Abstract
Background and purpose:
The cytochrome P450 2B6 (CYP2B6) enzyme metabolises a number of clinically important drugs. Drug-drug interactions resulting from inhibition or induction of CYP2B6 activity may cause serious adverse effects. The aims of this study were to construct a three-dimensional structure-activity relationship (3D-QSAR) model of the CYP2B6 protein and to identify novel potent and selective inhibitors of CYP2B6 for in vitro research purposes.
Experimental approach:
The inhibition potencies (IC50 values) of structurally diverse chemicals were determined with recombinant human CYP2B6 enzyme. Two successive models were constructed using Comparative Molecular Field Analysis (CoMFA).
Key results:
Three compounds proved to be very potent and selective competitive inhibitors of CYP2B6 in vitro (IC50<1 μM): 4-(4-chlorobenzyl)pyridine (CBP), 4-(4-nitrobenzyl)pyridine (NBP), and 4-benzylpyridine (BP). A complete inhibition of CYP2B6 activity was achieved with 0.1 μM CBP, whereas other CYP-related activities were not affected. Forty-one compounds were selected for further testing and construction of the final CoMFA model. The created CoMFA model was of high quality and predicted accurately the inhibition potency of a test set (n=7) of structurally diverse compounds.
Conclusions and implications:
Two CoMFA models were created which revealed the key molecular characteristics of inhibitors of the CYP2B6 enzyme. The final model accurately predicted the inhibitory potencies of several structurally unrelated compounds. CBP, BP and NBP were identified as novel potent and selective inhibitors of CYP2B6 and CBP especially is a suitable inhibitor for in vitro screening studies.
Keywords: CYP2B6, quantitative structure–activity relationship, CoMFA, 4-(4-chlorobenzyl)pyridine
Introduction
The cytochrome P450s (CYPs) are a superfamily of haem-containing mixed-function oxygenases that catalyse the regio- and stereoselective oxidation of a wide variety of xenobiotics, including numerous drugs. Early studies indicated that CYP2B6 levels were only approximately 0.2% of the total P450 content in human liver microsomes (Mimura et al., 1993; Shimada et al., 1994). However, later studies have demonstrated a greater frequency of detection and a higher percentage of CYP2B6 relative to the total P450 content (Ekins et al., 1998; Stresser and Kupfer, 1999).
Several investigations have also highlighted the wide range of interindividual variability in CYP2B6 protein levels and/or enzyme activity, possibly because of genetic polymorphisms and/or exposure to environmental inducers and inhibitors (Heyn et al., 1996; Lang et al., 2001). There are a number of important drugs metabolized by CYP2B6, including bupropion, efavirenz, cyclophosphamide, ifosamide, pethidine, artemisinin, propofol, ketamine and selegiline. Drug–drug interactions resulting from inhibition or induction of CYP2B6 can have serious consequences in the case of substrate drugs with a narrow therapeutic index, such as cyclophosphamide (Turpeinen et al., 2006).
Studies on the metabolic properties of drugs and chemicals often include in vitro tests with inhibitors of individual CYP forms. The use of chemical inhibitors is a routine approach when the relative contribution of CYPs to the metabolism of a particular compound needs to be evaluated in liver microsomal preparations (Hutzler et al., 2005; Pelkonen and Raunio, 2005). Several inhibitors of CYP2B6 have been described including ticlopidine, clopidogrel, thioTEPA, memantine and 2-phenyl-2-(1-piperidinyl)propane, but they do not adequately distinguish between different CYP forms (Turpeinen et al., 2006). Thus, more selective CYP2B6 inhibitors are needed for in vitro drug development and drug interaction studies. Some computational (in silico) approaches have been used to characterize the molecular properties of substrates of the CYP2B6 enzyme, but there are no reports on the application of pharmacophore or quantitative structure–activity relationship (QSAR) techniques to find new inhibitors or to predict the inhibitory potency of compounds. Therefore, the aims of this study were (1) to determine the CYP2B6 inhibition potencies of structurally diverse compounds to create a database for structure–activity relationship analysis, (2) to construct a comprehensive three-dimensional quantitative structure–activity relationship (3D-QSAR; comparative molecular field analysis (CoMFA)) model and (3) to identify novel, potent and selective inhibitors of CYP2B6.
In this study, we describe a 3D-QSAR model for CYP2B6 inhibitors using the IC50 values generated with cDNA-expressed CYP2B6. A total of 41 chemicals were screened. Some of the chemicals were selected from our extensive database and further expanded with structures related to CYP2B6 substrates and inhibitors. Three potent inhibitors were found and two of these compounds were also highly CYP2B6-selective. The CoMFA model created was also used to predict accurately the IC50 values of a test set of seven compounds not present in the training set.
Methods
Human liver microsomes and cDNA-expressed human P450s
Human liver samples used in this study were obtained from the University Hospital of Oulu as surplus material from kidney transplantation donors. The collection of surplus tissue was approved by the Ethics Committee of the Medical Faculty of the University of Oulu, Finland. All the donors were of Caucasian race. The livers were transferred to ice immediately after the surgical excision, cut into pieces, snap-frozen in liquid nitrogen and stored at −80°C until the microsomes were prepared by standard differential ultracentrifugation. Liver samples were extensively characterized (sufficient model activities, no known polymorphisms, expected effects of model inhibitors, quantitation of CYPs by immunoblotting) and a weight-balanced microsomal pool of 10 liver microsomal preparations was employed and used for these studies. The final microsomal pellet was suspended in 0.1 M phosphate buffer (pH 7.4). Baculovirus-insect cell expressed human CYP2B6 (containing recombinant P450 reductase) was purchased from BD Biosciences Discovery Labware (Bedford, MA, USA) and used according to the manufacturer's instructions.
Biochemical assays
Determinations of IC50 value of inhibitors/determination of inhibition potency
The 7-ethoxy-4-trifluoromethylcoumarin (EFC) O-deethylation activity assay is based on the detection of fluorescence emitted by the metabolite 7-hydroxy-4-trifluoromethylcoumarin. This assay is suitable only when pure recombinant CYP enzymes are used, since the substrate is not specific for CYP forms. The incubations were performed in 96-well plates. In each well, a 150 μl incubation volume contained 100 mM Tris–HCl buffer (pH 7.4), 4.2 mM MgCl2, 2.5 μM EFC, 0.75 pmol cDNA-expressed CYP2B6 and 0–1 mM inhibitor. The concentration of EFC varied between 1 and 17 μM in the determination of inhibition type and the inhibition constant Kic. All inhibitors were dissolved in dimethylsulphoxide (DMSO), and to ensure complete solubility of the compounds, a final concentration of 2% DMSO was used in the incubations. This concentration of DMSO caused a 17% inhibition in CYP2B6 catalytic activity, and this effect was taken into account in the experiments. The reaction was initiated by adding 50 μl nicotine adenine dinucleotide phosphate hydrogen (NADPH)-generating system after 10 min preincubation at 37°C. After a set incubation time of 30 min, the reactions were terminated by the addition of 110 μl 80% acetonitrile/20% 0.5 M Tris base. The formed fluorescence was measured with a Victor2 plate counter (Perkin-Elmer Life Sciences Wallac, Turku, Finland) at 405 nm excitation and 535-nm emission. The linearity of the reaction with respect to incubation time and protein concentration was determined. Several control incubations were carried out to determine the effect of quenching by the inhibitors and other interfering factors. Each inhibitor was prescreened using inhibitor concentrations ranging from 0.001 to 1000 μM. The actual IC50 values were determined using a narrower range of seven concentrations. All IC50 values were determined in two separate experiments. The apparent Km and Vmax of EFC was also determined. The IC50, the apparent Km and the apparent Vmax values were calculated using non-linear regression analysis with Prism 4.0 software (San Diego, CA, USA).
As the purpose of this study was to create a model for CYP2B6 enzyme inhibition, IC50 values (measuring relative inhibition potency) rather than the absolute Ki values were determined. This is justified because all measurements were made under standardized conditions and the results are thus comparable fully with each other.
Enzyme assays using human liver microsomes
To assess the CYP selectivity of novel inhibitors, a CYP screen was carried out using CYP-specific substrates and human liver microsomes as the enzyme source. The incubation conditions, analysis and instrumentation used to assess the enzyme activities of CYP1A1/2 (ethoxyresorufin O-deethylation), CYP2A6 (coumarin 7-hydroxylation), CYP2C9 (tolbutamide hydroxylation), CYP2D6 (dextromethorphan O-demethylation), CYP2E1 (chlorzoxazone 6-hydroxylation), CYP3A4/5 (midazolam 1′-hydroxylation) and multiple CYPs (7-ethoxycoumarin O-deethylation) have been described previously in detail by Taavitsainen et al. (2001). The bupropion hydroxylation assay and the analytical methods for CYP2B6 were described previously by Turpeinen et al. (2004). The amodiaquine N-desethylation assay for CYP2C8 was a modification from that described by Li et al. (2002): incubation mixtures contained 0.5 mg protein/ml and 30 μM amodiaquine and the incubation time was 20 min. Omeprazole 5-hydroxylation and sulfoxidation assays for CYP2C19 and CYP3A4, respectively, were adapted from Äbelö et al. (2000); incubation mixtures contained 0.5 mg of microsomal protein per millilitre and 40 μM omeprazole, and the incubation period was 20 min. Otherwise the incubations contained buffer and NADPH and the assay were performed as described above.
Each reaction mixture was preincubated for 2 min at +37°C in a shaking incubator block (Eppendorf Thermomixer 5436, Hamburg, Germany). The reaction was started by adding NADPH and terminated by adding 200 μl of ice-cold acetonitrile and subsequently cooled in an ice bath to precipitate the proteins. The mixture was vortex-mixed and centrifuged at 10 000 g for 15 min. Supernatants were collected and stored at −20°C until analysed. In experiments directed at revealing mechanism-based inhibition, the inhibitors were preincubated with NADPH for 15 min before the reaction was started by the addition of substrate.
Comparative molecular field analysis
CoMFA is a ligand-based predictive software with the ability to derive 3D-QSARs. Using regression analysis on the charges for each atom of each molecule in a 3D-aligned library, CoMFA can correlate electrostatic and steric properties with activities (e.g., IC50 values) obtained by biological experiments. The two uses of CoMFA analysis are to predict IC50 values (or other properties) of other ligands and to create contour plots that allow the visualization of predicted favourable and unfavourable properties of the ligands in 3D. Generally, these plots can be directly translated into interactions with the enzyme, allowing a more directed route of ligand design that exploits the desired properties (Cramer et al., 1988).
The construction of the molecules, superimposition and CoMFA modelling were performed using the Sybyl 6.9 (TRIPOS Associates Inc., St Louis, MO, USA) molecular modelling software. The biological data were transformed into pIC50 (−log IC50) values. Atomic point charges were calculated using the MMFF94 method. Standard CoMFA analysis included steric and electrostatic fields, which were calculated using the sp3 carbon probe atom with a +1 charge and a 2-Å grid spacing. The standard derivation threshold for exclusion of columns from the partial least-squares (PLS) analysis was set at 2 kcal/mol. The PLS method with five random group cross-validations was used for statistical analyses and this calculation was repeated 20 times to verify the stability of the obtained q2 values. The optimum number of components for the nonvalidated analyses was chosen according to the validation results: concerning the lowest SPRESS (standard error of prediction) or SDEP0* (corresponding value from progressive scrambling), highest q2 or Q0*2 (corresponding value from progressive scrambling). The predictive capacity of the generated model was tested using an external set of compounds.
Chemicals
Bupropion and hydroxybupropion were donated by Glaxo SmithKline, Inc. (Research Triangle, NC, USA), midazolam and α-hydroxymidazolam by F Hoffmann-La Roche (Basel, Switzerland) and omeprazole, 5-hydroxyomeprazole and omeprazole sulphone by Astra Zeneca (Mölndal, Sweden). Desethylamodiaquine was purchased from Sequoia Research Products (Pangbourne, UK) and other metabolite standards were from Ultrafine Chemical Company (Manchester, UK). 1-naphthol and 4-methoxybenzaldehyde were purchased from Sigma Chemical Co. (St Louis, MO, USA). γ-Heptalactone, γ-dodecanolactone, γ-caprolactone, ɛ-caprolactone, δ-hexalactone and α-tetralone were purchased from Sigma-Aldrich (St Louis, MO, USA). Undecanoic-γ-lactone, γ-phenyl-γ-butyrolactone, 2-methoxynaphthalene, 2-(p-tolyl) ethylamine, 2-naphthol, 2,4-dimethylquinoline, δ-decanolactone, quinaldine, 2-indanone, 2-coumarone, 2-benzoxazolinone, 3-methylisoquinoline, 3-methylquinoline, 2,7-dimethylquinoline, 2,6-dimethylquinoline, 2,3-dihydrobenzofuran, butylbenzyl, 4-(4-chlorobenzyl)pyridine (CBP), 4-benzylpyridine (BP), 2-benzylaniline, 4-hydroxydiphenylmethane, 4-chlorobenzophenone, 3,4-dihydroxybenzaldehyde and 2-(4-aminophenyl)-ethanol were purchased from Aldrich-Chemie GmbH & Co. (Heidenheim, Germany). 4-benzoylpyridine, 4-(4-chlorobenzoyl)pyridine, 4-(4-nitrobenzyl)pyridine (NBP), 4,4-diamino-diphenyl-methane bromodiphenylmethane, 4-bromo-benzaldehyde, 4-hydroxybenzophenone, 4-bromobenzylbromide and 4-hydroxybenzaldehyde were purchased from Acros Organics (New Jersey, NJ, USA). Other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA) and Boehringer (Ingelheim, Germany). The purity of all compounds used was higher than 95% according to the manufacturers.
Results
Inhibition of CYP2B6 activity in vitro and initial CoMFA model
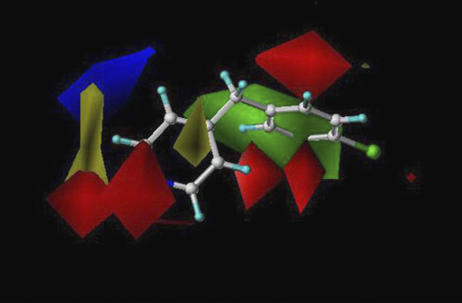
First, 25 compounds were analysed for CYP2B6 inhibition potency (IC50 values) (Table 1). The IC50 values of these compounds were measured using recombinant CYP2B6 catalysed EFC O-deethylation as the test reaction. The Km value of EFC was 4.0 μM (1.6–6.4 μM, 95% confidence interval) and the Vmax value was 2.4 nmol/(min × nmol CYP) (1.8–3.0 nmol/(min × nmol CYP)). The enzymatic activity was assayed under constant conditions using seven different concentrations of each inhibitor. These compounds were selected from our own structural database. This database consists of compounds used in our previous studies on CYP2A6 and CYP1A2 (Korhonen et al., 2005; Rahnasto et al., 2005). Based on this series of compounds, an initial CoMFA model was created to find out the key structural features, which increase inhibition potency. This CoMFA model revealed the location of a hydrophobic bulky group and an acceptor atom that affect inhibition potency (Figure 1). On the basis of this information and the 2D structure of a known potent CYP2B6 inhibitor, ticlopidine, 16 new pyridine and phenyl derivatives were selected and tested.
Table 1.
Inhibition potencies of compounds towards CYP2B6
Figure 1.
Colour contour maps of the initial CYP2B6 CoMFA model. Red and green colours represent areas where more negative partial charge and bulkier groups increase inhibition potency, respectively. Blue and yellow represent areas where more negative partial charge and bulkier groups decrease inhibition potency, respectively. The reference structure is CBP.
The lowest IC50 values were obtained with pyridine and diphenylmethane derivatives (Table 1). The heterocyclic nitrogen of benzylpyridine and benzoylpyridine increased the inhibitory potency. Benzylpyridines were found to be more potent than benzoylpyridines, as the sp2 oxygen decreased inhibitory potency. Of the polyaromatic compounds, 1-naphthol was the most potent inhibitor (IC50=9.1 μM). In contrast, 2-naphthol was more than seven-fold weaker as an inhibitor. The other polyaromatic compounds were even less potent, 2-benzoxalinone having the highest IC50 value (1600 μM). The inhibitory potencies of 11 lactones were also tested. The simple lactone ring structures were very weak inhibitors, ɛ-caprolactone being the least potent (IC50 value=28 mM). An increase in the length of side chain in lactone correlated with an increase in the inhibitory potency.
The inhibition by BP, CBP and NBP was studied in more detail because of their high potency (Figure 2). According to these experiments they all are competitive inhibitors for deethylation of EFC by CYP2B6 as the apparent Vmax was not affected and the apparent Km increased in the presence of these compounds. The apparent Km values correlated linearly and significantly with the concentration of inhibitors. According to this analysis, a Kic value of 0.15 μM (0.01–0.67 μM 95% confidence interval) was obtained for BP, 0.002 μM (0–0.11 μM) for CBP and 0.04 μM (0–0.10 μM) for NBP.
Figure 2.
Competitive inhibition of CYP2B6 by BP (a), CBP (b) and NBP (c). EFC deethylation to 7-hydroxy-4-trifluoromethylcoumarin via recombinant CYP2B6 was inhibited at different concentrations of the substrate and the inhibitors. The upper panels show non-linear regression analysis of 1 out of 3–5 experiments with each inhibitor. The lower panels show the effect of the inhibitor concentrations on the apparent Km values. The Km values were obtained from experiments shown in the upper panels. The Vmax value was not affected by the inhibitor concentration.
The final CoMFA model
Forty-one compounds listed in Table 1 were analysed using the CoMFA method (including the compounds used in the initial CoMFA model). The created CoMFA model was of high quality with the following statistical values with two components: q2=0.71, SPRESS=0.64, r2=0.85 and predictive r2=0.80 (Figure 3). The CYP2B6 CoMFA model is represented as 3D contour maps with CBP as the reference structure (Figure 4). Red and green maps represent areas where more negative partial charge and bulkier groups increase inhibitory potency, respectively. Blue and yellow maps represent areas where more negative partial charge and bulkier groups decrease inhibition potency, respectively. The sterically favoured green area was located around substitution at carbon 4 of the benzyl ring of benzylpyridine in the CoMFA model. A broad partial negative charge near the nitrogen atom and above and below the plane of the benzyl ring tended to increase the inhibitory potency.
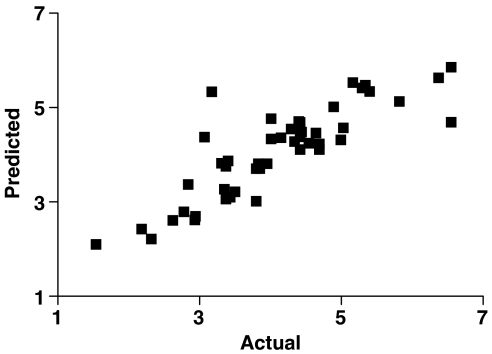
Figure 3.
Plot for the training set (actual/predicted IC50 values) of the CoMFA model (r2=0.85).
Figure 4.
Colour contour maps of final CYP2B6 CoMFA model. Red and green represent areas where more negative partial charge and bulkier groups increase inhibition potency, respectively. Blue and yellow represent areas where more negative partial charge and bulkier groups decrease inhibition potency, respectively. The reference structure is CBP.
The prediction power of the CoMFA model was evaluated by estimating pIC50 values for an external test set of seven compounds (Table 2). Two of these compounds are known substrates of CYP2B6 (selegiline and bupropion) and one is an inhibitor of this enzyme (thioTEPA). Four compounds were chosen on the basis that they possess structural similarities to compounds in the training set but also contain features that differ from the training set compounds (piperonylalcohol, 4-chlorobenzylamine, p-dimethylamino-benzaldehyde and anisalcohol). As summarized in Table 2, the predictions of pIC50 values for the external test set of molecules agreed well with the actual values. This is evidence that the created CoMFA exhibits a high predictive power.
Table 2.
Validation of the CYP2B6 model
CYP2B6 selectivity
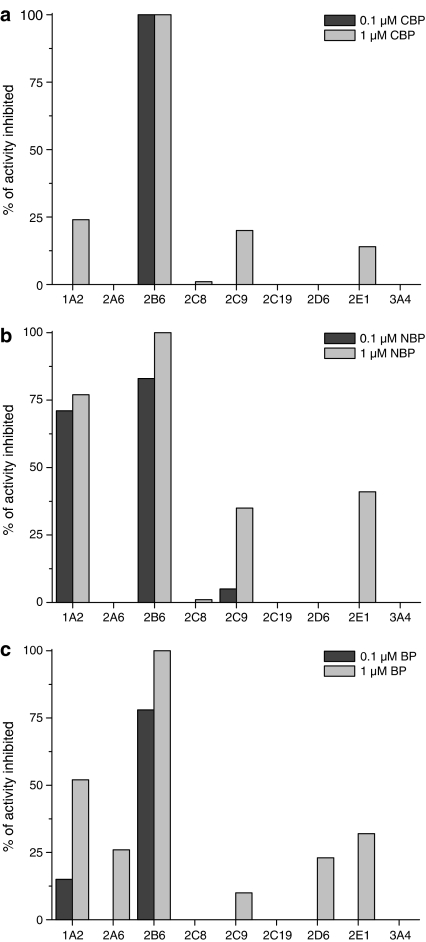
The CYP selectivity of the three most potent inhibitors, BP, CBP and NBP, were further characterized using the CYP2B6 selective bupropion as the substrate and human liver microsomes as the enzyme source. The IC50 values for the CYP2B6-mediated bupropion hydroxylation reaction were 0.02, 0.03 and 0.03 μM for CBP, NBP and BP, respectively (Table 3). No changes of IC50 values were observed with any of these three inhibitors when the experiments were carried out to reveal mechanism-based (suicide) inhibition. With CBP and BP, the IC50 values with other CYPs were >1 μM. The next sensitive CYP form for CBP and BP inhibition was CYP1A2 with IC50 values of 3.8 and 1.2 μM, respectively. In addition to inhibiting CYP2B6, NBP was also a potent inhibitor of CYP1A2 with an IC50 value of 0.02 μM (Table 3). At a 0.1 μM concentration of CBP, complete inhibition of CYP2B6 activity was observed. At this concentration, CBP did not affect any other CYP-related activities, and μM CBP (Figure 5a). Both NBP and BP were less selective, inhibiting also CYP1A2 at both 0.1 and 1.0 μM (Figure 5b–c).
Table 3.
IC50 values of CBP, NBP and BP on CYP-specific model activities in human liver microsomes in vitro
| CYP | Substrate |
IC50 (μM) |
||
|---|---|---|---|---|
| CBP | NBP | BP | ||
| Multiple | 7-Ethoxycoumarin (10 μM) | 7.8/7.8 | 0.05/0.06 | 2.8/2.8 |
| 1A2 | 7-Ethoxyresorufin (1 μM) | 3.8 | 0.02 | 1.2 |
| 2A6 | Coumarin (10 μM) | 31.3 | 24.3 | 3.0 |
| 2B6 | Bupropion (50 μM) | 0.02/0.02 | 0.03/0.025 | 0.03/0.02 |
| 2C8 | Amodiaquine (30 μM) | >100 | >100 | >100 |
| 2C9 | Tolbutamide (200 μM) | 5.4 | 6.8 | 7.2 |
| 2C19 | Omeprazole (40 μM) | 24.0 | 31.0 | 64.0 |
| 2D6 | Dextromethorphan (10 μM) | 17.5 | 13.3 | 5.1 |
| 2E1 | Chlorzoxazone (100 μM) | >100 | 3.8 | 8.5 |
| 3A4 | Midazolam (10 μM) | >100 | >100 | >100 |
| 3A4 | Testosterone (100 μM) | >100 | >100 | >100 |
| 3A4 | Omeprazole (40 μM) | >100 | >100 | >100 |
Abbreviations: BP, 4-benzylpyridine; CBP, 4-(4-chlorobenzyl)pyridine; CYP, cytochrome P450; NBP, 4-(4-nitrobenzyl)pyridine.
Mechanism-based inhibition experiments for ethoxycoumarin O-deethylation (multiple CYPs) and bupropion hydroxylation (CYP2B6) are also presented (2/15 min pre-incubation). Substrate concentrations for each model reaction are in parenthesis.
Figure 5.
CYP inhibition selectivity of CBP (a), NBP (b) and BP (c). CYP screen was carried out using CYP-specific substrates and human liver microsomes as the enzyme source.
Discussion and conclusions
The main achievement of this study was to find two novel potent and selective inhibitors of CYP2B6: CBP and BP. For this purpose the inhibition potencies of 41 compounds were determined and a comprehensive 3D-QSAR (CoMFA) model was created with this data set. In this way, a precise view of the key molecular characteristics of CYP2B6 inhibitors was obtained. The prediction power of this CoMFA model was evaluated by estimating the pIC50 values for an external test set of compounds. The CoMFA model yielded novel structural information about CYP2B6 inhibitors. The developed model accurately predicted the inhibitory potencies of several structurally unrelated compounds.
An important aim of this study was to find new potent and selective inhibitors of the CYP2B6 enzyme. Ticlopidine is a potent mechanism-based inhibitor of CYP2B6 (IC50=0.32 μM), but it is also an inhibitor of CYP2C19 and it also impairs activities associated with CYP1A2 and CYP2D6 (Ha-Duong et al., 2001; Richter et al., 2004; Turpeinen et al., 2004). ThioTEPA has been shown to be a very selective CYP2B6 inhibitor, but it is not as potent as ticlopidine (Rae et al., 2002; Turpeinen et al., 2004). In this study, we demonstrated that CoMFA modelling can be used to identify new inhibitors. Three novel potent inhibitors were found: CBP, NBP and BP, all displaying an IC50 <1 μM. CBP was the most selective inhibitor. It was 190 times more potent towards CYP2B6 than towards the next sensitive form, namely CYP1A2. For BP, the next sensitive CYP form was also CYP1A2, but the difference in potency was only 40-fold. Therefore, the chloro substituent in the benzyl ring in CBP must influence the selectivity. In addition to inhibiting CYP2B6, NBP was also a potent inhibitor of CYP1A2. The NO2 group in the NBP benzyl ring may be the reason for its inhibitory properties. Mechanism-based enzyme inhibition, by definition, requires metabolism of a substrate into a reactive intermediate that can bind to the enzyme irreversibly, resulting in the loss of enzyme activity (Kent et al., 2001). Ticlopidine is a mechanism-based inhibitor and its structure bears resemblances to these new potent molecules. However, none of the three new compounds act as a mechanism-based inhibitor.
The substrates of CYP2B6 are usually non-planar molecules, neutral or weakly basic, fairly lipophilic with one or two hydrogen-bond acceptors (Lewis, 2000). CYP2B6 has been studied using homology modelling, protein docking and molecular dynamics approaches (de Graaf et al., 2005). The crystal structure of CYP2B6 has not yet been published but several homology models have been created. The 3D-QSAR approach is mostly used, that is descriptors of essential functional groups and topologies of the molecules are superimposed. Ekins et al. (1999) published two different 3D-QSAR models based on 16 CYP2B6 substrates. A pharmacophore model was built using the program Catalyst, which was compared with a PLS model using molecular surface-weighted holistic invariant molecular (MS-WHIM) descriptors. The pharmacophore model obtained with Catalyst consisted of three hydrophobes and one hydrogen bond acceptor region. The cross-validated PLS MS-WHIM model gave a good q2 value of 0.607. Molecular size, positive electrostatic potential, hydrogen bonding acceptor capacity and hydrophobicity were found to be the most relevant descriptors for the model. Wang and Halpert (2002) published a homology model of CYP2B6 based on the crystal structure of CYP2C5. Also 3D-QSAR models were generated for 16 structurally diverse CYP2B6 substrates with Catalyst. Both include two hydrophobes and one hydrogen-bond acceptor. The pharmacophores were combined with the CYP2B6 model by docking the substrates in the active site of CYP2B6 and the pharmacophores were used to predict the Km value for test set molecules in conjunction with the CYP2B6 model.
To our knowledge there are no reports on the application of pharmacophore or QSAR techniques to find new inhibitors or to predict the inhibitory potency of compounds. The models described in the previous studies are based on the Km values obtained from the literature and were based on a limited number of structurally diverse compounds. The model presented here is based on a large training set, which was generated with internally fully consistent methods. The results in our present 3D-QSAR model are consistent with previous studies with the CYP2B6 enzyme since the importance of the hydrophobic and electronic characteristic in the binding of inhibitors was demonstrated. In the present study, both electrostatic and steric interactions were found to account for the differences in inhibitory potencies. In CoMFA, electrostatic fields described the areas where hydrogen bond acceptors or donors should be located in an inhibitor. The significance of hydrophobicity for inhibitory potency was evident in the CoMFA steric fields. The final CoMFA model pinpointed the location of the partial negative charge that increases the inhibition potency. In practice, an acceptor atom correctly located in the structure yields a more potent inhibitor. The CoMFA model also predicted accurately the inhibitory potencies of structurally diverse compounds. The predictions for selegiline, bupropion, 4-chlorobenzylamine and p-dimethylamino-benzaldehyde were successful because the residuals between predicted and tested pIC50 values were 0.08–0.33 log units.
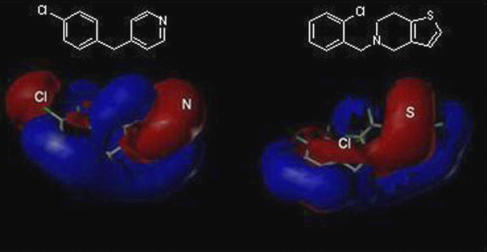
The CoMFA electrostatic and steric fields of CYP2B6 are consistent with the structure of benzylpyridine, which contains the optimal steric, hydrophobic and acceptor features. In particular, the benzyl structure and nitrogen acceptor atom render benzylpyridines more potent inhibitors than, for instance, lactones. It is interesting to compare the structural features of ticlopidine and CBP as these two compounds act via different inhibitory mechanisms. Figure 6 shows the electrostatic fields for these two structures. The red colour in the colour contour map of electrostatic fields means that this region has a partial negative charge, whereas the blue colour describes a partial positive charge. The most notable difference between these two structures is that CBP includes a partial negative region near to the electronegative chlorine atom in the benzyl ring. Also the nitrogen acceptor atom and the sulphur atom in ticlopidine exhibited a partial negative charge in the colour contour map.
Figure 6.
Electrostatic fields and 2D-structures of CBP (left) and ticlopidine (right). The electrostatic maps are shown in red and blue areas in which more negative charge increase and decrease inhibition potency, respectively.
As shown earlier, CoMFA modelling can predict very well the inhibitory potencies of a test set of compounds towards the human CYP2A6 and mouse CYP2A5 enzymes and human CYP1A2 (Korhonen et al., 2005; Rahnasto et al., 2005). In conclusion, the CYP2B6 CoMFA model was created, which revealed the key molecular characteristics of inhibitors of the CYP2B6 enzyme. Three novel potent inhibitors were discovered. Of these, especially CBP was very potent and selective. Thus, CBP is a suitable agent for in vitro studies directed at defining the relative contribution of CYP2B6 in the metabolism of chemical compounds, including novel drugs.
Acknowledgments
We thank Ms Hannele Jaatinen for expert technical assistance and Dr Ewen MacDonald for comments on the paper.
Abbreviations
- BP
4-benzylpyridine
- CBP
4-(4-chlorobenzyl)pyridine
- CoMFA
comparative molecular field analysis
- CYP
cytochrome P450
- NBP
4-(4-nitrobenzyl)pyridine
- PLS
partial least squares
- QSAR
quantitative structure–activity relationship
- 3D-QSAR
three-dimensional quantitative structure–activity relationship
Conflict of interest
The authors state no conflict of interest.
References
- Äbelö A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28:966–972. [PubMed] [Google Scholar]
- Cramer RI, III, Patterson D, Bunce J. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc. 1988;110:5959–5960. doi: 10.1021/ja00226a005. [DOI] [PubMed] [Google Scholar]
- de Graaf C, Vermeulen NP, Feenstra KA. Cytochrome P450 in silico: an integrative modeling approach. J Med Chem. 2005;48:2725–2755. doi: 10.1021/jm040180d. [DOI] [PubMed] [Google Scholar]
- Ekins S, Bravi G, Ring BJ, Gillespie TA, Gillespie JS, Vandenbranden M, et al. Three-dimensional quantitative structure activity relationship analyses of substrates for CYP2B6. J Pharmacol Exp Ther. 1999;288:21–29. [PubMed] [Google Scholar]
- Ekins S, Vandenbranden M, Ring BJ, Gillespie JS, Yang TJ, Gelboin HV, et al. Further characterization of the expression in liver and catalytic activity of CYP2B6. J Pharmacol Exp Ther. 1998;286:1253–1259. [PubMed] [Google Scholar]
- Ha-Duong NT, Dijols S, Macherey AC, Dansette PM, Mansuy D. Inhibition by ticlopidine and its derivatives of human liver cytochrome P450. Mechanism-based inactivation of CYP 2C19 by ticlopidine. Adv Exp Med Biol. 2001;500:145–148. doi: 10.1007/978-1-4615-0667-6_18. [DOI] [PubMed] [Google Scholar]
- Heyn H, White RB, Stevens JC. Catalytic role of cytochrome P4502B6 in the N-demethylation of S-mephenytoin. Drug Metab Dispos. 1996;24:948–954. [PubMed] [Google Scholar]
- Hutzler JM, Messing DM, Wienkers LC. Predicting drug–drug interactions in drug discovery: where are we now and where are we going. Curr Opin Drug Discov Dev. 2005;8:51–58. [PubMed] [Google Scholar]
- Kent UM, Juschyshyn MI, Hollenberg PF. Mechanism-based inactivators as probes of cytochrome P450 structure and function. Curr Drug Metab. 2001;2:215–243. doi: 10.2174/1389200013338478. [DOI] [PubMed] [Google Scholar]
- Korhonen LE, Rahnasto M, Mahonen NJ, Wittekindt C, Poso A, Juvonen RO, et al. Predictive three-dimensional quantitative structure–activity relationship of cytochrome P450 1A2 inhibitors. J Med Chem. 2005;48:3808–3815. doi: 10.1021/jm0489713. [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Lewis DF. On the recognition of mammalian microsomal cytochrome P450 substrates and their characteristics: towards the prediction of human P450 substrate specificity and metabolism. Biochem Pharmacol. 2000;60:293–306. doi: 10.1016/s0006-2952(00)00335-x. [DOI] [PubMed] [Google Scholar]
- Li XQ, Bjorkman A, Andersson TB, Ridderstrom M, Masimirembwa CM. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther. 2002;300:399–407. doi: 10.1124/jpet.300.2.399. [DOI] [PubMed] [Google Scholar]
- Mimura M, Baba T, Yamazaki H, Ohmori S, Inui Y, Gonzalez FJ, et al. Characterization of cytochrome P-450 2B6 in human liver microsomes. Drug Metab Dispos. 1993;21:1048–1056. [PubMed] [Google Scholar]
- Pelkonen O, Raunio H. In vitro screening of drug metabolism during drug development: can we trust the predictions. Expert Opin Drug Metab Toxicol. 2005;1:49–59. doi: 10.1517/17425255.1.1.49. [DOI] [PubMed] [Google Scholar]
- Rae JM, Soukhova NV, Flockhart DA, Desta Z. Triethylenethiophosphoramide is a specific inhibitor of cytochrome P450 2B6: implications for cyclophosphamide metabolism. Drug Metab Dispos. 2002;30:525–530. doi: 10.1124/dmd.30.5.525. [DOI] [PubMed] [Google Scholar]
- Rahnasto M, Raunio H, Poso A, Wittekindt C, Juvonen RO. Quantitative structure–activity relationship analysis of inhibitors of the nicotine metabolizing CYP2A6 enzyme. J Med Chem. 2005;48:440–449. doi: 10.1021/jm049536b. [DOI] [PubMed] [Google Scholar]
- Richter T, Murdter TE, Heinkele G, Pleiss J, Tatzel S, Schwab M, et al. Potent mechanism-based inhibition of human CYP2B6 by clopidogrel and ticlopidine. J Pharmacol Exp Ther. 2004;308:189–197. doi: 10.1124/jpet.103.056127. [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- Stresser DM, Kupfer D. Monospecific antipeptide antibody to cytochrome P-450 2B6. Drug Metab Dispos. 1999;27:517–525. [PubMed] [Google Scholar]
- Taavitsainen P, Juvonen R, Pelkonen O. In vitro inhibition of cytochrome P450 enzymes in human liver microsomes by a potent CYP2A6 inhibitor, trans-2-phenylcyclopropylamine (tranylcypromine), and its nonamine analog, cyclopropylbenzene. Drug Metab Dispos. 2001;29:217–222. [PubMed] [Google Scholar]
- Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O. Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab Dispos. 2004;32:626–631. doi: 10.1124/dmd.32.6.626. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- Wang Q, Halpert JR. Combined three-dimensional quantitative structure–activity relationship analysis of cytochrome P450 2B6 substrates and protein homology modeling. Drug Metab Dispos. 2002;30:86–95. doi: 10.1124/dmd.30.1.86. [DOI] [PubMed] [Google Scholar]