Abstract
Background and purpose:
Mature endothelial cells and their progenitors are dysfunctional in diabetes, resulting in deficient neovascularisation following arterial occlusion. This study aimed to evaluate the therapeutic activity of a nitric oxide (NO) releasing statin in the setting of experimental diabetes and peripheral ischaemia.
Experimental approach:
The effects of NCX 6550, an NO-releasing pravastatin derivative, on angiogenesis in ischaemic limbs was studied in normoglycaemic mice or mice made diabetic by treatment with streptozotocin (STZ). Control mice received an equimolar dosage of the parent statin compound, pravastatin. The therapeutic action of NCX 6550 was also tested in mice lacking the gene for endothelial nitric oxide synthase (eNOS).
Key Results:
In normoglycaemic or STZ-diabetic CD1 mice, only NCX 6550 stimulated skeletal muscle revascularisation. In addition, NCX 6550 induced greater improvement in limb reperfusion and salvage, than pravastatin. The number of circulating endothelial progenitor cells was decreased in STZ-diabetic mice, this defect being prevented by NCX 6550 and, to a lesser extent by pravastatin. In vitro, high glucose concentrations reduced the migratory capacity of endothelial progenitor EPCs, which was partly reversed by preincubation with pravastatin and completely reversed by NCX 6550. The postischaemic recovery of eNOS knockout mice was severely impaired as a consequence of depressed angiogenesis and this recovery was improved by treatment with NCX 6550, but not with pravastatin.
Conclusions and implications:
These findings indicate that incorporation of a bioactive NO moiety improves the therapeutic profile of statins for the treatment of peripheral vascular disease.
Keywords: ischaemia, nitric oxide, statin, diabetes, peripheral vascular disease
Introduction
Clinical evidence indicates that the cholesterol-lowering drugs, statins, alleviate ischaemic symptoms (Mohler et al., 2003) and reduce cardiovascular-related morbidity and mortality in patients with or without hypercholesterolaemia (Lewis et al., 1998; Athyros et al., 2002; MRC/BHF, 2002; Collins et al., 2003; Sever et al., 2003). Benefit could derive from the improvement of endothelial function, inhibition of inflammation and modulation of cardiovascular remodelling. In addition, statins promoted arterial collateral growth in response to acute ischaemia in normocholesterolaemic (Kureishi et al., 2000; Sata et al., 2001) as well as in atherosclerotic mice (Sata et al., 2004), reports which are contradicted by a recent finding that these drugs do not increase collateral-dependent perfusion in hypercholesterolaemic pigs with chronic myocardial ischaemia (Boodhwani et al., 2006).
The mechanisms by which statins may influence neovascularization are under intense investigation. Besides acting as competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Edwards and Ericsson, 1999; Maron et al., 2000), statins activate protein kinase B (Akt), facilitate the Akt-endothelial nitric oxide synthase (eNOS) interaction in endothelial caveolae and thereby promote the activation/phosphorylation of eNOS and nitric oxide (NO)-mediated angiogenesis (Dimmeler et al., 1999; Fulton et al., 1999; Kureishi et al., 2000; Brouet et al., 2001). They also enhance eNOS mRNA stability by blocking the geranylgeranylation of the GTPase Rho (Laufs and Liao, 1998). Interestingly, statins mobilise endothelial progenitor cells (EPCs) from bone marrow via the phosphatidylinositol 3 kinase/Akt pathway and thereby facilitate their incorporation into the neovasculature of ischaemic tissues (Dimmeler et al., 2001; Llevadot et al., 2001).
Because they are highly susceptible to vascular complications, patients with diabetes mellitus require more effective therapy for the consequences of ischaemia. Therapeutic angiogenesis reportedly improves peripheral microangiopathic complications in type I, insulin-dependent diabetes (Rivard et al., 1999; Emanueli et al., 2004a, 2004b, 2004c) and statins improve coronary collateral development in patients with type II, insulin-resistant diabetes, when given in association with other cardioprotective drugs (Dincer et al., 2006). Yet, to the best of our knowledge, whether or not statins are proangiogenic in type I diabetes remains undetermined.
Because the proangiogenic action of statins is dependent on the Akt/eNOS pathway, impairment of this pathway could theoretically prevent or reduce statin-induced vascular benefit. Importantly, eNOS was found to be dysfunctional in type I, streptozotocin (STZ)-diabetic mice, due to reduced bioavailability of tetrahydrobiopterin (BH4) (Cai et al., 2005). Furthermore, we have shown that the migratory and NO-releasing capacity of EPCs is impaired by diabetes (Kraenkel et al., 2005).
Based on this background, we proposed that the therapeutic profile of statins would be improved by adding an NO-releasing moiety to the statin molecule. Specifically, this study aimed to evaluate the healing potential of pravastatin and its nitrostatin derivative, NCX 6550 (Ongini et al., 2004; Presotto et al., 2005; Rossiello et al., 2005; Dever et al., 2006) in normoglycaemic and STZ-induced diabetic mice, in which hindlimb ischaemia was induced. We also tested the therapeutic potential of the two compounds in eNOS knockout mice, which show inadequate reparative neovascularization in response to ischaemia. Finally, we evaluated whether the nitrostatin NCX 6550 could improve the mobilization and migratory activity of bone marrow-derived EPCs in a high-glucose environment.
Methods
Mice and type-I diabetes induction and assessment
All procedures complied with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, MD, USA, 1996) and approved by the Italian Minister of Health and by the INBB ethical committee. Experiments were conducted in 4-month-old CD1 male mice (Charles River, Calco, Milan, Italy) with or without superimposed diabetes. Mice were made diabetic using STZ (40 mg kg−1, i.p. per day for 5 days; Sigma, Milan, Italy) (Emanueli et al., 2004a). Mice were considered diabetic only if they developed glycaemia >2.5 mg ml−1 and overt glycosuria at 14 days from the first STZ injection. Persistence of diabetes was determined at the end of the study.
In addition, the therapeutic potential of pravastatin and NCX 6550 was tested vs vehicle in normoglycaemic eNOS knockout mice (eNOS−/−, Jackson Laboratories, Bar Harbour, ME, USA) and wild-type controls (eNOS+/+) of the same genetic background (C57/Bl6, Charles River).
Experimental protocol
Tail-cuff blood pressure (BP) was measured by a plethysmography apparatus (Visitech Systems, Apex, NC, USA) during three basal sessions and then once a week during the experimental period. Body weight was recorded at the same occasion. Then, animals were assigned randomly to receive regular chow (vehicle group), or the same chow containing pravastatin (10 mg kg−1 daily) or equimolar NCX 6550 (12 mg kg−1 daily), which is fourfold less than that previously shown to exert antithrombotic effects in rodents (Rossiello et al., 2005). As statins are thought to be pro-angiogenic at low dosages (Sata et al., 2001, 2004), we wished to evaluate whether the addition of a NO-releasing moiety could potentiate this effect in vivo. Drug treatment was started 4 days before surgical induction of unilateral limb ischemia (Emanueli et al., 2004a) and maintained for the following 2 weeks (experimental period).
The following end points were evaluated: (i) clinical outcome, (ii) hindlimb blood flow (BF), (iii) muscular neo-angiogenesis, (iv) circulating EPCs, (v) expression of angiogenesis modulators and (vi) levels of NO derivatives (NOx) in limb muscle.
Clinical outcome
The number of necrotic toes and the occurrence of foot auto-amputation were recorded.
Hindlimb BF
The superficial BF of both feet was sequentially assessed by a perfusion imager system (Lisca colour laser Doppler, Perimed, Stockholm, Sweden). At 14 days after femoral artery occlusion, the BF of ischaemic and contralateral adductors was measured by means of an OxyLite/OxyFlo probe (Oxford Optronix Ltd, Oxford, UK). Perfusion values were expressed in Doppler Arbitrary Units (DAU).
Histological procedures
Hindlimb muscles were harvested following in situ perfusion fixation at physiological pressure and then processed for paraffin embedding. Transverse sections (5 μM) of adductor and gastrocnemius muscles were stained with haematoxylin/eosin (for capillary counting) or submitted to α-smooth muscle actin immunohistochemistry (to recognize arterioles). Capillary density was expressed as the absolute capillary number per millimetre square of transverse section (ncap mm−2) or normalised to myofibre density (ncap nfiber−1). Arteriolar density was expressed as arteriole number per millimetre square (nart mm−2) (Emanueli et al., 2001, 2002a, 2002b, 2004b).
Circulating EPCs
At 2 weeks after ischaemia, peripheral blood mononuclear cells (MNCs) were isolated from 500 μl of blood by density gradient centrifugation with Histopaque-1083 (Sigma). After 4 days of culture on rat vitronectin, cells were stained with acetylated LDL (acLDL)-DiI (Biomedical Technologies, Villalba, Madrid, Spain) and fluorescein isothiocyanate (FITC)-conjugated with Bandeiraea simplicifolia lectin I (Vector Laboratories, Burlingame, CA, USA) and examined using fluorescent microscopy. EPCs, identified as double-positive cells, were automatically counted in six randomly selected microscopic fields (at × 100).
Migration of EPCs
Human MNCs were isolated by density gradient centrifugation (Histopaque 1077; Sigma) from the bone marrow of patients undergoing hip replacement surgery. Written consent was obtained from all patients and the procedure complied with the institutional guidelines of the University of Bristol and was approved by the local ethics committee. After isolation, MNC were cultured on fibronectin-coated (Sigma) six-well plates at a density of 1 × 106 MNC cm−2 in EBM-2 medium (Cambrex, Verviers, Belgium) supplemented with 10% foetal calf serum (Biochrom, Cambridge, UK), endothelial growth factors (Cambrex), and 15 mM D-glucose (High glucose (HG)) for 4 days. In control samples, D-glucose was not supplemented (normal glucose (NG)). After 4 days, the medium was changed and vehicle (dimethylsulphoxide), pravastatin (100 μM) or NCX 6550 (10 μM) was added to the medium for 24 h before assessment of migratory capacity. The percentage of migrating EPCs was assessed as described previously (Kraenkel et al., 2005). Briefly, 2 × 104 EPCs of each group were applied per well to the upper chamber of a 96-well transmigration chamber (Neuroprobe; membrane pore size: 8 μM). The assay was conducted in serum- and growth factor-free EBM-2 (Cambrex) overnight in a cell culture incubator using SDF-1 (10 ng ml−1; Serotec, Oxford, UK) as the migratory stimulus. Migrated cells, attached to the lower side of the membrane, were stained for the uptake of DiI-acLDL and the binding of FITC-conjugated Ulex europaeus lectin I. EPC in five randomly chosen microscopic fields were counted (ImagePro PLUS, Media Cybernetics Inc., Silver Spring, MD, USA).
Immunoblotting
Western blot analyses of eNOS, Ser-473-phosphorylated and total Akt and vascular endothelial growth factor-A (VEGF-A) were performed, as described previously (Emanueli et al., 2004b), on homogenates of limb adductors obtained at 3 days after induction of ischemia. β-actin served as loading control. Specific protein was detected by chemiluminescence reaction, followed by software-assisted analysis of immunoblot density (Scion Corporation, Frederick, MD, USA).
Determination of NO metabolites (NOx)
The muscular content of NO metabolites was measured by a colorimetric non-enzymatic assay (Invitrogen, Paisley, UK), as described previously (Emanueli et al., 2004c). Tissue NOx were normalized by protein levels which were determined using Lowry's method.
Statistics
All results are expressed as mean±standard error (s.e.m.). Multivariate repeated-measures analysis of variance (ANOVA) was performed to test for interaction between time and grouping factor. In multiple comparisons in which ANOVA indicated significant differences, the statistical value was determined according to Bonferroni's method. Differences within and between groups were determined using paired or unpaired Student's t-test, respectively. The comparative incidence of toe necrosis and the ratio of foot auto-amputation was evaluated by Wilcoxon/Kruskal–Wallis tests (rank sums) for non-parametric distribution. A P-value <0.05 was taken as showing statistically significant differences between means.
Results
NCX 6550 improves postischaemic healing
As illustrated in Figure 1a and d, NCX 6550-treated normoglycemic CD1 mice showed an improved clinical outcome compared with control mice given pravastatin or vehicle (20 mice per group). Similarly, NCX 6550 improved foot salvage in STZ-diabetic CD1 mice, compared with vehicle or pravastatin (n=15 per group, Figure 1b and e).
Figure 1.
Bar graphs show the effects of vehicle, pravastatin or NCX 6550 on the postischaemic clinical outcome, which was assessed by recording the number of necrotic toes (left panels) and percent of foot auto-amputation (right panels). (a and d): normoglycaemic CD1 mice; (b and e): STZ-induced diabetic CD1 mice; (c and f): mice with eNOS gene knockout (eNOS−/−). NCX 6550 was able to improve the clinical outcome in all conditions, including the eNOS−/− mice, which normally showed a high rate of auto-amputation. Values are mean±s.e.m.; §P<0.05 and §§P<0.01 vs vehicle; +P<0.05 and ++P<0.01 vs pravastatin.
The clinical outcome of vehicle-treated eNOS+/+ mice (C57BL/6 strain) was similar to that of CD1 mice (data not shown). In the vehicle-treated eNOS−/− strain, we observed an increased incidence of toe necrosis and auto-amputation. In this strain, treatment with NCX 6550, but not with pravastatin, prevented the adverse outcome (n=8 per group, Figure 1c and f).
Both NCX 6550 and pravastatin reduce BP in hypertensive eNOS−/−, but not in normotensive mice
Diabetes did not affect BP in CD1 mice (data not shown). Neither pravastatin nor NCX 6550 altered the BP of normoglycaemic or STZ-diabetic CD1 mice (data not shown). In hypertensive eNOS−/− mice, pravastatin reduced BP from 132±1 to 117±3 mm Hg). A hypotensive effect was also observed with NCX 6550 (from 134±2 to 126±1 mm Hg), whereas in animals maintained on a control diet, BP increased from 136±1 to 148±2 mm Hg.
NCX 6550 accelerates the recovery of limb BF
BF was assessed by measuring the shift of a laser beam produced by erythrocytes passing through a blood vessel, either in the superficial or intramuscular microcirculations. Owing to the additional discomfort provoked by invasive measurement of muscular BF, this parameter was measured at only one occasion, just before the animals were killed.
In normoglycaemic mice treated with NCX 6550, we observed that, at 1 week after ischaemia induction, the recovery of superficial BF was improved compared with vehicle or pravastatin (Figure 2a). The superficial BF regained normal levels at 2 weeks from ischaemia, with no significant difference among treatments (data not shown). However, measurement of muscular BF by the OxyLite/OxyFlo probe showed a persistent perfusion deficit in vehicle- or pravastatin-treated mice (ischaemic to contralateral BF ratio: 0.61±0.04 and 0.62±0.03, respectively), whereas the recovery was improved in NCX 6550-treated mice (0.98±0.05; P<0.05 vs vehicle or pravastatin).
Figure 2.
The effects of drug treatments on the superficial BF of feet are shown. (a): Normoglycaemic CD1 mice; (b): STZ-induced diabetic CD1 mice; (c): eNOS−/−. Photographs show typical laser doppler images of superficial BF in lower limbs. The dotted squares include the area of interest (the feet) in which average perfusion was computed by the Perimed software. Colour scale from blue to brown indicates progressive increases in BF. Bar graphs illustrate the average BF, 1 week postsurgery, at the level of the ischaemic (I, filled columns) or contralateral foot (C, open columns). Values (expressed as DAU) are mean±s.e.m. +P<0.05 and ++P<0.01 vs C; *P<0.05 vs vehicle; §P<0.05 vs pravastatin, °°P<0.01 vs eNOS+/+ mice.
Similarly, in STZ-diabetic mice, NCX 6550 was the only agent able to improve BF at the level of the foot skin (Figure 2b). At 2 weeks postischaemia, the BF of the ischaemic adductor (measured by the intramuscular OxyLite/OxyFlo probe) was higher in NCX 6550-treated diabetic mice (0.97±0.03 DAU) than in vehicle (0.32±0.02 D.A.U.) or pravastatin (0.51±0.10 DAU), leading to significantly higher ischaemic to contralateral BF ratios in mice treated with NCX 6550 (1.02±0.05) compared with vehicle (0.51±0.03) or pravastatin (0.54±0.04).
As shown by Figure 2c, at 1 week postischaemia, in vehicle-treated eNOS−/− mice, BF to the ischaemic foot was compromised, as compared with vehicle-treated eNOS+/+ animals. The BF to the ischaemic foot was not influenced by pravastatin, but was significantly improved by NCX 6550. No further measurement was possible at 2 weeks, owing to the high rate of auto-amputation that imposed early termination of the experiment.
NCX 6550 stimulates angiogenesis and arteriogenesis
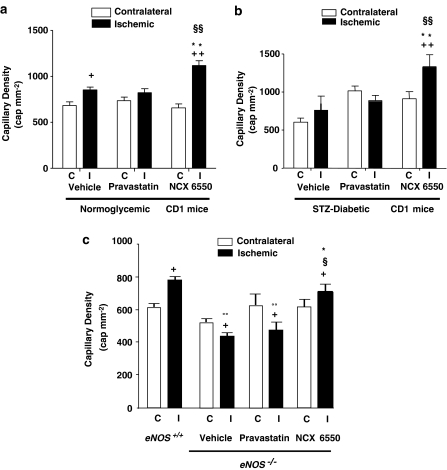
As shown by Figure 3a, in normoglycaemic CD1 mice, NCX 6550 enhanced capillary density in ischaemic adductor muscles, above the values for vehicle or pravastatin mice, but not in contralateral, normally perfused muscles. Comparison between treatments indicates that NCX 6550 is more effective than pravastatin in promoting reparative angiogenesis, either expressed as capillary density (Figure 3a) or as capillaries per myofibre unit (1.80±0.06 vs 1.30±0.06 in pravastatin group; P<0.001). Furthermore, NCX 6550 significantly increased arteriole density in ischaemic adductor muscles (8.1±1.8 vs 3.5±0.6 arterioles mm−2 in vehicle and 4.1±0.6 arterioles mm−2 in pravastatin treatment groups; P<0.05 for both comparisons).
Figure 3.
Bar graphs show the effects of vehicle, pravastatin or NCX 6550 on the capillary density of ischaemic (I, filled columns) and contralateral (C, open columns) adductor muscles. (a): Normoglycaemic CD1 mice; (b): STZ-induced diabetic CD1 mice; (c): data from eNOS−/−- and vehicle-treated eNOS+/+ mice for reference. Values are mean±s.e.m.; +P<0.05 and ++P<0.01 vs C; *P<0.05 and **P<0.01 vs vehicle; §P<0.05 and §§P<0.01 vs pravastatin, °°P<0.01 vs eNOS+/+.
Figure 3b illustrates the effect of NCX 6550 on angiogenesis in STZ-diabetic mice, whereas Figure 3c shows the response to treatment with NCX 6550 in eNOS−/− mice. In both models, NCX 6550, but not pravastatin, improved the reparative capillarization of the ischaemic adductor muscle.
NCX 6550 increases the number of circulating EPCs in STZ-diabetic mice
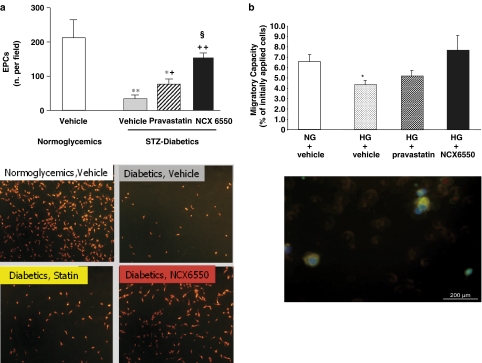
As shown in Figure 4a (upper panel), the numbers of circulating EPCs were significantly reduced in diabetic mice, relative to those in the normoglycaemic mice. Pravastatin partially counteracted this diabetes-induced EPC deficit. However, NCX 6550 exerted a greater improvement, restoring EPC numbers to a level not significantly different from that in normoglycaemic mice. Typical microphotographs of EPCs (identified in yellow by acLDL-DiI/lectin I costaining) are presented in lower panels of Figure 4a.
Figure 4.
(a) Bar graphs show the effects of vehicle, pravastatin or NCX 6550 on the number of circulating EPCs (number of cells per microscopic field) in STZ-diabetic CD1 mice with limb ischaemia. The number of EPC in vehicle-treated normoglycaemic CD1 mice are shown for reference. Values are mean±s.e.m.; *P<0.05 and **P<0.01 vs vehicle-treated normoglycaemic mice; +P<0.05 and ++P<0.01 vs vehicle-treated diabetics; §P<0.05 vs pravastatin-treated diabetics. In the lower panel, microphotographs show EPCs identified in yellow by acLDL-DiI/lectin I costaining. (b) The effect of preincubation with pravastatin or NCX 6550 on migratory capacity of hyperglycaemic EPC. HG leads to a reduction in EPC migratory capacity, which is partially reversed by pravastatin and completely reversed by NCX 6550. The lower panel shows migrating cells positive for acLDL uptake and UEAI binding. *P<0.05 vs NG.
NCX 6550 enhances EPC migratory capacity in HG concentrations
As shown in Figure 4b, incubation of EPCs in HG media (15 mM glucose) led to a significant decrease in the migratory capacity of EPCs, compared with those incubated with NG levels. This decrease was partly reversed by 24 h preincubation with pravastatin and completely reversed by preincubation with NCX 6550. The lower panel of Figure 4b shows migrating EPCs, stained with FITC-conjugated Ulex europaeus lectin I.
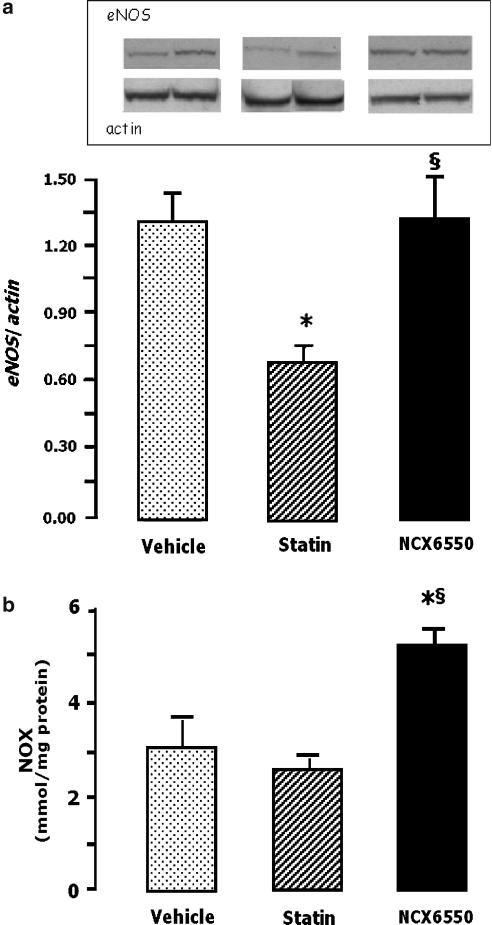
NCX 6550 preserves eNOS, phospho-Akt and VEGF-A levels in ischaemic muscles
As shown by Figure 5a, Western blot analysis showed reduced levels of eNOS in ischaemic muscles of normoglycaemic CD1 mice treated with pravastatin, but not in those given NCX 6550. The treatment with NCX 6550 also increased NOx levels in ischaemic muscles (Figure 5b), but neither NCX 6550 nor pravastatin affected NOx levels in the contralateral, non-ischaemic muscles (NCX 6550: 2.8±0.2 μmol NOx mg−1 protein; pravastatin: 3.5±0.4 μmol NOx mg−1 protein; and vehicle: 2.3±0.3 μmol NOx mg−1 protein).
Figure 5.
Bar graphs show the effect of vehicle, pravastatin or NCX 6550 on: (a) eNOS protein level and (b) NOx in ischemic adductor muscles. Western blot analyses served to quantify eNOS content after normalization against β-actin levels. Muscles were harvested from CD1 mice at 3 days after ischaemia induction. Values are mean±s.e.m. *P<0.05 vs vehicle, §P<0.05 vs pravastatin. A representative Western blot is shown.
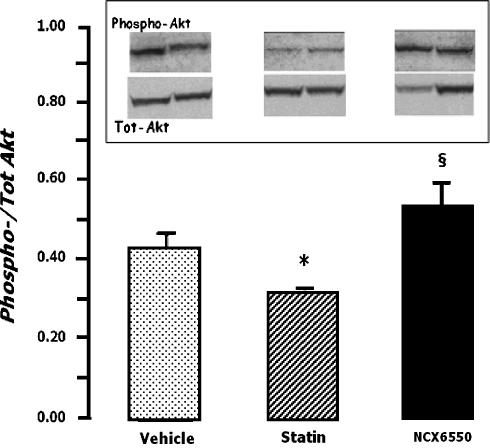
In Figure 6, the data show that whereas treatment with pravastin decreased Ser-473-phosphorylation of Akt in ischaemic muscles of CD1 mice, treatment with NCX 6550 maintained this variable at normal levels.
Figure 6.
Bar graph shows the effect of vehicle, pravastatin or NCX 6550 on the Ser473-phosphorylation of Akt in ischaemic adductor muscles. Western blot analyses served to quantify the ratio of phosphorylated Akt vs total Akt. Values are mean±s.e.m. *P<0.05 vs vehicle, §P<0.05 vs pravastatin. A representative Western blot is shown.
VEGF-A content in ischaemic muscles was also reduced by treatment of the mice with pravastatin (VEGF-A to β-actin ratios: 1.08±0.24 with vehicle and 0.74±0.08 after pravastatin), but remained unchanged in mice given NCX 6550 (1.01±0.24; P=NS vs vehicle and P<0.05 vs pravastatin).
Discussion
The potential advantages of incorporating an NO-releasing moiety into the statin structure are supported by recent studies, showing that nitrostatin is more effective than the respective parent compound in inhibiting vascular smooth muscle proliferation, platelet aggregation and the expression of iNOS and tissue factor in lipopolysaccharide-stimulated cells (Ongini et al., 2004; Rossiello et al., 2005).
Here, we show for the first time that the nitropravastatin derivative, NCX 6550, promotes therapeutic neovascularization, thus improving postischaemic healing in normoglycaemic mice as well as in STZ-induced type I diabetic mice. Importantly, NCX 6550, but not pravastatin, was able to rescue the impaired muscular capillarisation in ischaemic limbs of eNOS−/− mice, strongly suggesting that the therapeutic proangiogenic action of NCX 6550 is mainly mediated by the added NO donor part of the the molecule.
The potential therapeutic benefit of increasing bioactive NO is outlined by previous studies showing that, in experimental models of limb ischaemia, eNOS gene therapy successfully promotes neovascularization and accelerates haemodynamic recovery (Smith et al., 2002; Namba et al., 2003). It has been argued that the effectiveness of eNOS gene transfer could be compromised under pathological circumstances owing to the shortage of the eNOS cofactor, BH4 (Cai et al., 2005). Furthermore, uncontrolled production of NO may be toxic for the vascular endothelium (Heller et al., 1999). Compounds such as NCX 6550, which releases NO directly and in a predictable way, could avoid these limitations.
In the setting of limb ischaemia, pravastatin alone was unable to induce angiogenesis, which is at variance with previous reports (Kureishi et al., 2000; Sata et al., 2001, 2004). However, the proangiogenic activity of statins is still controversial. It has been suggested that high doses of statins inhibit the proliferation of endothelial cells in vitro and angiogenesis in vivo (Feleszko et al., 1999; Vincent et al., 2001, 2002; Weis et al., 2002), presumably via inhibition of prenylation of G-protein and G-protein subunits leading to decreased Akt activity (Edwards and Ericsson, 1999). This is consistent with our findings of reduced phospho-Akt in ischaemic muscles of pravastatin-treated mice. Interestingly, we found that the NO-releasing statin NCX 6550 restored normal levels of Akt phosphorylation, which in keeping with the fact that NO, beside being downstream to Akt (Dimmeler et al., 1999; Fulton et al., 1999; Brouet et al., 2001), also acts upstream to the kinase to influence its activity (Tsurumi et al., 1997). In hypercholesterolaemic pigs with chronic myocardial ischaemia, high doses of statins reportedly inhibited myocardial angiogenesis, reduced VEGF-A and increased endostatin expression in the myocardium (Boodhwani et al., 2006). We found similarly reduced levels of VEGF-A expression in ischaemic muscles of pravastatin-treated mice, with this reduction being prevented by NCX 6550. Thus, the NO donor might counteract the suppressive effect of statins on VEGF-A and Akt.
We demonstrated that the BP of normotensive mice was unaltered by pravastatin or NCX 6550. In contrast, in hypertensive eNOS−/− mice, both compounds exerted BP-lowering effects, but only NCX 6550 improved reparative angiogenesis. Therefore, it seems unlikely that NO-induced vasodilation is responsible for the proangiogenic action of the nitrostatin. This is in keeping with the lack of therapeutic utility of systemic vasodilators in the setting of peripheral artery disease (Bendermacher et al., 2005).
Circulating progenitor cells derived from the bone marrow contribute to the revascularization of ischaemic tissue. However, this regenerative mechanism is impaired in type I diabetes, owing to the negative effect of hyperglycaemia on EPC mobilization, migration and integration into neovasculature. In addition, exposure to high levels of glucose reduced eNOS phosphorylation and NO production by EPCs (Loomans et al., 2004; Kraenkel et al., 2005). Statins improve the functionality of EPCs via Akt (Dimmeler et al., 2001; Llevadot et al., 2001) and NO may be involved in the mobilisation of EPCs from the bone marrow change nitrosylation and activation of metalloproteinase-9 (Aicher et al., 2003). In agreement with the above findings, we found a striking reduction of EPCs in the blood of STZ-diabetic mice. Importantly, pravastatin partially prevented diabetes- and HG-induced reduction of EPC number and migratory capacity. However, NCX 6550 was more effective in counteracting the deficit in EPC liberation and function.
Statins are widely used in the clinic because of protective actions that seem to be independent of the capacity to decrease cholesterol levels. The results of our study do not support a proangiogenic action of statins in animals with type I diabetes or in those lacking the eNOS gene, although we cannot exclude the possibility that doses of pravastatin different from those used in the present study may still be effective in ischaemic disease. On the other hand, adding an NO-releasing moiety to the pravastatin molecule resulted in prohealing effects that appeared to be related to potentiation of vascular regeneration. The benefit obtained in this murine model of limb ischaemia encourages clinical investigation to determine if NO-releasing statins could be of therapeutic value in patients suffering from peripheral ischaemic disease.
Acknowledgments
This research was partially supported by a FIRB project grant from the Italian Ministry of Scientific Research and Universities. The University of Bristol and INBB are members of the European Genomic Vascular Network (EVGN) funded by the European Community. Dr Emanueli holds a British Heart Foundation (BHF) Basic Science Senior Lectureship. Dr Kraenkel holds a Marie Curie Transfer of Knowledge Fellowship from the European Community.
Abbreviations
- acLDL-DiI
acetylated LDL
- Akt
protein kinase B
- BH4
tetrahydrobiopterin
- DAU
Doppler arbitrary units
- eNOS
endothelial nitric oxide synthase
- eNOS−/−
eNOS knockout mice
- eNOS+/+
wild-type controls
- EPCs
endothelial progenitor cells
- MNC
mononuclear cells
- NOx
NO derivatives
- STZ
streptozotocin
- VEGF-A
vascular endothelial growth factor-A
Conflict of interest
Angela Monopoli and Ennio Ongini are employed by Nicox Research Institute Srl (Via Ariosto 21, 20091 Bresso, Milan, Italy), a pharmaceutical company that develops the drugs described in this paper. Both hold stock in the NicOx company.
There is no conflict of interest for other authors.
References
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou W, Symeonidis AN, Basayannis EO, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual' care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- Axel DI, Riessen R, Runge H, Viebahn R, Karsch KR. Effects of cerivastatin on human arterial smooth muscle cell proliferation and migration in transfilter cocultures. J Cardiovasc Pharmacol. 2000;35:619–629. doi: 10.1097/00005344-200004000-00016. [DOI] [PubMed] [Google Scholar]
- Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Medical management of peripheral arterial disease. J Thromb Haemost. 2005;3:1628–1637. doi: 10.1111/j.1538-7836.2005.01368.x. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Nakai Y, Voisine P, Feng J, Li J, Mieno S, et al. High-dose atorvastatin improves hypercholesterolemic coronary endothelial dysfunction without improving the angiogenic response. Circulation. 2006;114 Suppl I:I402–I408. doi: 10.1161/CIRCULATIONAHA.105.000356. [DOI] [PubMed] [Google Scholar]
- Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric-oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- Dever G, Spickett CM, Kennedy S, Monopoli A, Wainwright CL.The NO-donating pravastatin derivative (NCX 6550) reduces splenocyte adhesion and ROS generation in normal and atherosclerotic mice JPET 2006(in press) [DOI] [PubMed]
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fisslthaler B, Fleming I, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells via Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dincer I, Ongun A, Turhan S, Ozdol C, Ertas F, Erol C. Effect of statin treatment on coronary collateral development in patients with diabetes mellitus. Am J Cardiol. 2006;97:772–774. doi: 10.1016/j.amjcard.2005.09.124. [DOI] [PubMed] [Google Scholar]
- Edwards PA, Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–185. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Graiani G, Salis MB, Gadau S, Desortes E, Madeddu P. Prophylactic gene therapy with human tissue kallikrein ameliorates limb ischemia recovery in type 1 diabetic mice. Diabetes. 2004a;53:1096–1103. doi: 10.2337/diabetes.53.4.1096. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, et al. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in a mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, et al. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002a;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Salis B, Stacca T, Pinna A, Gaspa L, Madeddu P. Angiotensin AT(1) receptor signalling modulates reparative angiogenesis induced by limb ischaemia. Br J Pharmacol. 2002b;135:87–92. doi: 10.1038/sj.bjp.0704461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Van Linthout S, Meloni M, Desortes E, Silvestre JS, et al. Akt/protein kinase B and endothelial nitric oxide synthase mediate muscular neovascularization induced by tissue kallikrein gene transfer. Circulation. 2004b;110:1638–1644. doi: 10.1161/01.CIR.0000142051.36244.83. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Van Linthout S, Salis MB, Monopoli A, Del Soldato P, Ongini E, et al. Nitric oxide-releasing aspirin derivative, NCX 4016, promotes reparative angiogenesis and prevents apoptosis and oxidative stress in a mouse model of peripheral ischemia. Arterioscler Thromb Vasc Biol. 2004c;24:2082–2087. doi: 10.1161/01.ATV.0000144030.39087.3b. [DOI] [PubMed] [Google Scholar]
- Feleszko W, Balkowiec EZ, Sieberth E, Marczak M, Dabrowska A, Giermasz A, et al. Lovastatin and tumor necrosis factor-alpha exhibit potentiated antitumor effects against Ha-ras-transformed murine tumor via inhibition of tumor-induced angiogenesis. Int J Cancer. 1999;81:560–567. doi: 10.1002/(sici)1097-0215(19990517)81:4<560::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Polack T, Grabner R, Till U. Nitric oxide inhibits proliferation of human endothelial cells via a mechanism independent of cGMP. Atherosclerosis. 1999;144:49–57. doi: 10.1016/s0021-9150(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Kraenkel N, Adams V, Linke A, Gielen S, Erb S, Lenk K, et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25:698–703. doi: 10.1161/01.ATV.0000156401.04325.8f. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Moye LA, Sacks FM, Johnstone DE, Timmis G, Mitchell J, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. 1998;129:681–689. doi: 10.7326/0003-4819-129-9-199811010-00002. [DOI] [PubMed] [Google Scholar]
- Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- Mohler ER, Hiatt WR, Creager MA, The Study Investigators Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral disease. Circulation. 2003;108:1481–1486. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- MRC/BHF Heart protection study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Heart Protection Study Collaborative Group. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- Muscara M, McKnight W, Del Soldato P, Wallace J, Lovren F, Dicay M, et al. Vasorelaxant effects of a nitric-oxide-releasing aspirin derivative in normotensive and hypertensive rats. Br J Pharmacol. 2001;133:1314–1322. doi: 10.1038/sj.bjp.0704209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, et al. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003;108:2250–2257. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]
- Negre-Aminou P, van Vliet AK, van Erck M, van Thiel GC, van Leeuwen RE, Cohen LH. Inhibition of proliferation of human smooth muscle cells by various HMG-CoA reductase inhibitors: comparison with other human cell types. Biochim Biophys Acta. 1997;1345:259–268. doi: 10.1016/s0005-2760(96)00184-1. [DOI] [PubMed] [Google Scholar]
- Ongini E, Impagnatiello F, Bonazzi A, Guzzetta M, Govoni M, Monopoli A, et al. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc Natl Acad Sci USA. 2004;101:8497–8502. doi: 10.1073/pnas.0401996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presotto C, Miglietta R, Olivieri A, Monopoli A. The nitropravastatin derivative, NCX 6550, improves endothelial dysfunction in spontaneously hypertensive rats. Circulation. 2005;112 Suppl II:17. [Google Scholar]
- Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiello MR, Momi S, Caracchini R, Giannini S, Guglielmini G, Monopoli A, et al. A novel nitric oxide-releasing statin derivative exerts an antiplatelet/antithrombotic activity and inhibits tissue factor expression. J Thrombosis Haemostasis. 2005;3:2554–2562. doi: 10.1111/j.1538-7836.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- Sata M, Nishimatsu H, Osuga J, Tanaka K, Ishizaka N, Ishibashi S, et al. Statins augment collateral growth in response to ischemia but they do not promote cancer and atherosclerosis. Hypertension. 2004;43:214–1220. doi: 10.1161/01.HYP.0000126186.29571.41. [DOI] [PubMed] [Google Scholar]
- Sata M, Nishimatsu H, Suzuki E, Sugiura S, Yoshizumi M, Ouchi Y, et al. Endothelial nitric oxide synthase is essential for the HMG-CoA reductase inhibitor cerivastatin to promote collateral growth in response to ischemia. FASEB J. 2001;15:2530–2532. doi: 10.1096/fj.01-0415fje. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;36:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- Smith RS, Jr, Lin KF, Agata J, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–12850. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- Vincent L, Chen W, Hong L, Mirshahi F, Mishal Z, Mirshahi-Khorassani T, et al. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett. 2001;495:159–166. doi: 10.1016/s0014-5793(01)02337-7. [DOI] [PubMed] [Google Scholar]
- Vincent L, Soria C, Mirshahi F, Opolon P, Mishal Z, Vannier JP, et al. Cerivastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme a reductase, inhibits endothelial cell proliferation induced by angiogenic factors in vitro and angiogenesis in in vivo models. Arterioscler Thromb Vasc Biol. 2002;22:623–629. doi: 10.1161/01.atv.0000012283.15789.67. [DOI] [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glass AJ, Cooke JP. Statins have a biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]