agonists (Teresi et al., 2006) and the tumour suppressor p53 (Wang et al., 2005). The regulation of PTEN activity is complex and is as yet not completely understood (Figure 2). One of the mechanisms of its inactivation is via phosphorylation. The main enzyme responsible for this process is considered to be casein kinase 2 (CK2) (Torres and Pulido, 2001), although, interestingly, LKB1 (PJS; serine/threonine protein kinase 11) – a kinase implicated in metformin signaling via AMP-activated protein kinase – has also been demonstrated to phosphorylate PTEN, at least in an in vitro model (Mehenni et al., 2005). There is the opinion that in its phosphorylated state PTEN is inactive and as such is more stable against proteasomal degradation (Vazquez et al., 2000, 2001). PTEN can also be reversibly inactivated through oxidation induced by free radicals (reactive oxygen species, ROS) (Leslie et al., 2003). This seems to be the main process that regulates PTEN activity in the acute setting. The ROS responsible for this inactivation may have different sources. Recent data have shown that insulin, which is known to activate PI3K/Akt, may induce, as a primary effect, the activation of nicotinamide adenine dinucleotide phosphate oxidase which in turn releases ROS. These ROS could be responsible for the inhibition of PTEN (Seo et al., 2005), followed by Akt activation due to PIP3 accumulation. The same mechanisms of ROS production may also explain the action of some growth factors which have been shown to inhibit PTEN (Kwon et al., 2004). Further, it has been demonstrated that hydrogen peroxide produced at the mitochondrial level can also inhibit PTEN (Connor et al., 2005).
Figure 2.
PTEN regulation. PTEN is a constitutively active phosphatase. In summary, its activity can be unregulated by increased synthesis and downregulated by phosphorylation, oxidation and proteasomal degradation. The mechanisms through which its activity is regulated are complex and not yet elucidated completely.
Problems associated with the study of PTEN
PTEN is a small but significant switch in the balance between cell survival and death, but, like Achilles' heel, it is very difficult to target in the experimental setting. With regard to potential pharmacological manipulation of PTEN, the difficulty in investigating this phosphatase relates to the lack of highly specific commercially available activators or inhibitors.
It has been demonstrated that PTEN can be inhibited by vanadium compounds (Schmid et al., 2004; Wu et al., 2006) or zinc (Wu et al., 2003). Based on the homology of the active site between PTEN and protein tyrosine phosphatases (PTPases), it has been shown that PTPases inhibitors such as bisperoxovanadium molecules can also inhibit PTEN, this inhibition occurring at very low concentrations (up to 100-fold lower than necessary for PTPases inhibition) (Schmid et al., 2004). However, such positive results obtained in NIH3T3 cells (mouse embryonic fibroblast cell line) could not be reproduced in whole organs. Encouragingly, sodium orthovanadate was shown to protect against cerebral ischaemia by increasing the tyrosine phosphorylation of PTEN (Wu et al., 2006). All these phosphatase inhibitors need more investigation because they may be toxic in physiological settings and are certainly not very specific. It was also documented that zinc ions downregulate PTEN expression in airway epithelial cells in a dose- and time-dependent fashion, via increased proteasome-mediated degradation and reduced PTEN messenger RNA expression (Wu et al., 2003).
The activities of the few kinases known to regulate PTEN phosphorylation (e.g. mainly protein kinase CK2 (Torres and Pulido, 2001) and, potentially, LKB1 (Mehenni et al., 2005)) are, again, difficult to modulate either due to the lack of specific activators/inhibitors or due to the large spectrum of substrates they may act upon, making any result difficult to interpret.
Genetically engineered animals are not easy to breed. The homozygous PTEN−/− mouse is not viable and the heterozygous PTEN+/− develops numerous tumours, with the females becoming infertile at a very young age (Ghebranious and Donehower, 1998). However, the recently created organ targeted PTEN deletion mouse, may offer a more promising model (Sun et al., 2006) for further study. SiRNA (small-interfering RNA) can also be a promising tool for investigating the role played by PTEN in cardiovascular development and pathophysiology (Hamada et al., 2005).
Therefore, in spite of the increasing interest in PTEN downregulation as a mean for improving myocardial survival following an ischaemia/reperfusion episode (Oudit et al., 2004), the progress in experimentation has been slow and, as a consequence, the body of data reported has so far been limited.
PTEN and ischaemia/reperfusion injury
Taking into account all the limitations discussed above, it is not surprising that there is almost no data correlating myocardial ischaemia/reperfusion injury with PTEN levels, with one exception which will be discussed later (Cai and Semenza, 2005). Whereas in the area of cancer research, for example, the interest has focused on restoring the PTEN levels, in other areas, in which survival is the goal (such as in the myocardium undergoing ischaemia/reperfusion where Akt activation is beneficial) the interest has been directed toward PTEN downregulation (preferable in a reversible fashion). Some data have been obtained on the brain ischaemia demonstrating that PTEN phosphorylation, and therefore inactivation is induced by ischaemia (as an intrinsic protective mechanism) (Omori et al., 2002; Choi et al., 2005). Additionally, the pharmacological inhibition of PTEN has been reported to be associated with reduced injury (Lee et al., 2004; Wu et al., 2006). In cardiac tissues a reduced PTEN level is associated with hypertrophy and remodeling (Schwartzbauer and Robbins, 2001). PTEN has also been shown to play a role in the regulation of the size and contractile function in cardiomyocytes (Crackower et al., 2002) as well as in the regulation of the L-type calcium currents (Sun et al., 2006). Taking into consideration the overwhelming importance of the upregulation of the PI3K/Akt pathway in myocardial survival following ischaemia/reperfusion, it is surprising how little is known about the role of PTEN in this process.
Interestingly, in spite of the paucity of data regarding the role played by PTEN in cardiovascular pathophysiology, a recent review (Oudit et al., 2004) stressed the importance of PI3K/PTEN signaling, based on data available from other cell systems. The authors of this review stressed that therapeutic manipulation of this interaction may be of interest in myocardial survival. It has also been shown in other cell lines that insulin and other growth factors (already demonstrated to protect the ischaemic/reperfused myocardium by activating PI3K/Akt (Yellon and Baxter, 1999; Jonassen et al., 2001) may well increase survival not only by activating this signaling pathway but also by inhibiting PTEN via local ROS production (Kwon et al., 2004; Seo et al., 2005). All these data indicate that these protective effects may relate to the inhibition of PTEN, which could possibly be exploited in the acute setting of ischaemia/reperfusion. However, it is important to note that a reversible inhibition is preferred, bearing in mind that long-term absence of this phosphatase from the myocardium is associated with myocardial hypertrophy (Schwartzbauer and Robbins, 2001; Crackower et al., 2002).
PTEN and ischaemic preconditioning
Ischaemic preconditioning, one of the most powerful endogenous protective procedures against ischaemia/reperfusion injury, has already been demonstrated to be associated with the activation of the PI3 kinase/Akt pathway (Tong et al., 2000; Mocanu et al., 2002; Hausenloy et al., 2005). Recently, interesting data linking the protection seen using ischaemic preconditioning with a reduction in PTEN activity has been published (Cai and Semenza, 2005). Using an isolated perfused rat heart the authors showed that PTEN is downregulated after 15 min ischaemia and 30 min reperfusion in an isolated rat heart. Although these data are of potential importance, it should be appreciated that the protocol of ischaemic preconditioning used in this study could be questioned (Hausenloy et al., 2006). It involved using 15 min of myocardial ischaemia followed by reperfusion which has been regarded more as a model of mild, reversible stunning than of ischaemic preconditioning (Palmer et al., 2004). Moreover, in the study quoted above (Cai and Semenza, 2005) the protocol used for preconditioning was not validated using infarct size as the end point of injury. Therefore, without diminishing the importance of their data in investigating PTEN in the setting of myocardial ischaemia/reperfusion injury for the first time, it is worth noting the similarity between their results with the data obtained in the ischaemic brain (Choi et al., 2005). Choi et al. showed an increased phosphorylation of PTEN and Akt in the hippocampus after an ischaemic insult. These data support the hypothesis that in the ischaemic/reperfused tissues the PTEN downregulation is an endogenous protective mechanism, which may eventually be augmented for the benefit of the injured tissue.
PTEN as a downregulator of the cardio protection induced by pharmacological agents known to protect via PI3K/Akt activation
Recently, we reported the relevance of PTEN in a model of chronic versus acute treatment with an agent that induces protection against myocardial infarction via PI3K/Akt activation. In this respect we were unable to demonstrate protection if the agent, namely atorvastatin, was given chronically to rats for a 2-week period, this lack of protection being associated with an increase in PTEN levels. However, protection was observed with atorvastatin when given acutely (1–3 days) with no change in PTEN levels (Mensah et al., 2005). Recent studies have also confirmed the loss of protection following a chronic administration of another statin, lovastatin (Teresi et al., 2006) in a cell-based model. Therefore, it seems imperative to investigate further, in a chronic model, the relationship between PTEN and other pharmacological agents capable of activating the PI3K/Akt pathway and protecting against myocardial ischaemia/reperfusion injury in an acute setting. It would be interesting to examine whether such agents are able to sustain protection after chronic treatment, or whether, by upregulating PTEN, their effect will be abolished.
PTEN in the diabetic heart
PI3k/Akt signaling pathway can be impaired in some pathological conditions such as diabetes (Kondo and Kahn, 2004; Schinner et al., 2005; Zdychova and Komers, 2005) and interestingly, this state can be improved by downregulating PTEN (Jiang and Zhang, 2002; Wijesekara et al., 2005). The malfunction of PI3K/Akt pathway can affect not only insulin sensitivity but also any potential protection induced by PI3k/Akt activation against myocardial ischaemia/reperfusion injury. For instance, we have shown that, unlike its normoglycaemic Wistar parent strain, the diabetic Goto Kakizaki rat heart could not be preconditioned using a single cycle of sublethal ischaemia/reperfusion. To achieve protection, three cycles of preconditioning ischaemia/reperfusion were required (Tsang et al., 2005). Importantly, although the level of total Akt in the diabetic rat heart was not different from that in the normoglycaemic rat heart, the susceptibility of this enzyme to phosphorylation, hence activation, by protective mechanisms (in this case preconditioning) was reduced (Tsang et al., 2005). We have further shown that this decrease in the level of Akt phosphorylation is associated with an increased level of PTEN present in myocardial tissue (Mocanu et al., 2006). As a negative regulator of the insulin signaling pathway, PTEN is seen as a possible target for improving insulin sensitivity in type II diabetes (Butler et al., 2002; Jiang and Zhang, 2002). In this regard, it has already been demonstrated that the inhibition of PTEN expression in diabetic mice is associated with a reduction in blood glucose (Butler et al., 2002) and that PTEN can also affect the pancreatic islet development (Kushner et al., 2005). These data suggest that diabetes treatment may benefit from PTEN inhibition with subsequent PI3K/Akt upregulation. In addition, the balance between PTEN and prosurvival kinases may further protect the diabetic myocardium, which is known to be more susceptible to ischaemic heart disease.
Conclusions and perspectives
In summary, PTEN plays a significant role in regulating the balance between survival and death in many cell types, including cardiomyocytes. The activity of PTEN can be decreased either by affecting the balance between its synthesis and its degradation or by enzymatic inactivation via phosphorylation or oxidation. However, at present, the feasibility of modulating PTEN activity is impaired due to a lack of sufficient understanding about how it is regulated, in addition to the unavailability of appropriate pharmacological agents to target these processes. Although PTEN downregulation may seem potentially harmful because it could promote unwanted growth and malignancies, acute PTEN inhibition could ultimately prove to be significant in improving myocardial survival following ischaemia/reperfusion injury. A reversible inhibition of PTEN may be enough to upregulate the prosurvival PI3K/Akt pathway to reduce the cell death associated with such injury, without the negative hypertrophic consequences. Interestingly, this inhibition could also bring additional benefits in diabetes by augmenting the insulin sensitivity (Wijesekara et al., 2005). Finally, it may be crucial that all the drugs proven to protect against myocardial ischaemia/reperfusion by activating PI3K/Akt when given acutely, to be tested in chronic settings, in order to establish if any protective effect has not been lost due to PTEN upregulation.
Acknowledgments
We are grateful to British Heart Foundation for continuing support.
Abbreviations
- Akt
protein kinase B, PKB
- CK2
casein kinase 2
- eNOS
endothelial nitric oxide synthase
- LKB1
PJS, serine/threonine protein kinase 11
- MDM2
mouse double minute
- NIH 3T3
mouse embryonic fibroblast cell line
- PDK-1
3-phosphoinositide-dependent kinase 1
- PI3K
phosphoinositide-3 kinase
- PIP2
phosphatidylinositol (4,5)-bisphosphate, PtdIns(4,5)P2
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate, PtdIns(3,4,5)P3
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- PTPases
protein tyrosine phosphatases
- ROS
reactive oxygen species, free radicals
- siRNA
small-interfering RNA
Conflict of interest
The authors state no conflict of interest.
References
- Bell RM, Yellon DM. Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol. 2003a;41:508–515. doi: 10.1016/s0735-1097(02)02816-4. [DOI] [PubMed] [Google Scholar]
- Bell RM, Yellon DM. Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol. 2003b;35:185–193. doi: 10.1016/s0022-2828(02)00310-3. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11. doi: 10.1007/s10557-005-6892-4. [DOI] [PubMed] [Google Scholar]
- Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol. 2002;34:483–492. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397–403. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- Choi JS, Park HJ, Kim HY, Kim SY, Lee JE, Choi YS, et al. Phosphorylation of PTEN and Akt in astrocytes of the rat hippocampus following transient forebrain ischemia. Cell Tissue Res. 2005;319:359–366. doi: 10.1007/s00441-004-1033-0. [DOI] [PubMed] [Google Scholar]
- Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, et al. Regulation of myocardial contractility and cell size by distinct PI3K–PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Donehower LA. Mouse models in tumor suppression. Oncogene. 1998;17:3385–3400. doi: 10.1038/sj.onc.1202573. [DOI] [PubMed] [Google Scholar]
- Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Mocanu MM, Yellon DM. Is this truly ischemic preconditioning. Circ Res. 2006;99:e11. doi: 10.1161/01.RES.0000240435.97872.00. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hlobilkova A, Knillova J, Bartek J, Lukas J, Kolar Z. The mechanism of action of the tumour suppressor gene PTEN. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:19–25. [PubMed] [Google Scholar]
- Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Jiang G, Zhang BB. Pi 3-kinase and its up- and down-stream modulators as potential targets for the treatment of type II diabetes. Front Biosci. 2002;7:d903–d907. doi: 10.2741/A820. [DOI] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem. 2004;279:37997–38006. doi: 10.1074/jbc.M401339200. [DOI] [PubMed] [Google Scholar]
- Kushner JA, Simpson L, Wartschow LM, Guo S, Rankin MM, Parsons R, et al. Phosphatase and tensin homolog regulation of islet growth and glucose homeostasis. J Biol Chem. 2005;280:39388–39393. doi: 10.1074/jbc.M504155200. [DOI] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim KY, Lee YK, Park SY, Kim CD, Lee WS, et al. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther. 2004;308:896–903. doi: 10.1124/jpet.103.061853. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Mehenni H, Lin-Marq N, Buchet-Poyau K, Reymond A, Collart MA, Picard D, et al. LKB1 interacts with and phosphorylates PTEN: a functional link between two proteins involved in cancer predisposing syndromes. Hum Mol Genet. 2005;14:2209–2219. doi: 10.1093/hmg/ddi225. [DOI] [PubMed] [Google Scholar]
- Mensah K, Mocanu MM, Yellon DM. Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten. J Am Coll Cardiol. 2005;45:1287–1291. doi: 10.1016/j.jacc.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34:661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Field DC, Yellon DM. A potential role for PTEN in the diabetic heart. Cardiovasc Drugs Ther. 2006;20:319–321. doi: 10.1007/s10557-006-8876-4. [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Yellon DM. p53 down-regulation: a new molecular mechanism involved in ischaemic preconditioning. FEBS Lett. 2003;555:302–306. doi: 10.1016/s0014-5793(03)01260-2. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, et al. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta 1. Am J Physiol Heart Circ Physiol. 2006;291:H748–H755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- Omori N, Jin G, Li F, Zhang WR, Wang SJ, Hamakawa Y, et al. Enhanced phosphorylation of PTEN in rat brain after transient middle cerebral artery occlusion. Brain Res. 2002;954:317–322. doi: 10.1016/s0006-8993(02)03366-8. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Palmer BS, Hadziahmetovic M, Veci T, Angelos MG. Global ischemic duration and reperfusion function in the isolated perfused rat heart. Resuscitation. 2004;62:97–106. doi: 10.1016/j.resuscitation.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaoka T, Wada T, Tsuneki H. Lipid phosphatases as a possible therapeutic target in cases of type 2 diabetes and obesity. Pharmacol Ther. 2006;112:799–809. doi: 10.1016/j.pharmthera.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Schinner S, Scherbaum WA, Bornstein SR, Barthel A. Molecular mechanisms of insulin resistance. Diabet Med. 2005;22:674–682. doi: 10.1111/j.1464-5491.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- Seo JH, Ahn Y, Lee SR, Yeol YC, Chung HK. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, et al. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- Teresi RE, Shaiu CW, Chen CS, Chatterjee VK, Waite KA, Eng C. Increased PTEN expression due to transcriptional activation of PPARgamma by Lovastatin and Rosiglitazone. Int J Cancer. 2006;118:2390–2398. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, et al. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res. 2005;65:6601–6611. doi: 10.1158/0008-5472.CAN-04-4184. [DOI] [PubMed] [Google Scholar]
- Wetzker R, Rommel C. Phosphoinositide 3-kinases as targets for therapeutic intervention. Curr Pharm Des. 2004;10:1915–1922. doi: 10.2174/1381612043384402. [DOI] [PubMed] [Google Scholar]
- Wijesekara N, Konrad D, Eweida M, Jefferies C, Liadis N, Giacca A, et al. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol Cell Biol. 2005;25:1135–1145. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DN, Pei DS, Wang Q, Zhang GY. Down-regulation of PTEN by sodium orthovanadate inhibits ASK1 activation via PI3-K/Akt during cerebral ischemia in rat hippocampus. Neurosci Lett. 2006;287:28258–28263. doi: 10.1016/j.neulet.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Wu W, Wang X, Zhang W, Reed W, Samet JM, Whang YE, et al. Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J Biol Chem. 2003;278:28258–28263. doi: 10.1074/jbc.M303318200. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Baxter GF. Reperfusion injury revisited: is there a role for growth factor signaling in limiting lethal reperfusion injury. Trends Cardiovasc Med. 1999;9:245–249. doi: 10.1016/s1050-1738(00)00029-3. [DOI] [PubMed] [Google Scholar]
- Zdychova J, Komers R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications 6. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhu M, Feng J, Lucchinetti E, Fischer G, Xu L, Pedrazzini T, et al. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]