Abstract
Background and purpose:
Postsystolic wall thickening (PSWT) is part of thickening that occurs after end-systole and represents wasted effort as it does not contribute to ejection. The effects of antianginal drugs on PSWT remain to be established. We compared the effects on PSWT of two agents that reduce heart rate, the β-blocker atenolol and the selective inhibitor of If current, ivabradine.
Experimental approach:
Six dogs were prepared to measure wall thickening by sonomicrometry in the conscious state, at rest and during exercise, after administration of saline, atenolol (1mg.kg-1) or ivabradine (1mg.kg-1).
Key results:
Atenolol and ivabradine similarly reduced heart rate vs saline at rest (about 10-20%) and during exercise (about 30%). Atenolol but not ivabradine decreased dP/dtmax. Concomitantly, PSWT increased with atenolol vs saline at rest (0.35±0.07 vs 0.21±0.03mm, respectively) and during exercise (0.30±0.04 vs 0.15±0.04mm, respectively). In contrast, ivabradine did not alter PSWT. Importantly, atenolol but not ivabradine increased the ratio of postsystolic to systolic wall thickening by 80±23%. This enhanced thickening during diastole with atenolol was accompanied by impeded isovolumic relaxation of the left ventricle, as illustrated by the significant correlation between the isovolumic relaxation time constant τ and the postsystolic to systolic wall thickening ratio. None of these effects of atenolol were abolished when heart rate was controlled with atrial pacing.
Conclusion and implications:
For a similar heart rate reduction at rest and during exercise, ivabradine, but not atenolol, did not alter PSWT and preserved the part of thickening contributing to ejection.
Keywords: heart rate reduction, postsystolic, If inhibition, β-blockade
Introduction
The effects of antianginal drugs on contractile function have been extensively investigated but their impact on one important component of cardiac motion remains to be established, that is, postsystolic wall thickening or shortening (Brown et al., 1987; Takayama et al., 1988; Rose et al., 1993). This parameter has gained interest with the clinical use of echocardiographic tissue Doppler imaging for quantification of myocardial function (Voigt et al., 2003). Postsystolic motion represents the part of contraction that occurs after closure of the aortic valve (Takayama et al., 1988). It is a marker of left ventricular asynchrony (Ehring and Heusch, 1990) and it has been extensively described during experimental myocardial ischemia (Brown et al., 1987; Takayama et al., 1988; Rose et al., 1993; Birkeland and Hexeberg 1994; Derumeaux et al., 2000; Pislaru et al., 2001; Pislaru et al., 2004) as well as in clinical settings (Jamal et al., 1999; Hosokawa et al., 2000; Kukulski et al., 2003; Song et al., 2004; Zwanenburg et al., 2004). Measurement of postsystolic wall motion has also been proposed as a tool to assess the viability of the ischemic myocardium and to predict the recovery of left ventricular function during ischemic episodes (Brown et al., 1987; Takayama et al., 1988; Hosokawa et al., 2000). In heart failure, this measure is useful in predicting reverse remodeling after cardiac resynchronization therapy (Yu et al., 2004). However, the interpretation of postsystolic wall motion remains still highly debated (Birkeland and Hexeberg 1994; Sutherland, 2004) and part of this debate might result from the different effects on postsystolic wall motion of the pharmacological agents that are used when ischemic patients are treated with antianginal drugs. Therefore, interpretation of this parameter should take into account the relevant effects of pharmacological agents on this parameter. In this context, investigation of the effects of antianginal drugs on postsystolic wall motion can be considered as new and important information for their pharmacological profile.
Antianginal drugs such as β-blockers or ivabradine, a selective If channel inhibitor, share a common mechanism leading to their beneficial effects, that is, they reduce heart rate (Thollon et al., 1994, 1997; Colin et al., 2002, 2003; Borer, 2004; Tardif et al., 2005), which is a major determinant of the balance between myocardial oxygen supply and demand. β-blockers are well known to exert negative inotropic and lusitropic effects (Simon et al., 1995; Colin et al., 2002) contrasting with ivabradine, which is devoid of any intrinsic negative effect on systolic and diastolic functions (Simon et al., 1995; Colin et al., 2002; Vilaine et al., 2003). Accordingly, we compared the effects of heart rate reduction induced by atenolol and ivabradine on postsystolic wall thickening at rest and during treadmill exercise in conscious dogs, under conditions of both spontaneous and controlled heart rate with atrial pacing.
Methods
The animal instrumentation and the experiments were performed in accordance with the official regulations of the French Ministry of Agriculture.
Instrumentation
A left thoracotomy was performed in six dogs as described previously (Lucats et al., 2005). Fluid-filled Tygon catheters were placed in the descending thoracic aorta and the left atrium for measurement of blood pressure. A Silastic catheter was implanted in the pulmonary artery. A solid-state pressure transducer (P7A, Konigsberg Instruments, Pasadena, CA, USA) was introduced into the apex of the left ventricle (LV). A pair of ultrasonic crystals was used for measurement of LV wall thickening. One crystal was implanted within the endocardium and the other was sutured to the epicardium of the posterior free wall of the LV. Proper alignment of the crystals was ensured by visualizing the signal on an oscilloscope. A pair of electrodes was sutured on the right atrium to allow pacing. All catheters and wires were exteriorized between the scapulae and the pneumothorax was evacuated. Cefazolin (1 g intravenous (i.v.)) and gentamicin (40 mg i.v.) were administered before and during the first week after surgery. Postoperative analgesia was provided with morphine. The correct position of the crystals was verified at autopsy.
Hemodynamic measurements
All hemodynamic data were analyzed using the data acquisition software HEM v3.5. (Notocord Systems, Croissy-sur-Seine, France). Aortic and left atrial pressures were measured with a Statham P23 ID strain-gauge transducer (Gould-Nicolet, Courtaboeuf, France). LV pressure was measured using the Konigsberg gauge and the change in LV pressure over time (LV dP/dt) was computed from the LV pressure signal. LV pressure was calibrated in vitro with a mercury manometer and in vivo with the left atrial and aortic pressures.
Measurements of regional contractility
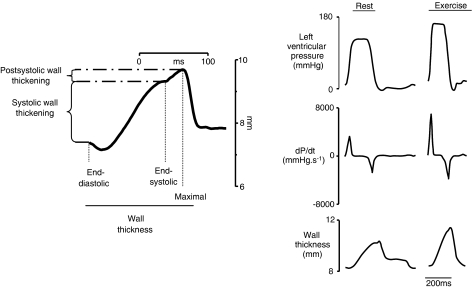
Wall thicknesses were obtained by using an ultrasonic transit-time dimension gauge (Module 201, System 6, Triton Technology Inc., San Diego, CA, USA). As illustrated in Figure 1, systolic wall thickening was defined as the difference between end-diastolic and end-systolic wall thicknesses, that is, the wall thickening (expressed in millimeter) that occurs during the ejection period. Maximal wall thickness was defined as the maximal distance between crystals, measured after end-systole. Postsystolic wall thickening was defined as the maximal minus end-systolic wall thicknesses, that is, the wall thickening that occurs after the ejection period. Rate of wall thickening was computed from the wall thickness signal and its maximal value (dW/dtmax) was measured during systole.
Figure 1.
Left panel: typical waveform representing the evolution of myocardial wall thickness and postsystolic wall thickening during a single beat: systolic wall thickening was defined as the difference between end-diastolic and -systolic wall thicknesses; maximal wall thickness was defined as the maximal distance between the implanted crystals measured after end-systole; postsystolic wall thickening was defined as the maximal minus end-systolic wall thicknesses. Right panel: representative recordings of left ventricular pressures, left ventricular pressure first derivative (LV dP/dt) and left ventricular posterior wall thickness measured at rest and during exercise in control conditions.
Calculation of isovolumic relaxation time constant (τ)
As previously described (Colin et al., 2002), the isovolumic relaxation period was defined as the period elapsed from the time at the peak of negative LV dP/dt to the time when LV pressure fell to a value of 5 mm Hg above LV end-diastolic pressure of the following beat. Using a best fit monoexponential decay model with non-zero asymptote, the left ventricular relaxation time constant, tau (τ), was calculated by the Levenberg–Marquart nonlinear regression algorithm to obtain the least squares best fit curve to the measured LV pressure.
Experimental protocol
The experiments were conducted at least 3 weeks after surgery. While the dogs were standing quietly on the treadmill, ‘baseline' parameters were recorded. A second set of measurements was performed 15 min after the onset of drug administration (saline, atenolol or ivabradine), both at spontaneous rate (so-called ‘rest') and during a sequence of 5 min of atrial pacing at a rate of 125 beats min−1 (so-called ‘paced rest'). Treadmill exercise (10 km h−1, slope 13%, 10 min) was then started. The first 5 min of exercise was performed at spontaneous heart rate, with a set of measurements when a hemodynamic steady state was achieved (so-called ‘exercise'). The last 5 min of exercise was performed under atrial pacing at a rate of 250 beats min−1 (so-called ‘paced exercise'), with a last set of measurements being performed at the end of this stage.
Each dog underwent three sequences (treatment with saline, atenolol and ivabradine), which were performed in a random order a week apart. Ivabradine (1 mg kg−1), atenolol (1 mg kg−1) or saline were administered through the pulmonary artery catheter as an intravenous bolus. We have previously demonstrated that hemodynamic changes are reproducible when the exercises are repeated (Berdeaux et al., 1994).
Statistical analysis
Data are reported as mean±s.e.m. Comparisons were performed using two-way analysis of variance for repeated measures. If needed, individual comparisons were then conducted using a paired Student's t-test with Bonferroni's correction. Regression lines were calculated with the last-squares method. A value of P<0.05 was considered significant.
Drugs
Ivabradine was obtained from Laboratoire Servier Neuilly-sur-Seine, France and atenolol from Sigma-Aldrich, Saint Quentin Fallavier, France.
Results
Hemodynamics
As. shown in Table 1, baseline hemodynamic values were not significantly different among the three sequences of the protocol and none of these parameters was altered at rest after saline administration. Atenolol and ivabradine decreased heart rate at rest as compared to saline (−11±5 and −19±5%, respectively) and similarly limited its increase during exercise. Atenolol, but not ivabradine, significantly reduced LV dP/dtmax as compared to saline both at rest and during exercise. These effects of atenolol were not abolished by atrial pacing.
Table 1.
Hemodynamic effects of saline, atenolol and ivabradine at rest and during exercise
| Baseline | Rest | Paced rest | Exercise | Exercise paced | |
|---|---|---|---|---|---|
| HR (beats/min) | |||||
| Saline | 112±4 | 112±4 | 125±0 | 218±6 | 249±1 |
| Atenolol | 107±7 | 99±5* | 126±1 | 152±5* | 246±4 |
| Ivabradine | 108±5 | 91±5* | 125±0 | 147±5* | 249±1 |
| MAP (mm Hg) | |||||
| Saline | 106±5 | 106±5 | 109±3 | 113±6 | 109±5 |
| Atenolol | 106±10 | 112±12 | 113±11 | 111±14 | 112±16 |
| Ivabradine | 110±5 | 110±6 | 119±7 | 116±10 | 122±9 |
| LV pressure (mm Hg) | |||||
| Saline | 135±5 | 135±5 | 136±8 | 173±12 | 169±12 |
| Atenolol | 131±7 | 130±9 | 130±7 | 146±10* | 141±11* |
| Ivabradine | 137±8 | 140±11 | 143±10 | 181±13 | 178±17 |
| LV dP/dtmax (mmHg s−1) | |||||
| Saline | 3518±335 | 3518±335 | 3578±440 | 7162±1116 | 7282±905 |
| Atenolol | 3232±341 | 2529±219* | 2530±223* | 3351±298* | 4163±589* |
| Ivabradine | 3480±336 | 3622±459 | 3586±450 | 6740±971 | 8042±1591 |
| τ (ms) | |||||
| Saline | 22±2 | 22±2 | 21±2 | 15±2 | 16±2 |
| Atenolol | 22±2 | 27±7* | 26±2* | 23±2* | 20±2* |
| Ivabradine | 22±2 | 22±2 | 21±1 | 15±2 | 16±1 |
Abbreviations: HR, heart rate; LV, left ventricle; LV dP/dtmax,maximum change in left ventricular pressure over time; MAP, mean arterial pressure; τ, left ventricular relaxation time constant.
Values are means±s.e.m., n=6 dogs in all sequences. Doses for atenolol and ivabradine were 1 mg kg−1.
P<0.05 vs saline.
Regional contractility
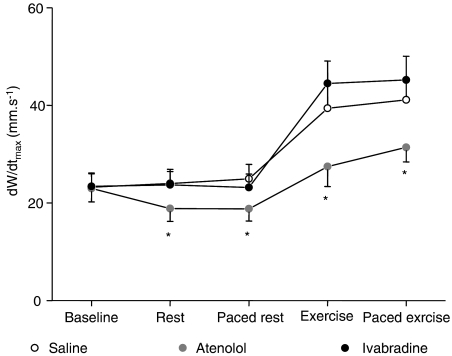
As shown in Table 2, baseline regional contractile parameters were not significantly different among the three sequences of the protocol and none of these parameters was altered at rest after saline administration. As illustrated in Figure 2, atenolol, but not ivabradine, significantly decreased the maximal rate of systolic wall thickening (dW/dtmax), both at rest and during exercise as compared to saline (−21±7 and −29±7%, respectively). This effect persisted when heart rate was controlled with atrial pacing.
Table 2.
Effects of saline, atenolol and ivabradine on myocardial wall thickening at rest and during exercise
| Baseline | Rest | Paced rest | Exercise | Exercise paced | |
|---|---|---|---|---|---|
| EDWT (mm) | |||||
| Saline | 8.9±0.5 | 8.8±0.5 | 9.0±0.6 | 8.7±0.5 | 9.0±0.6 |
| Atenolol | 8.9±0.6 | 8.6±0.5 | 8.7±0.5 | 8.3±0.5* | 9.3±0.6 |
| Ivabradine | 8.8±0.5 | 8.7±0.6 | 8.9±0.6 | 8.5±0.5 | 9.2±0.6 |
| SWT (mm) | |||||
| Saline | 2.6±0.2 | 2.6±0.2 | 2.5±0.2 | 3.7±0.5 | 3.4±0.6 |
| Atenolol | 2.6±0.1 | 2.4±0.3 | 2.2±0.2 | 3.4±0.4 | 2.3±0.2 |
| Ivabradine | 2.6±0.2 | 2.6±0.2 | 2.3±0.2 | 4.4±0.5* | 3.5±0.6 |
| PSWT (mm) | |||||
| Saline | 0.25±0.02 | 0.21±0.03 | 0.21±0.04 | 0.15±0.04 | 0.16±0.05 |
| Atenolol | 0.23±0.04 | 0.35±0.07* | 0.38±0.07* | 0.30±0.04* | 0.37±0.07* |
| Ivabradine | 0.24±0.06 | 0.22±0.07 | 0.22±0.03 | 0.11±0.04 | 0.17±0.05 |
Abbreviations: EDWT, end-diastolic wall thickness; PSWT, postsystolic wall thickening; SWT, systolic wall thickening.
Values are mean±s.e.m., n=6 dogs in all sequences. Doses for atenolol and ivabradine were 1 mg kg−1.
P<0.05 vs saline.
Figure 2.
Maximal rate of systolic wall thickening (dW/dtmax) measured at baseline, at rest, at paced rest, during exercise and paced exercise with saline, atenolol (1 mg kg−1 i.v.) and ivabradine (1 mg kg−1 i.v). *P<0.05 vs saline.
As shown in Table 2, postsystolic wall thickening was significantly increased with atenolol as compared to saline whereas ivabradine did not alter this parameter. This effect was observed both at rest and during exercise and was still present while heart rate was controlled with atrial pacing. Interestingly, a part of thickening devoted to ejection was also altered by atenolol but not by ivabradine as illustrated in Figure 3, that is, postsystolic to systolic wall thickening ratio (PS/S) was significantly increased by atenolol whereas ivabradine did not alter this parameter as compared to saline. This was observed both at spontaneous and controlled heart rate.
Figure 3.
PS/S measured at baseline, at rest and during exercise at spontaneous heart rate (left panel) and at controlled heart rate with atrial pacing (right panel) under saline, atenolol (1 mg kg−1) and ivabradine (1 mg kg−1). *P<0.05 vs saline.
Relationship between LV isovolumic relaxation and postsystolic wall thickening
As shown in Table 1, the left ventricular relaxation time constant, τ, was similar among the three sequences at baseline. Atenolol significantly increased this parameter by 27±10 and 52±9% as compared to corresponding saline value at rest and during exercise, respectively. In contrast, τ was not altered by ivabradine. When heart rate was controlled with atrial pacing, this effect of atenolol on τ persisted both at rest and during exercise.
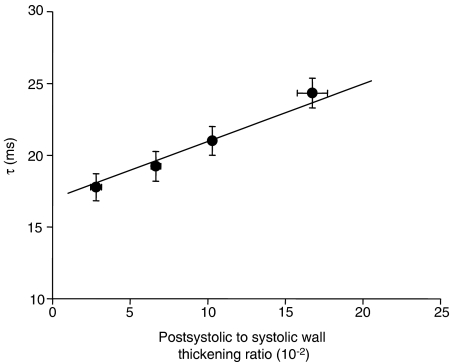
As illustrated in Figure 4, τ was significantly correlated with PS/S when all individual data from the three sequences were plotted together (τ=0.40 × PS/S+16.94, r=0.44, P<0.05).
Figure 4.
Correlation between postsystolic to systolic wall thickening ratio and left ventricular relaxation time constant. All individual data collected during the three sequences were pooled and in these conditions, a significant correlation between the two parameters was found (τ=0.40 × PS/S+16.94, τ=0.44, P<0.05). Individual values were pooled into the four ranges that are represented in the figure.
Discussion
This study compared the effects of two antianginal drugs, the β-blocker atenolol and the selective If current inhibitor, ivabradine, on postsystolic wall thickening at rest and during exercise. For similar heart rate reduction during exercise, these two agents exhibited marked differences in the patterns of regional contractility. Although atenolol depressed systolic function, it significantly increased postsystolic wall thickening both at rest and during exercise. These effects were independent of heart rate reduction as they were not abolished by atrial pacing. Furthermore, atenolol increased PS/S, and therefore, further compromised systolic function as it decreased the part of regional thickening devoted to ejection. This effect was probably the consequence of its negative inotropic properties as it was still observed with atrial pacing. In contrast, selective heart rate reduction with ivabradine did not alter postsystolic wall thickening and preserved the part of thickening that contributed to ejection. Finally, as demonstrated by the positive correlation between τ and PS/S, this enhanced paradoxical wall motion observed with atenolol but not with ivabradine, paralleled the impairment of left ventricular isovolumic relaxation.
Postsystolic wall thickening is a part of regional thickening that occurs after aortic valve closure and is thus occurring during diastole (Rose et al., 1993; Takayama et al., 1988; Skulstad et al., 2002; Voigt et al., 2003; Sutherland, 2004). This paradoxical motion does not contribute to ejection and therefore represents a ‘waste' of cardiac effort. Postsystolic wall thickening has also been described in the normal heart (Voigt et al., 2003; Zwanenburg et al., 2004). In this study, administration of atenolol significantly increased postsystolic wall thickening both at rest and during exercise as compared to saline. As demonstrated by the increase in PS/S, the part of thickening devoted to ejection was reduced by atenolol and this paradoxical wall motion was therefore wasted. This effect of atenolol was independent of heart rate reduction as it was not corrected by atrial pacing. Furthermore, ivabradine altered neither postsystolic wall thickening nor the PS/S; therefore, preserving systolic function during heart rate reduction. In agreement with previous reports, we did not observe significant negative inotropic effects with ivabradine (Simon et al., 1995; Colin et al., 2002; Monnet et al., 2004).
Postsystolic wall thickening occurs after end-systole, that is, it occurs at the same time as left ventricular isovolumic relaxation. Therefore, this paradoxical wall thickening occurring during diastole impedes the normal process of relaxation at the time when the myocardial wall needs to become thin to achieve relaxation. As previously described (Colin et al., 2002), the relaxation time constant, τ, was increased by atenolol, demonstrating that β-blockade decreased the physiological acceleration of left ventricular pressure fall at rest and during exercise, despite a limited increase in heart rate. In contrast, the effects of ivabradine and saline on τ were similar, that is, reduction in heart rate per se did not counteract the acceleration process during left ventricular isovolumic relaxation as described previously (Colin et al., 2002). Interestingly, the increase in τ caused by atenolol was accompanied by the increase in postsystolic wall thickening. We observed a positive correlation between τ and PS/S, suggesting that left ventricular isovolumic relaxation was impeded when an enhanced part of thickening initially devoted to ejection was finally wasted during diastole. In addition to previously reported mechanisms for the increase in τ during β-blockade (Colin et al., 2002), one could speculate that postsystolic wall thickening may participate in the negative lusitropic properties of atenolol. Conversely, during exercise, postsystolic wall thickening, τ and PS/S were reduced. This is an important issue as during exercise, the marked increase in left ventricular filing rate in early diastole mainly depends on the ability of the LV to relax rapidly and completely (Cheng et al., 1992).
In conclusion, this study demonstrates that during exercise, atenolol increased postsystolic wall thickening and PS/S both at rest and during exercise whereas isovolumic relaxation was impaired. In contrast, heart rate reduction with ivabradine did not alter these parameters and preserved that part of wall thickening that was contributing to ejection. As coronary artery perfusion occurs during diastole, this might have implications in the context of myocardial ischemia regarding oxygen supply. Further studies are needed to extend these findings during myocardial ischemia and post-ischemic dysfunction.
Acknowledgments
We thank Drs F Mahlberg, P Gluais, JP Vilaine and G Lerebours for fruitful discussions during the preparation of this manuscript. Laurence Lucats was a recipient of support from the Académie Nationale de Médecine.
Abbreviations
- dW/dtmax
maximal rate of thickening
- i.v.
intravenous
- LV
left ventricle
- LV dP/dt
first derivative over time of left ventricular pressure
- PS/S
postsystolic to systolic wall thickening ratio
- PSWT
postsystolic wall thickening
- τ
isovolumic relaxation time constant
Conflict of interest
This study was supported by the Institut de Recherche International Servier (Neuilly-sur-Seine, France).
References
- Berdeaux A, Ghaleh B, Dubois-Rande JL, Vigue B, Drieu La Rochelle C, Hittinger L, et al. Role of vascular endothelium in exercise-induced dilation of large epicardial coronary arteries in conscious dogs. Circulation. 1994;89:2799–2808. doi: 10.1161/01.cir.89.6.2799. [DOI] [PubMed] [Google Scholar]
- Birkeland S, Hexeberg E. Is postsystolic shortening area always a marker of myocardial ischaemia. Acta Physiol Scand. 1994;151:269–277. doi: 10.1111/j.1748-1716.1994.tb09746.x. [DOI] [PubMed] [Google Scholar]
- Borer JS. Drug insight: if inhibitors as specific heart-rate-reducing agents. Nat Clin Pract Cardiovasc Med. 2004;1:103–109. doi: 10.1038/ncpcardio0052. [DOI] [PubMed] [Google Scholar]
- Brown MA, Norris RM, Takayama M, White HD. Post-systolic shortening: a marker of potential for early recovery of acutely ischaemic myocardium in the dog. Cardiovasc Res. 1987;21:703–716. doi: 10.1093/cvr/21.10.703. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res. 1992;70:9–19. doi: 10.1161/01.res.70.1.9. [DOI] [PubMed] [Google Scholar]
- Colin P, Ghaleh B, Hittinger L, Monnet X, Slama M, Giudicelli JF, et al. Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 2002;282:H672–H679. doi: 10.1152/ajpheart.00547.2001. [DOI] [PubMed] [Google Scholar]
- Colin P, Ghaleh B, Monnet X, Su J, Hittinger L, Giudicelli JF, et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284:H676–H682. doi: 10.1152/ajpheart.00564.2002. [DOI] [PubMed] [Google Scholar]
- Derumeaux G, Ovize M, Loufoua J, Pontier G, Andre-Fouet X, Cribier A. Assessment of nonuniformity of transmural myocardial velocities by color-coded tissue Doppler imaging: characterization of normal, ischemic, and stunned myocardium. Circulation. 2000;101:1390–1395. doi: 10.1161/01.cir.101.12.1390. [DOI] [PubMed] [Google Scholar]
- Ehring T, Heusch G. Left ventricular asynchrony: an indicator of regional myocardial dysfunction. Am Heart J. 1990;120:1047–1057. doi: 10.1016/0002-8703(90)90116-f. [DOI] [PubMed] [Google Scholar]
- Hosokawa H, Sheehan FH, Suzuki T. Measurement of postsystolic shortening to assess viability and predict recovery of left ventricular function after acute myocardial infarction. J Am Coll Cardiol. 2000;35:1842–1849. doi: 10.1016/s0735-1097(00)00634-3. [DOI] [PubMed] [Google Scholar]
- Jamal F, Kukulski T, D'Hooge J, De Scheerder I, Sutherland G. Abnormal postsystolic thickening in acutely ischemic myocardium during coronary angioplasty: a velocity, strain, and strain rate doppler myocardial imaging study. J Am Soc Echocardiogr. 1999;12:994–996. doi: 10.1016/s0894-7317(99)70154-9. [DOI] [PubMed] [Google Scholar]
- Kukulski T, Jamal F, Herbots L, D'Hooge J, Bijnens B, Hatle L, et al. Identification of acutely ischemic myocardium using ultrasonic strain measurements. A clinical study in patients undergoing coronary angioplasty. J Am Coll Cardiol. 2003;41:810–819. doi: 10.1016/s0735-1097(02)02934-0. [DOI] [PubMed] [Google Scholar]
- Lucats L, Chalvignac V, Bize A, Monnet X, Zini R, Hittinger L, et al. Rapid ventricular pacing induces delayed cardioprotection against myocardial stunning. J Mol Cell Cardiol. 2005;39:849–855. doi: 10.1016/j.yjmcc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Monnet X, Colin P, Ghaleh B, Hittinger L, Giudicelli JF, Berdeaux A. Heart rate reduction during exercise-induced myocardial ischaemia and stunning. Eur Heart J. 2004;25:579–586. doi: 10.1016/j.ehj.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Pislaru C, Belohlavek M, Bae RY, Abraham TP, Greenleaf JF, Seward JB. Regional asynchrony during acute myocardial ischemia quantified by ultrasound strain rate imaging. J Am Coll Cardiol. 2001;37:1141–1148. doi: 10.1016/s0735-1097(01)01113-5. [DOI] [PubMed] [Google Scholar]
- Pislaru C, Bruce CJ, Seward JB, Greenleaf JF. Distinctive changes in end-diastolic wall thickness and postsystolic thickening in viable and infarcted myocardium. J Am Soc Echocardiogr. 2004;17:855–862. doi: 10.1016/j.echo.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Rose J, Schulz R, Martin C, Heusch G. Post-ejection wall thickening as a marker of successful short term hibernation. Cardiovasc Res. 1993;27:1306–1311. doi: 10.1093/cvr/27.7.1306. [DOI] [PubMed] [Google Scholar]
- Simon L, Ghaleh B, Puybasset L, Giudicelli JF, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275:659–666. [PubMed] [Google Scholar]
- Skulstad H, Edvardsen T, Urheim S, Rabben SI, Stugaard M, Lyseggen E, et al. Postsystolic shortening in ischemic myocardium: active contraction or passive recoil. Circulation. 2002;106:718–724. doi: 10.1161/01.cir.0000024102.55150.b6. [DOI] [PubMed] [Google Scholar]
- Song JK, Song JM, Kang DH, Haluska B, Marwick TH. Postsystolic thickening detected by Doppler myocardial imaging: a marker of viability or ischemia in patients with myocardial infarction. Clin Cardiol. 2004;27:29–32. doi: 10.1002/clc.4960270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR. Do regional deformation indexes reflect regional perfusion in all ischemic substrates. J Am Coll Cardiol. 2004;44:1672–1674. doi: 10.1016/j.jacc.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Takayama M, Norris RM, Brown MA, Armiger LC, Rivers JT, White HD. Postsystolic shortening of acutely ischemic canine myocardium predicts early and late recovery of function after coronary artery reperfusion. Circulation. 1988;78:994–1007. doi: 10.1161/01.cir.78.4.994. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- Thollon C, Bidouard JP, Cambarrat C, Lesage L, Reure H, Delescluse I, et al. Stereospecific in vitro and in vivo effects of the new sinus node inhibitor (+)−S 16257. Eur J Pharmacol. 1997;339:43–51. doi: 10.1016/s0014-2999(97)01364-2. [DOI] [PubMed] [Google Scholar]
- Thollon C, Cambarrat C, Vian J, Prost JF, Peglion JL, Vilaine JP. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaine JP, Bidouard JP, Lesage L, Reure H, Peglion JL. Anti-ischemic effects of ivabradine, a selective heart rate-reducing agent, in exercise-induced myocardial ischemia in pigs. J Cardiovasc Pharmacol. 2003;42:688–696. doi: 10.1097/00005344-200311000-00016. [DOI] [PubMed] [Google Scholar]
- Voigt JU, Lindenmeier G, Exner B, Regenfus M, Werner D, Reulbach U, et al. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr. 2003;16:415–423. doi: 10.1016/s0894-7317(03)00111-1. [DOI] [PubMed] [Google Scholar]
- Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- Zwanenburg JJ, Gotte MJ, Kuijer JP, Heethaar RM, van Rossum AC, Marcus JT. Timing of cardiac contraction in humans mapped by high-temporal-resolution MRI tagging: early onset and late peak of shortening in lateral wall. Am J Physiol Heart Circ Physiol. 2004;286:H1872–H1880. doi: 10.1152/ajpheart.01047.2003. [DOI] [PubMed] [Google Scholar]