Abstract
Background and purpose:
The role of nitric oxide (NO) in cardiac pathophysiology remains controversial. According to data from several studies using rat and rabbit isolated hearts, NO is an endogenous cardioprotectant against reperfusion-induced ventricular fibrillation (VF). Thus, if cardiac NO production is abolished by perfusion with L-NG-nitro-L-arginine methylester (L-NAME) (100 μM) there is a concomittant increase in the incidence of reperfusion-induced VF, with L-NAME's effects on NO and VF prevented by L- (but not D-) arginine co-perfusion. To make a better estimate of the clinical relevance of these findings, 100 μM L-NAME was tested in primate hearts under similar conditions.
Experimental approach:
Marmoset (Callithrix jaccus) hearts, isolated and perfused, were subjected to 60 min left regional ischaemia followed by 10 min reperfusion in vitro. The ECG was recorded and NO in coronary effluent measured by chemiluminescence.
Key results:
L-NAME (100 μ M) decreased NO in coronary effluent throughout ischaemia and reperfusion (e.g. from 3720±777 pmol min−1 g−1 in controls to 699±98 pmol min−1 g−1 after 5 min of ischaemia) and, during ischaemia, lowered coronary flow and reduced heart rate, actions identical to those seen in rat and rabbit hearts. However, the incidence of reperfusion-induced VF was unchanged (20%, with or without L-NAME).
Conclusions and implications:
A species difference exists in the effectiveness of endogenous NO to protect hearts against reperfusion-induced VF. The present primate data, which presumably take precedence over rat and rabbit data, cast doubt on the clinical relevance of NO as an endogenous, antiarrhythmic, cardioprotectant.
Keywords: antiarrhythmic agents, endogenous cardioprotection, ischaemia, nitric oxide, reperfusion, ventricular arrhythmias
Introduction
The role played by endogenous nitric oxide (NO) during myocardial ischaemia, infarction and heart failure remains controversial, and it remains questionable whether NO should be augmented, mimicked or ablated as a therapeutic approach. Bolli has presented a strong argument that NO is a ubiquitous endogenous cardioprotectant, especially when oxidant stress is present (see Jones and Bolli, 2006). Likewise, Downey, Ferdinandy and others have described endogenous NO as a mediator of preconditioning (Ferdinandy and Schulz, 2003; Cohen et al., 2006). On the other hand, cardiac NO synthase (NOS) isoform 3 (NOS3) may contribute to cardiac dysfunction, especially when oxidant stress is present (Takimoto et al., 2005), and Zweier has suggested that preconditioning is mediated by its ability to reduce NO (and peroxynitrite) generation (Zweier and Talukder, 2006). These data are not consistent with the notion that NO is a ubiquitous endogenous cardioprotectant.
The present study concerns the role of NO as an endogenous antiarrhythmic cardioprotectant during reperfusion. L-NG-nitro-L-arginine methylester (L-NAME) (100 μM) is a specific NOS probe which blocks endothelium-dependent NO release in isolated hearts under basal conditions (Pabla and Curtis, 1995, 1996a) and after agonist stimulation (Ellwood and Curtis, 1996). In previous studies, using rat and rabbit hearts, L-NAME (100 μM) caused up to a seven-fold increase in the incidence of reperfusion-induced ventricular fibrillation (VF) (Curtis and Pabla, 1997). This effect was surmounted by co-perfusion with L- (but not D-) arginine (Pabla and Curtis, 1995, 1996a) and all rhythm effects were related in an all-or-none fashion to the level of NO detected contiguously in the coronary effluent by chemiluminescence, indicating a tonic cardioprotective effect of endogenous NO (Pabla and Curtis, 1995, 1996a). The pro-arrhythmic effect of L-NAME was not the result of alteration of cardiac necrosis (Pabla and Curtis, 1996b), but it was mimicked by methylene blue in a zaprinast-surmountable manner with effects dependent on cardiac cGMP content (Pabla et al., 1995). The cardioprotective effect of endogenous NO was most prominent in hearts reperfused after a duration of 60 min ischaemia (Pabla and Curtis, 1995, 1996a), which is equivalent to the earliest times that elective reperfusion is successfully achieved in humans (Brener et al., 2005). Therefore, these findings had potential clinical relevance.
However, not all studies support a role for NO as an antiarrhythmic cardioprotectant (other studies include Naseem et al., 1995; Sun and Wainwright, 1997 and Swissa et al., 2002; Looi et al., 2006). Even when restricting consideration to VF induced by reperfusion in studies of a similar type, there are apparent species differences, such as the tissue source of protective NO (Pabla and Curtis, 1996a), which limit the confidence one may have in the clinical relevance of the data.
In view of this, we have examined whether the key effects of 100 μM L-NAME we found previously in rat and rabbit hearts could be replicated in primate hearts.
Methods
All experiments were performed according to the United Kingdom Home Office ‘Guide on the operation of the Animals (Scientific Procedures) Act 1986'.
Technique for perfusion
Anaesthesia, cardiac excision and perfusion of hearts from male Callithrix jaccus marmosets (Fisons, UK: 300-350 g) followed a procedure identical to that used previously (Rees and Curtis, 1993). Animals were anaesthetized with pentobarbitone (60 mg kg−1, intraperitoneal). Once reflex responses to paw pinch or corneal touching had ceased, the heart was arrested and exsanguinated in vivo by thoracotomy and insertion of a 23G needle into the left ventricle for infusion of ice cold modified Krebs solution (constituents below). This procedure was used because these animals were used jointly by others in the Institute in separate studies which required in vivo exsanguination of the brain. The interval between the opening of the thorax and cold cardiac arrest was less than 1 min.
Hearts were excised, immersed in ice-cold perfusion solution, then Langendorff-perfused with solution delivered at 37°C and pH 7.4. The initial perfusion solution contained (in mM): 118.5 NaCl, 25.0 NaHCO3, 1.2 MgSO4, 1.2 NaH2PO4, 1.4 CaCl2, 4.0 KCl and 11.1 glucose. The same constituents were used in the control solution during the main experiment. All solutions were filtered before use (5 μm pore filters) to remove particulate matter. Solutions were continuously gassed with carbogen (95% O2–5% CO2).
Perfusion pressure was constant and equivalent to 100 cm H2O. Changes in coronary flow were measured by timed collection of coronary effluent. A unipolar electrogram (ECG) was recorded (Curtis and Hearse, 1989a). A traction-type coronary occluder was used for coronary occlusion and reperfusion (Heimburger, 1946; Kannengeisser et al., 1975; Curtis and Hearse, 1989a).
Verification of coronary occlusion and reperfusion
Two independent methods based on changes in coronary flow and the use of a dye were employed to verify occlusion and reperfusion and to delineate the involved (occluded/reperfused) zone from uninvolved tissue, as described previously (Curtis and Hearse, 1989a; Rees and Curtis, 1993). Values of coronary flow in the uninvolved zone and the reperfused zone (the latter expressed as recovery of coronary flow, as shown in Table 1) were calculated from the total coronary flow and the weight of the involved zone and the uninvolved zone, as described previously (Curtis and Hearse, 1989a). Similar to that of other non-human primates, including Macaca mulatto and Papio anubis (Lavallee and Vatner, 1984; Shen et al., 1996), the marmoset has a heart that is coronary collateral deficient, permitting regional flow calculation as described (Rees and Curtis, 1993).
Table 1.
Effect of L-NAME on heart rate, coronary flow and recovery of flow in the marmoset isolated heart
| Group |
Heart rate (beats min−1) |
Coronary flow (ml min−1 g−1) I–5 min |
Recovery of flow (ml min−1 g−1) |
|||
|---|---|---|---|---|---|---|
| l–5 min | l–1 min | l–5 min | l–1 min | R+l min | R+5 min | |
| Control | 207±67 | 231±17 | 9±1 | 11±2 | 10±2 | 8±1 |
| L-NAME | 192±11 | 195±12* | 7±1 | 6±1* | 9±1 | 7±1 |
Abbreviation: L-NAME, L-NG-nitro-L-arginine methylester.
Time refers to min before (–) onset of ischaemia (l) and (+) refers to min after the onset of reperfusion (R). Values are mean±s.e.m. n=10/group.
P<0.05 vs controls. Values before introduction of L-NAME were not significantly different between groups (data not shown).
Diagnosis and quantification of arrhythmias
All ECG analysis and arrhythmia detection, diagnosis and quantification methods have been described previously (Walker et al., 1988; Tsuchihashi and Curtis, 1991).
Experimental protocol
Hearts (n=10 per group) were perfused with control solution and, after 10 min, this was switched in a blinded randomized fashion to an identical solution (control) or a similar solution containing 100 μM L-NAME. After a further 10 min perfusion, the left main coronary artery was occluded close to its origin to induce regional ischaemia. After 60 min of ischaemia the occluder was released to allow reperfusion for 10 min, sufficient to allow detection of reperfusion-induced arrhythmias.
Measurement of coronary effluent NO content
The NO content of coronary effluent was measured by chemiluminescence using a Sievers Nitric Oxide Analyser (NOA) model 270B (Dyson Instruments, Tyne and Wear, UK) and expressed as pmol min−1 g−1 wet weight of perfused tissue exactly as described in previous studies (Pabla and Curtis, 1995, 1996a; Ellwood and Curtis 1996).
Basis for choice of drug concentration and duration of ischaemia
Owing to limited availability of marmoset hearts, the protocol was chosen carefully so as to maximize scope for detection of NO-mediated actions with minimal animal use. We chose to use 100 μM L-NAME as the tool for studies because of the extensive available database concerning its effects and mechanism of action derived from rat and rabbit studies (see Introduction). The 60 min duration of ischaemia was chosen because it was found to be the most strongly associated with statistically detectable, L-NAME-susceptible, baseline NO-mediated, endogenous protection against reperfusion-induced VF in rat and rabbit hearts (Pabla and Curtis, 1995, 1996a). With shorter durations of ischaemia the baseline incidence of reperfusion-induced VF is too high to allow scope for detection of endogenous cardioprotection which, if present, is overwhelmed by the influence of a prevailing arrhythmogenic milieu (Rees and Curtis, 1993), whereas, with longer durations of ischaemia, the myocardium of any species becomes irreversibly injured and reperfusion is associated with ultrastructural injury incompatible with the involved zone being capable of functioning as a substrate for the initiation of VF (i.e., it becomes electrically quiescent) (Ravingerova et al., 1995).
Exclusion criteria
Hearts were excluded and immediately replaced if arrhythmias were present before ischaemia, if sinus rate was less than 200 beats min−1 or if coronary flow was more than 18 ml min−1 or less than 8 ml min−1 10 min before the switch to test solution, if sinus rhythm was not present throughout the 2 s before reperfusion, or if involved zone size was less than 30% or greater than 55% of ventricular weight (Rees and Curtis, 1993).
Statistical analysis
In concordance with the Lambeth Conventions (Walker et al., 1988), Gaussian-distributed variables were expressed as mean±s.e.m. and compared by unpaired t-test, whereas Mainland's contingency tables (Mainland et al., 1956) were used for non-parametric analyses of binomially-distributed variables (such as VF incidence). A P value <0.05 was taken as threshold for significance.
Drugs, chemicals and their sources
Drugs and chemicals were obtained from the following sources: calcium chloride, D(+)-glucose, magnesium sulphate, patent blue V sodium salt, potassium chloride, sodium chloride, sodium dihydrogen orthophosphate, sodium hydrogen carbonate (BDH Chemicals Ltd., Dagenham, Essex, UK); pentobarbitone sodium, heparin sodium (Veterinary Drug Company plc, Colnbrook, UK) and water for preparing drug and perfusion solutions was supplied using a reverse-osmosis system (Milli-RO 10 and Milli-Q, Millipore Ltd, Watford, UK).
Results
A total of 24 hearts were used in this study of which four were excluded owing to arrhythmias before the start of ischaemia.
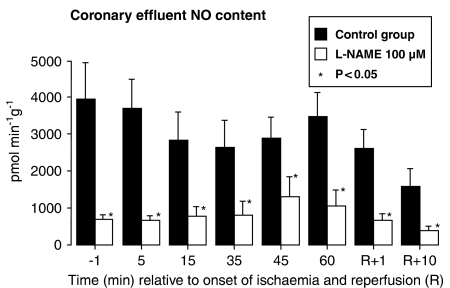
L-NAME slowed heart rate and reduced coronary flow (Table 1), but had no effect on QT interval (e.g., 116±4 msec in controls and 115±15 msec in L-NAME perfused hearts 1 min before reperfusion). L-NAME had a large inhibitory effect on cardiac NO production with the NO content of coronary effluent significantly reduced at all times in the experiment (Figure 1). Coronary flow was reduced by L-NAME during ischaemia but the recovery of coronary flow during reperfusion was not significantly impaired by L-NAME (Table 1). Involved zone sizes were identical between the two groups; 46±2% in control hearts and 46±2% in L-NAME perfused hearts.
Figure 1.
NO levels in coronary effluent from control and L-NAME perfused marmoset isolated hearts during ischaemia and reperfusion. Values are mean±s.e.m. recorded before ischaemia (−1 min), during ischaemia (5–60 min) and during reperfusion (R+1, R+10 min). All values in the L-NAME group are lower than time-matched values in controls (P<0.05). Values before switching solution to perfusion with L-NAME (not shown) were not significantly different between groups.
Despite these findings, L-NAME did not increase reperfusion-induced VF incidence (20%, versus 20% in control). Less severe arrhythmias were likewise unaffected by L-NAME; reperfusion-induced ventricular tachycardia (VT) incidence was 40% in each group, and ventricular premature beat (VPB) incidence was 90% in the control group and 80% with L-NAME. Therefore, it is clearly evident that L-NAME neither exacerbated nor inhibited reperfusion-induced arrhythmias in the marmoset heart.
Ischaemia-induced arrhythmias did not differ between the control and L-NAME group, with incidences of VPBs being 70 and 90%, VT 10 and 20% and VF 0 and 0%, respectively.
Discussion
Endogenous NO does not protect against reperfusion-induced VF in primate hearts
L-NAME produced effects on heart rate and coronary flow during ischaemia that were identical to those shown to be NO-dependent in rat and rabbit hearts (Pabla and Curtis, 1995, 1996a). L-NAME also strongly suppressed cardiac NO production to an extent similar to that seen in rat and rabbit hearts (Pabla and Curtis, 1995, 1996a). However, L-NAME did not exacerbate reperfusion-induced VF, in contrast to its effect in rat and rabbit hearts (Pabla and Curtis, 1995, 1996a). This identifies a potentially important species difference.
The incidence of VF in controls in the present study (20%) was similar to that previously reported in rat and rabbit hearts reperfused after the same duration of regional ischaemia (Pabla and Curtis, 1995, 1996a), indicating that there is scope for VF susceptibility to be increased in the marmoset heart. Likewise, there was a substantial generation of NO in controls, which was similar to that seen previously in control rat and rabbit hearts (Pabla and Curtis, 1995, 1996a). This means that L-NAME did not fail to increase reperfusion-induced VF susceptibility in the marmoset because basal NO production was already low, or because of a failure to inhibit NO production. Instead, it means that reperfusion-induced VF susceptibility in the marmoset heart differs in its sensitivity to altered cardiac NO production compared with reperfusion-induced VF in the rat and rabbit heart.
Comparison with other arrhythmia study outcomes
There are surprisingly few studies focused specifically on NO's actions on reperfusion-induced VF and fewer still that measured NO generation contiguously with VF. Using the rat heart in vitro, the NOS inhibitor, NG-nitro-L-arginine (LNNA), was reported to reduce the duration of VF, an apparent antiarrhythmic effect (Naseem et al., 1995), which differs from our findings in the same model with L-NAME (Pabla and Curtis, 1995). Only the latter study measured VF and NO contiguously. The basis for this difference has not been resolved (Curtis and Pabla, 1997). There are additionally some in vivo rat studies on ischaemia-induced VF susceptibility; in a direct comparison with control rats, L-NAME and LNNA had no effect on VF (Sun and Wainwright, 1997; Looi et al., 2006) suggesting that endogenous NO does not influence baseline ischaemia-induced VF susceptibility (being neither protective nor provocative). These data accord with in vitro data from a range of species that have failed to show any relation between ischaemia-induced VF and coronary effluent NO (e.g., Pabla and Curtis, 1995). However, VF mechanisms differ according to setting (Clements-Jewery et al., 2005) so the role of NO may also vary according to setting.
When considering susceptibility to arrhythmias induced by a range of different triggers, between-lab differences in outcome cannot be attributed to fundamental differences between the in vivo and in vitro settings (such as differences between crystalloid perfusate and blood oxygen content) because apparent protection and provocation of different types of VF by NO have been reported in each setting (Naseem et al., 1995; Pabla and Curtis, 1995, 1996a; Sun and Wainwright 1997; Curtis and Pabla, 1997; Swissa et al., 2002; Looi et al., 2006). Indeed, the most extreme differences in outcome occurred between two in vitro studies that used the same species and the same VF trigger (reperfusion; Naseem et al., 1995; Pabla and Curtis, 1995). The most recent large animal studies on NO and ventricular arrhythmias have tested opposing hypotheses and reached opposing conclusions with nitroglycerin reducing ischaemia-induced VF in the pig (Kumar et al., 2003), whereas sildenafil and NO donors facilitated electrically induced VF in the same species (Swissa et al., 2002).
Overall, the outcomes of studies on the relationship between reperfusion-induced VF and NO have been variable, although studies in which cardiac NO has been measured suggest it is protective (Curtis and Pabla, 1997), albeit with the present study being the exception.
Implications
The simplest explanation for the present outcome is that NO does not function as an endogenous cardioprotectant against reperfusion-induced arrhythmias in the marmoset heart, meaning that the marmoset heart differs from the rat and rabbit heart in this regard. There are other examples of species dependence relating to NO biology in ischaemia and reperfusion. These include (i) the tissue source of cardioprotective NO, which is neuronal in the rat but not in the rabbit (Pabla and Curtis, 1996a) and (ii) the ability of reperfusion to evoke NO-independent hyperaemia, which is insubstantial in the rat heart (Pabla and Curtis, 1995) but of sufficient strength to surmount L-NAME-induced impairment of recovery of coronary flow in the rabbit heart (Pabla and Curtis, 1996a), with the marmoset heart (according to the present study) resembling the rabbit. This species variation has potential consequences for drug discovery beyond the narrow issue of endogenous protection against reperfusion-induced arrhythmias.
In the 13 years since NO was first proposed as an endogenous cardioprotectant (Parratt, 1993) there have been no drugs introduced to the clinic that seek to limit susceptibility to reperfusion-induced VF by augmenting the levels of endogenous NO or by exogenously supplementing NO levels (or the converse). Indeed, with regard to the general concept that NO is beneficial in the cardiovascular system (Jones and Bolli, 2006) there is evidence that oxidant stress, as occurs during cardiac ischaemia and reperfusion, uncouples the homodimeric, isoform of NOS which is predominant in cardiac tissue (NOS3) to generate a monomer that, instead of generating NO, now generates primarily the cardiotoxic superoxide radical (Kuzkaya et al., 2003). This adds to the evidence that NO itself may have deleterious properties in the heart including negative inotropy (Champion et al., 2004) and, during reperfusion, cytotoxic actions, owing to its ability to combine with superoxide to generate peroxynitrite (Xia et al., 1996; Zweier and Talukder, 2006). If this is correct, it would seem to be incompatible with the other prevailing notion that NO mediates endogenous cardioprotection against infarct expansion, myocardial remodelling and heart failure (Jones and Bolli, 2006). In this wider context of cardioprotection, again, there have been no new drugs introduced clinically in the last 13 years that selectively mimic, augment or block NO.
There are several possible explanations for the lack of progress in NO-related drug development, full discussion of which is beyond the scope of this article (see Curtis and Pabla, 1997). However, one issue that is germane to the present study is the possibility that the animal species that have been used for establishing the existence of endogenous cardioprotection by NO may have generated data that is not accurately predictive of clinical outcome. Certainly, if the tissue source of endogenous protective NO differs between rabbit and rat as suggested (Curtis and Pabla, 1997) then it is not unreasonable to postulate that the role of NO may also be different between species.
To test the true role of NO or any other putative biological mediator requires clinical data. Short of this, a better picture can be sought by extending the range of species used in preclinical studies and, in particular, extending the data base to include the outcomes of studies in non-human primates.
In view of these considerations we propose that endogenous NO, which appears to play no role as an endogenous cardioprotectant against reperfusion-induced VF in the marmoset, probably plays little or no role as an endogenous antiarrhythmic cardioprotectant in the human, despite an apparent protective role in non-primate species. This appears to vitiate ‘proof of concept'.
Finally, if species is a critical determinant of the role of NO in cardiovascular health and disease, and if non-primate species are less fit for purpose than primates (as the present data imply), one may question the wisdom of the increasing reliance on the mouse to establish proofs of concept, for example, concerning the role of NO in cardiac contractile function (Champion et al., 2004) and hypertrophic remodelling (Takimoto et al., 2005).
Limitations
The trend in rat studies was for NO to play an increasing cardioprotective role with increasing durations of preceding ischaemia (Pabla and Curtis, 1995, 1996a). To strengthen the conclusion from the present studies (that NO is unlikely to play a substantial role as an anti-arrhythmic cardioprotectant in humans) will require extensive further studies using primates such as the marmoset. For example, these would need to include time-response studies to show that NO does not become increasingly relevant if reperfusion takes place at times later than 60 min after the onset of ischaemia. If it transpires that L-NAME does exacerbate reperfusion-induced VF in the marmoset when ischaemia is significantly extended, then further work will need to be performed to link the effect to endogenous NO, e.g., by comparing L- and D-arginine for their abilities to surmount the proarrhythmic effect of L-NAME, etc, as in rat and rabbit studies (Pabla and Curtis, 1995, 1996a). It would then be of interest to explore whether L-NAME fails to unmask VF in the marmoset when reperfusion takes place 60 min after the onset of ischaemia owing to the presence, for example, of a second endogenous cardioprotectant that can be shown to be more abundant in the marmoset versus rat or rabbit hearts. Although these issues are interesting, the feasibility of conducting all the necessary studies is limited by cost and ethical considerations.
Conclusion
The present study questions the role of NO as an endogenous cardioprotectant and illustrates the importance of not extrapolating NO biology findings directly from any one species (especially non-primate species) to man.
Acknowledgments
The study was funded by The Wellcome Trust. We thank Catherine Stables, Ellen Rossouw and Metin Avkiran (all KCL London) for their helpful comments when preparing and revising the paper.
Abbreviations
- CF
coronary flow
- ECG
electrogram
- i.p.
intraperitoneal
- i.u.
international units
- L-NAME
L-NG-nitro-L-arginine methylester
- LNNA
NG-nitro-L-arginine
- NOA
NO analyzer
- NOS
nitric oxide synthase
- NOS3
NOS isoform 3
- R-1
1 min before the start of reperfusion
- R+1
reperfusion plus 1 min
- VPB
ventricular premature beat
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Conflict of interest
These authors state no conflict of interest.
References
- Brener SJ, Lincoff AM, Bates ER, Jia G, Armstrong PW, Guetta V, et al. The relationship between baseline risk and mortality in ST-elevation acute myocardial infarction treated with pharmacological reperfusion: insights from the global utilization of strategies to open occluded arteries (GUSTO) V trial. Am Heart J. 2005;150:89–93. doi: 10.1016/j.ahj.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang W, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res. 2004;94:657–663. doi: 10.1161/01.RES.0000119323.79644.20. [DOI] [PubMed] [Google Scholar]
- Clements-Jewery H., Hearse DJ, Curtis MJ. Phase 2 ventricular arrhythmias in acute myocardial infarction: a neglected target for therapeutic drug development and safety pharmacology evaluation. Br J Pharmacol. 2005;145:551–564. doi: 10.1038/sj.bjp.0706231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MV, Yang X-M, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res. 2006;70:231–239. doi: 10.1016/j.cardiores.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hearse DJ. Reperfusion-induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J Mol Cell Cardiol. 1989a;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Pabla R. Nitric oxide supplementation or synthesis block - which is the better approach to treatment of heart disease. Trends Pharmacol Sci. 1997;18:239–244. doi: 10.1016/s0165-6147(97)01080-8. [DOI] [PubMed] [Google Scholar]
- Ellwood AJ, Curtis MJ. Mechanism of 5-Hydroxytryptamine-induced coronary vasodilatation assessed by direct detection of nitric oxide production in guinea pig isolated heart. Br J Pharmacol. 1996;119:721–729. doi: 10.1111/j.1476-5381.1996.tb15732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburger RF. Injection into pericardial sac and ligation of coronary artery of rat. Arch Surg. 1946;52:677–689. doi: 10.1001/archsurg.1946.01230050686004. [DOI] [PubMed] [Google Scholar]
- Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kannengeisser GJ, Lubbe WF, Opie LH. Experimental myocardial infarction with left ventricular failure in the isolated perfused rat heart: Effects of isoproterenol and pacing. J Mol Cell Cardiol. 1975;7:135–151. doi: 10.1016/0022-2828(75)90015-2. [DOI] [PubMed] [Google Scholar]
- Kumar K, Nguyen K, Waxman S, Nearing BD, Wellenius GA, Zhao SX, et al. Potent antifibrillatory effects of intrapericardial nitroglycerin in the ischemic porcine heart. J Am Coll Cardiol. 2003;41:1831–1837. doi: 10.1016/s0735-1097(03)00340-1. [DOI] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acide, and thiols: implications for uncoupling endothelial nitric oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- Lavallee M, Vatner SF. Regional myocardial blood flow and necrosis in primates following coronary occlusion. Am J Physiol. 1984;246:H635–H639. doi: 10.1152/ajpheart.1984.246.4.H635. [DOI] [PubMed] [Google Scholar]
- Looi YH, Kane KA, McPhaden AR, Wainwright CL. Adrenomedullin acts via nitric oxide and peroxynitrite to protect against myocardial ischaemia-induced arrhythmias in anaesthetized rats. Br J Pharmacol. 2006;148:599–609. doi: 10.1038/sj.bjp.0706771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland D, Harrera L, Sutcliffe MI. Statistical Tables for Use With Binomial Samples: Contingency Tests, Confidence Limits and Sample Size Estimates. New York University College of Medicine Publications: New York; 1956. [Google Scholar]
- Naseem SA, Koutos MC, Rao PS, Jesse RL, Hess ML, Kukreja RC. Sustained inhibition of nitric oxide by NG-nitro-L-arginine improves myocardial function following ischemia/reperfusion in isolated perfused rat heart. J Mol Cell Cardiol. 1995;27:419–426. doi: 10.1016/s0022-2828(08)80038-7. [DOI] [PubMed] [Google Scholar]
- Pabla R, Bland-Ward P, Moore PK, Curtis MJ. An endogenous protectant effect of cardiac cGMP against reperfusion-induced ventricular fibrillation in the rat heart. Br J Pharmacology. 1995;116:2923–2930. doi: 10.1111/j.1476-5381.1995.tb15946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Effects of nitric oxide modulation on cardiac arrhythmias in the rat isolated heart. Circulation Res. 1995;77:984–992. doi: 10.1161/01.res.77.5.984. [DOI] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Endogenous protection against reperfusion-induced ventricular fibrillation: role of neuronal versus non-neuronal sources of nitric oxide and species-dependence in the rat versus rabbit isolated heart. J Molec Cell Cardiol. 1996a;28:2097–2110. doi: 10.1006/jmcc.1996.0202. [DOI] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Effect of endogenous nitric oxide on cardiac systolic and diastolic function during ischemia and reperfusion in the rat isolated heart. J Mol Cell Cardiol. 1996b;28:2011–2021. doi: 10.1006/jmcc.1996.0203. [DOI] [PubMed] [Google Scholar]
- Parratt JR. Endogenous myocardial protective (antiarrhythmic) substances. Cardiovasc Res. 1993;27:693–702. doi: 10.1093/cvr/27.5.693. [DOI] [PubMed] [Google Scholar]
- Ravingerova T, Tribulova N, Slezak J, Curtis MJ. Brief, intermediate and prolonged ischemia in the isolated crystalloid perfused rat heart: relationship between susceptibility to arrhythmias and degree of ultrastructural injury. J Mol Cell Cardiol. 1995;27:1937–1951. doi: 10.1016/0022-2828(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Specific IKI blockade: A new antiarrhythmic mechanism?. Effect of RP 58866 on ventricular arrhythmias in rat, rabbit and primate. Circulation. 1993;87:1979–1989. doi: 10.1161/01.cir.87.6.1979. [DOI] [PubMed] [Google Scholar]
- Shen Y-T, Fallon JT, Iwase M, Vatner SF. Innate protection of baboon myocardium: effects of coronary artery occlusion and reperfusion. Am J Physiol. 1996;270:H1812–H1818. doi: 10.1152/ajpheart.1996.270.5.H1812. [DOI] [PubMed] [Google Scholar]
- Sun W., Wainwright CL. The role of nitric oxide in modulating ischaemia-induced arrhythmias in rats. J Cardiovasc Pharmacol. 1997;29:554–562. doi: 10.1097/00005344-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Swissa M, Ohara T, Lee M-H, Kaul S, Shah PK, Hayashi H, et al. Sildenafil-nitric oxide donor combination promotes ventricular tachyarrhythmias in the swine right ventricle. Am J Physiol. 2002;282:H1787–H1792. doi: 10.1152/ajpheart.00607.2001. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure overload. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi K, Curtis MJ. Influence of tedisamil on the initiation and maintenance of ventricular fibrillation: chemical defibrillation by Ito blockade. J Cardiovasc Pharmacol. 1991;18:445–456. doi: 10.1097/00005344-199109000-00018. [DOI] [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischemia, infarction and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6670–6674. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Talukder MAH. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]