Abstract
Background and purpose:
Nitrergic neurons are important for erectile responses in the corpus cavernosum and impaired signalling results in erectile dysfunction, today treated successfully by oral administration of the selective phosphodiesterase 5 (PDE 5) inhibitors sildenafil, tadalafil and vardenafil. Although the importance of nitrergic neurons in urogenital function has become evident, it has not been investigated if the PDE 5 inhibitors affect the nerve-induced release of nitric oxide (NO). In a previous study we found that the soluble guanylate cyclase (sGC)/cyclic guanosine 3',5'–monophosphate (cGMP) pathway might modulate nerve-induced release of NO in isolated cavernous tissue.
Experimental approach:
Electrical field stimulation (EFS 5 Hz, 40 V, 0.3 ms pulse duration, 25 pulses at intervals of 2 min) of rabbit isolated cavernous tissue elicited reproducible, nerve-mediated relaxations in the presence of scopolamine (10−5 M), guanethidine (10−5 M) and phenylephrine (3 × 10−6 M). In superfusion experiments, nerve stimulation (20 Hz, 40 V, 1 ms) of the cavernous tissue evoked release of NO/NO2 −, measured by chemiluminescence.
Key results:
Sildenafil, tadalafil and vardenafil decreased the muscular tone and prolonged the relaxations to nerve stimulation. The evoked release of NO decreased to 72±11%, 55±16% and 61±14% of control, respectively after addition of sildenafil, tadalafil or vardenafil (all 10−4 M, n=6–8, p<0.05).
Conclusions and Implications:
Selective PDE 5 inhibitors influence the nerve-induced release of NO, probably via cGMP-mediated negative feedback. This negative feedback might explain why priapism is not seen during monotherapy with the PDE inhibitors.
Keywords: rabbit, corpus cavernosum, nitric oxide, neurotransmission, cGMP, PDE, sildenafil, tadalafil, vardenafil, zaprinast
Introduction
Nitric oxide (NO) is an important neurotransmitter in the autonomic nervous system and evidence for involvement of the L-arginine/NO pathway in the urogenital tract has been found. In the corpus cavernosum, it was suggested that non–adrenergic non–cholinergic (NANC) inhibitory responses to nerve stimulation were mediated by NO (Ignarro et al., 1990; Holmquist et al., 1991). Further, in bovine retractor penis (Liu et al., 1991) and rat anococcygeus muscles (Li and Rand, 1989; Liu et al., 1991) NO was shown to mediate relaxations in response to nerve stimulation. These findings were supported by the identification of NO synthase (NOS) in neurons of the rat and canine penis (Burnett et al., 1992; Keast, 1992). Also, NOS positive neurons were found in the human penis where nerve stimulation of the corpus cavernosum elicited release of NO in parallel with reproducible relaxations (Leone et al., 1994). Previously, our group has quantified the release of NO from autonomic neurons of rabbit corpus cavernosum. The release of NO decreased in presence of the NOS inhibitor Nw-nitro-L-arginine methyl ester (L-NAME) and originated from neurons as indicated by the decreased release in the presence of tetrodotoxin (TTX) (Hallén et al., 2005).
In the cavernous tissue of the rabbit penis, the most likely sources for NO-synthesis would be the neurons and endothelium as indicated by their content of NOS (Andersson and Wagner, 1995; Dail et al., 1995). Stimulation of nitrergic neurons results in release of NO that will diffuse to surrounding smooth muscle cells. On the postsynaptic side, NO binds to soluble guanylate cyclase (sGC) and the conversion of GTP to cyclic guanosine 3′,5-monophosphate (cGMP) will be facilitated. The increase in cGMP concentration results in diminished levels of intracellular calcium and alterations in protein phosphorylation, thereby causing relaxation of smooth muscle cells (Andersson and Wagner, 1995). Further, the concentration of cGMP in a cell is controlled by phosphodiesterases (PDEs) that catalyse the degradation of cGMP by hydrolysis to GMP. As of today, 11 different subtypes of PDE are characterized in mammalian tissues. Upon the discovery that the PDE inhibitor zaprinast, with some preference for phosphodiesterase 5 (PDE 5), enhances NANC-mediated relaxations of human and rabbit cavernous tissue, it was suggested that defects of the cGMP pathway may cause some forms of impotence (Bush et al., 1992; Rajfer et al., 1992). Indeed, further studies have revealed the importance of intact NO/cGMP signalling for erectile responses and selective PDE 5 inhibitors have proven to be a pharmacological approach in treatment of erectile dysfunction (Corbin et al., 2002).
PDE 5 is present in corpus cavernosum and of main importance in the breakdown of cGMP, especially in the rabbit (Qiu et al., 2000). Other PDEs are present in the cavernous tissue (Waldkirch et al., 2005), but do not appear to significantly modulate changes in cGMP levels associated with the ability to achieve penile erection (Corbin et al., 2002; Weeks et al., 2005). Recently, several different selective PDE 5 inhibitors have been developed. First came Viagra (sildenafil) (Boolell et al., 1996), then Cialis (tadalafil) (Eardley and Cartledge, 2002), and recently Levitra (vardenafil) (Klotz et al., 2001) was launched. In common, they all facilitate erection as a consequence of stimuli by increasing the cellular content of cGMP in the cavernous tissue (Francis and Corbin, 2003). Viagra, Cialis and Levitra are now widely used in treatment of male erectile dysfunction.
Previously, it has been described that cGMP might affect the release of NO from the autonomic neurons in guinea-pig colon (Hallén et al., 2001) and in rabbit corpus cavernosum (Hallén et al., 2005). Application of modulators of the sGC/cGMP pathway (the analog 8-Br-cGMP, the sGC stimulator 3-(5′-hydroxymethyl-2-furyl)-1-benzylindazole (YC-1), the sGC inhibitor 1-H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) and the non-selective PDE 5 inhibitor zaprinast) suggested that cGMP enhances the release of NO from autonomic neurons in guinea-pig colon and in rabbit corpus cavernosum, but the effect of zaprinast was variable. In light of the previous findings, this study was designed to investigate if the commercially available selective PDE 5 inhibitors sildenafil, tadalafil and vardenafil affected the nerve–induced release of NO from cavernous tissue. This would also give an opportunity to study the effect of modulating endogenous cGMP levels, which might give more detailed information compared to applying cGMP analogues to the entire tissue as in our previous study (Hallén et al., 2005).
Methods
The experiments were approved by the local ethics committee for experimentation on animals.
Tissue preparation
Male New Zealand rabbits (2.5–3.1 kg) were anaesthetized by a slow injection of sodium pentobarbital (60 mg kg−1) in the marginal vein of the ear and exsanguinated by a cut in the heart. The abdominal aorta was cannulated and perfused with 150 ml 0.9% NaCl at 37°C in order to remove the hemoglobin from the tissue. The corpora cavernosa were removed and placed in ice–cold modified Krebs solution (136.9 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 0.6 mM MgSO4, 11.9 mM NaHCO3, 0.5 mM KH2PO4, 11.5 mM glucose), prepared from ultrafiltrated water (α-Q, Millipore, Bedford, MA, USA). All buffer salts were of pro-analysis grade. The paired corpora cavernosa were cleaned from adjacent muscle and connective tissues. Two types of experiments were performed, organ bath experiments for analysis of muscle responses and superfusion experiments for analysis of NO/nitrite -release.
Organ bath experiments
In organ bath experiments the paired corpora cavernosa had the tunica albuguinea removed, were divided into a total of 10–14 strips, 10–12 mm in length, and tied between thin threads. The preparations were suspended in 5.5–6.5 ml organ baths containing Krebs solution at 20°C, and after 20 min were heated to 37°C during continuous aeration with 6.5% CO2 in O2. The muscle preparations received transmural electrical field stimulation (EFS) (5 Hz, 40 V, 0.3 ms pulse duration, 25 pulses at intervals of 2 min), applied through 10 mm long platinum electrodes in the wall of the organ bath, 10 mm apart. The stimulation parameters were chosen to obtain stable and reproducible muscular response over time and in accordance with stimulation parameters utilized in previous work (Cellek and Moncada, 1997). The EFS was applied from a Grass S44 stimulator (Quincy, MA, USA) and the mechanical muscular activity was recorded isometrically by Grass force–displacement transducers (FT03) and displayed with a Grass model 7D Polygraph. For observations of the relaxing effects mediated by EFS and PDE inhibitors, a combination of adrenergic blockers and agonist were added to precontract the tissues (see Results). Pharmacological substances were added directly into the organ baths between periods of EFS and each substance was tested in six preparations from at least three rabbits.
Superfusion experiments for measurements of NO/NO2−
In experiments where the release of NO was measured, the cavernous tissue with part of the tunica albuginea was divided longitudinally to obtain two similar strips (35 × 6 mm). A cut was made longitudinally in each strip in order to increase the exposure of the tissue to the superfusate. Each half was tied between monofilic nylon threads and mounted in an open jacket-type glass chamber at a load of 6–10 mN, with continuous superfusion of Krebs solution at 1 ml/min at a temperature of 37°C. The superfusion experiments were performed without any precontraction of the tissue. The NOS substrate, L-arginine, (10−5 M) was added to the Krebs solution and the tissue was left for approximately 120 min in order to equilibrate before pharmacological substances were applied.
Needle shaped electrodes were placed on the cavernous tissue, approximately 3 cm apart. The electrodes used were made of silver since platinum electrodes are unsuitable for NO/NO2−-release experiments due to generation of a false NO/NO2−-signal through electrolytic conversion of compounds released from tissues as well as from amino acids (e.g. L-arginine) added to the tissue bath (Wiklund et al., 1993). The tissue received transmural electrical stimulation (20 Hz, 40 V biphasic, pulse duration 1 ms) for 1 min at 30 min intervals. The stimulation parameters were chosen to obtain a stable nerve-induced release of NO over time and in accordance to the stimulation parameters chosen in previous work (Leone et al., 1994; Cellek and Moncada, 1997; Hallén et al., 2005). The EFS was applied through a Grass S88 stimulator and the muscle activity of the tissue was monitored isometrically by Grass force–displacement transducer (FT03) and displayed with a Grass model 7D Polygraph.
The tissue superfusate was collected during 1 min periods in freshly rinsed tubes before, and during electrical stimulation, and was immediately analyzed for their content of NO/NO2−. The sampled superfusate corresponded to the fluid being in contact with the tissue exactly during the electrical stimulation. This was chosen since it previously has been shown that release of NO from nitrergic nerves falls by 75% in the first 20 s after a 60 s stimulation period (Wiklund et al., 1997). This was also confirmed in development of the methods for the present study (data not shown). For determination of NO and its oxidation product NO2−, aliquots of the superfusate were injected into a thermostated glass vessel, containing 150 ml of 1% sodium iodide in concentrated and deoxygenated hot acetic acid (90°C), continuously purged with N2. In the vessel, NO2− is reduced to NO in the acidic and reducing milieu and thereafter carried further by the stream of N2 into a reaction chamber where NO reacts with ozone under vacuum, with emission of photons. A photomultiplier tube counted the photons. The system was calibrated on a daily basis by injecting aliquots of NaNO2 solution and using peak heights for construction of standard curves (Iversen et al., 1994). The detection limit was 0.5–1 pmol NaNO2 per ml. The background signal from Krebs solution was subtracted from each sample. Four periods of stimulations (30 min intervals, see above) were applied, to determine whether the evoked release of NO/NO2− was stable and then the fourth stimulation was applied in the presence or absence of the selective PDE 5 inhibitor. Experiments without a sufficient or stable nerve induced release of NO/NO2− upon electrical stimulation during the first three stimulations were excluded (18% were excluded). This was possible to determine on-line in each experiment since the NO/NO2− analysis was made immediately after each stimulation. Drug treatments were applied in a randomized fashion. The concentrations of sildenafil, tadalafil and vardenafil used were chosen in accordance to their effect on inhibitory nerve stimulation evoked relaxations in organ bath experiments. Pharmacological substances were present for 20 min before the nerve stimulation and each tissue was exposed to only one concentration of the selective PDE 5 inhibitor. Also, a different PDE inhibitor was used within the two preparations obtained from each animal.
Pharmacological substances
Control experiments were performed to exclude the possibility that the pharmacological substances used would release NO/NO2− or that NO2− would be converted to NO3− upon electrical stimulation. Also, another possibility is that NO/NO2− could be lost or generated by an unspecific reaction with L-arginine or the PDE inhibitors used. To avoid the above-mentioned scenarios, a nylon thread was mounted in the open-jacket chamber instead of the corpus cavernosum and the thread was continuously superfused with Krebs solution. Electrical stimulation of the thread in absence or presence of the substances used (L-Arg, sildenafil, tadalafil and vardenafil) did not alter the release of NO/NO2−. Hence it can be assumed that none of the substances per se released NO/NO2− and that NO2− was not converted to NO3− upon electrical stimulation.
L–Arginine, and dimethyl sulphoxide (DMSO) were purchased from Sigma (St Louis, MO, USA). Sildenafil, tadalafil and vardenafil were a gift from the producers, Pfizer, Lilly and Bayer, respectively. Sildenafil, tadalafil and vardenafil were dissolved in DMSO, while L–arginine was prepared in α-Q ultrafiltrated water. The substances were diluted 1:1000 (concentrations 10−7–10−5 M) or 1:100 (10−4 M) in the organ bath or tissue superfusate. DMSO 1:100 to the tissue superfusate did not alter the amount of NO released (data not shown) despite showing an inhibitory effect on smooth muscle responses.
Statistical analysis
Experimental data are expressed as mean±s.e.m. Statistical significance of changes in NO/NO2−-release was tested according to Student's t–test for paired observations, comparing the release during the fourth stimulation to the third stimulation. For calculation of statistical differences in organ bath experiments on muscle tone and responses to nerve stimulation, and the effect thereupon by the selective PDE 5 inhibitors, repeated measurements One–way ANOVA was used followed by Tukey's test. All statistical calculations were done by using a computer program (SigmaStat, Jandel, San Rafael, CA, USA). n indicates the number of experiments, significant difference between treatment groups was accepted for P<0.05.
Results
Organ baths
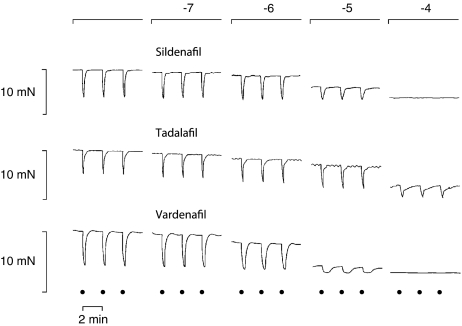
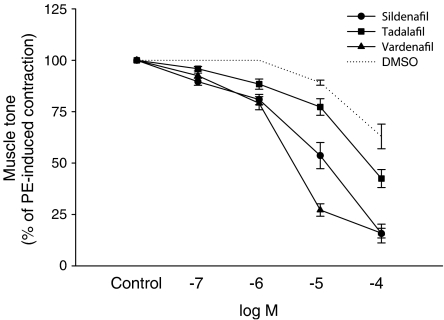
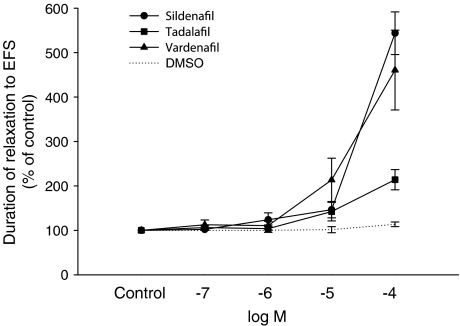
Reproducible contractions of the corpus cavernosum could be observed upon EFS (5 Hz, 40 V, 0.3 ms pulse duration, 25 pulses at intervals of 2 min). In the presence of scopolamine (10−5 M), guanethidine (10−5 M) and phenylephrine (3 × 10−6 M), a constant degree of contraction was achieved and EFS elicited reproducible relaxations over time (Figure 1). The relaxations could be totally abolished in the presence of TTX (10−6 M) (data not shown). The tone of the cavernous tissue was significantly lowered by addition of sildenafil, tadalafil and vardenafil in a dose dependent fashion (Figures 1 and 2). Furthermore, the relaxing phase induced by electrical stimulation was prolonged with increasing concentrations of sildenafil, tadalafil and vardenafil (Figure 3). The solvent, DMSO, caused some degree of relaxation, which however was significantly smaller than the combined effect of a PDE inhibitor and the solvent (Figure 2).
Figure 1.
Organ bath experiments. Three representative tracings of nitrergic relaxations of three rabbit corpus cavernosum preparations upon electrical stimulation (5 Hz, 40 V, 0.3 ms pulse duration, 25 pulses at intervals of 2 min, as indicated by dots) in the presence of phenylephrine (3 × 10−6 M), scopolamine (10−5 M) and guanethidine (10−5 M). Application of sildenafil, tadalafil and vardenafil (all at 10−7–10−4 M; as shown at top of figure) lowered the tone of the tissue in a dose-dependent fashion and prolonged the duration of the EFS-induced relaxations.
Figure 2.
The effects of sildenafil, tadalafil and vardenafil (all at 10−7–10−4 M), on phenylephrine-induced tone in preparations of rabbit corpus cavernosum. The tissue was pretreated with scopolamine (10−5 M) and guanethidine (10−5 M). Results are shown as percentage of the contractile response induced by phenylephrine (means±s.e.m.). For controls, the error bars were hidden within the symbol.
Figure 3.
The effects of sildenafil, tadalafil or vardenafil (all at 10−7–10−4 M), on nitrergic responses in preparations of rabbit corpus cavernosum. The tissue received EFS (5 Hz, 40 V, 0.3 ms pulse duration, 25 pulses at intervals of 2 min) and was pretreated with phenylephrine (3 × 10−6 M), scopolamine (10−5 M) and guanethidine (10−5 M). Results are shown as percentage of the duration of the relaxation response induced by EFS (means±s.e.m.).
Release of NO/NO2−
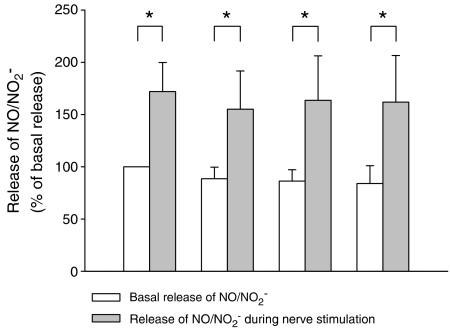
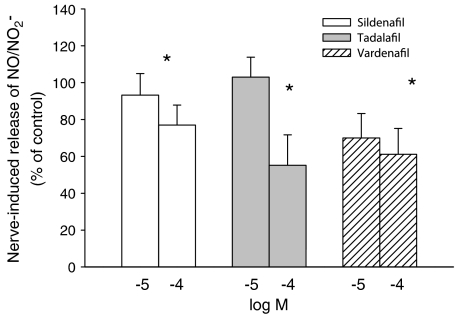
Upon addition of sildenafil (10−5 and 10−4 M), tadalafil (10−5 and 10−4 M) and vardenafil (10−5 M) to the tissue superfusate, the basal overflow of NO was not altered (Table 1). However, addition of vardenafil (10−4 M) decreased the overflow of NO. Electrical stimulation (20 Hz, 40 V, 1 ms) of the rabbit corpus cavernosum for 1 min, every 30 min, evoked a stable release of NO (Figure 4), as well as a muscular contraction (not shown), as described previously (Hallén et al., 2005). In the presence of sildenafil (10−5 M) and tadalafil (10−5 M) the nerve-induced release of NO was not altered (93±12% of controls, n=6 and 103±11% of controls, n=6, respectively, all NS) (Figure 5). In the presence of vardenafil (10−5 M) there was a small but not significant decrease in the nerve induced release of NO (Figure 5). At 10-fold higher concentration of the PDE inhibitors, the nerve stimulation evoked release of NO decreased after addition of sildenafil, tadalafil or vardenafil (all at 10−4 M; Figure 5). The solvent, DMSO, despite having inhibitory effects on muscle tone (Figure 2), did not modify the release of NO (data not shown).
Table 1.
Basal release of NO/NO2− from the cavernous tissue of the rabbit penis before (control) and during addition of sildenafil, tadalafil or vardenafil (all at 10−5 and 10−4 M)
| Sildenafil | |||||||
|---|---|---|---|---|---|---|---|
| 10−5 M | Control | 10−4 M | Control | ||||
| 8±1 | 7±1 | n=7 | NS | 10±2 | 12±2 | n=11 | NS |
| Tadalafil | |||||||
| 10−5 M | Control | 10−4 M | Control | ||||
| 10±3 | 10±3 | n=7 | NS | 17±4 | 16±3 | n=7 | NS |
| Vardenafil | |||||||
| 10−5 M | Control | 10−4 M | Control | ||||
| 8±1 | 9±1 | n=7 | NS | 10±2 | 15±2 | n=10 | ** |
Abbrevations: NO, Nitric oxide; NS, not significant, PDE, phosphodiesterase.
Statistically significant change of NO/NO2−-release is denoted by **(P<0.01). Results are shown as pmol/min, mean±s.e.m. where n denotes the number of animals and tissues studied at each concentration of PDE inhibitor.
Figure 4.
NO/NO2−-release from isolated cavernous tissue of the rabbit penis before and during nerve-induced stimulation (transmural electrical stimulation, at 40 V, 20 Hz, pulse duration 1 ms). Electrical stimulation was repeatedly applied for 1 min, 30 min apart. Each period of electrical stimulation elicited-release of NO/NO2− and a muscular contraction (not shown). The release of NO/NO2− was statistically significantly increased compared to controls. Results are shown as percentage of NO/NO2−-release at basal conditions prior to the first electrical stimulation, Columns show mean and bars indicate±s.e.m.; n=5–7; *P<0.05.
Figure 5.
Effect of sildenafil, tadalafil and vardenafil (all at 10−5 and 10−4 M), on nerve induced NO/NO2−-release from the cavernous tissue of the rabbit penis. Each column represents the evoked release during a period of transmural nerve stimulation (40 V, 20 Hz, pulse duration 1 ms) for 1 min. Application of sildenafil, tadalafil or vardenafil, at 10−4 M, decreased the nerve–induced NO/NO2−-release, whereas the lower concentration (10−5 M) did not alter nerve–induced NO/NO2−-release. Results are shown as percentage of nerve-induced NO/NO2−–release in controls. Columns show means and bars indicate±s.e.m.; n=6–8 for each group; *P<0.05.
Discussion
This study confirms that the commercially available selective PDE 5 inhibitors sildenafil, tadalafil and vardenafil may relax cavernous tissue of the rabbit penis. As expected, the responses of inhibitory nitrergic NANC neurons were enhanced, but unexpectedly the PDE inhibitors had an inhibitory effect on release of NO. Further, in organ bath experiments it was found that electrical stimulation of isolated preparations of rabbit corpus cavernosum elicited relaxation in the presence of phenylephrine, scopolamine and guanethidine. The relaxations were reproducible over time and totally blocked by TTX, indicating that the relaxations were nerve mediated. By application of selective PDE 5 inhibitors, sildenafil, tadalafil and vardenafil, a dose dependent reduction of the tone of the tissue was achieved. The nerve-induced relaxations were totally abolished in the presence of sildenafil and vardenafil at the concentration of 10−4 M, most likely due to an almost complete loss of muscular tone. Tadalafil was less potent in decreasing muscle tone in our in vitro situation, which is in agreement with reported data on IC50 values of these drugs on PDE 5 (Kim, 2003). Further, the selective PDE 5 inhibitors used in this study mediated a prolongation of the nerve-induced relaxing phase. This effect was also most pronounced for sildenafil and vardenafil. Taken together our results suggest that sildenafil, tadalafil and vardenafil were increasing the muscular relaxations in response to nerve stimulation in a dose-dependent fashion. Our results are in line with previous findings (Stief et al., 1998; Giuliano et al., 2003) and higlight the ability of these PDE 5 inhibitors to modulate the muscular responses induced by nitrergic neurons.
In experiments where the release of NO from the cavernous tissue was measured, it was found that electrical stimulation of the erectile tissue resulted in release of NO/NO2− as previously described (Hallén et al., 2005). The release is initiated by nerve-induced activation of NOS since it can be reduced by NOS inhibitors and the neurotoxin TTX (Hallén et al., 2005) in agreement with previous findings (Leone et al., 1994; Cellek et al., 1996). We report here the novel observation that the nerve–induced release of NO was subject to modulation by the selective PDE 5 inhibitors, sildenafil, tadalafil and vardenafil, and that this modulation was inhibitory. Neither sildenafil, tadalafil nor vardenafil at the lower concentration used (10−5 M) affected the basal release of NO, whereas a slight effect was observed with vardenafil at 10−4 M. This may indicate slight differences in potencies or cooperative interaction between vardenafil and cGMP as described recently (Blount et al., 2004), but the limited number of experiments does not allow any conclusions at this point.
The concentrations of the PDE 5 inhibitors necessary to evoke the modulatory effect on NO/NO2− release were considerably higher than those found in clinical use. This is likely to be due to suboptimal penetration of the substance in the in vitro situation, where no blood perfusion is at hand. The concentrations were only slightly above those necessary for direct relaxation of the tissues (Figure 1). We performed these experiments to find out the concentration range needed for biological effects in vitro in our model. We are therefore of the opinion that the results in this study might be of relevance for in vivo conditions where drug delivery to the tissue will be much more efficient.
Comparison with earlier observations on NO/NO2− release
The observations presented in this study, suggesting a negative cGMP-mediated feedback on nerve-induced NO release, may on initial inspection appear to be in contradiction with two previous studies from our laboratory. In the longitudinal smooth muscle layer of guinea-pig colon (Hallén et al., 2001) and in rabbit corpus cavernosum (Hallén et al., 2005) it was found that agents mimicking or stimulating the formation of cGMP led to increases of the nerve-induced release of NO. Thus, in both studies, enhancing effects on nerve-induced NO release were obtained by addition of the cGMP analog 8-Br-cGMP or by YC-1, a stimulator of cGMP synthesis. In the study on colon tissue, the moderately selective PDE inhibitor zaprinast increased the nerve-induced release of NO.
First, we will consider the discrepancy with our previous results in the colon. In intestinal tissue, an excitatory effect of exogenous NO was observed, leading to smooth muscle contraction (Olgart et al., 1997). This excitatory effect was shown to be due to NO-induced activation of cholinergic nerves and nerves releasing substance P-like peptides. The intestinal excitatory effect is likely to be exerted proximal to the most peripheral nerve endings since it was amenable to blockade by TTX, and was exerted via the cGMP pathway due to the blockade of the excitation by the guanylate cyclase inhibitor ODQ (Olgart et al., 1997). Therefore, in our previous study in colon, an inhibitory feedback by cGMP on NO release in the nerve terminals of the intestinal neurons might have been masked by a simultaneous excitatory effect by the applied NO/cGMP pathway modulators on intestinal neurons or their axons. The presently observed negative feedback by cGMP on nerve-induced NO release in the corpus cavernosum, as indicated by the effect of the selective PDE 5 inhibitors, might therefore motivate further in-depth studies on the possibility of a negative cGMP-mediated feedback in the terminal arborizations of the intestinal neurons.
Second, in our earlier report on the corpus cavernosum of rabbits (Hallén et al., 2005), neither the basal release of NO nor the nerve-induced release of NO was altered in the presence of zaprinast. The lack of effect of zaprinast contrasting with the presently observed significant effects of the more selective PDE 5 inhibitors might be due to less selectivity of zaprinast as a PDE inhibitor. Zaprinast is an inhibitor of PDE 1, 5 and 6 (Francis and Corbin, 2003) and inhibition of, for example, PDE 1 might lead to increments in cAMP, which in turn might act to facilitate neurotransmitter release (Lefkowitz, 1996).
Third, in our previous report on corpus cavernosum, we observed stimulating effects of 8-Br-cGMP and YC-1 on NO/NO2− release (Hallén et al., 2005). Application of these substances might lead to a more general activation of cGMP mediated mechanisms (Olgart et al., 1997) in the tissue, perhaps even activating or sensitizing nerves to stimulation as observed by us in the intestinal tissue, whereas the selective PDE 5 inhibitors might only enhance the action of cGMP generated locally upon nerve-stimulation induced neuronal activity.
Mechanism of modulation of NO/NO2− release
Previously it has been shown that cGMP-dependent phosphorylation of neuronal NOS leads to inhibition of the enzyme (Dawson et al., 1993; Dinerman et al., 1994). Thus, the inhibition of NO release by the selective PDE 5 inhibitors we found in this study could be effected via phosphorylation of neuronal NOS.
In their studies on NOS phosphorylation, Dinerman et al. found that both cAMP- and cGMP-dependent phosphorylation in living cells mainly occurred at a serine site, with some phosphorylation at a threonine site. Furthermore, Dinerman et al. found that NOS-dependent NO generation was inhibited, whereas NO generation from an NO-donor (sodium nitroprusside, SNP) was maintained (Dinerman et al., 1994). The exact locations of the phosphorylated amino acids were not determined. Subsequent studies have indicated that phosphorylation of both serine 847 and 1412 in nNOS may inhibit the enzyme (Mount et al., 2005). A recent study shows that phosphorylation of threonine 1296 of nNOS also inhibits the enzyme in living cells (Song et al., 2005), an effect observed also with LPS-induced tyrosine phosphorylation of nNOS in an astrocytoma cell line (Colasanti et al., 1999). In contrast, endothelial NOS is activated by cyclic nucleotide-dependent phosphorylation on two serine and one threonine sites (Butt et al., 2000). Interestingly, calcium/calmodulin-dependent phosphorylation of nNOS also can inhibit the enzyme, but by phosphorylation at serine 741 (Zoche et al., 1997). Thus, there is ample evidence for phosphorylation-induced regulation of NOS activity lending support to the hypothesis that the presently observed inhibitory effect on NO-release by PDE 5 inhibitors might be through such a mechanism.
The nNOS in the urogenital tract occurs also as a splice variant, PnNOS, which is elongated by 34 amino acids (http://www.expasy.org/uniprot.P29476; Magee et al., 1996). As a database simulation (http://www.cbs.dtu.dk/services/NetPhos) indicated that several amino acids in the elongation segment are possible sites of serine phosphorylation it is obvious that further studies are required to elucidate how the presently observed effects are exerted at the protein level. Studies in the kidney show that changes in nNOS phosphorylation can occur in physiological conditions (Mount et al., 2005). We would thus favour the view that the presently observed effects by the phosphodiesterase inhibitors are exerted via a change in the phosphorylation of an isoform of NOS, most probably nNOS.
Theoretically, the selective PDE 5 inhibitors, we have used might exert their effect through other phosphodiesterases than PDE 5, since expression of a multitude of PDEs has been observed in the urogenital tract and the corpus cavernosum (Kuthe et al., 2001; Makhlouf et al., 2006). Although PDE 11 has attracted considerable attention in these organs (Makhlouf et al., 2006) it seems less likely as a target for the presently used inhibitors (Weeks et al., 2005). Augmentation, by the phosphodiesterase inhibitors, of inhibitory effects of NO on neuronal calcium entry is another, perhaps less likely but still possible, explanation for the effects observed by us, since NO has been shown to decrease calcium entry into CNS neurons (Snider et al., 2000).
The physiological response to neuronal release of NO in the corpus cavernosum is increased blood flow to the cavernous tissue, a prerequisite for achieving and maintaining erection (Burnett, 2002). Indeed, lack of what is now known to be nitrergic neurons in human erectile tissue leads to an inability to maintain erections (Saenz de Tejada et al., 1989), and in diabetic impotent rats it was found that nitrergic neurons in the penis undergo degeneration (Cellek et al., 1999). Apart from the neurons, NO is also released from the endothelium upon stimulation by acetylcholine (ACh). However, administration of ACh does not result in reliably induced erections (Dorr and Brody, 1967) and intracavernosally injected atropine fails to inhibit erections induced by nerve stimulation (Brindley, 1986; Burnett et al., 1992). Thus, it was suggested that nitrergic neurons would be required to maintain erections of the penis. This is further supported by our previous finding that blockade of the cholinergic pathway did not affect the release of NO (Hallén et al., 2005).
Possible clinical implications
If there was only a positive feedback on nitrergic neurons, through NO-stimulated cGMP formation (Hallén et al., 2001, 2005), an uninhibited activation of erection might be evoked, theoretically leading to priapism. The present study proposes that increasing concentrations of cGMP generated via inhibition of PDE 5, results in a negative action on nerve-induced release of NO. The proposed negative feedback might be of considerable physiological importance, preventing induction of priapism. If an exogenous NO donor were administered concurrently with a PDE 5 inhibitor, there would be enhancement of the smooth muscle effects of NO but no cGMP dependent feedback on NO generation, since it does not involve NO generation through nNOS. The reports we have found in the literature on priapism with PDE 5 inhibitors involve either the use of selective PDE 5 inhibitors at a dosage above the recommended level or their use together with other pro-erectile substances by oral or intracavernous administration (Aoyagi et al., 1999; McMahon, 2003; Yonessi and Saeedi, 2005). At least the latter are situations where a negative cGMP-dependent feedback on NO formation might be expected to be bypassed.
Conclusion
Today, a common pharmacological approach for treatment of erectile dysfunction is to prescribe sildenafil (Viagra), tadalafil (Cialis) or vardenafil (Levitra). All three will inhibit PDE 5, thereby increasing the cellular concentration of cGMP (Jeremy et al., 1997; Ballard et al., 1998; Francis and Corbin, 2003). The present study provides indications that the local nerve-induced release of NO is under modulation in a negative feedback fashion as indicated by the effects of selective PDE 5 inhibitors. However, at present, this modulation is not fully elucidated and further studies are required, bearing in mind that a positive effect of NO, via cGMP might be also be present for neurotransmission in enteric nerve plexa (Olgart et al., 1998; Hallén et al., 2001). A negative feedback by NO, via cGMP, on NOS activity might explain why PDE 5 inhibitors rarely cause priapism as long as other pro-erectile compounds are not used.
Acknowledgments
Sildenafil, tadalafil and vardenafil were generously provided by the producers Pfizer, Lilly and Bayer, respectively. This work was supported by Gunvor och Josef Anérs stiftelse, Stiftelsen Johanna Hagstrand och Sigfrid Linnérs minne, Stiftelsen Lars Hiertas minne, Stiftelsen Sigurd och Elsa Goljes minne, Swedish Society for Medical Research and the Swedish Science Council grants (14285 and 7919).
Abbreviations
- ACh
acetylcholine
- cGMP
cyclic guanosine 3′,5-monophosphate
- EFS
electrical field stimulation
- GMP
guanosine 3′,5′-monophosphate
- L-NAME
Nw-nitro-L-arginine methyl ester
- NANC
non–adrenergic non–cholinergic
- NOS
NO synthase
- PDE 5
phosphodiesterase 5
- sGC
soluble guanylate cyclase
- TTX
tetrodotoxin
- YC-1
3-(5-hydroxymethyl-2′-furyl)-1-benzylindazole
Conflict of interest
The authors state no conflict of interest.
References
- Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Hayakawa K, Miyaji K, Ishikawa H, Hata M. Sildenafil induced priapism. Bull Tokyo Dent Coll. 1999;40:215–217. doi: 10.2209/tdcpublication.40.215. [DOI] [PubMed] [Google Scholar]
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, et al. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol. 2004;66:144–152. doi: 10.1124/mol.66.1.144. [DOI] [PubMed] [Google Scholar]
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- Brindley GS. Pilot experiments on the actions of drugs injected into the human corpus cavernosum penis. Br J Pharmacol. 1986;87:495–500. doi: 10.1111/j.1476-5381.1986.tb10191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AL. Nitric oxide regulation of penile erection: biology and therapeutic implications. J Androl. 2002;23:S20–S26. [PubMed] [Google Scholar]
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Bush PA, Aronson WJ, Buga GM, Rajfer J, Ignarro LJ. Nitric oxide is a potent relaxant of human and rabbit corpus cavernosum. J Urol. 1992;147:1650–1655. doi: 10.1016/s0022-5347(17)37671-1. [DOI] [PubMed] [Google Scholar]
- Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, et al. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- Cellek S, Kasakov L, Moncada S. Inhibition of nitrergic relaxations by a selective inhibitor of the soluble guanylate cyclase. Br J Pharmacol. 1996;118:137–140. doi: 10.1111/j.1476-5381.1996.tb15376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellek S, Moncada S. Nitrergic control of peripheral sympathetic responses in the human corpus cavernosum: a comparison with other species. Proc Natl Acad Sci USA. 1997;94:8226–8231. doi: 10.1073/pnas.94.15.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellek S, Rodrigo J, Lobos E, Fernandez P, Serrano J, Moncada S. Selective nitrergic neurodegeneration in diabetes mellitus – a nitric oxide-dependent phenomenon. Br J Pharmacol. 1999;128:1804–1812. doi: 10.1038/sj.bjp.0702981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti M, Persichini T, Cavalieri E, Fabrizi C, Mariotto S, Menegazzi M, et al. Rapid inactivation of NOS-I by lipopolysaccharide plus interferon-gamma-induced tyrosine phosphorylation. J Biol Chem. 1999;274:9915–9917. doi: 10.1074/jbc.274.15.9915. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Francis SH, Webb DJ. Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction. Urology. 2002;60:4–11. doi: 10.1016/s0090-4295(02)01686-2. [DOI] [PubMed] [Google Scholar]
- Dail WG, Barba V, Leyba L, Galindo R. Neural and endothelial nitric oxide synthase activity in rat penile erectile tissue. Cell Tissue Res. 1995;282:109–116. doi: 10.1007/BF00319137. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Steiner JP, Dawson VL, Dinerman JL, Uhl GR, Snyder SH. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci USA. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinerman JL, Steiner JP, Dawson TM, Dawson V, Snyder SH. Cyclic nucleotide dependent phosphorylation of neuronal nitric oxide synthase inhibits catalytic activity. Neuropharmacology. 1994;33:1245–1251. doi: 10.1016/0028-3908(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Dorr LD, Brody MJ. Hemodynamic mechanisms of erection in the canine penis. Am J Physiol. 1967;213:1526–1531. doi: 10.1152/ajplegacy.1967.213.6.1526. [DOI] [PubMed] [Google Scholar]
- Eardley I, Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int J Clin Pract. 2002;56:300–304. [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep. 2003;4:457–465. doi: 10.1007/s11934-003-0027-x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, Alexandre L, Niewoehner U, Haning H, Bischoff E. Pro-erectile effect of vardenafil: in vitro experiments in rabbits and in vivo comparison with sildenafil in rats. Eur Urol. 2003;44:731–736. doi: 10.1016/s0302-2838(03)00377-4. [DOI] [PubMed] [Google Scholar]
- Hallén K, Gustafsson LE, Wiklund NP. Nerve-induced release of nitric oxide from the rabbit corpus cavernosum is modulated by cyclic guanosine 3′,5′-monophosphate. Neuroscience. 2005;133:169–174. doi: 10.1016/j.neuroscience.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Hallén K, Olgart C, Gustafsson LE, Wiklund NP. Modulation of neuronal nitric oxide release by soluble guanylyl cyclase in guinea pig colon. Biochem Biophys Res Commun. 2001;280:1130–1134. doi: 10.1006/bbrc.2001.4241. [DOI] [PubMed] [Google Scholar]
- Holmquist F, Stief CG, Jonas U, Andersson KE. Effects of the nitric oxide synthase inhibitor NG-nitro-L-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Iversen HH, Wiklund NP, Gustafsson LE. Nitric oxide-like activity in guinea pig colon as determined by effector responses, bioassay and chemiluminescence analysis. Acta Physiol Scand. 1994;152:315–322. doi: 10.1111/j.1748-1716.1994.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Ballard SA, Naylor AM, Miller MA, Angelini GD. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- Keast JR. A possible neural source of nitric oxide in the rat penis. Neurosci Lett. 1992;143:69–73. doi: 10.1016/0304-3940(92)90235-y. [DOI] [PubMed] [Google Scholar]
- Kim NN. Phosphodiesterase type 5 inhibitors: a biochemical and clinical correlation survey. Int J Impot Res. 2003;15 Suppl 5:S13–S19. doi: 10.1038/sj.ijir.3901067. [DOI] [PubMed] [Google Scholar]
- Klotz T, Sachse R, Heidrich A, Jockenhovel F, Rohde G, Wensing G, et al. Vardenafil increases penile rigidity and tumescence in erectile dysfunction patients: a RigiScan and pharmacokinetic study. World J Urol. 2001;19:32–39. doi: 10.1007/s003450000168. [DOI] [PubMed] [Google Scholar]
- Kuthe A, Wiedenroth A, Magert HJ, Uckert S, Forssmann WG, Stief CG, et al. Expression of different phosphodiesterase genes in human cavernous smooth muscle. J Urol. 2001;165:280–283. doi: 10.1097/00005392-200101000-00079. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors and receptor kinases: from molecular biology to potential therapeutic applications. Nat Biotechnol. 1996;14:283–286. doi: 10.1038/nbt0396-283. [DOI] [PubMed] [Google Scholar]
- Leone AM, Wiklund NP, Hokfelt T, Brundin L, Moncada S. Release of nitric oxide by nerve stimulation in the human urogenital tract. Neuroreport. 1994;5:733–736. doi: 10.1097/00001756-199402000-00019. [DOI] [PubMed] [Google Scholar]
- Li CG, Rand MJ. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Liu XR, Gillespie JS, Gibson IF, Martin W. Effects of NG-substituted analogues of L-arginine on NANC relaxation of the rat anococcygeus and bovine retractor penis muscles and the bovine penile artery. Br J Pharmacol. 1991;104:53–58. doi: 10.1111/j.1476-5381.1991.tb12384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Fuentes AM, Garban H, Rajavashisth T, Marquez D, Rodriguez JA, et al. Cloning of a novel neuronal nitric oxide synthase expressed in penis and lower urinary tract. Biochem Biophys Res Commun. 1996;226:145–151. doi: 10.1006/bbrc.1996.1324. [DOI] [PubMed] [Google Scholar]
- Makhlouf A, Kshirsagar A, Niederberger C. Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res. 2006;18:501–509. doi: 10.1038/sj.ijir.3901441. [DOI] [PubMed] [Google Scholar]
- McMahon CG. Priapism associated with concurrent use of phosphodiesterase inhibitor drugs and intracavernous injection therapy. Int J Impot Res. 2003;15:383–384. doi: 10.1038/sj.ijir.3901046. [DOI] [PubMed] [Google Scholar]
- Mount PF, Fraser SA, Watanabe Y, Lane N, Katsis F, Chen ZP, et al. Phosphorylation of neuronal and endothelial nitric oxide synthase in the kidney with high and low salt diets. Nephron Physiol. 2005;102:p36–p50. doi: 10.1159/000089092. [DOI] [PubMed] [Google Scholar]
- Olgart C, Hallén K, Wiklund NP, Iversen HH, Gustafsson LE. Blockade of nitrergic neuroeffector transmission in guinea-pig colon by a selective inhibitor of soluble guanylyl cyclase. Acta Physiol Scand. 1998;162:89–95. doi: 10.1046/j.1365-201X.1998.0274f.x. [DOI] [PubMed] [Google Scholar]
- Olgart C, Wiklund NP, Gustafsson LE. Blockade of nitric oxide-evoked smooth muscle contractions by an inhibitor of guanylyl cyclase. Neuroreport. 1997;8:3355–3358. doi: 10.1097/00001756-199710200-00032. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Kraft P, Lombardi E, Clancy J. Rabbit corpus cavernosum smooth muscle shows a different phosphodiesterase profile than human corpus cavernosum. J Urol. 2000;164:882–886. doi: 10.1097/00005392-200009010-00066. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989;320:1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Snider BJ, Choi J, Turetsky DM, Canzoniero LM, Sensi SL, Sheline CT, et al. Nitric oxide reduces Ca(2+) and Zn(2+) influx through voltage-gated Ca(2+) channels and reduces Zn(2+) neurotoxicity. Neuroscience. 2000;100:651–661. doi: 10.1016/s0306-4522(00)00311-0. [DOI] [PubMed] [Google Scholar]
- Song T, Hatano N, Kume K, Sugimoto K, Yamaguchi F, Tokuda M, et al. Inhibition of neuronal nitric-oxide synthase by phosphorylation at Threonine1296 in NG108-15 neuronal cells. FEBS Lett. 2005;579:5658–5662. doi: 10.1016/j.febslet.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Stief CG, Uckert S, Becker AJ, Truss MC, Jonas U. The effect of the specific phosphodiesterase (PDE) inhibitors on human and rabbit cavernous tissue in vitro and in vivo. J Urol. 1998;159:1390–1393. [PubMed] [Google Scholar]
- Waldkirch E, Uckert S, Yildirim H, Sohn M, Jonas U, Stief CG, et al. Cyclic AMP-specific and cyclic GMP-specific phosphodiesterase isoenzymes in human cavernous arteries – immunohistochemical distribution and functional significance. World J Urol. 2005;23:405–410. doi: 10.1007/s00345-005-0026-2. [DOI] [PubMed] [Google Scholar]
- Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. High biochemical selectivity of tadalafil, sildenafil and vardenafil for human phosphodiesterase 5A1 (PDE5) over PDE11A4 suggests the absence of PDE11A4 cross-reaction in patients. Int J Impot Res. 2005;17:5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- Wiklund CU, Wiklund NP, Gustafsson LE. Modulation of neuroeffector transmission by endogenous nitric oxide: a role for acetylcholine receptor-activated nitric oxide formation, as indicated by measurements of nitric oxide/nitrite release. Eur J Pharmacol. 1993;240:235–242. doi: 10.1016/0014-2999(93)90904-v. [DOI] [PubMed] [Google Scholar]
- Wiklund NP, Cellek S, Leone AM, Iversen HH, Gustafsson LE, Brundin L, et al. Visualisation of nitric oxide released by nerve stimulation. J Neurosci Res. 1997;47:224–232. doi: 10.1002/(sici)1097-4547(19970115)47:2<224::aid-jnr11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Yonessi M, Saeedi M. A double-blind placebo-controlled evaluation of the effect of topical sildenafil on erectile dysfunction. J Appl Res. 2005;5:289–294. [Google Scholar]
- Zoche M, Beyermann M, Koch KW. Introduction of a phosphate at serine 741 of the calmodulin-binding domain of the neuronal nitric oxide synthase (NOS-1) prevents binding of calmodulin. Biol Chem. 1997;378:851–857. doi: 10.1515/bchm.1997.378.8.851. [DOI] [PubMed] [Google Scholar]