Abstract
Background and purpose:
Clinical studies demonstrate that aspirin consumption reverses the gastrointestinal (GI) benefits of coxibs, by an undefined mechanism.
Experimental approach:
Rodent models were employed to investigate the effects of combinations of celecoxib and aspirin on gastric ulcerogenesis, bleeding, surface hydrophobicity (by contact angle analysis) and ulcer healing. We also evaluated the effects of phosphatidylcholine (PC)-associated aspirin in these rodent models and confirmed its cyclooxygenase (COX)-inhibitory activity by measuring mucosal prostaglandin E2 (PGE2) concentration.
Key results:
We present evidence that aspirin's ability to induce gastric injury and bleeding in rats, was exacerbated in the presence of a coxib and was dependent on its ability to reduce gastric surface hydrophobicity. In contrast, co-administration of phosphatidylcholine (PC)-associated aspirin and celecoxib induced little or no gastric injury/bleeding and maintained the stomach's hydrophobic properties. Interestingly, aspirin and aspirin/PC equally inhibited gastric mucosal PGE2 concentration. Aspirin in combination with a coxib retarded the healing of experimentally induced gastric ulcers, whereas healing rates of rats treated with celecoxib in combination with aspirin/PC were comparable to controls.
Conclusions and implications:
Aspirin's gastric toxicity in combination with a coxib can be dissociated from its ability to inhibit COX-1 and appears to be dependent, in part, on its ability to attenuate the stomach's surface hydrophobic barrier. This adverse drug interaction between aspirin and coxibs, which impacts the treatment of osteoarthritic and cardiac patients requiring cardiovascular prophylaxis, can be circumvented by the administration of phosphatidylcholine (PC)-associated aspirin, to maintain the stomach's hydrophobic properties.
Keywords: NSAID, aspirin, Coxib, celecoxib, COX, ulcers, stomach, hydrophobicity, phosphatidylcholine, prostaglandins
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) induce injury to the gastrointestinal (GI) tract by a multifactorial process involving: inhibition of both cyclooxygenase-I (COX-I) and COX-2; topical injury to the mucosa; inhibition of local blood flow, leukocyte activation/adhesion to the endothelium and the induction of the apoptotic pathway of epithelial cells (Wallace, 1997; Rainsford, 1999; Wolfe et al., 1999; Hawkey, 2000; Lichtenberger, 2001; Perini et al., 2004; Whittle, 2004). In recent years the pharmaceutical industry has focused their efforts on the development of selective COX-2 inhibitors (Coxibs), based upon the evidence of Vane and others that constitutive COX-1 was protective of the GI mucosa, and COX-2 played a central role in the inflammatory process (Whittle et al., 1980; Mitchell et al., 1993; Masferrer et al., 1994; Vane and Botting, 1998). Laboratory and clinical endoscopic studies appeared to support this concept and led to the development and great success of the first Coxibs to be launched, celecoxib (Cel) (Celebrex) and rofecoxib (Vioxx). As will be discussed in more detail later, post-marketing clinical outcome studies of these two Coxibs which followed (CLASS and VIGOR) produced conflicting results on the GI safety of this class of drugs, which may have been partially attributable to the use of aspirin (ASA) in certain subjects (Bombardier et al., 2000; Silverstein et al., 2000), owing to its known efficacy to protect against a number of diseases including stroke, heart disease, a number of cancers and Alzheimer's disease (Hebert and Hennekens, 2000; Veld et al., 2001; Suleiman et al., 2002). The therapeutic benefit of using Coxibs became further confounded in recent years, as it became apparent that the chronic use of Coxibs and other NSAIDs may increase a subject's risk of developing a number of life-threatening cardiovascular/thrombotic adverse events (Mukherjee et al., 2001; Bresalier et al., 2005; Nussmeier et al., 2005; Solomon et al., 2005). These observations led to the withdrawal of several Coxibs from the market, and to the recommendation by some clinicians that these drugs be taken in combination with ASA, in spite of both preclinical and clinical evidence that ASA's use obliterates the GI safety of these drugs.
The observation that the combined use of COX-1 selective NSAID and a Coxib can lead to severe gastric injury was originally described by Wallace et al. (2000) who attributed this potentiating interaction to coincident inhibition of both COX-1 and COX-2. As will be discussed later, subsequent experiments by Fiorucci et al. (2002) provided evidence for the role of lipoxin A4 (LXA4) in the mechanism of this drug interaction.
In the present study, we investigated an alternative mechanism by which ASA and Coxibs may synergize to induce increased injury to the mucosa of the upper GI tract. This postulated mechanism, which would be independent of COX inhibition, is based upon the ability of ASA (and other conventional NSAIDs) to attenuate the hydrophobic surface barrier of the stomach (Goddard and Lichtenberger, 1987; Goddard et al., 1990; Lichtenberger, 1995; Lichtenberger et al., 1995, 2001; Darling et al., 2004). This transformation of the gastric mucosal surface from a non-wettable to a wettable state appears to be linked to the ability of ASA and related NSAIDs to interact with and destabilize an extracellular lining of zwitterionic phospholipids, and specifically phosphatidylcholine (PC), which are present within and on the surface of the mucus gel layer (Goddard et al., 1990; Lichtenberger, 1995, 2001; Lichtenberger et al., 1995; Giraud et al., 1999). We also investigated whether this form of surface injury could be prevented by the administration of ASA that had been pre-associated with soy PC, as we had previously reported in COX knockout mice (Darling et al., 2004). In this study, we confirm that coadministration of ASA and Cel induces severe gastric injury, bleeding and a delay of the healing of experimentally induced ulcers, using rodent model systems. In contrast, co-administration of an equivalent dose of phosphatidylcholine-associated aspirin (ASA/PC) with Cel induced little or no GI injury or bleeding and promoted ulcer healing in rats. The benefit of this new drug appears to relate more closely to maintenance of the stomach's hydrophobic surface barrier properties than COX inhibition.
Methods
Rodent model of NSAID-induced gastric lesion formation
All animal protocols described in this study were previously reviewed and approved by our institution's Animal Welfare Committee and determined to meet or exceed guidelines of the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health (NIH) on the proper care and treatment of laboratory animals. A modification of the technique previously described (Wallace et al., 2000) was used to study the acute effect of NSAIDs and Coxibs to induce gastric ulceration and bleeding. To study NSAID-induced gastric lesion formation, fasted male Sprague–Dawley rats (150–200 g) were intragastrically administered: Celebrex (15 mg Cel kg−1), ASA (40 mg kg−1), ASA/PC (40 mg NSAID kg−1) individually or in combination or an equivalent volume of saline (controls). A dose of Cel of 15 mg kg−1 was used, as it was previously demonstrated to be a COX-2 selective dose (Wallace et al., 2000). Furthermore, ASA was used at a dose of 40 mg kg−1 as it was determined to be the lowest dose that reduced gastric surface hydrophobicity without inducing gastric injury. The rats were killed by CO2 inhalation 3 h later at which time the stomach was excised for the assessment of haemorrhagic damage under a dissecting microscope by an observer unaware of the treatment groups and biopsies of the oxyntic mucosa were taken for contact angle and prostaglandin analyses as described below.
Rodent model of NSAID-induced gastric bleeding
To study NSAID-induced gastric bleeding, in a separate experiment, rats were treated as described above, except 15 min before the end of the experiment they were intragastrically administered 1 ml of 0.6 N HCl and at death the stomach was removed and flushed with 2 ml of chilled deionized distilled water and the perfusate collected for haemoglobin (Hb) analysis as described previously (Lichtenberger et al., 1995; Darling et al., 2004).
Rodent models of gastric ulcer healing
To study the effects of NSAID treatment on the healing of experimentally induced gastric ulcers, we employed a modification of the method of Tsukimi and Okabe (1994); Kurinetz and Lichtenberger (1998) as described previously. Briefly, this entails performing a laparotomy on fasted rats under isoflurane anaesthesia and clamping the dependent portion of the stomach with ringed forceps. Kissing gastric ulcers were then experimentally induced by injecting 0.2 ml of acetic acid (60%) through the gastric wall within the region circumscribed by the forceps and removed 45 s later. After the abdominal incision was closed with suture and treated with local anaesthetic, the rats were then returned to their cages where they had ad libitum access to food and water and the next day randomly distributed among the control and treatment groups that were daily administered Cel (15 mg kg−1) in combination with ASA (40 mg kg−1) or ASA/PC (40 mg of NSAID kg−1), or an equivalent volume of saline (control). Ten days later, the rats were killed as described above and the stomachs were removed and the surface area of the ulcers measured by caliper, as described previously (Kurinetz and Lichtenberger, 1998), by an observer unaware of the treatment groups.
Surface hydrophobicity measurement
Gastric surface hydrophobicity was measured by contact angle analysis as described previously (Goddard and Lichtenberger, 1987; Goddard et al., 1990; Lichtenberger et al., 1995; Darling et al., 2004). Briefly, this entails excising a gastric biopsy from the greater curvature that was rinsed in saline and placed upon the stage of a goniometer where the surface was lightly blotted with filter paper. The tissue was then air-dried for 30 min and a microlitre droplet of water applied to the mucosal surface and the contact angle at the air/liquid/solid triple point measured under a telescopic eye-piece by an observer under coded conditions.
Prostaglandin measurement
Gastric mucosal concentration of prostaglandin E2 (PGE2) was measured in accordance to a method described previously (Anand et al., 1999; Darling et al., 2004). Briefly, this entails extraction of tissue in methanol followed by drying and resuspension in phosphate-buffered saline. The samples were analysed by radioimmunoassay using anti-PGE2 antibody (Sigma Chemical Co., St Louis, MO, USA) according to the manufacturer's instructions.
Statistical analysis
One-way analysis of variance (ANOVA) was used to assess a statistically significant difference between multiple treatment groups. Post hoc analysis of sample means utilized the Fisher's least significant difference (LSD) test with α=0.01 which corresponds to a P-value⩽0.01.
Preparation of test NSAIDs
PC-associated ASA was prepared, as described previously, in which we reacted ASA (purchased from Sigma Chemical Company, St Louis, MO, USA) with an equal weight of triple strength lecithin, a PC-enriched soybean oil (Phosal 35 SB purchased from American Lecithin Co., Oxford, CT, USA) at 40°C until the oil changed its physical state, becoming clear and less viscous. ASA and Celebrex were purchased at a pharmacy (the latter under a prescription) and the tablets pulverized and homogenized in the required volume of deionized distilled water before intragastric administration.
Results
Gastric lesion formation
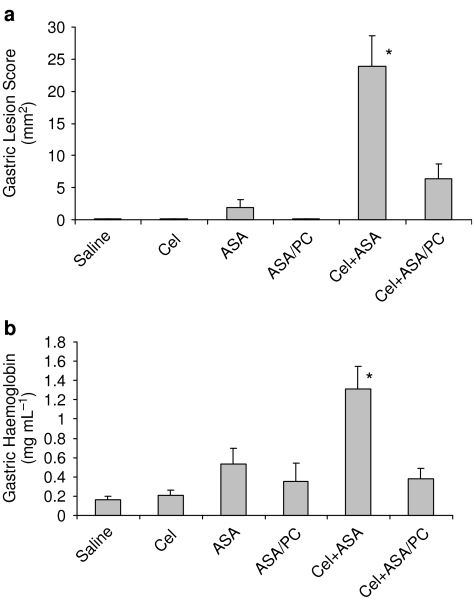
In the initial experimental series, we employed a modification of the rodent model system, described previously (Wallace et al., 2000), to study the effects of intragastrically administered Cel at a COX-2 selective dose of 15 mg kg−1 alone and in combination with a COX-1 selective dose of ASA or an ASA/PC formulation (intragastrically administered at an NSAID dose of 40 mg kg−1), on gastric lesions, mucosal prostaglandin concentration and surface hydrophobicity of the rodent gastric mucosa, 3 h after administration of the test drugs alone or in combination. The results, which are shown in Figure 1a, demonstrate that, as previously reported (Wallace et al., 2000), when administered individually, Cel at 15 mg kg−1 had little or no gastric toxicity. It can also be seen that ASA at a dose of 40 mg kg−1 had a modest, but nonsignificant tendency to induce acute gastric injury in fasted rats, which was not observed in rats that were administered an equivalent dose of ASA/PC. It also can be seen that Cel, in combination with ASA, induced a significant increase in gastric lesion formation that was greater than the additive effects of the test drugs when administered individually. Most importantly, this drug interaction between the Coxib and ASA was markedly attenuated if PC-associated ASA was intragastrically administered in combination with Cel.
Figure 1.
Acute effects of intragastrically administered Cel (15 mg kg−1), ASA (40 mg kg−1) and ASA/PC (40 mg of NSAID kg−1) alone and in combination in rodent model systems on (a) gastric haemorrhagic lesions, where the surface area of lesions was measured by caliper, with n=7–8 rats/group and on (b) gastric bleeding as measured by Hb concentration, with n=7–9 rats/group. In this and the subsequent figures, we used the following abbreviations: celecoxib (Cel); aspirin (ASA); phosphatidylcholine (PC). * Indicates a significant difference (P⩽0.01) vs Saline, Cel, ASA, ASA/PC and Cel+ASA/PC.
Gastric bleeding
In a separate experiment, we evaluated the same test drugs using a similar experimental design, but in this case the rats were challenged with a supra-physiological dose of HCl, 15 min before euthanasia, to induce gastric bleeding, which was determined by measuring the Hb concentration of the gastric perfusate. The results in Figure 1b indicate that similar to their effect on gastric lesions, when intragastrically administered separately at the above doses, neither Cel or ASA induced significant gastric bleeding, whereas the concomitant use of the two classes of drugs induced severe gastric haemorrhage, which was markedly reduced when ASA/PC was used in place of the conventional NSAID.
Gastric mucosal prostaglandin and surface hydrophobicity
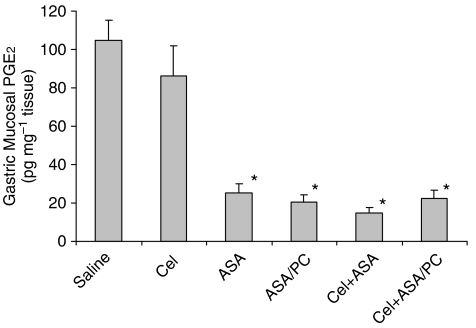
To elucidate the mechanism by which Coxibs interact with ASA and how that may be prevented by PC-associated ASA, we investigated the effects of these test drugs on gastric mucosal PGE2 concentration and surface hydrophobicity. Figure 2 demonstrates that mucosal prostaglandin concentration was not significantly reduced by the administration of Cel at a dose of 15 mg kg−1, confirming the conclusion of Wallace et al. (2000), indicating that it is indeed a COX-2 selective dose. In contrast, ASA at a dose of 40 mg kg−1 did significantly inhibit the PGE2 concentration of the gastric mucosa and this COX-1 inhibitory effect was also observed in rats administered an equivalent NSAID dose of the ASA/PC formulation.
Figure 2.
Acute effects of intragastrically administered Cel (15 mg kg−1), ASA (40 mg kg−1) and ASA/PC (40 mg of NSAID kg−1) alone and in combination on gastric mucosal PGE2 concentration as measured by radioimmunoassay, with n=4–8 rats/group. * Indicates a significant difference (P⩽0.01) vs Saline and Cel.
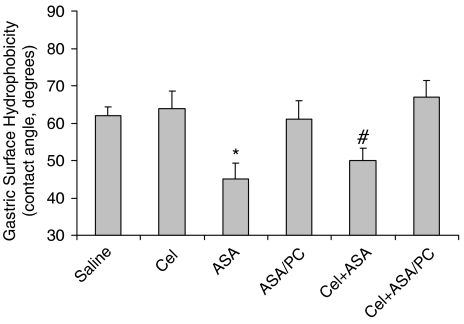
Figure 3 demonstrates the effects of the test drugs on mucosal surface hydrophobicity, as measured by contact angle analysis, from gastric biopsies collected from the above two ulcer experiments. Interestingly, it can be seen that in contrast to ASA (when administered individually or in combination with the Coxib), which induced a significant reduction on mucosal surface hydrophobic properties, as described previously, Cel had no effect on the surface wettability of the gastric mucosa. Furthermore, the ability of ASA to reduce the surface hydrophobic barrier properties of the stomach was not observed in rodents administered an equivalent dose of PC-associated ASA alone or in combination with Cel.
Figure 3.
Acute effects of intragastrically administered Cel (15 mg kg−1), ASA (40 mg kg−1) and ASA/PC (40 mg of NSAID kg−1) alone and in combination on gastric mucosal surface hydrophobicity as measured by contact angle analysis, with n=8–10 rats/group. * Indicates a significant difference (P⩽0.01) vs Saline, Cel, ASA/PC and Cel+ASA/PC. # indicates a significant difference (P⩽0.01) vs Cel+ASA/PC.
Ulcer healing
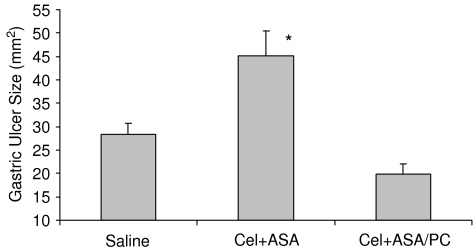
In the last study, we experimentally induced kissing gastric ulcers in rats using a modification of the acetic acid technique of Tsukimi and Okabe (1994) and Kurinetz and Lichtenberger (1998) and the next day randomized the injured rats among groups that were daily intragastrically administered saline (controls) or Cel (15 mg kg−1) in combination with either ASA or ASA/PC at an NSAID dose of 40 mg kg−1. Ten days later, the surface area of the gastric lesions was recorded. The results that are shown in Figure 4 demonstrate that the combination of ASA and Cel delayed the healing of experimentally induced gastric ulcers in comparison to controls, whereas the combination of the Coxib with PC-associated ASA appeared to significantly accelerate ulcer healing to values that were equivalent to the rate of healing observed in saline-treated control rats.
Figure 4.
Effect of intragastrically administered Cel (15 mg kg−1) in combination with either ASA (40 mg kg−1) or ASA/PC (40 mg NSAID kg−1) vs that of saline-treated controls, on the healing of acetic acid-induced gastric ulcers over a 10-day study period. Gastric ulcer size was measured by caliper, with n=6–7 rats/group. * Indicates a significant difference (P⩽0.01) vs Saline and Cel+ASA/PC.
Discussion and conclusions
Over the past decade a number of pharmaceutical companies have developed and commercialized Coxibs, as a safer alternative to conventional NSAIDs to treat pain and inflammation. These drugs have been designed to selectively inhibit COX-2 which is induced at sites of inflammation, and spare the COX-1 isoform that is constituitively expressed in the mucosa of the upper GI tract and plays a rate-limiting role in the biosynthesis of ‘cytoprotective' prostaglandins (Robert et al., 1979; Mitchell et al., 1993; Masferrer et al., 1994; Vane and Botting, 1998). The two major clinical outcome trials, CLASS and VIGOR, evaluating the GI safety of Celebrex (Cel) and Vioxx (rofecoxib) respectively vs conventional NSAIDs in osteoarthritic subjects obtained quite different results (Bombardier et al., 2000; Silverstein et al., 2000). The VIGOR trial demonstrated there were fewer episodes of GI bleeding, obstruction and perforation with Vioxx vs naproxen, whereas the CLASS study failed to show a statistically significant difference in the above outcome parameters when comparing Cel to diclofenac or ibuprofen at equivalent therapeutic doses. One explanation for the difference between the results of the two studies may have related to the facts that in the VIGOR study the subjects were not allowed to use ASA, even at low dose, whereas in the CLASS study this was not an exclusion criteria. In fact, post hoc analysis of the latter study revealed that Cel would have demonstrated significant increased GI safety if the ASA users were excluded from analysis (Silverstein et al., 2000). Similar results have recently been obtained in the TARGET study comparing the highly selective Coxib, Prexige (lumiracoxib) vs conventional NSAIDs in osteoarthritic subjects, where the GI benefit of the drug was lost if the patients concomitantly used ASA (Schnitzer et al., 2004). A potential explanation for this interaction of Coxibs and ASA can be found in a laboratory study by Wallace et al. (2000) who demonstrated in rodent model systems that both COX-1 and COX-2 need to be inhibited to induce gastric injury in rats, as the selective inhibition of only one isoform resulted in little or no GI injury. This adverse drug interaction between ASA and Coxibs to induce gastroduodenal injury has now been confirmed clinically in a number of endoscopic studies (Fiorucci et al., 2003; Laine et al., 2004; Goldstein et al., 2006). In spite of these observations, the use of ASA together with Cel is commonly being recommended for many high-risk individuals suffering from chronic inflammatory and cardiac diseases, as a means of mitigating cardiovascular/thrombotic events that are associated with long-term administration of a Coxib.
The mechanism by which Coxibs exacerbate ASA-induced gastric injury has yet to be determined. One mechanism suggested by Fiorucci et al. (2002) is that ASA acetylates COX-2 to produce 15(R)-hydroxy-eicosatetraenoic acid, which is further metabolized to 15(R)-epi-LXA4 that has potent anti-inflammatory actions. Thus, by co-administration of a Coxib with ASA the generation of this protective LXA4 would be blocked – thereby increasing the tissue's susceptibility to injury.
The results presented herein provide information on an alternative pathogenic mechanism involved in the induction of gastric injury and bleeding when ASA is concomitantly administered with a Coxib. We also provide information on the utility of PC-associated NSAIDs as a means of mitigating this form of GI injury. Our evidence that the reduction in surface hydrophobicity observed with ASA (in the presence or absence of Cel) can be reversed with a formulation of ASA/PC supports our previous evidence that conventional NSAIDs disrupt the mucosal surface barrier by interacting with intrinsic phospholipids within the mucus gel layer and that this damaging action can be circumvented if the NSAID is pre-associated with the exogenous PC (Lichtenberger et al., 1995; Darling et al., 2004). Furthermore, the maintenance of mucosal surface hydrophobicity, as is seen with animals treated with PC-associated ASA, not only protects against ASA-induced gastric injury but also against the severe injury and bleeding observed in rodents that received ASA and a Coxib in combination. Our data also suggest that ASA's ability to inhibit COX-1 and deplete the gastric mucosa of prostaglandins can be dissociated from gastric injury and bleeding under certain conditions, and that the ability of the NSAID to attenuate the surface hydrophobic barrier is a critical component of the pathogenic process. ASA/PC alone and in combination with Cel induced >80% inhibition in mucosal PGE2 concentration with little or no evidence of gastric injury or bleeding, similar to previously reported findings with NO-ASA (Fiorucci et al., 2003; Perini et al., 2004). It should be pointed out that as gastric mucosal prostaglandin concentration was not decreased by a Cel dose of 15 mg kg−1, that the Coxib was, indeed, used at a COX-2 selective dose as reported previously (Wallace et al., 2000). Furthermore, based upon the observation that ASA administration to rats at a dose of 50 mg kg−1 increased the COX-2 product, LXA4 (Souza et al., 2003), it is likely that in our experiment ASA (at 40 mg kg−1) was used at a COX-1 selective dose.
Our observations are also consistent with a previous report from our laboratory that ASA-induces gastric injury and bleeding in both COX-1 and COX-2 knockout mice that can also be prevented with PC-associated ASA (Darling et al., 2004). It is also important to note that our group has previously reported in a 4-day crossover study in healthy volunteers that ASA's ability to induce gastric erosions as observed by endoscopy was significantly reduced if the NSAID was associated with soy PC, although prostaglandin levels of gastric biopsy tissue were decreased by >80% during both arms of the study (Anand et al., 1999).
If ASA's ability to attenuate the surface barrier is an important component in the pathogenic mechanism, then how do Coxibs further exacerbate this process? There is evidence from a number of laboratories that COX-2 is induced at sites of mucosal injury and that Coxibs delay ulcer healing (Mizuno et al., 1997; Peskar, 2006). These findings, which were further corroborated by our study, suggest that the ability of Coxibs to disrupt the mucosal repair process is an important element in the pathogenic mechanism when these two classes of NSAIDs are administered in combination. Interestingly, when the surface barrier is maintained by the administration of PC-associated ASA, the delay in ulcer healing with Coxibs is no longer observed. These findings thereby suggest a potential therapeutic role for PC-associated ASA and perhaps other PC-NSAIDs in both the prevention of mucosal injury and treatment of pre-existent ulcers, when patients are placed on regimens that require the concomitant use of both a Coxib and a conventional NSAID.
Acknowledgments
The research described in this report was supported in part by a Technology Development and Transfer (TD&T) grant awarded by the Texas Higher Education Coordinating Board and by NIH Grants 2 R42 DK 063882 and P30 DK 56338.
Abbreviations
- ASA
aspirin
- ASA/PC
phosphatidylcholine-associated aspirin
- Cel
celecoxib
- COX
cyclooxygenase
- Coxib
COX-2 selective inhibitor
- GI
gastrointestinal
- LSD
least significant difference
- LXA
lipoxin A
- NSAID
nonsteroidal anti-inflammatory drug
- PC
phosphatidylcholine
- PGE
prostaglandin E2
- NIH
National Institutes of Health
Conflict of interest
This research was partially supported by NIH Small Business Technology Transfer (STTR) Phase II grant 2 R42 DK 063882 awarded to PLx Pharma Inc. of Houston TX with a subcontract to The University of Texas Health Science Center at Houston (UTHSCH). PLx Pharma Inc. has licensed the intellectual property related to PC-NSAID formulations from UTHSCH. Drs Lichtenberger and Dial have financial interests in PLx Pharma Inc. and Mr Romero is an employee of this university-based start-up company.
References
- Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM. Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am J Gastroenterol. 1999;94:1818–1822. doi: 10.1111/j.1572-0241.1999.01211.x. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Eng J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial (APPROVe) N Eng J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity and prostaglandin metabolism in COX knockout mice. Gastroenterology. 2004;127:94–104. doi: 10.1053/j.gastro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Menezes de Lima O, Jr, Mencarelli A, Plaazetti B, Distrutti E, McKnight W, et al. Cyclooxygenase-2-derived Lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del Soldato P, et al. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc Natl Acad Sci USA. 2003;100:10937–10941. doi: 10.1073/pnas.1933204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M-N, Motta C, Romero JJ, Bommelaer G, Lichtenberger LM. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: a possible mechanism for the gastric toxicity of NSAIDs. Biochem Pharm. 1999;57:247–254. doi: 10.1016/s0006-2952(98)00303-7. [DOI] [PubMed] [Google Scholar]
- Goddard PJ, Kao Y-CJ, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98:361–370. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- Goddard PJ, Lichtenberger LM. Does aspirin damage the canine gastric mucosa by reducing its surface hydrophobicity. Am J Physiology: Gastrointestinal Liver Physiol. 1987;15:G421–G430. doi: 10.1152/ajpgi.1987.252.3.G421. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Lowry SC, Lanza FL, Schwartz HI, Dodge WE. The impact of low-dose aspirin on endoscopic gastric and duodenal ulcer rates in users of a non-selective non-steroidal anti-inflammatory drug or a cyclo-oxygenase-2-selective inhibitor. Aliment Pharmacol Ther. 2006;23:1489–1498. doi: 10.1111/j.1365-2036.2006.02912.x. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastoenterology. 2000;119:521–535. doi: 10.1053/gast.2000.9561. [DOI] [PubMed] [Google Scholar]
- Hebert PR, Hennekens CH. An overview of the 4 randomized trials of aspirin therapy in the primary prevention of vascular disease. Arch Intern Med. 2000;160:3123–3127. doi: 10.1001/archinte.160.20.3123. [DOI] [PubMed] [Google Scholar]
- Kurinetz A, Lichtenberger LM. Phosphatidylcholine-associated aspirin accelerates healing of gastric ulcers in rats. Dig Dis Sci. 1998;43:786–790. doi: 10.1023/a:1018870131886. [DOI] [PubMed] [Google Scholar]
- Laine L, Maller ES, Yu C, Quan H, Simon T. Ulcer formation with low-dose enteric-coated aspirin and the effect of COX-2 selective inhibition: a double blind trial. Gastroenterology. 2004;127:395–402. doi: 10.1053/j.gastro.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Ann Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- Lichtenberger LM. Where is the evidence the cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited. Biochem Pharm. 2001;61:631–637. doi: 10.1016/s0006-2952(00)00576-1. [DOI] [PubMed] [Google Scholar]
- Lichtenberger LM, Wang Z-M, Romero JJ, Ulloa C, Perez JC, Giraud M-N, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Zioeifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, et al. Selective inhibition of inducible cyclo-oxygenase-2 in vivo is anti-inflammatory and non-ulcerogenic. Proc Nat Acad Sci USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Akarasreenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of NSAIDs as inhibitors of constitutive and inducible cyclo-oxygenase. Proc Nat Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi T, et al. Induction of COX-2 in gastric mucosal lesions and its inhibition by specific antagonists delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Eng J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- Perini R, Fiorucci S, Wallace JL. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Can J Gastroenterol. 2004;18:229–236. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- Peskar BM. Role of cyclooxygenase isoforms in gastric mucosal defense and healing. Inflammopharmacology. 2006;13:15–26. doi: 10.1163/156856005774423809. [DOI] [PubMed] [Google Scholar]
- Rainsford KD. Profile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs. Am J Med. 1999;107:27S–35S. doi: 10.1016/s0002-9343(99)00365-4. [DOI] [PubMed] [Google Scholar]
- Robert A, Nezamis JE, Lancaster C., Hanchar AJ. Cytoprotection by prostaglandins in rats: prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl and thermal injury. Gastroenterology. 1979;70:359–370. [PubMed] [Google Scholar]
- Schnitzer T, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vsnonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1081. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Souza MHLP, Menezes de Lima O, Jr, Zamuner SR, Fiorucci S, Wallace JL. Gastritis increases resistance to aspirin-induced mucosal injury via COX-2 mediated lipoxin synthesis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G54–G61. doi: 10.1152/ajpgi.00525.2002. [DOI] [PubMed] [Google Scholar]
- Suleiman S, Rex D, Sonnenberg A. Chemoprevention of colorectal cancer by aspirin: a cost effective analysis. Gastroenterology. 2002;122:78–84. doi: 10.1053/gast.2002.29689. [DOI] [PubMed] [Google Scholar]
- Tsukimi Y, Okabe S. Effect of anterior unilateral vagotomy on healing of kissing gastric ulcers in rats. Jpn J Pharmacol. 1994;66:105–114. doi: 10.1254/jjp.66.105. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:2S–8S. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal anti-inflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Nonsteroidal anti-inflammatory drugs and gastropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol. 2004;500:427–439. doi: 10.1016/j.ejphar.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Whittle BJR, Higgs GA, Eakins KE, Moncada S, Vane JR. Selective inhibition of prostaglandin production in inflammatory exudates and gastric mucosa. Nature. 1980;284:271–273. doi: 10.1038/284271a0. [DOI] [PubMed] [Google Scholar]
- Wolfe MM, Lichtenberstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]