Abstract
Background and purpose:
A bradykinin (BK) B2 receptor (B2R) antagonist, B-9870 (CU201), has been proposed to behave as a ‘biased agonist' at B2Rs and to exert anti-neoplasic effects. It was unclear whether these effects were determined by the activation of B2Rs by the drug. B-9870 was evaluated for antagonism or stimulation of several responses mediated by the rabbit B2R or B1 receptor (B1R); its anti-proliferative activity was also characterized.
Experimental approach and key results:
B-9870 was an insurmountable B2R antagonist in the rabbit jugular vein contractility assay, but a partial agonist in HEK 293 cells expressing the rabbit B2R or a green fluorescent protein (GFP) conjugate of the latter (ERK1/2 phosphorylation, [Ca2+]i, [3H]-arachidonate release, endocytosis). The agonist-like effects of B-9870 were inhibited by the B2R antagonist LF 16.0687 and absent in untransfected cells. In addition, B-9870 was a surmontable antagonist of the rabbit B1R in the aorta contractility assay, and blocked Lys-des-Arg9-BK-induced ERK1/2 phosphorylation in HEK 293 cells expressing a fluorescent B1R conjugate. B-9870 inhibited the growth of MDA-MB-231 cells. The latter effect was not influenced by B1R or B2R antagonists and was not apoptotic. MDA-MB-231 cells expressed a small population of B2Rs but no B1Rs; they responded to BK (small calcium transients) and B-9870 behaved as an antagonist.
Conclusion and implications:
B-9870 is a dual B1R and B2R antagonist with confirmed stimulating effects at the B2R in high expression systems only. Its cell type-specific anti-proliferative effect occurs at a high concentration, independently from kinin receptors and apoptosis.
Keywords: bradykinin, B2 receptor, B1 receptor, MDA-MB-231 cells, pharmacological antagonism, biased agonist, B-9870 (CU201)
Introduction
Bradykinin (BK)-related peptides (the kinins) are blood-derived peptides that are vasodilator, hypotensive, natriuretic and pro-inflammatory (Leeb-Lundberg et al., 2005). The role of the preformed B2 receptors (B2Rs) and the inducible B1 receptors (B1Rs) is prominent in the mediation of kinin effects in cell types such as the endothelial and smooth muscle cells, the afferent nerve terminals, the renal epithelium and others. B1Rs and B2Rs are G protein-coupled receptors primarily linked to phospholipase C activation, mainly via Gq proteins (De Weerd and Leeb-Lundberg, 1997; Leeb-Lundberg et al., 2005). Following agonist stimulation, B2Rs are redistributed to caveolae and endocytic vesicles that are not clathrin-coated (De Weerd and Leeb-Lundberg, 1997; Haasemann et al., 1998). Ser/Thr phosphorylation and dephosphorylation events precede the internalization and recycling to the surface of the B2R, respectively (Blaukat et al., 1996; Pizard et al., 1999). A fluorescent rabbit B2R-green fluorescent protein conjugate (B2R-GFP) is confined to a recyling endosome compartment, and it is completely recycled in agonist-stimulated HEK 293 cells (Bachvarov et al., 2001).
‘Biased agonists' have been defined as receptor ligands causing the activation of a subset of the signal transduction pathways activated by the reference agonist (MacKinnon et al., 2001; Chan et al., 2002a). This concept may differ from the definition of a partial agonist, a drug that causes a fraction of the maximal activation of all signalling pathways of the receptor. Synthetic drugs consisting of dimers of peptide B2R antagonists, linked at their N-terminus by the suberimidyl moiety (Stewart et al., 1997), are proposed to behave as biased agonists of the B2R. The most active drug in this respect, B-9870 (also called CU201), blocked the proliferation of tumour-derived cell lines, activated c-Jun kinase and caspase-3 while antagonizing BK-induced calcium signalling (Chan et al., 2002a). The monomer structure of B-9870, D-Arg-[Hyp3, Igl5, D-Igl7, Oic8]BK (B-9430) was essentially devoid of the biased agonist properties of the dimer, but was a potent B2R antagonist and, in previous investigations, a significant antagonist at the related B1R as well (Stewart et al., 1996; Chan et al., 2002a). B-9870 inhibits tumour cell growth both in vitro and in vivo (Stewart et al., 2005). The pharmacological effects of B-9870 on tumour cells have been postulated to be mediated by endogenously expressed B2Rs, although this has not been directly evaluated (Chan et al., 2002a, 2002b).
The major aim of the present work was to verify whether the antiproliferative effect of B-9870 is a generalized cellular response mediated by kinin receptors. To support this line of investigation, the pharmacological profile of B-9870 has been initially established at the natural or recombinant rabbit receptors of both B1 and B2 subtypes. Both rabbit kinin receptors exhibit a high degree of pharmacological convergence with the human orthologues (Leeb-Lundberg et al., 2005). The characterization of the pharmacological profile included testing of B2R stimulation by B-9870 and related constrained peptide antagonists. B-9870 actions have been compared with the possible similar effects of B-10346 (the first pass metabolite of B-9870 in which imide groups are hydrolysed into amides at both ends of the divalent linker; JM Stewart, L Gera and DC Chan, unpublished results), of Hoe 140 and of a dimer of the latter peptide, B-9872 (also composed of two monomers linked at their N-terminus by the suberimidyl moiety; Gera et al., 1996). Hoe 140 (icatibant, D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-BK) has been successfully used as a B2R antagonist in many systems (Leeb-Lundberg et al., 2005), but may exert some atypical effects reminiscent of the agonist, depending on the experimental system and the species. Hoe 140 activates the mitogen-activated kinases ERK1/2 and cell growth in some human tumour cell lines (Drube and Liebmann, 2000); a slow form of receptor endocytosis correlated with an insurmountable type of antagonism was observed at the rabbit B2R for Hoe 140 and the related drug NPC 17731 (D-Arg-[Hyp3, D-HypE(transpropyl)7, Oic8]-BK) (Houle et al., 2000), consistent with the finding that significant phospholipase C activation is elicited by both peptides in cells that express the recombinant human B2R (Fathy et al., 1999). Further, the expression and stimulation of the kinin B1R has been implicated in proliferation, migration and invasiveness of prostate cancer PC3 cells (Taub et al., 2003). Thus, we have verified whether B-9870 is a B1R ligand and of which functional type it belongs to. Finally, a tumour-derived cell line reportedly responsive to B-9870 has been evaluated for kinin receptor expression and the possible mediation of the antiproliferative effect by kinin receptors.
Methods
Cells
The derivation of HEK 293 cell lines stably expressing B2R-GFP or B1R-YFP and their properties are described elsewhere (Houle et al., 2000; Bachvarov et al., 2001; Sabourin et al., 2002). The conjugation with a fluorescent protein has no detectable effect on the pharmacological profile of either receptor, and allows the visual monitoring of constant receptor expression in a simple manner. Non-transfected HEK 293 cells were used in some control experiments. Confocal microscopy for B2R-GFP was applied to the live cells as described previously (Houle et al., 2000; Bachvarov et al., 2001), and supplemented with epifluorescence microscopy. An arachidonic acid release assay was performed, as described elsewhere, to evaluate the activation of the phospholipase A2 (PLA2) following a stimulation with ligands in HEK 293 cells stably expressing B2R-GFP (24-well plates, 20 min release period at 37°C) (Houle et al., 2000).
The effect of B-9870 on the proliferation of HEK 293 cells stably expressing B2R-GFP or not was tested in the following manner: 5 × 104 cells were seeded in 35 mm Petri dishes in the usual serum-containing culture medium, optionally supplemented with B-9870 (applied 24 h after seeding). After 4 days of culture (3 days of drug treatment), the cells were detached using trypsin-ethylenediaminetetraacetic acid (EDTA) and manually counted using a haemocytometer. A similar procedure to this protocol has been applied to evaluate the effect of several kinin receptor ligands on the proliferation of the human breast cancer cell line MDA-MB-231 (originally obtained from American Type Culture Collection); 5 × 104 cells were seeded in 35 mm dishes containing 2 ml of Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Burlington, ON, Canada) supplemented with 5% fetal bovine serum (FBS). After a 24-h incubation period, the medium was changed and the drugs were introduced for an additional 48 h incubation period. At 72 h post-seeding, the cells were detached with trypsin–EDTA and counted.
Binding assays
The binding of 2 nM [3H]-BK (Perkin Elmer Life Sciences; 90 Ci mmol−1) or 1 nM [3H]-Lys-des-Arg9-BK (Perkin Elmer Life Sciences, Shelton, CT, USA; 69–80 Ci mmol−1) to adherent intact HEK 293 cells stably expressing B2R-GFP or B1R-YFP (24-well plates), respectively, was evaluated as described previously (Houle et al., 2000; Sabourin et al., 2002). The assays were applied to construct-binding competition curves for a series of unlabelled antagonists. The same radioligands were used in binding experiments involving adherent, intact MDA-MB-231 cells grown in 24-well plates to monitor endogenous kinin receptor expression (protocols for each radioligand identical to those applied to HEK 293 cells; saturation curve in the 0.25–7 nM concentration range for [3H]-BK).
Immunoblots
For the analysis of B2R-GFP in total cell extracts, immunoblots were generally performed as described previously (based on an anti-GFP monoclonal from BD Biosciences Clontech, Palo Alto, CA, USA, Marceau et al., 2002). For the detection of phospho-ERK1/2 and total ERK1/2, cells maintained in reduced serum (0.5% FBS) were stimulated with BK-related and other drugs for definite periods of time; total cell extracts were prepared and submitted to electrophoresis (Marceau et al., 2002). Transferred proteins were revealed using two types of antibodies for each sample: phospho-ERK1/2 (monoclonal, New England Biolabs, Pickering, ON, Canada, dilution 1/1000) and total ERK1/2 (to show comparable loading; polyclonal, New England Biolabs, dilution 1/1000). Staining was revealed using the appropriate peroxidase-conjugated secondary antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, USA). Poly(ADP-ribose)polymerase I (PARP, 116 kDa) and cleaved PARP (89 kDa) immunoblots were applied as described by Morissette et al. (2004b). Briefly, total cell extracts (attached and detached cells) of MDA-MB-231 cells seeded in 25 cm2 flask, maintained in 5% serum for 24 h and treated for another 48 h, were migrated and blotted with anti-PARP antibodies (polyclonal, Cell Signalling Technologies, Inc., Pickering, ON, Canada; 1/1000). Actinomycin D 0.4 μM was used as a positive control treatment that induces PARP cleavage and apoptosis.
Contractility assays
A local ethics committee approved procedures based on rabbits and the specific pathogen-free animals (Charles River) were cared for in accordance with the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care). Rabbit jugular vein contractile responses to BK (mediated by B2Rs) or histamine (mediated by H1 receptors) were measured as generally described by Houle et al. (2000). Specifically, cumulative concentration–effect curves were established in each tissue at 1.5 h for BK and 3.5 h for histamine (in order to present all contraction results as a per cent of the maximal histamine-induced effect, a standard independent from BK receptors). In some tissues, B2R ligands tested as antagonists were applied 30 min before BK. Kinins contract rabbit aortic rings via B1Rs, and the effect of different concentrations of B-9870 on the cumulative concentration–effect curves of the B1R agonist des-Arg9-BK were determined and analysed as described previously (Morissette et al., 2004a).
Calcium transient imaging
HEK 293 cells (35 mm dishes) were co-transfected with a pcDNA3-based vector coding for the wild-type rabbit B2R (Bachvarov et al., 1995) and the pHcRed1-N1 vector (Clontech) coding for a red fluorescent protein (used to assess the efficacy of the transient transfection and identify transfected cells) using the Ex-Gen 500 transfection reagent (MBI Fermentas Inc., Flamborough, Canada) as directed by the manufacturer. Forty-eight hours later, cells were transferred in serum-free α-minimal essential medium (MEM) containing Fluo-3-AM (Molecular Probes, Eugene, OR, USA; 10 μM), bovine serum albumin (BSA) (0.1%), Pluronic F-127 (Sigma-Aldrich, St Louis, MO, USA; 0.01%) and dimethylsulphoxide (DMSO) (0.1%). The cells were incubated for 20 min at room temperature, then thoroughly rinsed with serum-free α-MEM and further incubated in this medium for 1 h at 37°C. Cells were immediately observed at × 400 using a fluorescence microscope (blue excitation, green emission) equipped with a heated chamber (37°C) and containing a controlled atmosphere (humid air, 5% CO2). B2R ligands were added and the green fluorescence of Fluo-3 bound to calcium was observed as a function of time using time lapse photography (electronic camera); at the end of the recordings, the calcium ionophore A23187 (5 μM) was added to the cell dishes as a positive control. Fluo-3 imaging has been applied to cells expressing the wild-type receptor because the emission spectrum of this fluorophore overlaps with that of GFP (present in the B2R-GFP construction).
Calcium mobilization
To quantify the calcium mobilization induced or influenced by several BK receptor ligands, Fura-2 fluorometry (Molecular Probes, Invitrogen Detection Technologies) was applied to the non-transfected HEK 293, stably transfected B2R-GFP HEK 293 or MDA-MB-231 cells. The cells were detached using trypsin–EDTA, suspended in complete culture medium containing FBS (to neutralize the trypsin), counted, centrifuged for 5 min at 1100 r.p.m. at room temperature and resuspended in Hank's balanced salt solution (HBSS) (1 × , pH 7.4, prepared from 10 × concentrate, Multicell Wisent, St Bruno, Canada) with 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid) and 1.6 mM CaCl2. At this point, Fura-2-AM was added to cell suspensions (final concentration 1 μM), and they were incubated for 30 min in a 37°C bath with agitation. After the incubation, cells were centrifuged and rinsed twice. Calcium mobilization was read with a thermostated (37°C) spectrofluorimeter (SLM8000C; excitation 340 nm and emission 510 nm) in 2 ml suspension of cells loaded with Fura-2 (2.5 × 106 cells ml−1 for the two HEK 293 cell lines, 5 × 106 for MDA-MB-231) with optional drug pretreatments 15 min before stimulations. After the readings, the maximum mean fluorescence (Fmax) was measured by adding 15 μl of 10% Triton X-100 and the minimum mean fluorescence (Fmin) with further addition of 15 μl of NaOH 1 N and 100 μl of ethylene glycol bis(β-aminoethylether)-N,N,N′,N′,-tetraacetic acid (EGTA) 100 mM. Calcium mobilization concentrations were established with the following equation ([Ca2+]=224((y−Fmin)/(Fmax−y)), where y represents the fluorescence reading from the sample, as described by Burelout et al. (2004).
Drugs
BK was purchased from Bachem (Plymouth Meeting, PA, USA). The non-peptide LF 16.0687 (1-[[2,4-dichloro-3-[(2,4-dimethylquinolin-8-yl)oxy]methyl]phenyl]sulphonyl]-N-[3-[[4-(aminoiminomethyl]-phenyl]carbonylamino]propyl]-2(S)-pyrrolidinecarboxamide, mesylate salt) and the peptide Hoe 140 (icatibant), antagonists of BK at the rabbit and human B2R (Pruneau et al., 1999; Houle et al., 2000), were gifts from Laboratoires Fournier (Daix, France). Compound 11 (2-{(2R)-1-[(3,4-dichlorophenyl)sulphonyl]-3-oxo-1,2,3,4-tetrahydroquinoxalin-2-yl}-N-{2-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]ethyl}acetamide), a powerful non-peptide antagonist at the rabbit and human B1R (Su et al., 2003; Morissette et al., 2004a), was a gift from Dr DJ Pettibone Merck Research Labs (West Point, PA, USA). The other kinin receptor antagonists B-9870, B-10346 (suberyl-(D-Arg-Arg-Pro-Hyp-Gly-Igl-Ser-Digl-Oic-Arg)2.TFA; the N-terminal amide analogue of B-9870) and B-9872 were synthesized using general methods described elsewhere (Gera et al., 1996; Stewart et al., 2002). PD 98059 (2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one) was purchased from Research Biochemicals International (Natick, MA, USA). The other drugs were purchased from Sigma-Aldrich (St Louis, MO, USA).
Data and statistical analyses
Data points and values are presented as means±s.e. of n determinations. Where appropriate, experimental groups were compared by use of Student's t-test or analysis of variance (ANOVA) followed by Dunnett's test (GraphPad Instat, version 3, San Diego, CA, USA). P<0.05 was considered to be statistically significant.
Results
Effect of B-9870 and related compounds at the recombinant rabbit B2Rs
We tested whether a set of five compounds that are nominally B2R antagonists could displace the specific binding of [3H]-BK (2 nM) from HEK 293 cells stably expressing B2R-GFP (Figure 1). It can be seen that the non-peptide antagonist LF 16.0687, the peptide antagonist Hoe 140, its dimer B-9872, the anti-tumoral ligand B-9870 and its metabolite B-10346 possess comparable affinities for the rabbit B2R, with IC50 values in the range 50–75 nM.
Figure 1.
Competitive inhibition of [3H]-BK (2 nM) binding to HEK 293 cells stably expressing B2R-GFP by a panel of cold drugs. Values are the means±s.e.m. (vertical lines) of 3–6 experiments composed of duplicate determinations. Average specific binding without competitor (100%) was 116±20 fmol/well.
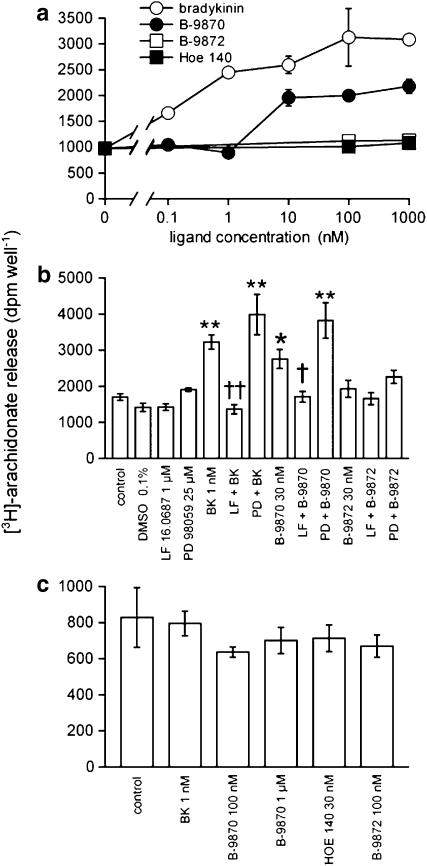
A phosphorylation assay of the mitogen-activated protein (MAP) kinases ERK1/2 was applied to HEK 293 cells stably expressing B2R-GFP in search of agonist activity (Figure 2a). Cells were stimulated with drugs at concentrations known to occupy the receptors. The stimulant effect of the reference agonist, BK, is shown. Other peptides exerted stimulative actions in the following decreasing order of effect amplitude: the proposed biased agonist B-9870 (Figure 2a and c), Hoe-140 (Figure 2c), its dimer B-9872 (data not shown) and B-10346 (10 min treatments, Figure 2b). The non-peptide antagonist LF 16.0687 had no agonist effect, but could antagonize both BK- and B-9870-induced ERK1/2 activation (Figure 2a) as well as the smaller effects of B-10346 (Figure 2b), Hoe 140 or B-9872 (data not shown). Non-transfected HEK 293 cells were unresponsive to BK, B-9870 or B10346, further showing that the stimulant effect of these peptides is receptor-mediated (Figure 2a and b). The pharmacological inhibitor of the upstream MAP kinase MEK-1, PD 98059 (Dudley et al., 1995), profoundly inhibited the effect of BK, B-9870, Hoe 140 (Figure 2c) and also B-9872 (data not shown). In the ERK phosphorylation assay, B-9870 exhibited an active threshold concentration of 10 nM (higher than that of BK, Figure 2d), and the effect of B-9870 was generally lower in amplitude and not more durable than that of BK (Figure 2e), indicating that the desensitization of B2R-mediated MAP kinase signalling applies to both agents.
Figure 2.
Immunoblots for phospho-ERK1/2 and total ERK1/2 in HEK 293 cells stably expressing B2R-GFP in response to BK, B-9870, its metabolite B-10346, Hoe-140 or its dimer B-9872 (10 min treatments). (a) B2R ligand-induced ERK1/2 phosphorylation in HEK 293 cells is receptor-dependent, as shown by the lack of effect of BK (10 nM; 10 min) or B-9870 (100 nM 10 min) on untransfected cells and by the antagonist effect of LF 16.0687 (5 μM; 30 min) on the activation of ERK1/2 induced by BK or B-9870 in cells stably expressing B2R-GFP. Total ERK1/2 is shown to compare loading in tracks. (b) Effect of B-10346, the B-9870 metabolite, on the phosphorylation of ERK1/2. Presentation as in (a). (c) PD 98059 (25 μM, 30 min), the inhibitor of the upstream MAP kinase MEK-1, prevents the effect of all B2R-GFP peptide ligands (last 10 min) on ERK1/2 phosphorylation in HEK 293 cells. (d) Concentration–effect relationship for BK and B-9870 (10 min). (e) Time–effect relationship for BK and B-9870 (the control track was common to both stimulants). In all panels, the number of replicates was 2–3.
PLA2 activation is a documented response to BK in HEK 293 cells stably expressing B2R-GFP (Houle et al., 2000). As found previously, Hoe 140 (0.1–1000 nM) did not stimulate PLA2 over baseline in these cells (Figure 3a); it was shown elsewhere that this drug antagonizes BK in this assay (Houle et al., 2000). B-9870 (10–1000 nM) clearly stimulated arachidonate release with a maximal effect inferior to that of BK, whereas the Hoe 140 dimer B-9872 was inactive in this respect. The non-peptide antagonist LF 16.0687 (1 μM) had no direct effect on arachidonate release by cells, but could antagonize the stimulant effects of BK (1 nM) and B-9870 (30 nM) (Figure 3b). BK and B-9870 failed to release [3H]-arachidonate from untransfected HEK 293 cells (Figure 3c).
Figure 3.
PLA2 assay applied to HEK 293 cells stably expressing B2R-GFP (a, b) or untransfected cells (c). (a) Concentration–effect relationship for PLA2 activation by some peptide B2R ligands. Values are the means±s.e.m. (vertical lines) of 4–5 determinations. (b) Effect of the MEK-1 inhibitor PD 98059 and of the B2R antagonist LF 16.0687 on arachidonate release induced by peptide antagonists/biased agonists. The effect of the DMSO vehicle for PD 98059 is also shown. Values are means±s.e.m. (vertical lines) of 5–6 determinations. Inhibitory drugs were applied 30 min before stimuli. Analysis of variance (ANOVA) indicated that the groups were heterogeneous (P<0.001). Dunnett's test for comparison with the control group: *P<0.05; **P<0.01. Comparison of the inhibitor-treated groups with the appropriate stimulant-treated group, t-test: †P<0.01; ††P<0.01. (c) Lack of effect of BK, B-9870 or other ligands on untransfected HEK 293 cells (n=4).
As the MEK-1-ERK1/2 signalling pathway activation seems to be somewhat correlated to the ability to stimulate arachidonate release in the series of compounds, we have verified whether the latter effect is dependent on MAP kinase signalling. The MEK-1 inhibitor PD 98059 had a slight, nonsignificant stimulative effect in the PLA2 assay, but did not prevent arachidonate release by BK or B-9870 (Figure 3b).
In confocal microscopy studies of live HEK 293 cells stably expressing B2R-GFP, cells were stimulated for 30 min with B2R ligands (Figure 4). Control cells generally exhibit sharply defined plasma membrane-associated fluorescence. The agonist BK promotes the endocytosis of fluorescent receptors in the form of vesicles of various sizes. The biased agonist B-9870 produces fluorescent receptor internalization in the form of very fine particles with a slow kinetic, in a manner similar to B-9872 (see Figure 4) or Hoe 140 (Figure 4a, Houle et al., 2000). None of these ligands promoted B2R-GFP degradation, as judged with immunoblot of total cell extracts based on anti-GFP antibodies (data not shown; positive control: extracellular trypsin produces GFP-sized fragments of the fusion protein in 10 min; Houle et al., 2003). Cell treatment with PD 98059 did not prevent peptide ligand-induced translocation (data not shown). LF 16.0687 does not promote B2R-GFP endocytosis (Houle et al., 2000). The non-peptide antagonist LF 16.0687 had no direct effect on the cellular fluorescence distribution, but decreased the specific dislocation pattern of the membrane fluorescence induced by BK or B-9870 (Figure 4b).
Figure 4.
(a) Confocal microscopy studies of live HEK 293 cells stably expressing B2R-GFP and stimulated for 30 min with B2R ligands at the indicated concentrations. Control cells generally exhibited sharply defined plasma membrane-associated fluorescence. All photomicrographs represent square fields with 40 μm sides. Typical results of at least two independent days of observation in multiple microscopic fields. (b) LF 16.087 pretreatment (10 min, 5 μM) decreased the dislocation of the membrane fluorescence induced by BK or B-9870 (30 min treatment). Epifluorescence imaging, original magnification × 1000.
HEK 293 cells expressing the wild-type rabbit B2R responded by a transient increase of calcium concentration in response to BK (10 nM, Figure 5, Fluo-3 fluorescence); this applied to some cells only, consistent with the fractional effect of transient transfections. The response, detected by the fluorescence of Fluo-3 bound to calcium, was maximal in 30 s and decreased afterwards. B-9870 (30 or 100 nM) was consistently observed to induce a small response in some cells and this response was delayed in some cases (Figure 5). This response appeared to be receptor-dependent, as it was not recorded in cells that did not exhibit the red fluorescence caused by co-transfection of the B2R with the HcRed1-coding vector (data not shown; each microscopic field contains numerous untransfected cells that can be taken as controls).
Figure 5.
Imaging of Fluo-3 fluorescence in HEK 293 cells transiently expressing rabbit B2Rs and stimulated with the indicated drugs. At the end of each sequence of time lapse photography, the cells were stimulated with the calcium ionophore (5 μM) in order to provide a positive control. Arrowheads indicate some of the responsive cells. Cells were not responsive to saline, the peptide vehicle (data not shown). Results are representative of several replicates performed over three separate days.
A more quantitative evaluation of calcium transients was based on Fura-2 in HEK 293 cells stably expressing B2R-GFP (Figure 6). This approach confirmed that BK (10 or 100 nM) induced a fast Ca2+ mobilization followed by decreasing signal (Figure 6a). B-9870 (30 or 100 nM) produced smaller and protracted responses with no clear dependence on the concentration (Figure 6a and b). LF 16.0687, which had no effect on calcium mobilization, prevented the effects of BK (10 nM) or B-9870 (100 nM) (Figure 6b). Non-transfected HEK 293 cells failed to respond to B-9870 and exhibited a small response to BK (100 nM only) that was inhibited by either B-9870 or LF 16.0687.
Figure 6.
Calcium mobilization in untransfected HEK 293 cells or in cells stably expressing B2R-GFP at high density. (a) Representative tracings of the Fura-2 fluorescence to show the time course of effects elicited by acute application of B2R ligands (conventionally at time 10 s). (b) Peak intracellular calcium measurements observed in cells as a function of drug treatment or co-treatments. Drug concentrations are indicated in nM, and that of LF 16.0687 was always 5 μM (5000 nM). Values are means±s.e.m. (vertical lines). The number of replicates is indicated close to each column.
Effect of constrained peptides on naturally expressed rabbit B2Rs
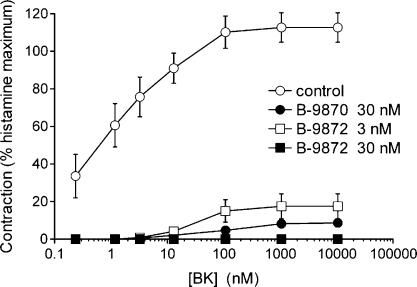
Both B-9870 and the Hoe 140 dimer, B-9872, behaved as insurmountable antagonists of BK in the rabbit jugular vein contractility assay (a tissue that expresses the wild type B2R) (Figure 7). The peptide analogues had no agonist contractile effects. This behaviour is similar to that of Hoe 140 in the same preparation (Houle et al., 2000).
Figure 7.
Effect of B-9870 and B-9872 on cumulative concentration–effect curve for BK in the rabbit jugular vein contractility assay. The peptide analogues had no direct contractile effects. Replicate numbers: control (4), B-9870 (5), B-9872 3 nM (5), B-9872 30 nM (2). Vertical lines show mean±s.e.m.
Effect of B-9870 at the rabbit B1R
Reminiscent of the effect of the monomer B-9430 (Stewart et al., 1996), the dimer B-9870 also is a surmountable B1R antagonist in the aortic contractility assay (Figure 8a). It antagonized, with a pA2 value of 7.40 (Schild plot; Figure 8b, regression slope −0.88), the rabbit aorta contraction induced by the B1R agonist des-Arg9-BK. B-9870, although at a higher concentration than compound 11, displaced Lys-des-Arg9-BK binding to HEK 293 cells stably expressing B1R-YFP (Figure 8c). Finally, in contrast with the B2R-GFP system, only the specific agonist Lys-des-Arg9-BK (10 nM) activated the phosphorylation of ERK1/2 in HEK 293 stably expressing B1R-YFP (Figure 8d). This phosphorylation was receptor-dependent as the highly selective non-peptide B1R antagonist compound 11 could prevent it; no activation was observed in the untransfected cells in response to any of the B1R ligands. Thus, B-9870 possesses a fair affinity for the B1R without a stimulative effect on ERK1/2 phosphorylation or on aortic contractility, suggesting that B-9870 behaved as a competitive antagonist on this receptor.
Figure 8.
(a) Effect of B-9870 on rabbit isolated aorta contractions induced by the B1R agonist des-Arg9-BK (values are means±s.e.m. of triplicate determinations). (b) Schild plot analysis based on the averaged data in (a). (c) Displacement of [3H]-Lys-des-Arg9-BK (1 nM) binding to HEK 293 cells stably expressing B1R-YFP by B-9870 or compound 11 (a highly selective non-peptide B1R antagonist). Values are the means of duplicate determinations. The average specific binding without competitor (100%) was 139 fmol/well. (d) Immunoblots for total or phosphorylated ERK1/2 in untransfected HEK 293 and HEK 293 cells stably expressing B1R-YFP and maintained for 24 h in reduced serum (0.5% FBS). Compound 11 applied 30 min before extraction, and the agonist 10 min.
Effect of B-9870 on cell proliferation
After 4 days of culture, cell wells seeded with 5 × 104 HEK 293 cells stably expressing B2R-GFP contained 3.79±0.51 × 105 cells (controls, n=4). Culture in the presence of LF 16.0687 (1 μM) or B-9870 (100 nM) did not significantly change the extent of proliferation, although a nonsignificant stimulative trend was observed for the latter drug (final cell counts of 5.05±0.50 × 105 and 5.36±0.78 × 105 cells, respectively; n=4). Higher concentrations of B-9870 (1 or 5 μM) had no consistent effect on the proliferation of HEK 293 cells expressing B2R-GFP; all these observations also applied to untransfected HEK 293 cells (data not shown).
MDA-MB-231 is one of the five breast cancer cell lines found by Chan et al. (2002a) to respond to B-9870 (CU201) by growth inhibition. In our model, a rather modest 30.6% inhibition of MDA-MB-231 proliferation was observed after 48 h of B-9870 treatment (5 μM, Figure 9). No significant inhibition was found at 1 μM. The N-terminal amide analogue and first pass metabolite of B-9870, B-10346 was less potent as it exerted no statistically significant effect on cell proliferation (Figure 9a). B-9870 has been proposed to exert its antiproliferative effects via a B2R biased agonist mechanism. Thus, we used the non-peptide antagonist LF 16.0687 (1 μM) to verify whether peptide ligands stimulate endogenous B2R to mediate proliferation inhibition. LF 16.0687 has a ∼10-fold greater affinity for the human B2R, relative to the rabbit orthologue (Pruneau et al., 1999; Houle et al., 2000). The inhibitory effect of B-9870 was not reversed by cell co-treatment with LF 16.0687 (Figure 9a). The highly selective non-peptide B1R antagonist, compound 11 (1 μM, Figure 9a), or this drug combined with LF 16-0687 (data not shown), also failed to reverse the inhibitory effect of B-9870 on proliferation. The non-peptide antagonists, alone (Figure 9a) or combined (not shown), failed to influence carcinoma cell proliferation. To further ascertain that the competitive antagonist LF 16.0687 was used optimally in the proliferation experiments against a high concentration of B-9870, experiments were repeated with a different ratio of the drugs (10 and 5 μM, respectively; Figure 9b). The results again failed to show an inhibition of the antiproliferative effect of the peptide by the non-peptide agent.
Figure 9.
(a) Effects of bradykinin receptor ligands on the proliferation of breast carcinoma MDA-MB-231 cells. Petri dishes were seeded with 5 × 104 cells at time 0; at time 24 h the media were changed and drug treatments were introduced for an additional 48 h period; cells were trypsinized and counted at time 72 h. Values are the means±s.e.m. (vertical lines) of the indicated number of replicates indicated in each column and are expressed as the percentage of cells relative to controls established for each day of the experiments (100% in absolute cell counts=156 000±12 000). ANOVA P<0.001; Dunnett's test relative to controls: *P<0.01. Adding the non-peptide antagonists LF 16.0687 or compound 11 to the peptide ligands B-9870 or B-10346 did not produce effects that were significantly different from those of the peptide ligands alone. (b) Effect of a different ratio of LF 16.0687 (10 μM) and B-9870 (5 μM) on the proliferation of MDA-MB-231 cells. (c) Immunoblot of PARP and cleaved PARP detected in the MDA-MB-231 extracts. Cells were seeded, stimulated and harvested (attached and detached cells) at time 0, 24 and 72 h, respectively. Drug concentrations as in (a). Representative results of two experiments.
Pre-apoptotic signalling has been shown to be activated by B-9870 in at least some responsive tumour-derived cell lines (Chan et al., 2002a). PARP is cleaved during apoptotic events. Actinomycin D (0.4 μM, 48 h treatment) strongly inhibited MDA-MB-231 proliferation (Figure 9a), and induced the typical apoptotic PARP cleavage (89 kDa; Figure 9c). However, no PARP cleavage was observed upon B-9870 or B-10346 treatment (48 h; Figure 9c).
The MDA-MB-231 cell line failed to exhibit specific binding sites for [3H]-Lys-des-Arg9-BK (1 nM, Figure 10a), but expressed some specific sites for [3H]-BK (Figure 10b). The specific binding was smaller than the nonspecific binding and low in absolute abundance in this system; applying a single site model was not attempted owing to the uncertainty of the specific binding at low ligand concentrations. Nevertheless, MDA-MB-231 cells exhibit a small calcium mobilization in response to BK (100 nM, Figure 11). This response is not mimicked by B-9870 (100 nM or 1 μM), but is prevented by pretreatment with this peptide or with LF 16.0687 (Figure 11b).
Figure 10.
Surface expression of receptors for kinins in MDA-MB-231 cells. (a) Total binding of a saturating concentration (1 nM) of [3H]-Lys-des-Arg9-BK to the tumour-derived cells. The unchanged amount of bound ligand in the presence of a large excess of agonist or antagonist drugs is consistent with the absence of B1Rs. (b) Total, nonspecific and specific binding of [3H]BK to cells. In both (a) and (b), values are means±s.e.m. (vertical lines) of two experiments composed of duplicate determinations.
Figure 11.
Calcium mobilization in MDA-MB-231 cells exposed to B2R agonists or antagonists. (a) Representative tracings of the Fura-2 fluorescence to show the time course of effects elicited by acute application of B2R ligands (conventionally at time 10 s). (b) Peak intracellular calcium measurements observed in cells as a function of drug treatment or co-treatments. Values are means±s.e.m. (vertical lines). The number of replicates is indicated close to each column.
Discussion
In the present experiments, the peptide B-9870 has been observed to behave either as a potent and insurmountable BK antagonist in the rabbit jugular vein contractility assay and in the calcium mobilization assay in MDA-MB-231 cells or as a partial agonist in other assays (ERK1/2 phosphorylation, PLA2 activity, calcium transients and receptor endocytosis in HEK 293 cells expressing high densities of recombinant rabbit B2Rs). These results extend the number of tested end points relative to a previous study (Chan et al., 2002a). The stimulative effects of B-9870 were strictly receptor-dependent, as shown by the lack of drug effect on untransfected cells or the antagonism exerted on the peptide drug effect on MAP kinase and calcium or PLA2 signalling by the non-peptide drug LF 16.0687. The lack of agonist effect in jugular vein contractility studies (Figure 7) and in calcium assays in MDA-MB-231 cells (a system with low receptor density) seems, at first sight, to conflict with the results obtained in HEK 293 cells expressing recombinant rabbit receptors, where B-9870 exerts agonist-like effects, but consistent with the previously reported antagonist effect of B-9870 on BK-induced calcium signalling in a tumour-derived cell line (Chan et al., 2002a). However, ligands with low efficacy are more likely to behave as pure antagonists if a pharmacological effect requires the stimulation of a very large proportion of the receptors on each cell (i.e., if there are no spare receptors; Leslie, 1987; Kenakin, 1987; Mathieu et al., 2005). As the recombinant receptors (either the wild-type or the GFP conjugate) are expressed at a high density (Bachvarov et al., 1995, 2001), these systems are more likely to reveal the agonist-like actions of a low efficacy partial agonist. Untransfected HEK 293 cells unexpectedly exhibited a small calcium response to BK, but no response in the ERK1/2 phosphorylation or PLA2 assays. B-9870 behaved as an antagonist of the small calcium response, consistent with a small population of B2Rs in untransfected HEK 293 cells. The biased agonist activity of B-9870 for a subset of signalling pathways activated by BK, as previously defined (see Introduction), is not necessary to account for the present data.
Although widely exploited as a B2 receptor antagonist, Hoe 140 may be a stimulator at the rabbit B2R (see Introduction). This type of effect may be analogous to the partial agonist actions of B-9870, but with an even lower efficacy (limited to a minor activation of endocytosis and ERK1/2 kinase activation). The same contention applies to the Hoe 140 dimer, B-9872 and to the first pass metabolite and amide analogue of B-9870, B-10346 (ERK1/2 phosphorylation Figure 2b). Hoe 140 has a large stimulative effect at the sheep B2R (Félétou et al., 1994) and is a full agonist at the ornithokinin receptor, a bird homologue of the mammalian B2R (Schroeder et al., 1997). The efficacy in this series is not related to affinity as these three synthetic peptides and B-10346, as well as the pure antagonist LF 16.0687, exhibit comparable affinities at B2R-GFP (Figure 1). Thus, a specific structure–activity relationship must exist for the partial agonist activity among the structurally constrained peptide B2R antagonists. Another property of the constrained peptide antagonists is the high resistance to metabolic breakdown; by comparison, BK is destroyed rapidly following endocytosis of the receptor–agonist complex (Munoz and Leeb-Lundberg, 1992) and also in serum-containing culture media (Bachvarov et al., 2001). Thus, even a low efficacy agonist activity may be amplified over time, as observed with the slow but very persistent endocytosis of B2R-GFP induced by Hoe 140 (Houle et al., 2000). Resistance to catabolism may explain why B-9870 was more persistent than BK as a function of time to mobilize Ca2+ (Figure 5 and 6a).
B-9870 (CU201) has been shown to exert antitumour cell line proliferation activity and to have a significant anti-neoplasic action when used alone or in combination with other drugs in animal models (Stewart et al., 2005). The effect of B-9870 on tumour-derived cell lines (including MDA-MB-231 Figure 9a; Chan et al., 2002a) has not been observed on HEK 293 cells, whether they expressed B2R-GFP or not (proliferation assay over 4 days). In addition, B-9870-induced proliferation inhibition observed in MDA-MB-231 cells was not blocked by the non-peptide B2R antagonist LF 16.0687 (Figure 9a and b). The latter non-peptide drug has no direct effect on carcinoma cell growth in vitro. Although the stimulation of kinin B1R has also been implicated in proliferation, migration and invasion of cancer cells (Taub et al., 2003), and despite the fact that B-9870 possesses a fair affinity for this receptor as an antagonist (rabbit aortic contractility, radioligand binding competition and ERK1/2 phosphorylation in HEK 293 stably expressing B1R-YFP; Figure 8), its inhibitory action on growth was not mediated by B1Rs, as evidenced by the lack of effect of compound 11 co-treatment (Figure 9a) and the absence of specific binding for [3H]-Lys-des-Arg9-BK (Figure 10a). Although MDA-MB-231 cells possess a small functional B2R population, the concentrations of B-9870 needed to inhibit proliferation in this or other studies (⩾5 μM) were much higher than those that efficiently inhibited either BK receptor subtype in the present pharmacological study. Cell type-dependent, kinin receptor-independent pathway(s) must exist for the antiproliferative effect of B-9870, which does not apply to HEK 293 cells and may vary from one tumour cell type to another. In contrast with the lung cancer line used in most signalling experiments described by Chan et al. (2002a), inhibition of MDA-MB-231 cell growth was not apoptotic (lack of PARP cleavage, Figure 9c; small sub-G1 peaks unaltered by B-9870 in ploidy experiments, data not shown) and did not involve JNK activation (lack of phospho-c-jun in cells treated with either B-9870 or BK, data not shown).
Arginine-rich peptides possessing six or more Arg or D-Arg residues are effective at infusing into the cytosol of several cell types (Tung and Weissleder, 2003). The dimer B-9870 has six of these residues (four Arg and two D-Arg). Two such Arg-rich sequences of physiological significance are found in the TAT domain from the transcription activating factor of HIV and ‘penetratin' sequence from the Drosophila homeodomain protein Antennapedia (Melikov and Chernomordik, 2005). Arg-rich peptide conjugates with a ‘cargo', for example, a cytotoxic drug, have been designed as cell permeant agents with a cytosolic site of action. It is not clear whether B-9870 has structural features that explain its cell type-specific effect on proliferation and the induction of apoptosis observed in specific cell types (Chan et al., 2002a). The linker region of the dimer may be critical for an effect of B-9870 on the cell cycle, because its metabolite that differs only in this region, B-10346, is not active at depressing MDA-MB-231 cell proliferation (Figure 9a). Thus, B-9870 may not be a pro-drug of a more active cytotoxic agent.
B-9870 is a dual rabbit B1R and B2R antagonist with confirmed significant stimulative effects at the latter receptor subtype only in systems where B2Rs are overexpressed; the compound may be a partial agonist of the B2R with low efficacy and be highly resistant to metabolic breakdown. In any event, the antiproliferative effect on the carcinoma cell line studied occurred at a relatively high concentration range and was not dependent on kinin receptors and apoptosis. The contention that cell type-specific, non-receptor-mediated effects are mediated by the direct entry of this peptide, rich in Arg or D-Arg residues, into specific cells remains an intriguing possibility.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research (CIHR; Grant MOP-14077). SH has been the recipient of a Studentship from the CIHR. GM is the recipient of a Canada Graduate Scholarships Doctoral Award from the CIHR. We thank Ms Johanne Bouthillier for technical help, Mr André Lévesque for guidance with the microscopic techniques and the Imaging Core facility (Centre de recherche de L'Hôtel-Dieu de Québec) and Dr Marc Pouliot and Dr Paul Naccache (CHUQ-CHUL) for facilitating the access to microscopic equipment and the luminescence spectrophotometer, respectively.
Abbreviations
- B1R
B1 receptor
- B2R
B2 receptor
- BK
bradykinin
- ERK1/2
extracellular signal regulated kinases 1/2
- GFP
green fluorescent protein
- Hyp
4-hydroxyproline
- Igl
α(2-indanyl)glycine
- Oic
L-(3aS,7aS)-octahydroindol-2-yl-carbonyl
- PARP
poly(ADP-ribose)polymerase I
- PLA2
phospholipase A2
- Tic
1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
- YFP
yellow fluorescent protein
Conflict of interest
The authors state no conflict of interest.
References
- Bachvarov DR, Houle S, Bachvarova M, Bouthillier J, Adam A, Marceau F. Bradykinin B2 receptor endocytosis, recycling, and down-regulation assessed using green fluorescent protein conjugates. J Pharmacol Exp Ther. 2001;297:19–26. [PubMed] [Google Scholar]
- Bachvarov DR, Saint-Jacques E, Larrivée JF, Levesque L, Rioux F, Drapeau G, et al. Cloning and pharmacological characterization of the rabbit bradykinin B2 receptor. J Pharmacol Exp Ther. 1995;275:1623–1630. [PubMed] [Google Scholar]
- Blaukat A, Abd Alla S, Lohse MJ, Müller-Esterl W. Ligand-induced phosphorylation/ dephosphorylation of the endogenous bradykinin B2 receptor from human fibroblasts. J Biol Chem. 1996;271:32366–32374. doi: 10.1074/jbc.271.50.32366. [DOI] [PubMed] [Google Scholar]
- Burelout C, Thibault N, Levasseur S, Simard S, Naccache PH, Bourgoin SG. Prostaglandin E2 inhibits the phospholipase D pathway stimulated by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Involvement of EP2 receptors and phosphatidylinositol 3-kinase γ. Mol Pharmacol. 2004;66:293–301. doi: 10.1124/mol.66.2.293. [DOI] [PubMed] [Google Scholar]
- Chan D, Gera L, Stewart J, Helfrich B, Verella-Garcia M, Johnson G, et al. Bradykinin antagonist dimer, CU201, inhibits the growth of human lung cancer cell lines by a ‘biased agonist' mechanism. Proc Natl Acad Sci USA. 2002a;99:4608–4613. doi: 10.1073/pnas.072077299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC, Gera L, Stewart JM, Helfrich B, Zhao TL, Feng WY, et al. Bradykinin antagonist dimer, CU201, inhibits the growth of human lung cancer cell lines in vitro and in vivo and produces synergistic growth inhibition in combination with other antitumor agents. Clin Cancer Res. 2002b;8:1280–1287. [PubMed] [Google Scholar]
- De Weerd WFC, Leeb-Lundberg LMF. Bradykinin sequesters B2 bradykinin receptors and the receptor-coupled Gα subunits Gαq and Gαi in caveolae in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1997;272:17858–17866. doi: 10.1074/jbc.272.28.17858. [DOI] [PubMed] [Google Scholar]
- Drube S, Liebmann C. In various tumour cell lines the peptide bradykinin B2 receptor antagonist, Hoe 140 (Icatibant), may act as mitogenic agonist. Br J Pharmacol. 2000;131:1553–1560. doi: 10.1038/sj.bjp.0703764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathy DB, Leeb T, Mathis SA, Leeb-Lundberg LMF. Spontaneous human B2 bradykinin receptor activity determines the action of partial agonists as agonists or inverse agonists. J Biol Chem. 1999;274:29603–29606. doi: 10.1074/jbc.274.42.29603. [DOI] [PubMed] [Google Scholar]
- Félétou M, Germain M, Thurieau C, Fauchère JL, Canet E. Agonistic and antagonistic properties of the bradykinin B2 receptor antagonist, Hoe 140, in isolated blood vessels from different species. Br J Pharmacol. 1994;112:683–689. doi: 10.1111/j.1476-5381.1994.tb13130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera L, Stewart JM, Whalley E, Burkard M, Zuzack JS. A new class of potent bradykinin antagonist dimers. Immunopharmacology. 1996;33:178–182. doi: 10.1016/0162-3109(96)00099-9. [DOI] [PubMed] [Google Scholar]
- Haasemann M, Cartaud J, Müller-Esterl W, Dunia I. Agonist-induced redistribution of bradykinin B2 receptor in caveolae. J Cell Sci. 1998;111:917–928. doi: 10.1242/jcs.111.7.917. [DOI] [PubMed] [Google Scholar]
- Houle S, Larrivée JF, Bachvarova M, Bouthillier J, Bachvarov DR, Marceau F. Antagonist-induced intracellular sequestration of rabbit bradykinin B2 receptor. Hypertension. 2000;35:1319–1325. doi: 10.1161/01.hyp.35.6.1319. [DOI] [PubMed] [Google Scholar]
- Houle S, Molinaro G, Adam A, Marceau F. Tissue kallikrein actions at the rabbit natural or recombinant kinin B2 receptors. Hypertension. 2003;41:611–617. doi: 10.1161/01.HYP.0000054971.03046.9B. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacological Analysis of Drug–receptor Interaction. Raven Press: New York; 1987. Agonist efficacy; pp. 183–203. [Google Scholar]
- Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International Union of Pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Leslie FM. Methods used for the study of opioid receptors. Pharmacol Rev. 1987;39:197–249. [PubMed] [Google Scholar]
- MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. Direct evidence for biased agonism. J Biol Chem. 2001;276:28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- Marceau F, Sabourin T, Houle S, Fortin JP, Petitclerc E, Molinaro G, et al. Kinin receptors: functional aspects. Int Immunopharmacol. 2002;2:1729–1739. doi: 10.1016/s1567-5769(02)00189-3. [DOI] [PubMed] [Google Scholar]
- Mathieu MC, Sawyer N, Greig GM, Hamel M, Kargman S, Ducharme Y, et al. The C3a receptor antagonist SB 290157 has agonist activity. Immunol Lett. 2005;100:139–145. doi: 10.1016/j.imlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Melikov K, Chernomordik LV. Arginine-rich cell penetrating peptides: from endosomal uptake to nuclear delivery. Cell Mol Life Sci. 2005;62:2739–2749. doi: 10.1007/s00018-005-5293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette G, Fortin JP, Otis S, Bouthillier J, Marceau F. A novel non-peptide antagonist of the kinin B1 receptor: effects at the rabbit receptor. J Pharmacol Exp Ther. 2004a;311:1121–1130. doi: 10.1124/jpet.104.071266. [DOI] [PubMed] [Google Scholar]
- Morissette G, Petitclerc E, Marceau F. Loss of function of vascular smooth muscle cells by nitric oxide-dependent and – independent interactions with tumorigenic cells. Int J Cancer. 2004b;112:830–839. doi: 10.1002/ijc.20495. [DOI] [PubMed] [Google Scholar]
- Munoz CM, Leeb-Lundberg FLM. Receptor-mediated internalization of bradykinin. DDT1 MF-2 smooth muscle cells process internalized bradykinin via multiple degradative pathways. J Biol Chem. 1992;267:303–309. [PubMed] [Google Scholar]
- Pizard A, Blaukat A, Müller-Esterl W, Alhenc-Gelas F, Rajerison RM. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three serine and two threonine residues at its carboxyl tail. J Biol Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- Pruneau D, Paquet JL, Luccarini JM, Defrêne E, Fouchet C, Franck RM, et al. Pharmacological profile of LF 16–0687, a new potent non-peptide bradykinin B2 receptor antagonist. Immunopharmacology. 1999;43:187–194. doi: 10.1016/s0162-3109(99)00128-9. [DOI] [PubMed] [Google Scholar]
- Sabourin T, Bastien L, Bachvarov DR, Marceau F. Agonist-induced translocation of the kinin B1 receptor to caveolae-related rafts. Mol Pharmacol. 2002;61:546–553. doi: 10.1124/mol.61.3.546. [DOI] [PubMed] [Google Scholar]
- Schroeder C, Beug H, Müller-Esterl W. Cloning and functional characterization of the ornithokinin receptor. Recognition of the major kinin receptor antagonist, HOE140, as a full agonist. J Biol Chem. 1997;272:12475–12481. doi: 10.1074/jbc.272.19.12475. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Gera L, Chan DC, Bunn PA, York EJ, Simkeviciene V, et al. Bradykinin-related compounds as new drugs for cancer and inflammation. Can J Physiol Pharmacol. 2002;80:275–280. doi: 10.1139/y02-030. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Gera L, Chan DC, Whalley ET, Hanson WL, Zuzack JS. Potent, long-acting bradykinin antagonists for a wide range of applications. Can J Physiol Pharmacol. 1997;75:719–724. [PubMed] [Google Scholar]
- Stewart JM, Gera L, Chan DC, York EJ, Simkeviciene V, Bunn PA, et al. Combination cancer chemotherapy with one compound: pluripotent bradykinin antagonists. Peptides. 2005;26:1288–1291. doi: 10.1016/j.peptides.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Gera L, Hanson W, Zuzak JS, Burkard M, McCullough R, et al. A new generation of bradykinin antagonists. Immunopharmacology. 1996;31:51–60. doi: 10.1016/0162-3109(96)00084-7. [DOI] [PubMed] [Google Scholar]
- Su DS, Markowitz MK, DiPardo RM, Murphy KL, Harrell CM, O'Malley SS, et al. Discovery of a potent, non-peptide bradykinin B1 receptor antagonist. J Am Chem Soc. 2003;125:7516–7517. doi: 10.1021/ja0353457. [DOI] [PubMed] [Google Scholar]
- Taub JS, Guo R, Leeb-Lundberg LMF, Madden JF, Daaka Y. Bradykinin receptor subtype 1 expression and function in prostate cancer. Cancer Res. 2003;63:2037–2041. [PubMed] [Google Scholar]
- Tung CH, Weissleder R. Arginine containing peptides as delivery vectors. Adv Drug Deliv Rev. 2003;55:281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]