Abstract
Background and purpose:
Progression of heart failure in hypertensive Dahl rats is associated with cardiac remodeling and increased cardiomyocyte apoptosis. This study was conducted to study whether treatment with a novel inotropic vasodilator compound, levosimendan, could prevent hypertension-induced cardiac remodeling and cardiomyocyte apoptosis.
Experimental approach:
6-week-old salt-sensitive Dahl/Rapp rats received levosimendan (0.3 mg kg-1 and 3 mg kg-1 via drinking fluid) and high salt diet (NaCl 7%) for 7 weeks, Dahl/Rapp rats on low-salt diet served as controls. Blood pressure, cardiac functions by echocardiography, cardiomyocyte apoptosis by TUNEL technique, tissue morphology, myocardial expression of calcium cycling proteins, and markers of neurohumoral activation were determined.
Key Results:
Untreated Dahl/Rapp rats on high salt diet developed severe hypertension, cardiac hypertrophy and moderate systolic dysfunction. 38% of Dahl/Rapp rats (9/24) survived the 7-week-follow-up period. Cardiomyocyte apoptosis was increased by 6-fold during high salt diet. Levosimendan improved survival (survival rates in low- and high-dose levosimendan groups 12/12 and 9/12, p<0.001 and p=0.05, respectively), increased cardiac function, and ameliorated cardiac hypertrophy. Levosimendan dose-dependently prevented cardiomyocyte apoptosis. Levosimendan normalized salt-induced increased expression of natriuretic peptide, and decreased urinary noradrenaline excretion. Levosimendan also corrected salt-induced decreases in myocardial SERCA2a protein expression and myocardial SERCA2a/NCX-ratio.
Conclusions and Implications:
Improved survival by the novel inotropic vasodilator levosimendan in hypertensive Dahl/Rapp rats is mediated, at least in part, by amelioration of hypertension-induced cardiac remodeling and cardiomyocyte apoptosis.
Keywords: inotropic agents, remodeling, hypertension, calcium (cellular), apoptosis
Introduction
Congestive heart failure is a complex syndrome, which includes disturbances in ventricular function, neurohumoral and cytokine activation and increased arrhythmias (Hasenfuss and Pieske, 2002; Jessup and Brozena, 2003). Accumulating evidence indicates a central role for myocyte mishandling of calcium in the pathogenesis of heart failure (del Monte and Hajjar, 2003). Defects in various steps of cardiac excitation–contraction coupling have been identified in both human and experimental models of heart failure, characterized as prolonged calcium transients with elevated end-diastolic and decreased systolic intracellular calcium concentrations and prolonged relaxation phases (Narula et al., 1996). Emerging evidence also suggests that myocyte loss owing to apoptosis plays an important pathophysiological role in the progression of myocardial dysfunction (Narula et al., 1996; Olivetti et al., 1997; Kang and Izumo, 2000).
Levosimendan is a novel inotropic agent used in the management of acute decompensated heart failure that mediates its cardiac effect mainly by the calcium sensitization of the contractile protein troponin C (Innes and Wagstaff, 2003). Levosimendan binds to the calcium-saturated N-terminal domain of troponin C in cardiac muscle and stabilizes the troponin molecule with subsequent prolongation of its effect on the contractile proteins (Haikala et al., 1995; Innes and Wagstaff, 2003). Besides increasing the strength of cardiac contractions, levosimendan also exerts vasodilatory effects through opening of the ATP-dependent K+ channels (KATP) (Kaheinen et al., 2001). Although levosimendan displays some structural similarities with phosphodiesterase inhibitors, it is believed that the inotropic action of levosimendan is due primarily to calcium sensitization (Haikala et al., 1995; Innes and Wagstaff, 2003). Previous clinical studies have provided evidence that short-term infusion of levosimendan improves the hemodynamic function and may improve long-term survival in patients with decompensated heart failure (Slawsky et al., 2000; Follath et al., 2002; Kivikko et al., 2003). It has also been suggested that levosimendan may exert antiapoptotic properties that are linked to activation of mitochondrial KATP channels (Parissis et al., 2004; Maytin and Colucci, 2005).
The inbred Dahl/Rapp rat strain provides a widely used animal model for salt-sensitive hypertension and hypertension-induced target organ damage (Rapp and Dene, 1985; Walder et al., 1996). Salt-sensitive Dahl/Rapp rats rapidly and uniformly develop fulminant hypertension and begin to die after only 3 weeks on high-salt diet, and the overall mortality is usually almost 100% when kept on high-salt diet for 8 weeks (Rapp and Dene, 1985; Walder et al., 1996). Dahl/Rapp rats develop severe target-organ damage such as cardiac hypertrophy, heart failure, as well as vascular and renal damage (Rapp and Dene, 1985; Walder et al., 1996). Previous studies have revealed only relatively modest alterations in myocardial calcium homeostasis during the transition from compensated left ventricular hypertrophy to heart failure (Kihara and Sasayama, 1997; Yoneda et al., 2001). Noguchi et al. (2003) identified a thin-filament defect that causes a reduction in calcium sensitivity in the failing hearts of Dahl salt-sensitive rats. Furthermore, Ikeda et al. (2002) reported increased cardiomyocyte apoptosis at the stage of heart failure in Dahl salt-sensitive rats. The aim of the present study was to investigate whether oral treatment with the novel inotropic vasodilator levosimendan could prevent hypertension-induced cardiac remodeling and salt-induced mortality in Dahl/Rapp rats. The influence of levosimendan on cardiomyocyte apoptosis, neurohumoral activation and myocardial expression of calcium cycling proteins were also examined.
Materials and methods
Experimental animals, dietary and drug regimens and sample preparation
Sixty-six 6-week-old male Dahl/Rapp salt-sensitive rats (SS/JrHsd) were purchased from Harlan (Indianapolis, IN, USA). Development and characteristics of this inbred strain of Dahl salt-sensitive rat has been described in detail previously (Rapp and Dene, 1985; Walder et al., 1996). The protocols were approved by the Animal Experimentation Committee of the University of Helsinki, Finland, whose standards correspond to those of the American Physiological Society. Dahl/Rapp rats were divided into four groups to receive the following diet and drug regimens for 7 weeks: (1) Dahl/Rapp rats on high-salt diet (n=36), (2) Dahl/Rapp rats on high-salt diet+low-dose levosimendan (0.3 mg kg−1) (n=12), (3) Dahl/Rapp rats on high-salt diet+high-dose levosimendan (mg kg−1) (n=12), and (4) Dahl/Rapp rats on low-salt diet (n=6). To examine the influence of levosimendan during the late-stage of hypertension, an additional study group (n=12) was treated with levosimendan (mg kg−1) from week 4 to week 7 for observational reasons only. In an additional pharmacokinetic experiment the diurnal blood drug concentrations of levosimendan and its long-lasting metabolite (OR-1896) was examined after 2 weeks oral treatment with low- and high-dose levosimendan (n=4 in both groups). High-salt diet was produced by adding NaCl (Riedel-de-Haen) to commercial low-salt diet (Na 0.3%, K 0.8%, Mg 0.2%; Harlan). Levosimendan (the (−)enantiomer of {[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl]-hydrazono}-propanedinitrile) (Orion Pharma, Finland) was given via drinking fluid at concentrations (1 and 10 mg l−1) that have produced beneficial effects in previous rat experiments (Levijoki et al., 2001). Fresh levosimendan solutions were prepared daily. The rats had free access to chow and drinking water. Systolic blood pressure was measured by using a tail cuff blood pressure analyzer (Apollo-2AB Blood Pressure Analyzer, Model 179-2AB, IITC Life Science, Woodland Hills, CA, USA) at weeks 3.5 and 7 by the same technician. Urine was collected over 2 consecutive days in metabolic cages for albumin and catecholamine measurements. Urine volumes and water intakes were measured gravimetrically. Rats were then anesthetized with CO2/O2 (AGA, Riihimäki, Finland) and decapitated. Blood samples were collected for plasma brain natriuretic peptide (BNP), aldosterone and levosimendan measurements using ethylenediaminetetraacetic acid as anticoagulant. The heart and kidneys were excised, washed with ice-cold saline, blotted dry and weighed. Tissue samples were snap-frozen in liquid nitrogen. All samples were stored at −80°C until assayed. Samples for conventional morphology were fixed with 10% formalin and processed in paraffin with routine techniques.
Echocardiography
Transthoracic echocardiography was performed using a Toshiba Ultrasound System and a 15-MHz linear transducer under light isoflurane anesthesia on all surviving rats at the end of the experimental period. Using two-dimensional imaging (Gibson method), a short-axis view of the left ventricle at the level of the papillary muscles was obtained and the two dimensionally guided M-mode recording through the anterior and posterior walls (PWs) of the left ventricle was obtained. Left ventricle (LV) end-systolic (LVESD) and end-diastolic (LVEDD) dimensions as well as interventricular septum (IVS) and PW thickness were measured from the M-mode tracings. LV shortening fraction (LVFS) and ejection fraction (EF), measures of LV systolic function, were calculated from the M-mode LV dimensions using the following equations:
 |
LVIDD is the diameter of the short-axis left ventricle in end diastole and LVIDS is the diameter of the short-axis left ventricle in end systole.
Heart morphology and immunohistochemistry
Histology of the myocardial samples was evaluated without the knowledge of the treatments, with conventional light microscopy. The severity of observed lesions was graded with numerical values denoting to the degree of damage at the whole tissue level. The following system of severity grading was used: ‘(0, no abnormalities detected) 1, minimal; 2, mild; 3, moderate; 4, marked; or 5, severe' (Herbert et al., 2002). In the cardiac samples, severity grading was performed on the coronary arteries and the myocardium. The observed lesions in the coronary arteries ranged from minimally thickened media and slight increase of connective tissue around the arteries to severe hyperplasia of intimal/medial layer and fibrinoid necrosis of the arterial wall with adventitial scarring and perivascular inflammation. Lesions in the myocardium ranged from a focal increase of slender connective tissue bundles to severe necrosis of the myocardium with inflammatory infiltrate and presence of older myocardial scars.
Perivascular monocyte/macrophage infiltration was examined by immunohistochemistry using rat ED-1 (Serotec Ltd, Oslo, Norway) as primary antibody, and cardiac nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression using p47phox (BD Biosciences, Pharmingen, USA) as primary antibody.
Cardiomyocyte apoptosis
Cardiomyocyte apoptosis was assessed by the terminal deoxynucleotide transferase mediated ddUTP nick end labeling (TUNEL) assay as described previously (Kytö et al., 2004). In brief, nuclear DNA strand breaks were end-labeled with digoxigenin-conjugated dideoxy-UTP by terminal transferase and visualized immunohistochemically with digoxigenin antibody conjugated to alkaline phosphatase. The assay was standardized with the use of adjacent tissue sections treated with DNase I to induce DNA fragmentation as a positive control for apoptosis. The percentages of TUNEL-positive cardiomyocytes were calculated in transverse sections of tissue from the left ventricle, using light microscopy (× 200 magnification) with an ocular grid. An average of 190 fields each containing on average 160 myocyte nuclei were studied. Cardiomyocytes were identified by the presence of myofilaments surrounding the nucleus.
Biochemical determinations
Plasma BNP (BNP-45, Peninsula Laboratories, USA), plasma renin activity (PRA) (Angiotensin I RIA kit, Diasorin, Italy), and aldosterone (Coat-a-Count Aldosterone RIA kit, DPC, USA) were determined by RIA according to the manufacturer's instructions. Plasma samples were analyzed for levosimendan and OR-1896 by liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Kivikko et al., 2003). Urinary albumin was measured by ELISA using rat albumin as a standard (Celltrend, Luckenwalde, Germany). Urinary noradrenaline was analyzed using the isocratic ion-pair reversed-phase high-performance liquid chromatography method with electrochemical detection.
Myocardial gene expression analysis by quantitative real-time reverse transcriptase PCR assay (RT-PCR)
The gene expressions of atrial natriuretic peptide (ANP), BNP, sarco/endoplasmic reticulum Ca2+–ATPase 2a (SERCA2), Na+–Ca2+ exchanger (NCX), osteopontin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by quantitative real-time RT-PCR (LightCycler, Roche Diagnostics, Neuilly Sur Seine, France). The following primers were used: ANP forward CCGATAGATTCTGCCCTCTTGAA, reverse CCCGAAGCAGCTTGATCTTC; BNP forward AACTTCTAAAAAGACTCCTTAGGTCTCAA, reverse GCCATCTTGCAATTTCGAAGTC; SERCA2 forward TTCCGTTACCTGGCTATT, reverse CATCGGATACGGGGAC; NCX forward ATGCTTCGACTAAGTCTCCCA, reverse ACAAAATACACAGTTGCTCTAG; GAPDH forward GGATGCAGGGATGATGTTCT, reverse GAAGGGCTCATGACCACAGT; osteopontin forward CCAGCACACAAGCAGACGTT, reverse TCAGTCCATAAGCCAAGCTATCAC. The quantities of ANP, BNP, SERCA2, NCX, osteopontin and GAPDH PCR products were quantified with an external standard curve amplified from purified PCR product.
Myocardial SERCA2 and NCX expressions by Western blot
Western blot analysis was performed by standard procedures. Myocardial samples (15 μg protein per lane) were electrophoretically separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8% acrylamide). The proteins were transferred onto a polyvinylidene fluoride membrane (Immobilon, Millipore, Bedford, MA, USA) and the membranes were then blocked overnight, at +4°C, in 5% milk powder–TBS−0.01% Tween solution. The membrane was washed and probed for 1 h at room temperature with the primary antibody (rabbit anti-NCX, 1:5000 AD). After washing, the membrane was probed with peroxidase-conjugated secondary antibody (anti-rabbit 1:5000; Chemicon). Detection was accomplished with an enhanced chemiluminescence kit (Advanced ECL, Amersham Biotech, Buckinghamshire, UK) and the blots were exposed to X-ray film (Hyperfilm-ECL; Amersham Pharmacia Biotech). The membrane was stripped from antibodies (Stripping Buffer; Pierce) and after washing it was reprobed with a second antibody (rabbit anti-Serca2, 1:5000 Abcam), probing with secondary antibody and detection were carried out as described above. The films were scanned in a densitometer (Syngene, Cambridge, UK) and a semi-quantitative measurement of the relative intensity of each protein band was performed using the ‘GeneSnap'-software program (Syngene).
Statistical analysis
Data are presented as means±s.e.m. Statistically significant differences in mean values were tested by ANOVA and Tukey's test. The differences were considered significant when P<0.05. The Kaplan–Meier test was used for survival analysis. The data were analyzed using SPSS statistical software (SPSS Inc., USA).
Results
Survival rates in Dahl/Rapp salt-sensitive rats
Only 38% of Dahl/Rapp rats on high-salt diet (9/24) survived the 7-week follow-up period (Figure 1a), significantly lower than the survival rate in Dahl/Rapp rats on low-salt controls. Levosimendan treatment when started in the early stages of hypertension, improved survival in Dahl/Rapp rats at both doses. None of the rats in the low-dose levosimendan group died and only three out of 12 rats died in the high-dose levosimendan group, yielding survival rates greater than that in Dahl/Rapp rats on high-salt diet. Interestingly, late-stage levosimendan treatment (3 mg kg−1 from weeks 4 to 7) also improved survival in Dahl/Rapp rats (Figure 1b). Untreated Dahl/Rapp rats on high-salt diet gained weight less compared to levosimendan-treated Dahl/Rapp rats on high-salt diet and to Dahl/Rapp rats low-salt controls (data not shown).
Figure 1.
Survival curves of Dahl/Rapp rats on high salt diet treated with levosimendan at two different doses compared to those for untreated Dahl/Rapp rats on high-salt diet and Dahl/Rapp rats on low-salt diet. Levosimendan was given either during the whole 7-week-follow-up period (a) or only during the late stage of hypertension, from week 4 to week 7 (b). The concentration of levosimendan in drinking water (given ad libitum) was 1 mg l−1 (low dose) or 10 mg l−1 (high dose) corresponding to daily doses of 0.3–3 mg kg−1, respectively. The log rank test was used to compare the Kaplan–Meier survival curves to each other. * denotes P<0.05 compared to Dahl/Rapp rats on high-salt diet.
Blood pressure, cardiac hypertrophy and cardiac morphology
Dahl/Rapp rats on high-salt diet developed pronounced hypertension (Figure 2a and b) with cardiac hypertrophy (Figure 3a), coronary damage (Figure 3b) and to a lesser extent myocardial damage (Figure 3c). This group of animals showed intimal hyperplasia, fibrinoid necrosis of the arteries with marked medial thickening and adventitial scarring, evident myocardial infarcts with inflammation (Figure 3d), and perivascular monocyte/macrophage infiltration (Figure 3e). The expression of reactive oxygen species (ROS)-generating NADPH oxidase, assessed by p47phox immunohistochemistry, was found both in perivascular space and in cardiomyocytes (Figure 3f).
Figure 2.
Bar graphs showing the effects of levosimendan treatment on systolic blood pressure (a and b) and heart rate (c and d) in Dahl/Rapp rats on high-salt diet. In the figure, Dahl HS denotes untreated Dahl/Rapp rats on high-salt diet; Dahl HS+LS 0.3 denotes Dahl/Rapp rats treated with low-dose levosimendan; Dahl HS+LS 3 denotes Dahl/Rapp rats treated with high-dose levosimendan; Dahl LS denotes Dahl/Rapp controls on low-salt diet. High-dose levosimendan produced a transient decrease in blood pressure (week 3.5), whereas low-dose levosimendan did not influence blood pressure or heart rate. Means±s.e.m. are given, n=6–12 in each group. * denotes P<0.05 compared to Dahl/Rapp rats on high-salt diet; # denotes P<0.05 compared to Dahl/Rapp rats on low-salt diet.
Figure 3.
Bar graphs showing the effects of the 7-week levosimendan treatment on cardiac hypertrophy measured as heart weight-to-body weight ratio (a), coronary damage score (b) and myocardial damage score (c) in Dahl/Rapp rats on high-salt diet. Representative photomicrograph from the heart of Dahl/Rapp rat on high-salt diet is shown in (d), perivascular monocyte/macrophage infiltration in (e), and myocardial p47phox expression in (f). For abbreviations, see Figure 2. Means±s.e.m. are given, n=6–12 in each group. * denotes P<0.05 compared to Dahl SS rats on high-salt diet; # denotes P<0.05 compared to Dahl/Rapp rats on low-salt diet.
High-dose levosimendan produced a transient decrease in blood pressure, whereas low-dose levosimendan did not influence blood pressure in Dahl/Rapp rats (Figure 2a and b). Levosimendan did not influence heart rate (Figure 2c and d). Both levosimendan doses equally prevent the development of cardiac hypertrophy when measured as heart weight-to-body weight ratio (Figure 3a). However, levosimendan treatments did not significantly decrease coronary or myocardial damage scores (Figure 3b and c).
Echocardiography
Dahl/Rapp rats on high-salt diet showed deterioration of cardiac functions and left ventricular hypertrophy when assessed by echocardiography. Ejection fraction and fractional shortening were decreased in Dahl/Rapp rats on high-salt diet (Table 1). Levosimendan dose-dependently improved EF and fractional shortening. Left ventricular diameter in systole was markedly decreased by levosimendan.
Table 1.
Echocardiographic parameters in Dahl/Rapp salt-sensitive rats
| Variable | Dahl/Rapp on high-salt diet | Dahl/Rapp on high-salt diet+Levo 0.3 | Dahl/Rapp on high-salt diet+Levo 3 | Dahl/Rapp on low-salt diet | ANOVA P-value |
|---|---|---|---|---|---|
| IVS (d), mm | 2.54±0.04 | 2.20±0.06* | 2.26±0.10* | 1.92±0.08* | <0.001 |
| LVD (d), mm | 7.42±0.08 | 6.86±0.14* #¤ | 7.67±0.14 | 8.21±0.11* | <0.001 |
| LVPW (d), mm | 2.45±0.04 | 2.29±0.09# | 2.13±0.06* | 1.88±0.08* | <0.001 |
| EF, % | 83.0±0.6 | 92.7±0.7*# | 95.1±0.5*# | 87.2±0.8* | <0.001 |
| FS, % | 50.9±0.8 | 68.0±1.7*# | 73.2±1.2*# | 56.9±1.2 | <0.001 |
Abbreviations: EF, ejection fraction; IVS, interventricular septum; LVD, left ventricle diastolic; LVPW, left ventricle posterior wall.
Four experimental groups of animals were used: Dahl/Rapp rats on high-salt diet with and without levosimendan (Levo, 0.3 or 3 mg kg−1 via drinking fluid for 7 weeks) and Dahl/Rapp controls receiving low-salt diet. Data shown are means±s.e.m., n=6–12 in each group.
P<0.05 compared to Dahl/Rapp rats on high-salt diet
P<0.05 compared to Dahl/Rapp rats on low-salt diet
P<0.05 compared to Dahl/Rapp rats on high-salt diet+Levo 3.
Cardiomyocyte apoptosis
In Dahl/Rapp rats on high-salt diet, cardiomyocyte apoptosis detected by TUNEL technique was increased by sixfold as compared to low-salt controls (Figure 4). Levosimendan dose-dependently decreased the number of apoptotic cardiomyocytes in Dahl/Rapp rats on high-salt diet.
Figure 4.
Bar graphs showing the effects of the 7-week levosimendan treatment on cardiomyocyte apoptosis in Dahl/Rapp rats on high-salt diet. For abbreviations, see Figure 2. Means±s.e.m. are given, n=6–12 in each group. * denotes P<0.05 compared to Dahl/Rapp rats on high-salt diet; # denotes P<0.05 compared to Dahl/Rapp rats on low-salt diet.
Myocardial SERCA2 and NCX protein and mRNA expressions
In Dahl/Rapp rats on high-salt diet, myocardial SERCA2 expression was decreased by 40%, NCX expression unaltered and the SERCA2-to-NCX-ratio decreased as compared to Dahl/Rapp controls on low-salt diet (Figure 5a–c). Both levosimendan doses prevented the decrease in SERCA2 expression as well as in the SERCA2-to-NCX-ratio in the heart.
Figure 5.
Bar graphs showing the effects of the 7-week levosimendan treatment on myocardial SERCA2 protein (a) and mRNA (d) expression, NCX protein (b) and mRNA (e) expression and SERCA2-to-NCX-protein (c) and mRNA (f) ratios in Dahl/Rapp rats on high-salt diet. For abbreviations, see Figure 2. Means±s.e.m. are given, n=6–12 in each group. * denotes P<0.05 compared to Dahl/Rapp rats on high-salt diet; # denotes P<0.05 compared to Dahl/Rapp rats on low-salt diet.
A similar trend was also found in SERCA2 and NCX mRNA expressions in the heart. However, the difference between high- and low-salt diet groups did not quite reach statistical significance, except in SERCA2-to-NCX mRNA ratio (Figure 5d–f).
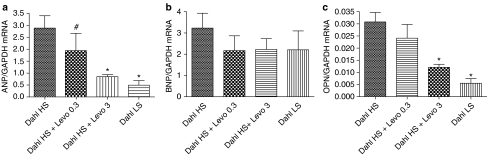
Myocardial ANP, BNP and osteopontin mRNA expressions
Myocardial ANP mRNA expression (Figure 6a) was increased by fivefold in Dahl/Rapp rats on high-salt diet and myocardial BNP mRNA expression nonsignificantly, by 50% (Figure 6b). High-dose levosimendan prevented the increase in ANP mRNA expression keeping it at the level found in the group of Dahl/Rapp rats maintained on low-salt diet.
Figure 6.
Bar graphs showing the effects of the 7-week levosimendan treatment on myocardial ANP (a), BNP (b) and osteopontin mRNA expressions (c) in Dahl/Rapp rats on high-salt diet. For abbreviations, see Figure 2. Means±s.e.m. are given, n=6–12 in each group. * denotes P<0.05 compared to Dahl/Rapp rats on high-salt diet.
High-dose levosimendan prevented the salt-induced increase in myocardial osteopontin mRNA expression in Dahl/Rapp rats (Figure 6c).
Biochemical and hormonal analyses
Dahl/Rapp rats on high-salt diet developed pronounced renal damage when assessed by 24-h urinary albumin excretion (Table 2). They also showed increased plasma BNP concentration as compared to Dahl/Rapp controls on low-salt diet. There was no difference in PRA or serum aldosterone level. Levosimendan did not influence albuminuria, but it decreased plasma BNP to levels found in Dahl/Rapp rats low-salt controls, and decreased urinary noradrenaline excretion (Table 2). PRA or serum aldosterone was not influenced by levosimendan.
Table 2.
Effects of levosimendan on some biochemical and hormonal markers of neurohumoral activation in Dahl/Rapp salt-sensitive rats
| Variable | Dahl/Rapp on high-salt diet | Dahl/Rapp on high-salt diet+Levo 0.3 | Dahl/Rapp on high-salt diet+Levo 3 | Dahl/Rapp on low-salt diet | ANOVA P-value |
|---|---|---|---|---|---|
| dU-Alb (mg days−1) | 100.5±14.8 | 106.8±31.2 | 117.0±12.3 | 52.4±16.3 | 0.26 |
| pl-BNP (pg ml−1) | 27.3±4.1 | 16.5±1.3* | 18.3±1.9* | 16.5±1.2* | 0.007 |
| PRA (ng) Ang I (ml−1 h−1) | 0.9±0.2 | 3.9±2.5 | 2.0±0.7 | 1.2±0.2 | 0.51 |
| s-Aldosterone (pg ml−1) | 116.0±15.4 | 75.0±18.3 | 118.8±46.6 | 117.3±10.4 | 0.64 |
| dU-NA (nmol days−1) | 1.92±0.22 | 1.49±0.26 | 1.08±0.28* | 1.73±0.15 | 0.04 |
Abbreviations: dU-Alb, 24-h urinary albumin excretion; pl-BNP, plasma brain natriuretic peptide concentration; PRA, plasma renin activity; s-aldosterone, serum aldosterone concentration; dU-NA, 24-h urinary noradrenaline excretion.
Four experimental groups of animals were used: Dahl/Rapp rats on high-salt diet with and without levosimendan (Levo, 0.3 or 3 mg kg−1 via drinking fluid for 7 weeks) and Dahl/Rapp controls receiving low-salt diet. Variables measured were as follows: dU-Alb, pl-BNP, PRA, s-aldosterone, dU-NA. Data shown are means±s.e.m., n=6–12 in each group.
P<0.05 compared to Dahl/Rapp rats on high-salt diet.
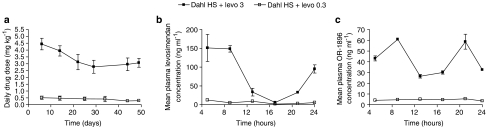
During the 7-week-experiment period the daily dosages of low- and high-dose levosimendan treatments averaged 0.36±0.01 and 3.6±0.1 mg kg−1, respectively (Figure 7a). Plasma levosimendan concentration exhibited typical diurnal variation with highest plasma concentrations during night when the rats are active (Figure 7b). The mean 24-h plasma concentrations of levosimendan and its active metabolite OR-1896 (Figure 7c) in Dahl/Rapp rats receiving the low-dose levosimendan treatment were 6.4±1.3 and 4.5±0.2 ng ml−1, and the corresponding drug concentrations in the high-dose levosimendan group were 79±19 and 42±15 ng ml−1, respectively. Plasma OR-1896 level did not correlate with BNP concentration (ANOVA P=0.334).
Figure 7.
Line graphs showing average daily levosimendan doses (a) calculated from water consumption during the 7-week experimental period. Diurnal profiles of plasma levosimendan and OR-1896 are shown in (b) and (c), respectively. Solid squares denote Dahl/Rapp rats treated with high-dose levosimendan, solid triangles rats treated with low-dose levosimendan. Means±s.e.m. are given. In (a), n=6–12 in each group, in (b and c), n=4 in each group. * denotes P<0.05 compared to Dahl/Rapp rats on high-salt diet.
Discussion
In the present study, the effects of oral levosimendan on cardiac remodeling and cardiomyocyte apoptosis were examined in Dahl/Rapp salt-sensitive rats with fulminant hypertension and severe target-organ damage. The important finding of our study was that levosimendan improved the survival in Dahl/Rapp rats on high-salt diet without major changes in systemic blood pressure or heart rate. Levosimendan improved systolic function, prevented cardiac remodeling and dose-dependently decreased the number of apoptotic cardiomyocytes.
In the present study, both low and high levosimendan doses improved survival in Dahl/Rapp rats without significantly influencing systolic blood pressure, heart rate, or albuminuria suggesting that the beneficial effect of levosimendan on salt-induced mortality was mediated, to a great extent, via mechanisms independent of systemic blood pressure or renal function. The finding that late levosimendan treatment, which was started when the rats already have developed manifest hypertension, also improved survival, supports this notion. In rats, levosimendan is absorbed rapidly and extensively (Orion Pharma, unpublished data). Whereas levosimendan has a relatively short elimination half-life in rats (0.76 h), the active metabolite OR-1896 is long-lasting (T½ 6.5 h) (Orion Pharma, unpublished data). At therapeutic concentrations the primary mechanism of action of levosimendan is calcium sensitization of the contractile protein troponin C (Haikala et al., 1995; Innes and Wagstaff, 2003). Consistently we noticed a significant dose-dependent improvement in cardiac function by oral levosimendan. The present study also confirmed the previous finding by Klotz et al. (2006) that development of heart failure in hypertensive Dahl rats is associated with a relatively well-preserved ejection fraction.
ATP-sensitive potassium channels (KATP) have been thought to be a mediator of cardioprotection in ischemic heart disease, and in particular, in ischemic–reperfusion injury (for reviews see Szewczyk and Marbán, 1999; Grover and Garlid, 2000; Ardehali and O'Rourke, 2005). It is believed that mitochondrial rather than sarcolemmal KATP channels mediate the physiological and molecular mechanisms of cardioprotection (Grover and Garlid, 2000). Although the signalling mechanisms that link mitochondrial KATP channel openers to cardioprotection are not completely characterized, Jaburek et al. (2006) provided compelling evidence that mitochondrial KATP channel and protein kinase C epsilon (PKCɛ) directly interact in the inner mitochondrial membrane, and that PKCɛ is required for the opening of mitochondrial KATP. Levosimendan activates myocardial KATP channels at therapeutic plasma concentrations (Yokoshiki et al., 1997a, 1997b; Kopustinskiene et al., 2001, 2004). Opening of KATP channels in ventricular cardiomyocytes has been shown to contribute to the inotropic action of levosimendan (Yokoshiki et al., 1997a). Levosimendan is a KATP channel opener of both plasma membranes and mitochondria. Hence, it is likely the vasodilatory and cardioprotective effects of oral levosimendan treatment found in the present study, are mediated partially via opening of the KATP channels.
Positive-inotropic agents such as phosphodiesterases (PDE) as well as β-adrenoceptor agonists are known to increase myocardial contractility through increased myocardial cyclic adenosine monophosphate (cAMP) levels and increased intracellular free calcium concentrations. Levosimendan displays structural similarities with a family of phosphodiesterase inhibitors and is also a very selective PDE III inhibitor (Szilagyi et al., 2004). In fact, high concentrations of levosimendan have been shown to inhibit PDE III and IV in human and guinea pig cardiomyocytes, and to stimulate L-type calcium current (Edes et al., 1995; Virag et al., 1996; Boknik et al., 1997; Ajiro et al., 2002; Szilagyi et al., 2004). Takahashi and Endoh (2005) have reported recently in experiments carried out in canine ventricular trabeculae that the muscarinic receptor agonist carbachol inhibited levosimendan-induced increase in calcium transients and abolished the positive-inotropic effect of the compound, suggesting that the increase in calcium transients induced by levosimendan is owing to its inhibition of PDE. On the contrary, Yokoshiki et al. (1997a) reported that levosimendan at concentrations varying from 0.2 to 10 μM did not affect calcium transients of rat cardiomyocytes. Such discrepancies might arise from the different species studied, which have different PDE isoenzymes. Taken together, it is possible that the positive inotropic action of levosimendan found in the present study, may have been partly mediated through phosphodiesterase inhibition.
Data from animal and cell culture experiments strongly support the notion that increased formation of ROS regulates apoptotic pathways in the heart (von Harsdorf et al., 1999; Giordano, 2005). Increased ROS formation plays an important role in the pathogenesis of target-organ damage in Dahl rats (Tojo et al., 2002). In good agreement with these findings we reported here that myocardial damage in Dahl rats is associated with increased perivascular inflammatory cell recruitment, expression of ROS-generating NADPH oxidase and induction of the gene expression of the redox-sensitive osteopontin. It is of particular interest, that opening of mitochondrial KATP channels inhibits ROS-induced apoptosis in myocytes (Akao et al., 2001). Kopustinskiene et al. (2004) have demonstrated that levosimendan activates potassium flux to the myocardial mitochondrial matrix at therapeutic plasma concentrations. Levosimendan also reduced the plasma levels of proinflammatory cytokines and soluble apoptosis mediators in patients with decompensated heart failure (Parissis et al., 2004). Furthermore, levosimendan, in vitro, protects cardiomyocytes from ROS-induced apoptosis via activation of KATP channels (Maytin and Colucci, 2005). It is of particular interest that PDE III inhibition with pharmacological agents, such as milrinone or cilostamide, promotes cardiomyocyte apoptosis (Ding et al., 2005). Taken together, our findings support the notion that the dose-dependent decrease in apoptotic cardiomyocytes by levosimendan found in the present study, was mediated by opening of the mitochondrial ATP-sensitive K+ channels and inhibition of mitochondrial apoptotic pathway, and not by PDE III inhibition.
There is compelling evidence to indicate that altered intracellular calcium handling plays a key role in the development of heart failure (Bers, 2002; Hasenfuss and Pieske, 2002; Yano et al., 2005). It has been shown that cellular acidosis, typical of myocardial ischemia, consistently decreases calcium sensitivity of the cardiomyocytes owing to a decrease in the affinity of troponin C for Ca2+ (Takahashi and Endoh, 2005). Takahashi and Endoh (2005) reported recently that acidosis suppressed the inotropic effect of levosimendan in vitro owing to an attenuation of the increase in Ca2+ transients, whereas the increase by levosimendan in Ca2+ sensitivity remained even during acidosis. Involvement of impaired calcium handling in the pathogenesis was also suggested by Seki et al. (2003), who reported markedly decreased protein expression of SERCA2 and SERCA2/NCX ratio in hypertrophied heart from Dahl salt-sensitive rats. In very good agreement with these findings we found in the present study that levosimendan improved cardiac function, decreased SERCA2 protein expression and decreased myocardial SERCA2/NCX ratio.
Levosimendan treatment normalized the salt-induced increases in plasma BNP concentration and myocardial ANP mRNA expression, and slightly decreased urinary noradrenaline excretion suggesting amelioration of neurohumoral activation in Dahl/Rapp rats on high-salt diet. As BNP concentration correlates with increased end-diastolic pressure and left ventricular wall tension, our findings suggest that levosimendan was able to decrease myocardial tension and the degree of cardiac overload in Dahl/Rapp salt-sensitive rats, possibly leading to improved microcirculation in the heart.
Levosimendan did not exhibit a clear dose dependency in all parameters examined. The limitation of the present experimental procedure, which is suitable for clarifying the effects of oral levosimendan treatment on cardiac remodeling and cardiomyocyte apoptosis, is that it is unable to dissect the influence of calcium sensitization, KATP channel opening and PDE III inhibition, and thus to provide the exact molecular mechanism of action related to the beneficial cardiovascular effects of oral levosimendan treatment found in the present study. Therefore, further studies comparing the cardiovascular effects of KATP channel openers, inotropic agents not acting through calcium sensitization and PDE III inhibitors, with levosimendan are warranted.
In conclusion, using Dahl/Rapp salt-sensitive rats as an animal model of fulminant hypertension and hypertension-induced heart disease, we here demonstrated that oral treatment with levosimendan improved survival and prevented hypertension-induced cardiac remodeling, cardiomyocyte apoptosis and neurohumoral activation. Our findings suggest a therapeutic role for oral levosimendan in the prevention of hypertension-induced heart failure.
Acknowledgments
This study was supported by grants from the Academy of Finland, University's Research Funds, and the Sigrid Jusélius Foundation. We are grateful to Ms Anneli von Behr and Ms Sari Laakkonen for expert technical assistance.
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- KATP
ATP-dependent K+ channel
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NCX
Na+–Ca2+ exchanger
- PKCɛ
protein kinase C epsilon
- PRA
plasma renin activity
- ROS
reactive oxygen species
- SERCA2a
sarco/endoplasmic reticulum Ca2+-ATPase 2a
- TUNEL
terminal deoxynucleotidyl transferase labeling
Conflict of interest
The authors state no conflict of interest.
References
- Ajiro Y, Hagiwara N, Katsube Y, Sperelakis N, Kasanuki H. Levosimendan increases L-type Ca2+ current via phosphodiesterase-3 inhibition in human cardiac myocytes. Eur J Pharmacol. 2002;435:27–33. doi: 10.1016/s0014-2999(01)01569-2. [DOI] [PubMed] [Google Scholar]
- Akao M, Ohler A, O'Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- Ardehali H, O'Rourke B. Mitochondrial KATP channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–204. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Boknik P, Neumann J, Kaspareit G, Schmitz W, Scholz H, Vahlensieck U, et al. Mechanisms of the contractile effects of levosimendan in the mammalian heart. J Pharm Exp Ther. 1997;280:277–283. [PubMed] [Google Scholar]
- del Monte F, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer. J Physiol. 2003;546:49–61. doi: 10.1113/jphysiol.2002.026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, et al. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis. Implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, et al. Effects of levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995;77:107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, et al. Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- Haikala H, Kaivola J, Nissinen E, Wall P, Levijoki J, Linden IB. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol. 1995;27:1859–1866. doi: 10.1016/0022-2828(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- Herbert RA, Hailey JR, Seely JC, Shackelford CC, Jokinen MP, Wolf JC, et al. Nomenclature Handbook of Toxicologic Pathology 2002Academic Press: San Diego; 157–167.In: Haschek WM, Rousseaux CG, Wallig MA (eds). [Google Scholar]
- Ikeda S, Hamada M, Qu P, Hiasa G, Hashida H, Shigematsu Y, et al. Relationship between cardiomyocyte cell death and cardiac function during hypertensive cardiac remodelling in Dahl rats. Clin Sci. 2002;102:329–335. [PubMed] [Google Scholar]
- Innes CA, Wagstaff AJ. Levosimendan. A review of its use in the management of acute decompensated heart failure. Drugs. 2003;63:2651–2671. doi: 10.2165/00003495-200363230-00009. [DOI] [PubMed] [Google Scholar]
- Jaburek M, Costa ADT, Burton JR, Costa CL, Garlid KD. Mitochondrial PKCɛ and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- Jessup M, Brozena S. Medical progress. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- Kaheinen P, Pollesello P, Levijoki J, Haikala H. Levosimendan increases diastolic coronary flow in isolated guinea-pig heart by opening ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 2001;37:367–374. doi: 10.1097/00005344-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Kang PM, Izumo S. Apoptosis and heart failure. A critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation. 2003;107:81–86. doi: 10.1161/01.cir.0000043245.00859.11. [DOI] [PubMed] [Google Scholar]
- Kihara Y, Sasayama S. Transition from compensatory hypertrophy to dilated failing left ventricle in Dahl-Iwai salt-sensitive rats. Am J Hypertens. 1997;10:78S–82S. [PubMed] [Google Scholar]
- Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- Kopustinskiene DM, Pollesello P, Saris NE. Levosimendan is a mitochondrial K(ATP) channel opener. Eur J Pharmacol. 2001;428:311–314. doi: 10.1016/s0014-2999(01)01350-4. [DOI] [PubMed] [Google Scholar]
- Kopustinskiene DM, Pollesello P, Saris NE. Potassium-specific effects of levosimendan on heart mitochondria. Biochem Pharmacol. 2004;68:807–812. doi: 10.1016/j.bcp.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Kytö V, Saraste A, Saukko P, Henn V, Pulkki K, Vuorinen T, et al. Apoptotic cardiomyocyte death in fatal myocarditis. Am J Cardiol. 2004;94:746–750. doi: 10.1016/j.amjcard.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Levijoki J, Pollesello P, Kaheinen P, Haikala H. Improved survival with simendan after experimental myocardial infarction in rats. Eur J Pharmacol. 2001;419:243–248. doi: 10.1016/s0014-2999(01)00997-9. [DOI] [PubMed] [Google Scholar]
- Maytin M, Colucci WS. Cardioprotection: a new paradigm in the acute management of acute heart failure syndromes. Am J Cardiol. 2005;96:26G–31G. doi: 10.1016/j.amjcard.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Kihara Y, Begin KJ, Gorga JA, Palmiter KA, LeWinter MM, et al. Altered myocardial thin-filament function in the failing Dahl salt-sensitive rat heart. Circulation. 2003;107:630–635. doi: 10.1161/01.cir.0000046267.16901.e9. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Adamopoulos S, Antoniades C, Kostakis G, Rigas A, Kyrzopoulos S, et al. Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol. 2004;93:1309–1312. doi: 10.1016/j.amjcard.2004.01.073. [DOI] [PubMed] [Google Scholar]
- Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7:340–349. [PubMed] [Google Scholar]
- Seki S, Nagai M, Takeda H, Onodera T, Okazaki F, Taniguchi M, et al. Impaired Ca2+ handling in perfused hypertrophic hearts from Dahl salt-sensitive rats. Hypertens Res. 2003;26:643–653. doi: 10.1291/hypres.26.643. [DOI] [PubMed] [Google Scholar]
- Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study Investigators. Circulation. 2000;102:2222–2227. doi: 10.1161/01.cir.102.18.2222. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Marbán E. Mitochondria: a new target for K+ channel openers. Trends Pharmacol Sci. 1999;20:157–161. doi: 10.1016/s0165-6147(99)01301-2. [DOI] [PubMed] [Google Scholar]
- Szilagyi S, Pollesello P, Levijoki J, Kaheinen P, Haikala H, Edes I, et al. The effects of levosimendan and OR-1896 on isolated hearts, myocyte-sized preparations and phosphodiesterase enzymes of the guinea pig. Eur J Pharmacol. 2004;486:67–74. doi: 10.1016/j.ejphar.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Endoh M. Dual regulation of myofilament Ca2+ sensitivity by levosimendan in normal and acidotic conditions in aequorin-loaded canine ventricular myocardium. Br J Pharmacol. 2005;145:1143–1152. doi: 10.1038/sj.bjp.0706292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo A, Onozato MT, Kobayashi N, Goto A, Matsuoka H, Fujita T. Angiotensin II and oxidative stress in Dahl salt-sensitive rat with heart failure. Hypertension. 2002;40:834–839. doi: 10.1161/01.hyp.0000039506.43589.d5. [DOI] [PubMed] [Google Scholar]
- Virag L, Hala O, Marton A, Varro A, Papp JG. Cardiac electrophysiological effects of levosimendan, an new calcium sensitizer. Gen Pharmacol. 1996;27:551–556. doi: 10.1016/0306-3623(95)02060-8. [DOI] [PubMed] [Google Scholar]
- von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- Walder RY, Morgan DA, Haynes WG, Sigmund RD, McClain AM, Stokes JB, et al. Genetic characterization of the ‘New' Harlan Sprague Dawley Dahl salt-sensitive rats. Hypertension. 1996;27:546–551. doi: 10.1161/01.hyp.27.3.546. [DOI] [PubMed] [Google Scholar]
- Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest. 2005;115:556–564. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer levosimendan activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther. 1997a;283:375–383. [PubMed] [Google Scholar]
- Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. Levosimendan, a novel Ca2+ sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocytes. Eur J Pharmacol. 1997b;333:249–259. doi: 10.1016/s0014-2999(97)01108-4. [DOI] [PubMed] [Google Scholar]
- Yoneda T, Kihara Y, Ohkusa T, Iwanaga Y, Inagaki K, Takeuchi Y, et al. Calcium handling and sarcoplasmic-reticular protein functions during heart-failure transition in ventricular myocardium from rats with hypertension. Life Sci. 2001;70:143–157. doi: 10.1016/s0024-3205(01)01383-2. [DOI] [PubMed] [Google Scholar]