Abstract
Background and purpose:
Extracts of Plumbago zeylanica containing suberosin exhibit anti-inflammatory activity. We purified suberosin from such extracts and studied its effects on a set of key regulatory events in the proliferation of human peripheral blood mononuclear cells (PBMC) stimulated by phytohemagglutinin (PHA).
Experimental approach:
Proliferation of PBMC in culture was measured by uptake of 3H-thymidine; production of cytokines and cyclins by Western blotting and RT-PCR. Transcription factors NF-AT and NF-κB were assayed by immunocytochemistry and EMSA.
Key results:
Suberosin suppressed PHA-induced PBMC proliferation and arrested cell cycle progression from the G1 transition to the S phase. Suberosin suppressed, in activated PBMC, transcripts of interleukin-2 (IL-2), interferon-γ (IFN-γ), and cyclins D3, E, A, and B. DNA binding activity and nuclear translocation of NF-AT and NF-κB induced by PHA were blocked by suberosin. Suberosin decreased the rise in intracellular Ca2+ concentration ([Ca2+]i) in PBMC stimulated with PHA. Suberosin did not affect phosphorylation of p38 and JNK but did reduce activation of ERK in PHA-treated PBMC. Pharmacological inhibitors of NF-κB, NF-AT, and ERK decreased expression of mRNA for the cyclins, IL-2, and IFN-γ and cell proliferation in PBMC activated by PHA.
Conclusions and Implications:
The inhibitory effects of suberosin on PHA-induced PBMC proliferation, were mediated, at least in part, through reduction of [Ca2+]i, ERK, NF-AT, and NF-κB activation, and early gene expression in PBMC including cyclins and cytokines, and arrest of cell cycle progression in the cells. Our observations provide an explanation for the anti-inflammatory activity of P. zeylanica.
Keywords: suberosin, PBMC, proliferation, NF-AT, NF-κB, [Ca2+]i, ERK
Introduction
Plumbago zeylanica Linn. belongs to the Plumbaginaceae family and is commonly used in traditional Chinese medicine for the treatment of tissue inflammation (Li, 1998). Both naphthoquinone and β-sitosterol components have been isolated from P. zeylanica and demonstrated to possess antitumor and antioxidative activities (Lin et al., 2003; Nguyen et al., 2004; Tilak et al., 2004). Although this plant has been utilized in Chinese herbal medicinal prescriptions for improvement of tissue inflammation for a long time, there has been a relative scarcity of definitive evidence to establish its immunopharmacological activity. In a previous study, we found that the ethanolic extracts of P. zeylanica inhibited proliferation and interleukin-2 (IL-2) production in peripheral T lymphocytes activated by phytohemagglutinin (PHA) (Yang et al., 1999). In order to identify the active ingredients in this plant that are responsible for its possible clinical effects, pure compounds from P. zeylanica were evaluated in immune response assays.
There is now convincing evidence that cytokines secreted by T lymphocytes such as IL-2 and interferon-γ (IFN-γ) in response to antigen stimulation play a role in tissue inflammation (Arai et al., 1990; Kountouras et al., 2004; Rathmann et al., 2004). One of the therapeutic objectives in tissue inflammation is to reduce the local inflammatory response through the reduction of the activation and proliferation of inflammatory cells and of the production of inflammatory cytokines. Blockade of the activation, proliferation and cytokine production by T lymphocytes is one such anti-inflammatory goal (Arai et al., 1990). The activation and proliferation of lymphocytes are highly regulated processes involving the ordered expression of a series of control genes such as IL-2 and IFN-γ (Cantrell, 1996). Proliferation of all eukaryotic cells is under strict regulation and is governed by checkpoints located at distinct points in the cell cycle (Norbury and Nurse, 1992). In cells, transition through G2/M is controlled by cyclins A and B. A distinct class of G1 cyclins involving cyclins D and E regulates the progression of the cell cycle at the G1/S transition (Parde, 1989; Lew et al., 1991). Following interaction with an antigen or a mitogen, a cascade of intracellular signal-transduction pathways involving Ca2+ mobilization, nuclear factor of activated T cells (NF-AT), nuclear factor κB (NF-κB) and mitogen-activated protein kinases (MAPK) is triggered in T lymphocytes, which leads to activation and transcription of a variety of different genes including cytokines and cyclins and entry into the cell cycle (Rao et al., 1997; Edward, 2000; Dong et al., 2002).
Calcium is a major second messenger that is triggered during the cell cycle and by growth factor stimulation. A reduction of the intracellular Ca2+ concentration ([Ca2+]i) would lead to impairment of IL-2 production in T lymphocytes and peripheral blood mononuclear cells (PBMC) (Cantrell, 1996). One of the downstream effects of increased [Ca2+]i is regulation of NF-AT dephosphorylation and its subsequent nuclear translocation (Rao et al., 1997). NF-AT is required for activation and proliferation of T lymphocytes (Rao et al., 1997). The transcription factor NF-κB exists in an inactive form, associated with IκB proteins, in the cytoplasm of quiescent cells. Phosphorylation of IκB and subsequent degradation enables NF-κB to translocate to the nucleus (Gilmore and Morin, 1993). NF-κB has been shown to participate in cell proliferation and transformation (Edward, 2000). By coordinated binding of NF-κB and NF-AT, the induction of a defined genetic program is initiated that leads to the production of cyclins and cytokines such as IL-2 and IFN-γ (Sica et al., 1997; Kennedy et al., 1999). Concurrently, the recruitment and assembly of a number of phosphorylated-protein complexes cause signal transduction of MAPK. In mammalian cells, MAPK comprise the extracellular signal-regulated protein kinase (ERK), p38 kinase and c-Jun NH2-terminal kinase (JNK) (Dong et al., 2002). The activation of ERK is involved in proliferation of T cells and PBMC (Arnaud et al., 2004). Inhibition of JNK and p38 causes inhibition of IFN-γ production (Yang et al., 1998). Activity of the MAPK phosphorylation system is essential for the entry of T cells and PBMC into the cell cycle (Zornig and Evan, 1996). Growth modulators or other external events that affect the proliferation of T cells and PBMC are ultimately likely to act by controlling the expression or function of these genes and signals (Ajchenbaum et al., 1993; Cantrell, 1996).
In the present study, suberosin was purified from ethanolic extracts of the roots of P. zeylanica and PHA-stimulated PBMC were used as target cells. The PBMC are a mixture of T cells, B cells and monocytes/macrophages (Charles et al., 1997). PHA is a mitogen for T lymphocytes. It binds to N-acetylgalactosamine glycoproteins expressed on the surface of T cells thus inducing the cells to proliferate (Charles et al., 1997). To elucidate the effects of suberosin on PBMC proliferation, the uptake of tritiated thymidine was utilized to detect total cellular DNA synthesis in the cultures. In addition, we determined the actions of suberosin on cell cycle progression, production and gene expression of cytokines and cyclins, Ca2+ mobilization and activation of NF-AT, NF-κB, and MAPK in PBMC induced by PHA and examined their roles in regulation of PBMC proliferation.
Methods
Source of P. zeylanica
P. zeylanica was collected at Wulai, Taiwan and identified by Dr Lie-Chwen Lin. A voucher specimen has been deposited in the herbarium of the Department of Botany of the National Taiwan University (no. 1732).
Suberosin purified from P. zeylanica
The roots of P. zeylanica (2.2 kg) were air-dried and cut into small pieces before grinding. The ground roots were then extracted with 95% ethanol three times at room temperature. The solvent was removed under reduced pressure and the residue was partitioned between H2O and n-hexane, followed by ethyl acetate (EtOAc) and n-butanol. The organic layers were combined and concentrated to yield the crude extracts (21 g). The sample, absorbed on silica gel (sample/adsorbent (v/v)=1/8), was subjected to dry flash column chromatography. Sufficient n-hexane was passed through the column to expel all the air. An extensive gradient elution was then employed using n-hexane and EtOAc to yield 13 fractions. The like fractions were combined to give seven main fractions with monitoring by the lymphoproliferation test and thin-layer chromatography (TLC) and the solvent was removed under reduced pressure. Each combined fraction was further purified by rechromatography and recrystalization. Suberosin (26.4 mg; C15H16O3; MW 244; Figure 1a) was isolated from fraction 3. The nuclear magnetic resonance (NMR) and mass spectral data for suberosin were: 1H NMR (CDCl3) δ 1.67 (3H, s, −Me), 1.73 (3H, s, −Me), 3.27/3.28 (each 1H, H2-1′), 3.86 (3H, s, OMe-7), 5.25 (1H, t, J=1.5Hz, H-2′), 6.19 (1H, d, J=9.5Hz, H-3), 6.74 (1H, s, H-8), 7.15 (1H, s, H-5), 7.59 (1H, d, J=9.5Hz, H-4); 13C NMR (CDCl3) δ 17.7 (3′-CH3), 25.8 (3′-CH3), 27.7 (C-1′), 55.8 (7-OCH3), 98.4 (C-8), 111.8 (C-4a), 112.7 (C-3), 121.3 (C-2′), 127.4 (C-5), 133.6 (C-3′), 143.6 (C-3), 154.4 (C-8a), 160.6 (C-7), 161.5 (C-2); APCIMS m/z 245 [M+H]+, that were identical with those previously reported for suberosin (Nayar and Bhan, 1972; Lin et al., 2003). The purity of suberosin was estimated at 96.038% with a high-performance liquid chromatography (HPLC) purity program. Suberosin was dissolved in dimethylsulfoxide (DMSO) to a concentration of 100 mM and then stored at 4°C for use.
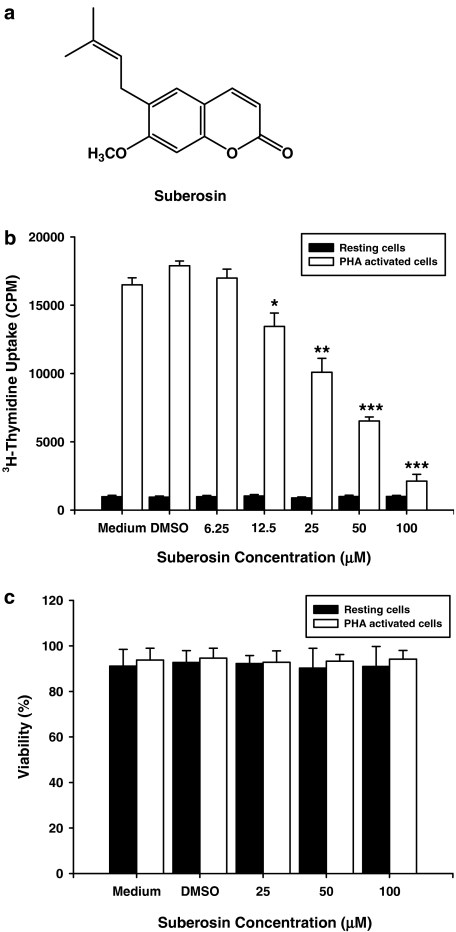
Figure 1.
Suberosin and its effect on PBMC proliferation and viability. (a) The structure of suberosin isolated from P. zeylanica. (b) PBMC (2 × 105 well−1) were treated with the indicated concentrations of suberosin with or without PHA (5 μg ml−1) for 3 days. The proliferation of cells was detected by tritiated thymidine uptake (1 μCi well−1). After a 16-h incubation, the cells were harvested by an automatic harvester, then radioactivity was measured by liquid scintillation counting. (c) PBMC (2 × 105) were stimulated with or without PHA (5 μg ml−1) and treated with medium, 0.1% DMSO, or the indicated concentration of suberosin for 4 days. Numbers of total, viable and non-viable cells were counted after trypan blue staining. Each bar represents the mean±s.d. of three independent experiments with PBMC from different individuals. *P<0.05, **P<0.01, ***P<0.0001: vs the cells treated with DMSO and PHA.
Human subjects
Twenty healthy male subjects (24–32 years, mean age 27 years) were chosen for this investigation. The experimental protocol had been reviewed and approved by the institutional human experimentation committee. Written informed consent was obtained from each and every subject.
Preparation of PBMC
Heparinized human peripheral blood (35 ml) were obtained from healthy donors. PBMC were isolated by the Ficoll–Paque (specific gravity 1.077) gradient density method as described previously (Kuo et al., 2000). The peripheral blood sample was centrifuged at 850 g, 4°C for 10 min to remove the plasma. Blood cells were diluted with phosphate-buffered saline (PBS; pH 7.2) then centrifuged in a Ficoll–Paque discontinuous gradient at 420 g for 30 min. The PBMC layer was collected and washed with cold distilled water and 10 × Hanks' buffer saline solution (HBSS) to remove red blood cells. The cells were resuspended to a concentration of 2 × 106 cells ml−1 in Rosewell Park Memorial Institute medium (RPMI)-1640 medium supplemented with 2% fetal calf serum (FCS), 100 U ml−1 penicillin and 100 μg ml−1streptomycin.
Lymphoproliferation test
The lymphoproliferation test was modified from previously described (Kuo et al., 2003). The DNA synthesis in proliferating cells was labeled with tritiated thymidine. The density of PBMC was adjusted to 2 × 106 cells ml−1 before use. 100 μl of cell suspension was applied into each well of a 96-well flat-bottomed plate (Nunc 167008, Nunclon, Raskilde, Denmark) with or without 5 μg ml−1 PHA (Sigma Chemical Co., St Louis, MO, USA) or anti-CD3 (2 μg ml−1) and anti-CD28 (1 μg ml−1) antibodies (BD Biosciences, San Diego, CA, USA). Suberosin (6.25–100 μM) or various pharmacological inhibitors including the NF-AT inhibitor cyclosporin A (2 μM), the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC; 25 μM) and the ERK inhibitor U0126 (12.5 μM) were added to the cells. The plates were incubated in 5% CO2–air humidified atmosphere at 37°C for 3 days. Subsequently, tritiated thymidine (1 μCi; NEN) was added to each well. After 16-h incubation, the cells were harvested on glass fiber filters by an automatic harvester (Dynatech, Multimash 2000, Billingshurst, UK). Radioactivity in the filters was measured by liquid scintillation counting. The inhibitory activity of suberosin on PBMC proliferation was calculated by the following formula:
Determination of IL-2 and IFN-γ production in PBMC
PBMC (2 × 105 cells well−1) were cultured with PHA alone, anti-CD3 (2 μg ml−1) and anti-CD28 (1 μg ml−1) antibodies, or in combination with varying concentrations of suberosin for 3 days. The cell supernatants were then collected and assayed for IL-2 and IFN-γ by enzyme immunoassays (EIA; R&D Systems, Minneapolis, MN, USA).
Extraction of total cellular RNA
PBMC (5 × 106) were activated with or without PHA and cocultured with 100 μM of suberosin or various pharmacological inhibitors. PBMC were collected and lysed by RNA-Bee (Tel-Test Inc., Friendswood, TX, USA). After centrifugation, the supernatants were extracted with a phenol–chloroform mixture. The extracted RNA was precipitated with 100% cold ethanol. The total cellular RNA was pelleted by centrifugation and redissolved in diethyl pyrocarbonate (DEPC)-treated H2O. The concentration of RNA was calculated by measuring the optical density at 260 nm.
Reverse transcription-polymerase chain reaction (RT-PCR)
The RT-PCR was performed by a method described previously (Kuo et al., 2003). Aliquots of 1 μg of RNA were reverse-transcribed to cDNA using the Advantage RT-for-PCR kit from Clontech according to the manufacturer's instructions. Briefly, 10 μl of cDNA was mixed with 0.75 μM primers, 4 U of Taq polymerase, 10 μl of reaction buffer (2 mM Tris-HCl, pH 8.0; 0.01 mM ethylenediaminetetraacetate, EDTA; 0.1 mM dithiothreitol, DTT; 0.1% Triton X-100; 5% glycerol; and 1.5 mM MgCl2), and 25 μl of water in a total volume of 50 μl. All primer pairs for the IL-2, IFN-γ, and cyclins D3, E, A, and B were designed from the published human cDNA sequence data (Gray et al., 1982; Taniguchi et al., 1983; Ajchenbaum et al., 1993; Parkar et al., 2004). The following specific primers were used: IL-2 (262 bp), forward 5′-GTCACAAACAGTGCACCTAC-3′, reverse 5′-GAAAGTGAATTCTGGGTCCC-3′; IFN-γ (320 bp), forward 5′-GCAGAGCCAAATTGTCTCCT-3′, reverse 5′-ATGCTCTTCGACCTC GAAAC-3′; cyclin D3 (587 bp), forward 5′-TGGATGCTGGAGGTCTGCGAGGAA-3′, reverse 5′-TAGCTTCGGGACGACCTCAGTTCG-3′; cyclin E (415 bp), forward 5′-CCTGTACTGAGCTGGGCAAATAGA-3′, reverse 5′- ACGAAGGTCCTGACACGTCACGTA-3′; cyclin A (150 bp), forward 5′-TGGAGAGTGTATGTTTGTTGTGGTTG-3′, reverse 5′-AAACTTAACAATACAACTAAACTACCCACC-3′; cyclin B (720 bp), forward 5′-AAGGCGAAGATCAAC ATGGC-3′, reverse 5′-AGTCACCAATTTCTGGAGGG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 983 bp), forward 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′, reverse 5′-CACCACCTGGAGTACCGGGTGTAC-3′. The PCR was performed by the following setting of the air thermocycler: denaturing temperature of 94°C for 1 min, annealing temperature of 60°C for 1 min and elongation temperature of 72°C for 80 s for the first 35 cycles and finally elongation temperature of 72 °C for 10 min. Following the reaction, the amplified products were taken out of the tubes and run on 2% agarose gel.
Real-time quantitative PCR
The real-time PCR was performed by TaqMan PCR assay using an ABI prism 7700 sequence detection system (Applied Biosystems, Foster City, CA, USA). The reaction conditions were 50°C for 2 min followed by 10 min at 95°C and 40 cycles of 15 s at 95°C and 1 min at 60°C. ΔCycle of threshold (ΔCT) was calculated by subtracting the CT of GAPDH mRNA from the CT of cyclins, IL-2 or IFN-γ mRNAs.
Cell cycle analysis
Procedures for cell cycle analysis have been described previously (Javier et al., 1997; Kuo et al., 2000). PBMC (2 × 106 cells) were divided into each well of a six-well flat-bottomed plate (Cellstar 657160, Greiner, Germany), with or without 5 μg ml−1 PHA and with suberosin (100 μM). The plates were incubated in 5% CO2–air humidified atmosphere at 37°C for 3 days. The cells were harvested by centrifugation, washed in PBS and then fixed in 70% ethanol for 30 min at −20°C. After washing the cells once with PBS, the DNA was stained with propidium iodide (4 μg ml−1) containing 100 μg ml−1 of ribonuclease A. Flow cytometry analysis was conducted using a Becton-Dickinson FASCan (Franklin Lakes, NJ, USA). The distribution of cells in the cell cycle was determined with WinMDI version 2.8 software.
Western blot analysis
The total cellular proteins were extracted from PBMC by a method described previously (Kuo et al., 2002). PBMC (1 × 107 cells) were applied into each well of a six-well flat-bottomed plate and treated with PHA (5 μg ml−1) in the presence or absence of suberosin (100 μM). The plates were incubated in 5% CO2-air humidified atmosphere at 37°C for 24 h. Cells were harvested and washed once with PBS containing 0.5 mM EDTA. Then the cells were lysed by a solution containing 20 mM Tris-HCl, 30 mM Na4P2O7, 50 mM NaF, 5 mM EDTA, 0.5% Triton X-100, 1 mM DTT, 10 μg ml−1 leupetin, 5 μg ml−1 aprotinin, and 10 mM β-glycerophosphate. The lysates were cleared of insoluble material by centrifugation. The proteins (50 μg) were dissolved in the dissociation buffer (2% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol, 0.05 M Tris-HCl, and 20% glycerol, pH 7.6) and boiled for 5 min. Then proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose filters. After blocking the filters with a solution containing 1% bovine serum albumin (BSA), the filters were incubated with mouse monoclonal antibodies raised against human cyclins (BD Biosciences, San Diego, CA, USA). Specific reactive proteins were detected by an enhanced chemiluminescence method, employing a rabbit anti-mouse Ig antibody linked to horseradish peroxidase (HRP) (Pierce, Rockford, USA).
Determination of [Ca2+]i in PBMC
PBMC were loaded with 5 μM fura-2-acetoxymethyl ester (fura-2-AM) at 37°C for 30 min. The cells were then resuspended in Ca2+-free RPMI-1640 medium without phenol red to a concentration of 4 × 106 cells ml−1. In each experiment, 0.5 ml of cells suspension was equilibrated with an equal volume of 2 mM Ca2+-containing medium at 37°C. Then DMSO (2.5 μl of 0.1%) or suberosin (25, 50 and 100 μM) were added to the cells at the 40th second and stimulated with 2.5 μl of PHA (5 μg ml−1). Changes in fluorescence with time were recorded. During the measurement, the cells suspension was kept at 37°C and continuously stirred. The fluorescent activity was recorded with excitation at 340, 380 nm and emission at 505 nm using an F-4500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) with a multi-wavelength time-scan program.
Determination of cell viability
Approximately 2 × 105 PBMC were cultured with 0.1% DMSO or suberosin (25, 50, and 100 μM) in the presence or absence of PHA (5 μg ml−1) for 4 days. Total, viable and non-viable cell numbers were counted under the microscope with the help of a hemocytometer following staining by trypan blue and cell viability was calculated.
Preparation of nuclear extracts
PBMC (5 × 107 cells) were treated with 100 μM suberosin in the presence or absence of PHA for 1 h, stop buffer was added (10 mM Tris-HCl, pH 7.4; 150 mM NaCl; 10 mM EDTA; 10 mM ethylene glycol bis(β-aminoethylether)-N,N,N',N',-tetraacetic acid; 2 mM Na3VO4; 0.4 mM phenylmethylsulfonylfluoride (PMSF); 50 μM NaF; 2 μM leupeptin; 1 μM pepstatin A] and samples centrifuged at 550 g, 4°C for 10 min. After discarding supernatants, the cell pellets were suspended in 400 μl of cold buffer A (10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT and 0.1 mM PMSF) plus NP-40 (0.25%) and incubated on ice for 15 min. After centrifugation at 6000 g, 4°C for 4 min, the nuclear pellets were resuspended in 75 μl of cold buffer B (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF and 2 μM leupeptin) and incubated on ice for 30 min. After centrifugation at 15000 g, 4°C for 5 min, the supernatants (nuclear extracts) were collected and the protein concentrations were measured by a Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, USA). All nuclear extracts were stored at −80°C until use.
Electrophoretic mobility shift assay (EMSA)
The oligonucleotide sequences corresponding to NF-AT and NF-κB binding sites in the human IL-2 promoter were 5′-ACGCCCAAAGAGGAAAATTTGTTTCATACA-3′ and 5′-AGTTGAGGGGACTTTCCCAGGC-3′, respectively. Reaction mixtures (15 μl) contained, together with 10 μg of nuclear extracts, 2 μg of poly (dI-dC), and 10 ng of biotin-labeled probes. For competition experiments, 100-fold excess of unlabeled competitor oligos were added to the extracts before the addition of labeled probes. The supershift analysis for NF-κB/DNA complex and NF-AT/DNA complex formation was determined with Abs to NF-ATc1 or NF-κBp65 (Santa Cruz, CA, USA), respectively. Biotin-labeled DNA–protein complexes were detected by the streptavidin conjugated with HRP (Panomics, Redwood, USA).
Nuclear translocation of NF-AT and NF-κB determined by confocal microscopy
PBMC (2 × 106 cells ml−1) were incubated with or without suberosin (100 μM) and stimulated by PHA for 1 h. Cytospins were prepared, fixed, blocked and labeled with antibodies to NF-ATc1 or NF-κBp65 according to the manufacturer's instructions. Detection was through a fluorescein isothiocyanate-conjugated secondary antibody, and slides were analyzed with a confocal microscope (Leica TCS SP2, Wetzler, Germany).
Statistical analysis
Data are presented as means±s.d., and the differences between groups were assessed with Student's t-test.
Materials
Reagents were obtained from Sigma or Merck (KGaA, Darmstadt, Germany). Antibodies were purchased from BD Biosciences (San Diego, CA, USA). Rabbit anti-mouse Ig antibody linked to HRP for detection in the Western blots was obtained from Pierce Inc. (Rockford, IL, USA). The kits for EIA of cytokines were from R&D Systems (Minneapolis, MN, USA). RNA-Bee reagent for RNA extraction was obtained from Tel-Test Inc. (Friendswood, TX, USA). The Advantage RT-for-PCR kit was from Clontech (Palo Alto, CA, USA). The TaqMan PCR reagents were bought from Applied Biosystems (Foster City, CA, USA).
Results
Effects of suberosin on PBMC proliferation
As shown in Figure 1b, treatment with PHA for 3 days stimulated cell proliferation as indicated by about 19-fold increase in tritiated thymidine uptake. Treatment with the vehicle (DMSO 0.1%) affected neither the tritiated thymidine uptake in the resting state nor that in the stimulated state. Although suberosin had little effect on tritiated thymidine uptake in resting PBMC, it significantly suppressed the enhanced uptake observable in activated cells. Furthermore, the inhibitory effects of suberosin on activated PBMC were concentration dependent, with a half-maximal inhibitory concentration (IC50) of 41.2±3.6 μM. However, the inhibitory effect of suberosin on PBMC was not related to direct cytotoxicity as the viabilities of resting and activated PBMC were not significantly decreased following treatment with 25, 50 or 100 μM of suberosin for 4 days (Figure 1c).
Effects of suberosin on the cell cycle
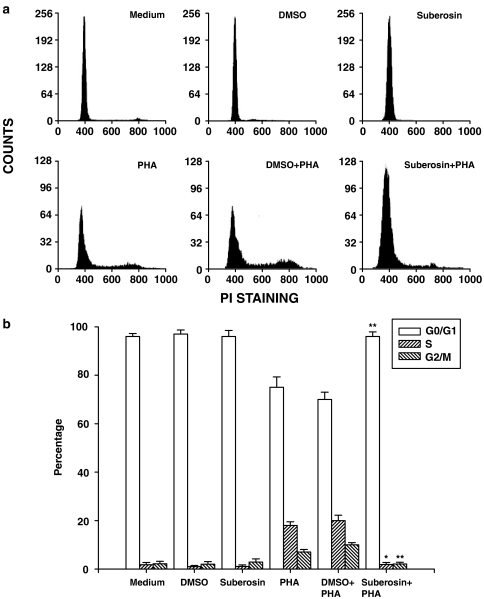
Because the above data suggested that suberosin inhibited PBMC proliferation after stimulation with PHA, we further examined where in the cell cycle of PBMC this inhibtion occurred. Following incubation with or without suberosin for 3 days, cell cycle analyses were performed using a commercially prepared propidium iodide reagent for staining nuclear DNA before flow cytometry analysis. As shown in Figure 2a, resting PBMC existed almost exclusively in the G0/G1 phase, which was not affected by DMSO or suberosin treatment. When the cells were stimulated with PHA and then induced into cell cycle, fluorescence intensity increased from that of the G0/G1 phase to the S phase and G2/M phase. DMSO did not affect this fluoresence change. By contrast, after adding suberosin to PHA-activated PBMC, almost all cells were still blocked at G0/G1 stage compared with the control group. A computer program (WinMDI version 2.8 software) was then used to determine the percentage of PBMC in the G0/G1, S and G2/M phases (Figure 2b). Results indicated that addition of suberosin significantly decreased the percentage of PBMC in the S and G2/M phases and increased the G0/G1 percentage.
Figure 2.
Ability of suberosin to block PBMC progression into the S phase of the cell cycle. PBMC (2 × 106) were treated by 100 μM of suberosin with or without PHA (5 μg ml−1) for 3 days. (a) For determining the cell counts that entered into the cell cycle, cells from a representative subject were stained with propidium iodide, and the DNA content of the cells was analyzed by flow cytometry as described in Methods. (b) A computer program was then used to determine the percentage of PBMC in the G0/G1, S, and G2/M phases. Each bar is the mean±s.d. of three independent experiments with PBMC from different individuals. *P<0.01, **P<0.001: vs the cells treated with DMSO and PHA.
Suberosin reduces production and mRNA expression of cyclins in PBMC
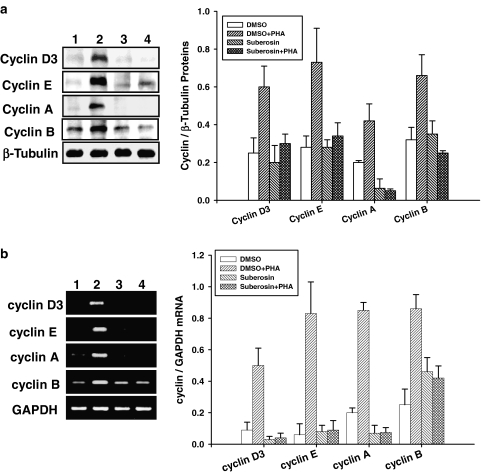
To study whether the blockade of PHA-induced PBMC cell cycle progression described above was related to expression of cyclins, the cells were incubated with or without suberosin for 24 h and total cellular proteins were extracted. The results of Western blotting are shown in Figure 3a. β-Tubulin was detectable in the samples treated with DMSO (lane 1), DMSO and PHA (lane 2), suberosin (lane 3), and suberosin and PHA (lane 4), respectively. Neither suberosin nor DMSO affected β-tubulin expression in PBMC. Although unstimulated PBMC expressed a low level of cyclin D3, E, A and B proteins, the levels of these cyclin proteins were significantly increased in the cells induced by PHA. The data also indicated that the increase in cyclins D3, E, A and B in PHA-treated PBMC was prevented by suberosin. Analysis of laser densitometry demonstrated that the ratios of those cyclins to β-tubulin in PHA-activated PBMC were significantly impaired by suberosin (P<0.01).
Figure 3.
Effects of suberosin on cyclins gene expression in PBMC detected by Western blotting and RT-PCR, respectively. PBMC (1 × 107) were activated with or without PHA in the presence or absence of 100 μM suberosin. (a) Lysates (50 μg of protein) were collected at 24 h and run on a 10% SDS-PAGE gel and analyzed by immunoblotting with anti-cyclin D3, E, A or B antibody. (b) The total cellular RNA was isolated from PBMC at 18 h and analyzed by RT-PCR. (lane1) PBMC treated with DMSO, (lane 2) PBMC treated with DMSO and PHA, (lane 3) PBMC treated with suberosin, (lane 4) PBMC treated with suberosin and PHA. Bar graphs indicates the ratio of cyclin D3, E, A or B to β-tubulin proteins or GAPDH mRNAs, respectively. Each bar represents the mean±s.d. of three independent experiments with PBMC from different individuals.
To determine whether the reduction in cyclin proteins brought about by suberosin was related to gene expression, the total cellular RNA was extracted from PBMC and analyzed by RT-PCR. The results are shown in Figure 3b. The mRNA for GAPDH was detectable in the samples treated with DMSO (lane 1), DMSO and PHA (lane 2), suberosin (lane 3), and suberosin and PHA (lane 4), respectively. Both suberosin and DMSO did not affect GAPDH mRNA expression in PBMC. After PHA stimulation, the levels of each cyclin mRNA were significantly increased in the cells. However, the PCR products for cyclins D3, E, A and B amplified from PHA-treated PBMC RNA preparations were attenuated by suberosin. The laser densitometry analysis demonstrated that the ratios of those cyclin mRNAs to GAPDH mRNA in PHA-activated PBMC were significantly decreased by suberosin. Furthermore, the effects of suberosin on cyclins D3, E, A and B mRNAs expression were confirmed with real-time PCR (Table 1). By comparison with the DMSO-treated group, the ΔCT values of those cyclins were significantly decreased by PHA (P<0.01). Suberosin reversed the ΔcT values of cyclins D3, E, A and B in activated PBMC (P<0.01). These data support that suberosin decreases the levels of cyclin protein by decreasing cyclin mRNA expression in PBMC.
Table 1.
ΔCT values for cyclins, IL-2, and IFN-γ in suberosin-treated PBMC
| DMSO | Suberosin | DMSO+PHA | Suberosin+PHA | |
|---|---|---|---|---|
| Cyclin D3 CT | 36.78±0.13 | 35.43±0.22 | 25.65±0.08 | 35.98±0.16 |
| GAPDH CT | 21.00±0.05 | 22.12±0.07 | 20.89±0.01 | 21.33±0.12 |
| ΔCT | 15.30±0.31 | 14.63±0.33 | 4.55±0.08# | 13.95±0.17* |
| Cyclin E CT | 33.21±0.02 | 34.18±0.12 | 23.85±0.04 | 33.00±0.05 |
| GAPDH CT | 22.36±0.12 | 22.05±0.09 | 21.49±0.07 | 21.30±0.06 |
| ΔCT | 10.32±0.18 | 12.08±0.33 | 2.30±0.04# | 11.75±0.19* |
| Cyclin A CT | 30.45±0.03 | 31.18±0.32 | 24.75±0.05 | 31.09±0.08 |
| GAPDH CT | 21.88±0.16 | 22.00±0.19 | 20.99±0.02 | 20.98±0.02 |
| ΔCT | 8.52±0.03 | 9.10±0.18 | 3.68±0.02# | 10.08±0.01* |
| Cyclin B CT | 30.20±0.01 | 29.89±0.21 | 23.75±0.03 | 30.77±0.01 |
| GAPDH CT | 23.26±0.07 | 23.15±0.01 | 21.87±0.05 | 22.55±0.10 |
| ΔCT | 7.01±0.23 | 6.86±0.19 | 1.85±0.02# | 8.03±0.09* |
| IL-2 CT | 34.99±0.21 | 35.90±0.32 | 27.22±0.11 | 32.77±0.08 |
| GAPDH CT | 24.36±0.02 | 25.03±0.01 | 22.36±0.01 | 24.78±0.01 |
| ΔCT | 10.64±0.23 | 10.87±0.33 | 4.86±0.10# | 7.99±0.07* |
| IFN-γ CT | 30.35±0.06 | 30.73±0.06 | 20.35±0.03 | 24.15±0.05 |
| GAPDH CT | 22.76±0.04 | 22.78±0.03 | 19.17±0.04 | 21.38±0.04 |
| ΔCT | 7.59±0.02 | 7.95±0.03 | 1.19±0.04# | 2.77±0.01* |
Abbreviations: DMSO, dimethyl sulfoxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; PBMC, human peripheral blood mononuclear cells; PHA, phytohemagglutinin.
PBMC (5 × 106 cells) were cultured with 100 μM suberosin in the presence or absence of PHA for 18 h. The cDNA was reverse-transcribed from cellular RNA and amplified by TaqMan PCR assay with an ABI prism 7700 sequence detection system. ΔCT was calculated by subtracting the CT of GAPDH mRNA from the CT of cyclin D3, cyclin E, cyclin A, cyclin B, IL-2, or IFN-γ mRNAs. The representative data from 3 independent experiments with PBMC from different individuals are shown.
P< 0.01, as compared with DMSO group;
P< 0.01, as compared with the DMSO+PHA group.
Inhibitory effects of suberosin on IL-2 and IFN-γ production in PBMC
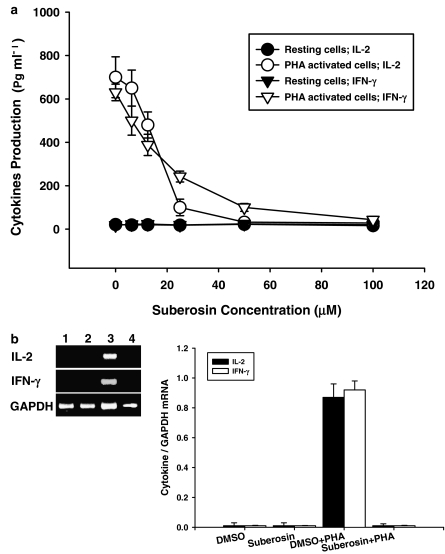
Production of IL-2 and IFN-γ is a hallmark of activated T cells and PBMC (Carter et al., 1998) and the suberosin-induced suppression of PHA-induced PBMC proliferation could be related to IL-2 and IFN-γ production. Therefore, PBMC were stimulated with PHA in the presence or absence of varying concentrations of suberosin (6.25–100 μM) for 3 days. Supernatants were then collected, and the production of IL-2 and IFN-γ were assayed by EIA, respectively. As shown in Figure 4a, the stimulated production of IL-2 and IFN-γ in activated PBMC was significantly suppressed by suberosin. Furthermore, the inhibitory activities of suberosin were concentration dependent. At 100 μM, the stimulated production of IL-2 or IFN-γ in activated PBMC was completely blocked by suberosin, with their concentrations returning to almost the same as those produced in resting cells.
Figure 4.
Cytokine production and mRNA expression in PBMC treated with suberosin. (a) PBMC (2 × 105 well−1) were treated by 0, 6.25, 12.5, 25, 50, and 100 μM of suberosin with or without PHA (5 μg ml−1) for 3 days. Then the cell supernatants were collected and IL-2 and IFN-γ concentration was determined by EIA. Each point is the mean±s.d. of three independent experiments with PBMC from different individuals. (b) PBMC (5 × 106) activated with or without PHA (5 μg ml−1) in the presence or absence of 100 μM suberosin for 18 h. The total cellular RNA was isolated from PBMC treated with DMSO (lane 1), suberosin (lane 2), DMSO and PHA (lane 3), or suberosin and PHA (lane 4). Aliquots of 1 μg of RNA were reverse-transcribed for synthesis of cDNA. Briefly, 10 μl of cDNA was applied in the PCR test. The PCR was carried out as described in Methods. After the reaction, the amplified product was taken out of the tubes and analyzed on 2% agarose gel. Graphical representation of laser densitometry of IL-2 and IFN-γ mRNA expression in resting or PHA-stimulated PBMC in the presence or absence of suberosin. Each band was quantitated using laser-scanning densitometer SLR-2D/1D (Biomed Instruments Inc., Fullerton, CA, USA). The ratio of each cytokine mRNA to GAPDH mRNA was calculated. Each bar is the mean±s.d. of three independent experiments with PBMC from different individuals.
Therefore, we examined whether expression of IL-2 or IFN-γ mRNA in activated PBMC was inhibited by suberosin. Total cellular RNA was extracted from activated PBMC in the presence or absence of suberosin and the results of RT-PCR analyses are shown in Figure 4b. The mRNA for GAPDH was detectable in the samples treated with DMSO (lane 1), suberosin (lane 2), DMSO and PHA (lane 3) and suberosin and PHA (lane 4), respectively. The results indicated that the levels of IL-2 and IFN-γ mRNA in PBMC were significantly induced by PHA. By contrast, PCR products for both cytokines amplified from activated PBMC RNA preparations were reduced by suberosin. The laser densitometry analysis demonstrated that the ratios of IL-2 and IFN-γ to GAPDH mRNAs in PHA-activated PBMC were significantly decreased by suberosin (P<0.001). The inhibitory actions of suberosin on IL-2 and IFN-γ mRNAs expression were also analyzed with the real-time PCR (Table 1). Comparison with the PHA-treated group, suberosin reversed the ΔCT values of IL-2 and IFN-γ in activated PBMC (P<0.01). These data were consistent with inhibition of IL-2 and IFN-γ production by suberosin.
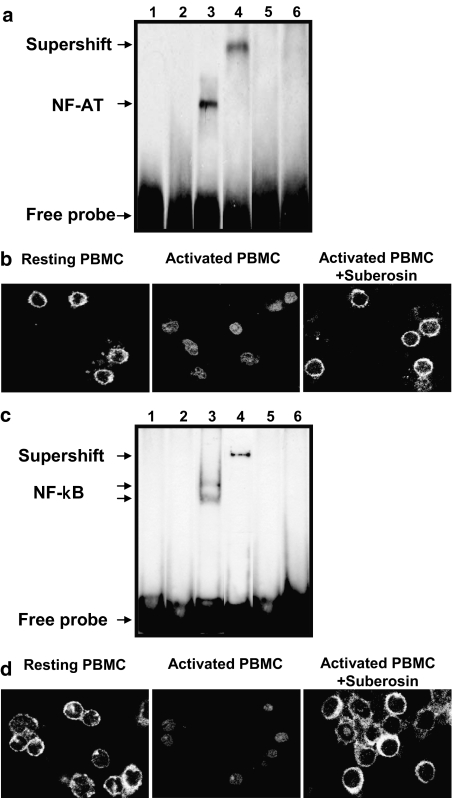
NF-AT and NF-κB DNA binding and nuclear translocation are inhibited by suberosin
Suppression of IL-2, IFN-γ and cyclin transcripts by suberosin could be related to inhibition of the initial transcriptional transactivation event. To assess this possibility, biotin-labeled oligonucleotide sequences corresponding to NF-AT and NF-κB binding sites were incubated with nuclear extracts from suberosin-treated PBMC. As shown in Figure 5a and c, loss of NF-AT/DNA and NF-κB/DNA complexes occurred in resting PBMC (lane 2), but not when activation was conducted in the presence of PHA (lane 3). A supershifted complex appeared when the anti-NF-ATc1 or anti-NF-κBp65 antibody was added in the reaction mixture, respectively (lane 4). The cold competition analysis showed a reduction in the amount of NF-AT/DNA and NF-κB/DNA complexes in activated cells (lane 5). However, the formation of the PHA-induced NF-AT/DNA and NF-κB/DNA complexes were markedly decreased in the case in which the nuclear extracts from suberosin-treated PBMC were used (lane 6). Furthermore, the effect of suberosin on nuclear translocation of NF-AT and NF-κB in PBMC was examined by immunocytochemistry. As shown in Figure 5b and d, NF-AT and NF-κB were predominantly cytoplasmic in resting PBMC (left panel), but these signals disappeared upon stimulation and accumulated in the nuclear (center panel). In the presence of suberosin, NF-AT and NF-κB remains cytoplasmic (right panel). Both the nuclear translocation and DNA-binding activity of NF-AT and NF-κB were blocked by suberosin. These results suggested that suberosin reduced expression of the mRNAs for IL-2, IFN-γ and cyclins by interfering with the activation of NF-AT and NF-κB.
Figure 5.
Effects of suberosin on DNA binding activity and nuclear translocation of NF-AT and NF-κB in PBMC detected by EMSA and immunofluorescent staining, respectively. The EMSA was performed as described in the Methods. PBMC (5 × 107) were treated by 100 μM of suberosin with or without PHA (5 μg ml−1) for 1 h. A biotin-labeled (a) NF-AT or (c) NF-κB probe was incubated with nuclear extracts from PBMC treated with 0.1% DMSO (lane 2), DMSO and PHA (lane 3), or suberosin and PHA (lane 6), respectively. The formations of NF-AT/DNA and NF-κB/DNA complexes were detected by streptavidin-HRP conjugate. Lanes 1, 4 and 5 represent the results of adding free probes, anti-NF-ATc1 or anti-NF-κBp65 antibody and a 100-fold excess of unlabeled probe to the reaction mixture, respectively. (b) NF-AT and (d) NF-κB levels in unstimulated PBMC (left panel), and those cultured with (right panel) and without (central panel) suberosin (100 μM) and stimulated with PHA for 1 h, were stained with anti-NF-ATc1 or anti-NF-κBp65 antibody and quantified by confocal microscopy (Leica TCS SP2, Wetzler, Germany).
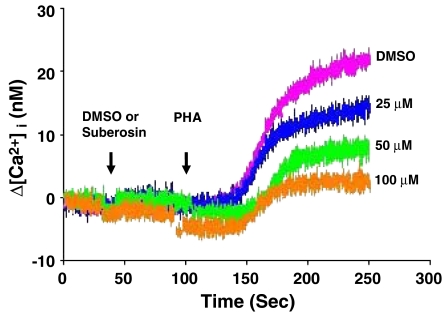
Calcium mobilization in PBMC affected by suberosin
Calcium mobilization is a very early event in the activation of T cells and PBMC that is necessary for activation of NF-AT (Buggins et al., 2001). To study whether the impairment of NF-AT activation in PBMC was related to Ca2+ mobilization, the cells were incubated with or without suberosin and [Ca2+]i in PBMC stimulated by PHA was determined. The results are shown in Figure 6. When PBMC were pretreated with DMSO and PHA added to cells at 100 s, [Ca2+]i began to increase about 50 s later and reached a maximum value at about 250 s. This patteren of change in [Ca2+]i was the same as that in PBMC stimulated without DMSO (data not shown). However, the rise in [Ca2+]i in PBMC was decreased by suberosin dose-dependently. Therefore, inhibition of activation of NF-AT might be explained by a failure to mobilize Ca2+ in suberosin-treated PBMC.
Figure 6.
Effects of suberosin on [Ca2+]i induced in PHA-treated PBMC. PBMC were loaded with 1 μM fura-2-AM at 37°C for 30 min. The cells were then resuspended in RPMI-1640 medium without phenol red to a concentration of 4 × 106 cells ml−1. In each experiment, to 0.5 ml of equilibrated PBMC suspension was added 2.5 μl of DMSO (0.1%) or suberosin (25, 50, or 100 μM) at 40 s, then stimulated with 2.5 μl of PHA (5 μg ml−1) at 100 s and the changes in fluorescence with time recorded. The fluorescent activity was recorded by an F-4500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) with multi-wavelength time scan program.
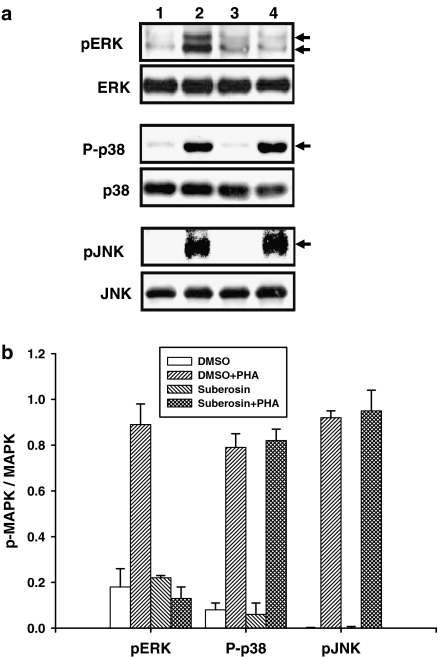
Effects of suberosin on MAPK activation in PBMC activated by PHA
To assess how suberosin might affect intracellular signaling in general in PBMC, total cellular proteins were extracted from PBMC and the MAPK family of signal transduction proteins was measured by Western blotting. Activation of ERK, p38 and JNK is dependent on phosphorylation at specific sites, which was assayed by probing the blots with phosphorylation site-specific antibodies. As shown in Figure 7a, the phosphorylation of ERK (pERK), p38 (P-p38) and JNK (pJNK) in unstimulated PBMC was not affected by DMSO (lane 1) or suberosin (lane 3) treated alone. After treatment with PHA for 1 h, the phosphorylation of ERK, p38 and JNK was detected in PBMC (lane 2). Although the presence of suberosin did not affect p38 and JNK activation, the phosphorylation of ERK was significantly impaired by suberosin (lane 4). As shown in Figure 7b, the laser densitometry analysis demonstrated that the ratio of pERK to ERK in PHA-activated PBMC were significantly decreased by suberosin (P<0.01). Taken together, these results indicate that suberosin affects the activation of ERK in PBMC induced by PHA.
Figure 7.
Effects of suberosin on the phosphorylation of ERK, p38, and JNK in PBMC. (a) PBMC (1 × 107 cells) were treated with 0.1% DMSO (lane 1), DMSO and 5 μg ml−1 PHA (lane 2), 100 μM suberosin (lane 3), or suberosin and PHA (lane 4), for 60 min. The total cellular proteins (50 μg) were run on a 10% SDS-PAGE gel and analyzed by immunoblotting with anti-pMAPK or MAPK antibody. The representative results from more than three independent experiments are shown. (b) Each band was quantitated by laser-scanning densitometer SLR-2D/1D (Biomed Instruments Inc., Fullerton, USA) and the ratio of pMAPK to MAPK was calculated. Each bar is the mean±s.d. of more than three independent experiments with PBMC from different individuals.
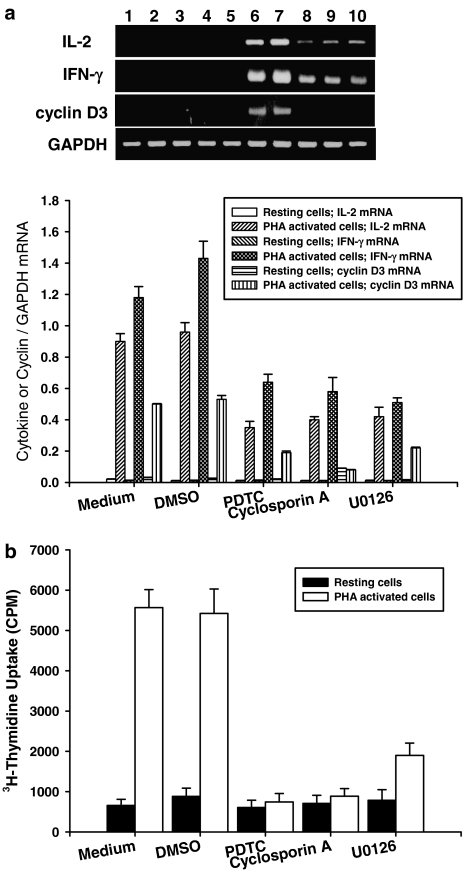
Inhibitory effects of suberosin on PBMC are related to NF-AT, NF-κB and ERK activation
To demonstrate the essential roles of NF-AT, NF-κB and ERK in PHA-activated PBMC, a range of pharmacological inhibitors including the NF-AT inhibitor cyclosporin A (2 μM) the NF-κB inhibitor PDTC (25 μM), and the ERK inhibitor U0126 (12.5 μM) were added to PBMC cultures and IL-2, IFN-γ and cyclin D3 mRNA transcripts and cell proliferation were determined by RT-PCR and tritiated thymidine uptake, respectively. As shown in Figure 8a, PHA significantly induced IL-2, IFN-γ and cyclin D3 mRNA expression in PBMC (lane 6) and DMSO did not affect these gene transcriptions in the cells (lane 7). However, PDTC, cyclosporin A and U0126 reduced IL-2, IFN-γ and cyclin D3 gene expression in PBMC activated with PHA (lanes 8–10). As shown in Table 2, these results were confirmed by real-time PCR. All three inhibitors decreased the levels of cyclin D3, IL-2, and IFN-γ mRNAs in activated PBMC. As shown in Figure 8b, all inhibitors also significantly suppressed the proliferation of PBMC induced by PHA (P<0.001). These data support the concept that NF-AT, NF-κB and ERK are regulators of IL-2, IFN-γ and cyclin D3 gene transcription in PBMC induced by PHA.
Figure 8.
Effects of pharmacological inhibitors on IL-2, IFN-γ and cyclin D3 mRNA expression and cell proliferation in PBMC induced by PHA. (a) 5 × 106 PBMC activated with or without PHA (5 μg ml−1) in the presence or absence of 25 μM PDTC, 2 μM cyclosporin A or 12.5 μM U0126, for 18 h. The total cellular RNA was isolated from PBMC and analyzed by RT-PCR as described in Methods. The lanes indicates PBMC treated with medium (lane 1), 0.1% DMSO (lane 2), PDTC (lane 3), cyclosporin A (lane 4), U0126 (lane 5), PHA (lane 6), DMSO and PHA (lane 7), PDTC and PHA (lane 8), cyclosporin A and PHA (lane 9), and U0126 and PHA (lane 10). Following the reaction, the amplified products were taken out of the tubes and run on 2% agarose gel. Each band was quantitated using laser-scanning densitometer SLR-2D/1D (Biomed Instruments Inc., Fullerton, USA). Graphical representation of laser densitometry of IL-2, IFN-γ and cyclin D3 mRNA expression in unstimulated or PHA-stimulated PBMC in the presence or absence of various inhibitors. (b) For PBMC proliferation, the cells (2 × 105 well−1) were treated by 25 μM PDTC, 2 μM cyclosporin A or 12.5 μM U0126 with or without PHA (5 μg ml−1) for 3 days. The proliferation of cells was detected by tritiated thymidine uptake (1 μCi well−1). After a 16-h incubation, the cells were harvested by an automatic harvester, then radioactivity was measured by liquid scintillation counting. Each bar represents the mean±s.d. of three independent experiments with PBMC from different individuals.
Table 2.
ΔCT for cyclin D3, IL-2, and IFN-γ in PBMC treated with pharmacological inhibitors
| CT |
ΔCT | CT |
ΔCT | CT |
ΔCT | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cyclin D3 | GAPDH | IL-2 | GAPDH | IFN-γ | GAPDH | ||||
| Medium | 32.89±0.05 | 20.32±0.03 | 12.60±0.04 | 32.77±0.02 | 21.65±0.05 | 11.10±0.05 | 31.44±0.02 | 22.17±0.06 | 9.20±0.05 |
| Medium+PHA | 24.88±0.02 | 21.59±0.01 | 3.32±0.02 | 26.32±0.03 | 21.50±0.04 | 4.80±0.06 | 22.65±0.01 | 20.97±0.10 | 1.62±0.09 |
| DMSO | 33.35±0.12 | 20.88±0.08 | 12.36±0.06 | 32.68±0.04 | 20.76±0.01 | 11.88±0.03 | 31.88±0.02 | 22.68±0.03 | 9.19±0.02 |
| DMSO+PHA | 24.28±0.03 | 21.01±0.02 | 3.30±0.03# | 26.44±0.01 | 21.55±0.02 | 4.91±0.02# | 21.83±0.01 | 20.32±0.04 | 1.50±0.03# |
| PDTC | 32.89±0.23 | 20.07±0.09 | 12.77±0.15 | 32.25±0.03 | 20.35±0.01 | 11.07±0.02 | 31.22±0.03 | 21.98±0.02 | 9.15±0.03 |
| PDTC+PHA | 28.01±0.02 | 19.87±0.21 | 8.00±0.10* | 30.22±0.06 | 20.62±0.02 | 9.53±0.05* | 27.43±0.01 | 21.00±0.06 | 6.35±0.04* |
| Cyc A | 33.16±0.08 | 21.05±0.02 | 12.05±0.05 | 32.78±0.02 | 20.88±0.03 | 11.50±0.02 | 31.06±0.02 | 22.01±0.02 | 9.00±0.02 |
| Cyc A+PHA | 30.05±0.01 | 20.88±0.01 | 9.12±0.01* | 30.10±0.01 | 21.00±0.01 | 9.10±0.01* | 28.01±0.01 | 21.07±0.05 | 6.90±0.04* |
| U0126 | 33.65±0.03 | 21.07±0.01 | 12.32±0.02 | 32.48±0.05 | 21.07±0.04 | 11.35±0.04 | 31.55±0.02 | 21.56±0.04 | 9.90±0.04 |
| U0126+PHA | 29.46±0.02 | 20.08±0.11 | 8.49±0.06* | 30.89±0.01 | 20.92±0.02 | 9.83±0.02* | 28.42±0.05 | 21.60±0.05 | 6.78±0.05* |
Abbreviations: DMSO, dimethyl sulfoxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; PBMC, human peripheral blood mononuclear cells; PHA, phytohemagglutinin.
PBMC (5 × 106 cells) were cultured with PDTC (25 μM), cyclosporin A (Cyc A; 2 μM),or U0126 (12.5 μM) in the presence or absence of PHA for 18 h. The cDNA was reverse-transcribed from cellular RNA and amplified by TaqMan PCR assay with an ABI prism 7700 sequence detection system. Each ΔCT was calculated by subtracting the CT of GAPDH mRNA from the CT of cyclin D3, IL-2 or IFN-γ mRNAs, respectively. The representative data from 3 independent experiments with PBMC from different individuals are shown.
P<0.01, as compared with DMSO group;
P<0.01, as compared with the DMSO+PHA group.
Suberosin decreases cell proliferation and IL-2 production in PBMC induced by anti-CD3 and anti-CD28 antibodies
To investigate whether suberosin has effects on PBMC proliferation and cytokine production generated by TCR/CD3 crosslinking, the cells proliferation and IL-2 production were determined in the presence of anti-CD3 and anti-CD28 antibodies. We found that suberosin (100 μM) resulted in a reduction of CD3/CD28-induced PBMC proliferation (12850±1355 vs 2079±209 CPM; n=3; P<0.001) and IL-2 production (525±101 vs 103±36 pg ml−1; n=3; P<0.01).
Discussion and conclusions
In the present study, we have shown for the first time that suberosin isolated from P. zeylanica has a profound inhibitory effect on the activation and proliferation of human PBMC stimulated with PHA. Results demonstrated that increase in total cellular DNA synthesis induced by PHA was inhibited by suberosin. The proliferation-suppressive actions of suberosin were not explained by a drug-induced reduction in cell viability. Cell cycle analysis revealed that suberosin inhibited the entry of PHA-stimulated PBMC into the S phase of the cell cycle, observations were consistent with data that suberosin suppressed PHA-driven PBMC proliferation. In addition, we observed that suberosin decreased production and mRNA expression of IL-2, IFN-γ, and cyclins D3, E, A and B in activated PBMC. The DNA-binding activity and nuclear translocation of NF-AT and NF-κB and phosphorylation of ERK in the activated cells were attenuated by suberosin. The mobilization of Ca2+ in PHA-activated PBMC was inhibited by suberosin. We suggest that suberosin interferes with some regulatory events required for entry of PHA-activated PBMC into the S phase and then cell proliferation is suppressed.
Suberosin is a coumarin and has also been isolated from Zanthoxylum rhesta and Citrus deliciosa (El-Shafae and Soliman, 1998; Ahsan et al., 2000). It has been reported that suberosin has antiproliferative effects on several cancer cell lines (Kawaii et al., 2001) and inhibits the aggregation of rabbit platelets induced by platelet-activating factor (Teng et al., 1992). Furthermore, the present results showed that suberosin suppressed proliferation and IL-2 and IFN-γ production of human PBMC activated with PHA. It suggests that suberosin has immunopharmacological activities. The possible inhibitory effect of DMSO on PBMC was studied in these experiments. DMSO did not change PBMC proliferation and viability. Therefore, the inhibitory function of suberosin was not related to DMSO. Results of cell viability tests indicated that there was no significant cell death in PBMC cultures after treatment with 100 μM suberosin for 4 days. We suggest that under 100 μM and during this time frame, the inhibitory effects of suberosin on PBMC were not through cytotoxic effects. In the present study, T cells were the major proliferating cells in PBMC cultures activated with PHA. On the other hand, suberosin could attenuate cell proliferation and IL-2 production in PBMC generated by TCR/CD3 cross-linking, which specifically stimulates T cells (Charles et al., 1997). Thus, inhibitory effects of suberosin on PHA-activated PBMC proliferation could reflect suppression of T cell proliferation.
The central event in generation of immune responses is the activation and clonal expansion of T cells (Charles et al., 1997). During the process of differentiation, T cells spontaneously arrest in G0 and may remain quiescent for long periods of time until exposed to specific antigen or mitogens. Interaction of T cells with antigens or PHA initiates a cascade of biochemical events that induces the resting T cells to enter the cell cycle then proliferate and differentiate (Kuo et al., 2003). In our studies, the results indicated that almost all unstimulated PBMC existed at the G0/G1 phase and after stimulation with PHA, entry into cell cycle was induced. It has been demonstrated in many previous studies with T cells that a series of genes such as those for IL-2, IFN-γ and cyclins are expressed in a carefully controlled order as the cells pass through G0, G1 and S phase (Ajchenbaum et al., 1993; Liu et al., 2004). Cell cycle activation is in part coordinated by D-type cyclins, which are rate limiting and are essential for the progression through the G1 phase of the cell cycle. Cyclin D3 normally occurs in mid-G1 and is one of the key regulators of cell cycle progression from G1 to S phase (Hleb et al., 2004). Subsequent G1 events and initiation of DNA synthesis are dependent on induction of the IL-2 receptor and on a supply of IL-2 from autocrine or external sources. Growth modulators that affect the PBMC proliferation are ultimately likely to act by controlling the expression or function of these gene products, although the molecular mechanisms involved in regulating passage through cell cycle in PBMC stimulated with PHA remain largely unknown (Ajchenbaum et al., 1993; Liu et al., 2004).
The effects of suberosin on PBMC can be grouped into two categories: first, inhibition of entry into the cell cycle; and, secondly, inhibition of IL-2 and IFN-γ production. The data indicated that suberosin suppressed PHA-activated PBMC proliferation and cell cycle progression from G1 to S phase. The results demonstrated that suberosin acted similarly to the immunosuppressive agent rapamycin, retaining T cells predominantly in either the G0/G1 phase or the early S phase of the cell cycle (Schreiber and Crabtree, 1992; Hleb et al., 2004). Suberosin decreased the protein levels of cyclin D3, E, A and B in PBMC induced by PHA. We also found that cyclin D3, cyclin E, cyclin A and cyclin B mRNA transcripts in PHA-activated PBMC were attenuated by suberosin. Therefore, we believe that suberosin reduces the production of cyclin D3, E, A and B in PBMC through decreasing of cyclin D3, E, A and B mRNA expression. It appears that treatment of PBMC with suberosin alone had resulted in the suppression of the cyclin A protein and mRNA expression as compared to the treatment with DMSO alone. We explain this result by the following possibilities: (1) the results from real-time PCR demonstrated that suberosin alone did not reduce cyclin A mRNA transcripts in PBMC as comparison with DMSO treated alone. However, both data from RT-PCR and Western blotting were calculated by the semi-quantitative method. We suggest that the inhibitory activities of suberosin on cyclin A expression are almost not significant although we cannot exclude the possibility that suberosin attenuates cyclin A by some other mechanism. (2) Suberosin may affect the stability of cyclin A mRNA or proteins and thus cause the levels of cyclin A transcripts and proteins to decrease. Data from primary human T lymphocytes indicate that cyclin D3 and cyclin E are rate limiting and essential for the progression through the G1 phase of the cell cycle (Charles, 1993; Hleb et al., 2004). Cyclin D3 is a key cyclin controlling T cells growth and apoptosis. Activation of T-cell hybridoma cells through the TCR has been shown to cause rapid reduction in both cyclin D3 mRNA and protein levels, which is accompanied by growth arrest in G1 phase (Truneh et al., 1985). The accumulation of cyclins A and B is a pre-requisite for mitotic initiation in the G2/M phase in mammalian cells. It might be expected that inhibition of cell cycle entry would prevent the production of cyclins A and B. Furthermore, cyclin-dependent kinase (cdk) 4 complexed with cyclin D plays important role in controlling cell proliferation (Ajchenbaum et al., 1993). Preliminary data indicated that the levels of cdk4 mRNA in PHA-activated PBMC were decreased by suberosin (data not shown), suggesting a possible regulatory effect of suberosin on cdk gene expression. Cell proliferation accompanies cell cycle entry, and we suggest that inhibitory effects of suberosin on PBMC proliferation are related to inhibition of cyclin gene expression and cell cycle progression in the cells.
Production of IL-2 and IFN-γ is important in coordinating the activation and proliferation of T lymphocytes (Cantrell, 1996, Liu et al., 2004). We demonstrated that production of these cytokines was inhibited by suberosin. Impairment of IL-2 and IFN-γ production was related to suberosin-suppressing transcription of their mRNA. When exogenous IL-2 was added to PHA-activated PBMC in the presence of suberosin, cell proliferation was determined in a preliminary study. Although, 30% proliferation of suberosin-treated PBMC could be restored by IL-2 at 10 U ml−1, other cytokines such as IL-4 and IFN-γ might help to restore it (data not shown). Another possibility is that higher concentration of IL-2 (above 10 U ml−1) might be required to enhance the recovery of suberosin-treated PBMC proliferation. We also can assume that the block occurs early during cell cycle entry from G0, before IL-2-dependent signals are required to stimulate entry from G1 into S phase. We predicted that inhibition of cell cycle entry would prevent the production of IL-2 and IFN-γ. As T-lymphocyte proliferation is primarily mediated by IL-2, inhibition of IL-2 production is a central mechanism of action of several immunosuppressants including cyclosporin A. However, these actions are similar to those of cyclosporin A, which induces arrest activation and proliferation of T-cells by inhibiting IL-2 transcription (Schreiber and Crabtree, 1992). It is likely that failure to produce IL-2 and IFN-γ is the reason that PBMC did not proliferate.
Two of the earliest signaling events induced by PHA in T cells are activation of NF-AT and NF-κB, essential for proliferation and activation of T cells (Charles et al., 1997). Many previous studies show that NF-AT is an inducible regulatory complex critical for transcriptional induction of many genes in activated T cells, containing IL-2 and IFN-γ (Rao et al., 1997). Inactivation of NF-AT in mice reduces T cells numbers and impairs the proliferation of PBMC (Yoshida et al., 1998). The transcription factor NF-κB is one of the key regulators of genes involved in the immune response as well as in survival from apoptosis (Ferreira et al., 1999). NF-κB has been shown to regulate cell growth and differentiation through transcriptional regulation of cyclin D (Sée et al., 2004). Abolition of NF-κB activity in the T cell lineage of mice caused a decrease in spleenic T cells and suppressed proliferation of peripheral T cells. Further, production of IFN-γ was reduced by inhibition of NF-κB (Ferreira et al., 1999). Therefore, inhibition of NF-κB by suberosin is sufficient to account for inhibition of IFN-γ production and entry into the cell cycle. Nevertheless, the coordinate induction and activation of the NF-AT and NF-κB are required to regulate IL-2, IFN-γ and cyclin gene expression (Ferreira et al., 1999). We have shown that the NF-AT inhibitor cyclosporin A and the NF-κB inhibitor PDTC can block IL-2, IFN-γ and cyclin D3 mRNA transcripts and cell proliferation in PBMC stimulated with PHA. Both EMSA and nuclear translocation analysis were applied to determine inhibitory effects of suberosin on NF-AT and NF-κB activation. Thus, the inhibition by suberosin of IL-2, IFN-γ and cyclin gene expression and of PBMC proliferation could be explained by suberosin inhibiting nuclear translocation and DNA-binding activity of NF-AT and NF-κB, thereby preventing entry of PBMC into the cell cycle.
As a consequence of an increase of [Ca2+]i levels, calcineurin, a Ca2+/calmodulin-dependent protein phosphatase, is activated, leading to dephosphorylation of NF-AT and its subsequent nuclear translocation (Sée et al., 2004). Although PKC activation sequentially results in the phosphorylation and in an incomplete degradation of IκB in T cell lines, co-activation of Ca2+-dependent pathways accelerates the rate of IκB phosphorylation and results in its complete degradation (Lewis, 2001). Therefore, the Ca2+-dependent pathways synergize with PKC-induced activation of nuclear factor NF-κB in T cell lines (Steffan et al., 1995). Whitaker and Larman (2001) have provided clear evidence for a role of [Ca2+]i in the G1/S and G2/M phases of the cell cycle . We have demonstrated that the magnitude of the Ca2+ signal in PBMC triggered by PHA was affected by suberosin. It is likely that this could affect activation of NF-AT and NF-κB in PBMC stimulated with PHA. Sustained activation of ERK is required for fibroblasts to pass the restriction point in G1 and enter S-phase (Pages et al., 1993). The activation of ERK results in the activation of a range of transcriptional factors and cyclin D expression is positively regulated by this ERK signal (Lavoie et al., 1996). Investigation of the activation of JNK, ERK and p38 pathways, as determined by assaying their phosphorylation, showed that ERK was affected by suberosin, whereas JNK and p38 were not affected. The ERK inhibitor U0126 decreased cyclin D3, IL-2 and IFN-γ gene expression in PHA-activated PBMC. We suggest that inhibitory effects of suberosin on gene expression of these cyclins and cytokines and on cell cycle entry are related to inhibition of ERK phosphorylation.
From the present results, we hypothesize that inhibitory mechanisms of suberosin on PHA-activated PBMC proliferation, at least in part, are related to: (1) suberosin affecting [Ca2+]i in the cells; (2) suppression of ERK phosphorylation; (3) reduction of NF-AT and NF-κB activation; (4) decreased production of cytokines and cyclins, as the entry into S phase of the cell cycle induced by PHA was blocked and (5) inhibition of entry into the S phase of the cell cycle causing the antiproliferative effect of suberosin on PBMC. It is believed that arthritis, asthma, cough, and rheumatism are related to overexpression of inflammatory responses (Goodman et al., 1996). Increased activity of NF-κB transcription factor system has been documented in chronic tissue inflammation; accordingly, pharmacologic blockade may become particularly important in the treatment of inflammatory diseases (Neurath et al., 1996). Thus, the results of the present study indicate that suberosin present in extracts of P. zeylanica may also have acted to reduce tissue inflammation in part by inhibiting PBMC proliferation, cytokines gene expression, and NF-κB activation. Our observations correlated with the putative pharmacological activities of P. zeylanica. The relative contributions of these activities to the potent immunosuppressive action of suberosin in vivo remain to be elucidated.
Acknowledgments
This study was partially supported by grant from National Science Council, Republic of China (NSC93-2320-B-030-001; NSC94-2320-B-030-001).
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- DEPC
diethyl pyrocarbonate
- DMSO
dimethyl sulfoxide
- EMSA
electrophoretic mobility shift assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFN-γ
interferon-γ
- IL-2
interleukin-2
- PBMC
human peripheral blood mononuclear cells
- PDTC
pyrrolidine dithiocarbamate
- PHA
phytohemagglutinin
- PMSF
phenylmethylsulfonylfluoride
- RT-PCR
reverse transcription-polymerase chain reaction
Conflict of interest
The authors state no conflict of interest.
References
- Ahsan M, Zaman TA, Hasan CM, Ito C, Islam SK. Constituents and cytotoxicity of Zanthoxylum rhesta stem bark. Fitoterapia. 2000;71:697–700. doi: 10.1016/s0367-326x(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Ajchenbaum F, Ando K, DeCaprio JA, Griffin JD. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- Arai K, Lee F, Miyajima A. Cytokines: Coordinators of immune and inflammatory response. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Arnaud M, Crouin C, Deon C, Loyaux D, Bertoglio J. Phosphorylation of Grb2-associated binder 2 on serine 623 by ERK MAPK regulates its association with phosphatase SHP-2 and decreases STAT5 activation. J Immunol. 2004;173:3962–3971. doi: 10.4049/jimmunol.173.6.3962. [DOI] [PubMed] [Google Scholar]
- Buggins AGS, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NSB, et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-κB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–6030. doi: 10.4049/jimmunol.167.10.6021. [DOI] [PubMed] [Google Scholar]
- Cantrell D. T cell antigen receptor signal transduction pathways. Ann Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- Carter LL, Zhang X, Dubey C, Rogers P, Tsui L, Swain SL. Regulation of T cell subsets from naive to memory. J Immunother. 1998;21:181–187. doi: 10.1097/00002371-199805000-00003. [DOI] [PubMed] [Google Scholar]
- Charles AJ, Jr, Paul T, Hunt S, Walport M. Immunobiology. Current Biology Ltd: New York; 1997. [Google Scholar]
- Charles JS. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinase in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Edward A. NF-κB activation. Crtit Care Med. 2000;28:100–104. [Google Scholar]
- El-Shafae AM, Soliman AS. A pyranocoumarin and two alkaloids (one with antispasmodic effect) from Citrus deliciosa. Pharmazie. 1998;53:640–643. [PubMed] [Google Scholar]
- Ferreira V, Sidenius N, Tarantino N, Hubert P, Chatenoud L, Blasi F, et al. In vivo inhibition of NF-kB in T-lineage cells leads to a dramatic decrease in cell proliferation and cytokine production and to increased cell apoptosis in response to mitogen stimuli, but not to abnormal thymopoiesis. J Immunol. 1999;162:6442–6450. [PubMed] [Google Scholar]
- Gilmore TD, Morin PJ. The IκB proteins: members of a multifunctional family. Trends Genet. 1993;9:427–433. doi: 10.1016/0168-9525(93)90106-r. [DOI] [PubMed] [Google Scholar]
- Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines inpatients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- Gray PW, Aggrawal BB, Benton CV, Bringman TS, Henzel WJ, Jarrett JA, et al. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982;295:503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Hleb M, Murphy S, Wagner EF, Hanna NN, Sharma N, Park J, et al. Evidence cyclin D3 as novel target of rapamycin in human T lymphocytes. J Biol Chem. 2004;279:31948–31955. doi: 10.1074/jbc.M400638200. [DOI] [PubMed] [Google Scholar]
- Javier AF, Bata-Csorgo Z, Ellis CN, Kang S, Voorhees JJ, Cooper KD. Rapamycin (sirolimus) inhibits proliferating cell nuclear antigen expression and blocks cell cycle in the G1 phase in human keratinocyte stem cells. J Clin Invest. 1997;99:2094–2099. doi: 10.1172/JCI119382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountouras J, Zavos C, Chatzopoulos D. Immunomodulatory benefits of cyclosporine A in inflammtory bowel disease. J Cell Mol Med. 2004;8:317–328. doi: 10.1111/j.1582-4934.2004.tb00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaii S, Tomono Y, Ogawa K, Sugiura M, Yano M, Yoshizawa Y, et al. Antiproliferative effect of isopentenylated coumarins on several cancer cell lines. Anticancer Res. 2001;21:1905–1911. [PubMed] [Google Scholar]
- Kennedy JS, Raab M, Rudd CE. Signaling scaffolds in immune cells. Cell Calcium. 1999;26:227–235. doi: 10.1054/ceca.1999.0069. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin LC, Tsai WJ, Chou CJ, Kung SH, Ho YH. Samaragenin B identified from Limonium sinense suppressed herpes simplex virus type 1 replication in Vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Ch. 2002;46:2854–2864. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YC, Weng SC, Chou CJ, Chang TT, Tsai WJ. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br J Pharmacol. 2003;140:895–906. doi: 10.1038/sj.bjp.0705500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YC, Yang NS, Chou CJ, Lin LC, Tsai WJ. Regulation of cell proliferation, gene expression, production of cytokines, and cell cycle progression in primary human T lymphocytes by piperlactam S isolated from Piper kadsura. Mol Pharmacol. 2000;58:1057–1566. doi: 10.1124/mol.58.5.1057. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, L'allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/44MAPK and negatively by p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Dulic V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Li HL.Plumbaginaceae Flora of Taiwan 1998Department of Botany, National Taiwan University: Taipei; 79–82.In: Editorial Committee of the Flora of Taiwan (eds)Vol. IV [Google Scholar]
- Lin LC, Yang LL, Chou CJ. Cytotoxic naphthoquinones and plumbagic acid glucosides from Plumbago zeylanica. Phytochemistry. 2003;62:619–622. doi: 10.1016/s0031-9422(02)00519-8. [DOI] [PubMed] [Google Scholar]
- Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF, Kuo YC. The extracts from Nelumbo nucrifera suppress cell cycle progression, cytokine genes expression, and cell proliferation in human peripheral blood mononuclear cells. Life Sci. 2004;75:699–716. doi: 10.1016/j.lfs.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Nayar MNS, Bhan MK. Coumarins and other constituents of Hesperethusa crenulata. Phytochemistry. 1972;11:3331–3333. [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local adminstration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Malonne H, Duez P, Vanhaelen-Fastre R, Vanhaelen M, Fontaine J. Cytotoxic constituents from Plumbago zeylanica. Fitoterapia. 2004;75:500–504. doi: 10.1016/j.fitote.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parde AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Parkar MH, Hussain F, Wickrmaratna A, Olsen I. The immunosuppressant and hyperplasia-inducing drug cyclosporin A regulates the cell cycle and cyclin B1 gene expression in gingival fibroblasts in vitro. Cell Tissue Res. 2004;317:221–225. doi: 10.1007/s00441-004-0909-3. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Rathmann S, Rajasalu T, Rosinger S, Schlosser M, Eiermann T, Boehm BO, et al. Preproinsulin-specific CD8+ T cells secrete IFN-γ in human type 1 diabetes. Ann N Y Acad Sci. 2004;1037:22–25. doi: 10.1196/annals.1337.004. [DOI] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclsporin A and FK 506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Sée V, Rajala NKM, Spiller DG, White MRH. Calcium-dependent regulation of cell cycle via a novel MAPK-NF-κB pathway in Swiss 3T3 cells. J Cell Biol. 2004;166:661–672. doi: 10.1083/jcb.200402136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica AL, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, et al. Interaction of NF-κB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- Steffan NM, Bren GD, Frantz B, Tocci MJ, O'Neill EA, Paya CV. Regulation of IkB alpha phosphorylation by PKC- and Ca(2+)-dependent signal transduction pathways. J Immunol. 1995;155:4685–4691. [PubMed] [Google Scholar]
- Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, et al. Structure and expression of a cloned cDNA for interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Teng CM, Li HL, Wu TS, Huang SC, Huang TF. Antiplatelet actions of some coumarin compounds isolated from plant sources. Thromb Res. 1992;66:549–557. doi: 10.1016/0049-3848(92)90309-x. [DOI] [PubMed] [Google Scholar]
- Tilak JC, Adhikari S, Devasagayam TP. Antioxidant properties of Plumbago zeylanica, and Indian medicinal plant and its active ingredient, plumbagin. Redox Rep. 2004;9:219–227. doi: 10.1179/135100004225005976. [DOI] [PubMed] [Google Scholar]
- Truneh A, Albert F, Golstein P, Schmitt-Verhulst AM. Early steps of lymphocytes activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985;313:318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Whitaker M, Larman MG. Calcium and mitosis. Semin Cell Dev Biol. 2001;12:54–58. doi: 10.1006/scdb.2000.0217. [DOI] [PubMed] [Google Scholar]
- Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ. Differentiation of CD4+ T cells to Th1 requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- Yang NS, Chou CJ, Lin LC, Tsai WJ, Kuo YC. Evalution of Chinese herbs that affect the cell-mediated immunity (III) J Chinese Med. 1999;10:179–188. [Google Scholar]
- Yoshida H, Nishina H, Takimoto H, Marengere LE, Wakeham AC, Bouchard D, et al. The transcription factor NF-Atc1 regulates lymphocytes proliferation and Th2 cytokine production. Immunity. 1998;8:15–24. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- Zornig M, Evan GI. Cell cycle: on target with Myc. Curr Biol. 1996;6:1553–1556. doi: 10.1016/s0960-9822(02)70769-0. [DOI] [PubMed] [Google Scholar]