Abstract
Background and purpose:
Adrenoceptors can associate with cardiac caveolae. To investigate the function of vascular caveolae, adrenoceptor-mediated effects were compared in the saphenous artery of caveolin-1 knockout (cav-1KO) and wild-type (WT) mice.
Experimental approach:
Electronmicroscopy was used to detect caveolae. Real-Time quantitative PCR was used for adrenoceptor subtypes. Catecholamine-evoked contractions and relaxations were studied in arterial segments.
Key results:
Caveolae were found in arterial smooth muscle from WT but not from cav-1KO mice. Arterial mRNA levels for the adrenoceptors α1A, α1B, α1D, β1, β2 and β3 were similar in cav-1KO and WT. (-)-Noradrenaline contracted cav-1KO (-log EC50M=7.1) and WT (-log EC50M=7.3) arteries through prazosin-sensitive receptors. Maximum (-)-noradrenaline-evoked contractions were greater in cav-1KO than WT arteries. (-)-Isoprenaline relaxed WT arteries (-log EC50M=7.3) more potently than cav-1KO arteries (-log EC50M=6.8); the effects were antagonized partially and similarly by the β2-selective antagonist ICI118551 (50 nM). The (-)-isoprenaline-evoked relaxation was partially antagonized by the β1-adrenoceptor-selective antagonist CGP20712 (300 nM) in WT but not cav-1KO arteries. The β3-adrenoceptor-selective antagonist L748337 (100nM) partially antagonized the relaxant effects of (-)-isoprenaline in cav-1KO but not in WT arteries. BRL37344 partially relaxed arteries through β3-adrenoceptors in cav-1KO but not WT. The relaxant effects of BRL37344 were decreased by the NO synthase inhibitor ΩL-nitroarginine.
Conclusions and implications:
The function of arterial α1- and β2-adrenoceptors is similar in cav-1KO and WT mice. β1-adrenoceptor-mediated relaxation in WT is lost in cav-1KO and replaced by the appearance of β3-adrenoceptors.
Keywords: caveolin-1 knockout, saphenous artery, α1-β1-β2- and β3-adrenoceptors
Introduction
Cell membrane invaginations (Palade, 1953), coined caveolae (Yamada, 1955), contain the scaffolding protein caveolin that modulates the function of colocalized proteins. Caveolin-regulated proteins include α1-adrenoceptors (Fujita et al., 2001; Bucci et al., 2004), β1- and β2-adrenoceptors (Schwencke et al., 1999; Ostrom et al., 2000; Rybin et al., 2000) and adenylyl cyclase (Ostrom et al., 2000, 2001, 2002). Mice with the disruption of the caveolin-1 gene (Razani et al., 2002), the most ubiquitous of three caveolins, are available (Drab et al., 2001; Razani et al., 2001; Park et al., 2003).
The scaffolding domain of caveolin-1 potentiates the constriction of murine aortic rings evoked by phenylephrine, presumably through inhibition of nitric oxide (NO) synthesis (Bucci et al., 2000). Conversely, the vasoconstrictor effect of phenylephrine is blunted in the aorta from cav-1 knockout (cav-1KO) mice but acetylcholine-induced relaxation is enhanced through increased NO synthesis and release (Drab et al., 2001; Razani et al., 2001). Although the effects of phenylephrine are presumably mediated through α-adrenoceptors, the nature of these receptors and their interaction with noradrenaline in a small artery are unknown. We have therefore compared the function of α-adrenoceptors, activated by (−)-noradrenaline, in the saphenous artery of cav-1KO and wild-type (WT) mice, in the absence and presence of the α1-adrenoceptor-selective antagonist prazosin.
β-Adrenoceptors appear associated with caveolae before but not after agonist treatment (Insel et al., 2005). Agonist stimulation has been found to cause a decline in the abundance of caveolar β2-adrenoceptors but not of β1-adrenoceptors in cardiomyocytes (Rybin et al., 2000). However, the role of caveolae in the vascular β-adrenoceptor function is unknown. We therefore investigated the function of β-adrenoceptor subtypes in the saphenous artery of cav-1KO and WT mice.
Methods
Mice
Black six mice (C57Black6, WT) and caveolin-1 knockout mice (cav-1KO), aged 9–18 months, were used. Cav-1KO mice (Drab et al., 2001) were bred at the animal facility of Dresden Technical University. The mice were genotyped before the experiment. Mice were killed by cervical dislocation. The local animal welfare officer supervised the health status, and all experiments were performed according to German legislation (permit no. 24-9168.24-1-2002-8 of the Dresden Regierungspräsidium).
Saphenous arteries
Both saphenous arteries were isolated, dissected and set up in physiological salt solution (PSS) of the following composition (mM): NaCl 119, KCl 4.7, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, glucose 5.5 and ethylenediaminetetraacetic acid (EDTA) 0.027. After the tissues had been mounted, 1.6 mM CaCl2 was added to the bath solution. Segments (2 mm long) were mounted on two wires (diameter of 40 μm) in microvascular myographs for isometric tension recordings, as described by Mulvany and Halpern (1977). One wire was attached to an isometric force transducer and the other to a displacement unit, permitting control of the internal circumference of the preparation. The organ bath contained PSS gassed with 5% CO2 in O2. Vessels were allowed to equilibrate in PSS at 37°C and set at a tension (T) corresponding to a pressure (P) of 70 mm Hg according to P=T2πU−1 (where U is the internal circumference). This passive tension was found to be optimal for maximal force development.
Electron microscopy
For transmission electron microscopy, the mouse tissues were fixed in 2.5% glutaraldehyde (in 0.1 M sodium cacodylate buffer, pH 7.4) overnight at 4°C. After being washed in sodium cacodylate buffer, sections were postfixed in buffered osmium tetroxide solution (1% osmium tetroxide and 0.1 M sodium cacodylate buffer, pH 7.4) for 2 h at 4°C, washed again, dehydrated in ethanol and embedded in Epon 812. Polymerization was carried out at 60°C. The specimens were viewed at 80 kV using a Zeiss EM 906 microscope (Zeiss, Oberkochen, Germany).
Measurement of vessel thickness
Vessel thickness was determined with a water immersion microscope, using a 40-times magnified scaled ocular.
Functional measurements
The viability of the preparations was examined by an initial exposure to 10 μM (−)-noradrenaline. Once contraction was stable 10 μM carbachol was added to test viability of the endothelium.
To investigate α-adrenoceptors, the vessels were exposed to the β-adrenoceptor antagonist (−)-propranolol (1 μM) and noradrenaline reuptake-inhibitor cocaine (3 μM) for at least 15 min. After this equilibrium phase the bath solution was renewed (still containing (−)-propranolol and cocaine). Concentration-effect curves for (−)-noradrenaline (10−11–10−4 M) were determined in the absence and presence of prazosin (10−8–10−6 M).
To investigate β-adrenoceptors, the vessels were precontracted by changing the bath solution to a PSS-60 solution, containing (mM) NaCl 63.7, KCl 60, CaCl2 1.6, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, glucose 5.5, phentolamine (10 μM) and EDTA 0.027. The vessels were incubated with β-adrenoceptor subtype-selective antagonists for at least 15 min before concentration-effect curves for (−)-isoprenaline (10−9–10−4 M) or for the β3-adrenoceptor-selective agonist BRL37344 were determined. The experiments were terminated with the administration of 30 μM sodium nitroprusside (SNP) to assess maximum relaxation.
Molecular biology
Saphenous arteries were isolated and frozen immediately. Total RNA was isolated using the EZNA Total RNA-Kit (Peqlab, Erlangen, Germany). Four arteries were used from two mice and pooled for mRNA isolation. The procedure was repeated three times, so that a total of six WT mice and six cav-1KO mice were used. Real-time PCR was performed using the QuantiTect SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) and Rotor Gene thermal cycler (Corbett Research, Mortlake, Australia). We used the primers shown in Table 1 (Chernogubova et al., 2005). RT-PCR and real-time PCR conditions were 50°C for 30 min, 95°C for 15 min, 30 s at 94°C, 30 s at 60°C for α1B, α1D and β1-adrenoceptors, respectively, and at 64°C for α1A, β2 and β3-adrenoceptors 30 s at 72°C and 15 s at 80°C. cRNA standards were produced by T7 in vitro transcription with the Message Machine Kit (Ambion, Austin, TX, USA) from RT-PCR products produced as above, but by using a modified forward primer containing an additional T7 promoter sequence (GGCCGCGG). Standards of known concentrations of RNA included in each dilution series (105–1012 mol μl−1) allowed calculation of cRNAs. Results were analysed by Rotorgene software version 4.6 (Corbett Research, Mortlake, Australia).
Table 1.
Primers used for real-time PCR of adrenoceptors
| Gene | Acc. No. | Primer sequence (5′–3′) | Size (bp) | |
|---|---|---|---|---|
| Adra1a | NM_013461 | Sense: | GGACAAGTCAGACTCAGAGCAAGTGACG | 28 |
| Antisense: | TATAGCCCAGGGCATGCTTGGAAGAC | 26 | ||
| Adra1b | NM_007416 | Sense: | TTTCATGAGGACACCCTCAGCAGTACC | 27 |
| Antisense: | CTGCCGCTGTCATCCAGAGAGTCC | 24 | ||
| Adra1d | NM_013460 | Sense: | GGCCAAAGGAAATCCAGGGACAC | 23 |
| Antisense: | CAGAGCGGAACTTATGGGACAGG | 23 | ||
| Adrb1 | NM_007419 | Sense: | CGCCTGCTACAACGACCCCAAG | 22 |
| Antisense: | AGCCAGTTGAAGAAGACGAAGAGGCG | 26 | ||
| Adrb2 | NM_007420 | Sense: | GGTTATCGTCCTGGCCATCGTGTTTG | 26 |
| Antisense: | TGGTTCGTGAAGAAGTCACAGCAAGTCTC | 29 | ||
| Adrb3 | NM_013462 | Sense: | TCTAGTTCCCAGCGGAGTTTTCATCG | 26 |
| Antisense: | CGCGCACCTTCATAGCCATCAAACC | 25 |
Data analysis and statistics
The mechanical responses were measured as force and expressed as wall tension, ΔT, which is the increase in measured force, ΔF, divided by twice the segment length (Mulvany and Halpern, 1977). Relaxations were expressed as a percentage of the response to SNP at the end of the concentration–relaxation response curves. By using a computer programme (Graph Pad Prism, San Diego, CA, USA), the concentration–effect curves were fitted to the Hill equation and in the case of biphasic curves they were fitted with the equation for two receptor populations. Sensitivity to the agonists was expressed as –log (EC50M). The results are expressed as mean±s.e.m.. Unless stated otherwise, number of experiments are expressed as arteries from n mice. Means of multiple groups were compared by t-tests. Probability levels less than 5% were considered significant. If variances were different, Mann–Whitney test was used to test statistical significance.
Drugs
The following drugs were used: BRL37344 (GlaxoSmithKline, Harlow, Essex, UK), ICI118551 (Tocris, Bristol, UK), L748337 (Lilly, IN, USA), (−)-isoprenaline (hydrochloride), (−)-noradrenaline (bitartrate), phentolamine, CGP20712, carbachol, cocaine hydrochloride, prazosin, (−)-propranolol, ΩL-nitroarginine and SNP were purchased from Sigma (Deisenhofen, Germany).
All drugs were dissolved in water, except (−)-noradrenaline and (−)-isoprenaline, which were dissolved in water containing 200 μM ascorbic acid and 40 μM EDTA.
Results
Carbachol-evoked relaxations
Carbachol (10 μM)-evoked relaxations of contractions induced by (−)-noradrenaline (10 μM) were not different (P=0.59) in WT (51.4±3.9%, n=57 arterial segments from 18 mice) and cav-1KO (48.7±3.1%, n=85/22) mice.
Vessel morphology
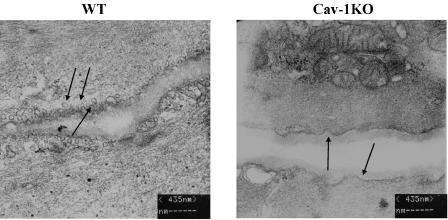
Electron microscopy revealed that vascular smooth muscle cells of WT mice are edged by caveolae (Figure 1), whereas membranes of cav-1KO mouse cells are smooth and caveolae are absent. Thus, the interruption of only the caveolin-1 gene is sufficient to prevent the appearance of caveolae in vascular smooth muscle cells. We also observed hypertrophy of cav-1KO saphenous arteries. Arterial walls appeared 39% thicker in cav-1KO (32±1 μm, n=4/2) than in WT (23±1 μm, n=4/2) mice.
Figure 1.
Electron microscopy of vascular smooth muscle cells (VSMC). WT: cell membranes of VSMC of WT mice exhibit numerous invaginations (caveolae, indicated by arrows). Caveolin-1 knockout mice (Cav-1KO): cell membranes of VSMC of cav-1KO mice show shallow indentations (arrows) but no caveolae.
Molecular analysis
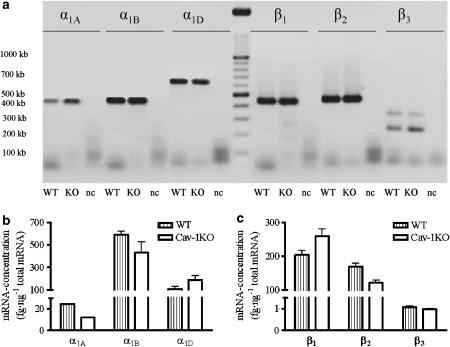
By real-time PCR, we could not find differences in expression of the mRNA levels for α1A, α1B, α1D, β1, β2 and β3 adrenoceptors between WT and cav-1KO mice (Figure 2). Interestingly, expression levels of α1A mRNA were extremely small compared with α1B and α1D-mRNA levels. The same was found for the level of β3-adrenoceptor mRNA, which was less than 1% of expression levels of β1- and β2-adrenoceptor mRNA.
Figure 2.
Expression of subunits of adrenoceptors in murine arteria saphena (Aa. saphena). Mean values from three experiments with pooled data from two mice each. (a) Agarose gel electrophoresis of the samples after OneStep RT real-time PCR: notice that there are two splice variants of β3-adrenoceptor. (b) Amount of α-adrenoceptor-mRNA normalized to total mRNA. (c) Amount of β-adrenoceptor-mRNA normalized to total mRNA. Data from the two β3-adrenoceptor splice variants were pooled.
(−)-Noradrenaline-evoked contractions
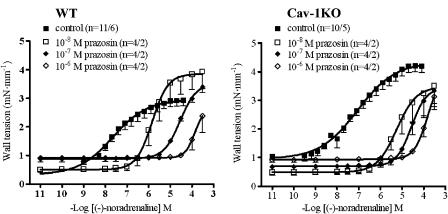
(−)-Noradrenaline caused concentration-dependent contractions in WT and cav-1KO arteries (Figure 3). The maximum wall tension induced by (−)-noradrenaline was significantly higher (P<0.0001; Figure 3) in cav-1KO (4.2±0.2 mN mm−1, n=10/5) than in WT vessels (2.9±0.2 mN mm−1, n=11/6). The concentration–effect curves for (−)-noradrenaline extended over 4 log units. Hill slopes were 0.67±0.06 in WT and 0.54±0.05 in cav-1KO. The potency of (−)-noradrenaline was similar for arteries of WT (−log EC50M=7.25±0.23) and cav-1KO arteries (−log EC50M=7.10±0.12).
Figure 3.
Concentration–effect curves for (−)-noradrenaline and antagonism by prazosin on Aa. saphena of WT and cav-1KO mice. Numbers refer to arterial preparations/number of mice.
Prazosin antagonized in a concentration-dependent manner the effects of (−)-noradrenaline in both WT and cav-1KO arteries (Figure 3). The curves for (−)-noradrenaline in the presence of prazosin 10−8 and 10−7 M were steeper than the curves in the absence of prazosin, with Hill slopes of 1.27±0.04 and 1.25±0.07, respectively. Owing to the high blocking potency of prazosin, only a partial concentration-effect curve was obtained for (−)-noradrenaline in the presence of prazosin (10−6 M) but the curve also tended to have a steep slope.
Effects of cocaine
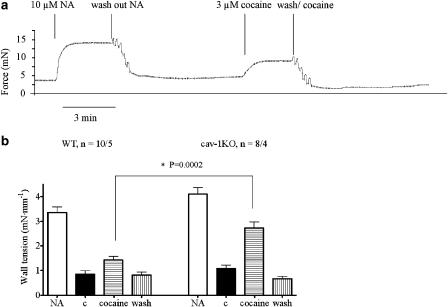
Cocaine 3 μM increased the wall tension significantly stronger in arteries from cav-1KO (53.4±2.4% of the effect of (−)-noradrenaline, n=8/4) than from WT (21.7±4.1%, n=10/5) mice (Figure 4). The effect of cocaine disappeared after washes with a PSS solution containing cocaine (Figure 4). Prazosin relaxed basal tension of arteries from cav-1KO but not from WT mice, consistent with the neuronal leak of noradrenaline; prazosin also prevented cocaine-induced contractions (results not shown, n=4/2).
Figure 4.
Effects of cocaine on Aa. Saphenae of WT and cav-1KO mice. (a) Original tracing of an experiment on a cav-1KO artery. (b) Addition of 3 μM cocaine leads to contraction of the vessels. This effect is significantly stronger in cav-1KO compared with WT mice (P=0.0002). In both groups of arteries, the effect was washed out with PSS containing 3 μM cocaine. c=control; NA=(−)-noradrenaline (10 μM). Numbers refer to arterial preparations/number of mice.
SNP-evoked relaxations
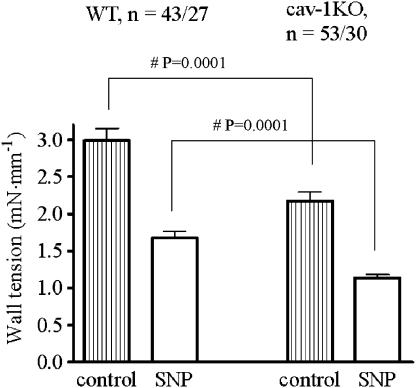
Maximum relaxation was determined with 30 μM SNP. Wall tension at 60 mM KCl was significantly greater in WT (2.99±0.16 mN mm−1, n=43/27) than in cav-1KO (2.18±0.12 mN mm−1, n=52/30) vessels. There was no difference in percentage relaxation caused by SNP between both groups (58±2% for WT and 57±2% for cav-1KO, Figure 5).
Figure 5.
Relaxant effects of SNP (30 μM) on Aa. saphenae precontracted with 60 mM KCl. Numbers refer to arterial preparations/number of mice.
(−)-Isoprenaline-evoked relaxations
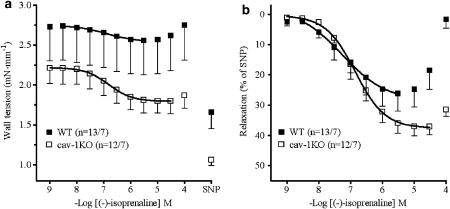
Vascular relaxation, mediated through β-adrenoceptors, was investigated with (−)-isoprenaline on arteries precontracted with 60 mM KCl. (−)-Isoprenaline relaxed arteries of cav-1KO and WT mice with similar maximum effects (Emax) but lower potency on cav-1KO than on WT (P<0.03) (Table 2: mean Emax values from curves fitted to single experiments; Figure 6: average curve).
Table 2.
Effect of β-AR antagonists on relaxation curves of (−)isoprenaline
|
WT |
KO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | −log EC50M | P | Emax | P | n | −log EC50M | P | Emax | P | |
| (−)-Isoprenaline | ||||||||||
| Control | 13/7 | 7.31±0.17 | 39.9±6.3 | 12/7 | 6.84±0.1 | 36.7±3.4 | ||||
| CGP20712A (300 nM) | 8/4 | 6.93±0.29 | NS | 19.6±3.7 | <0.02 | 6/3 | 7.21±0.06 | <0.03 | 51.3±2.9 | <0.02 |
| ICI118551 (50 nM) | 5/3 | 6.74±0.1 | <0.04 | 28.7±4.2 | NS | 6/3 | 6.21±0.09 | <0.002 | 29.7±4.5 | NS |
| L748337 (100 nM) | 4/2 | 6.62±0.34 | NS | 33.3±6.3 | NS | 10/5 | 6.85±0.07 | NS | 42.4±4.7 | NS |
| CGP20712A + ICI118551 | 8/4 | 5.24±0.12 | <0.0001 | 12.8±2.1 | <0.002 | 7/4 | 7.99±0.20H | <0.0001 | 8.2±2.0 | <0.0001 |
| 7/4 | 5.32±0.10L | <0.0001 | 36.0±3.4 | <0.0001 | ||||||
| CGP20712A+ICI118551+L748337 | 3/2 | 5.10±0.46 | <0.0004 | 25.4±8.8 | NS | 5/4 | 5.22±0.25 | <0.0001 | 17.0±7.9 | <0.02 |
| BRL37344 | ||||||||||
| Control | 3/2 | 5.18±0.41 | 8.6±4.5 | 7/4 | 8.73±0.35H | 6.25±2.5 | ||||
| 7/4 | 6.10±0.18L | 19.4±3.5 | ||||||||
| L748337 (100 nM) | 7/4 | 6.51±0.45 | NS | 18.9±4.3 | NS | |||||
| Control (L-NAME experiments) | 6/3 | 8.75±0.09H | 26.9±2.5 | |||||||
| 6/3 | 6.22±0.38L | 64.5±2.8 | ||||||||
| L-NAME (100 μM) | 6/3 | 8.29±0.27H | NS | 15.8±1.7 | <0.04 | |||||
| 6/3 | 5.74±0.33L | NS | 39.2±4.0 | <0.03 | ||||||
Abbreviation: NS, not significant.
P-values are with respect to the corresponding control in the WT and cav-1KO.
Emax: Maximum relaxation in the presence of the agonist compared with relaxation in the presence of 30 μM SNP.
: high-affinity component.
: low-affinity component.
Figure 6.
Concentration–effect curves of (−)-isoprenaline in Aa. saphenae of WT and cav-1KO mice. (a) Curves showing reduction in wall tension. (b) Curves normalized to SNP-evoked relaxation. Numbers refer to arterial preparations/number of mice.
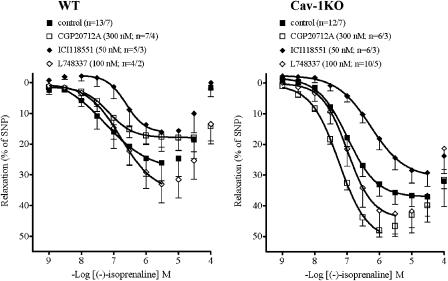
The β1-adrenoceptor-selective antagonist CGP20712A (300 nM) did not significantly affect the potency of (−)-isoprenaline but reduced the Emax in WT arteries (Figure 7). In vessels of cav-1KO mice, CGP20712 paradoxically appeared to slightly enhance Emax and to shift the concentration–relaxation curve of (−)-isoprenaline to the left (Table 2: mean Emax values from curves fitted to single experiments; Figure 7: average curve). The different effects of CGP20712 on the responses to (−)-isoprenaline appeared unrelated to the age of the mice, which was 80 weeks for WT and 74 weeks for cav-1KO.
Figure 7.
Comparison of the effects of β-blockers on (−)-isoprenaline-evoked relaxation in WT and cav-1KO arteries. For −log EC50M and Emax data, see Table 2.
The β2-adrenoceptor-selective antagonist ICI118551 (50 nM) shifted concentration–relaxation curves of (−)-isoprenaline similarly to the right in WT and in cav-1KO arteries (Figure 7). The β3-adrenoceptor-selective antagonist L748337 (100 nM) did not significantly affect the concentration–relaxation curves of (−)-isoprenaline in WT and cav-1KO arteries (Figure 7).
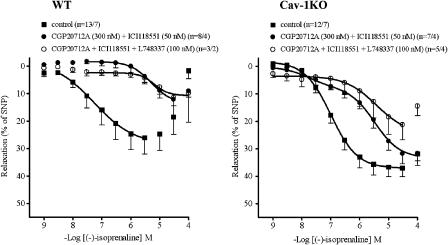
The combination of CGP20712A (300 nM) and ICI118551 (50 nM) caused a greater shift of the concentration–relaxation curve to (−)-isoprenaline of WT arteries than each antagonist, administered separately (Figure 8). The corresponding curve of cav-1KO arteries was shifted less to the right than with the WT arteries and the curve was biphasic. The different effects of the combination of CGP20712 and ICI118551 on the responses to (−)-isoprenaline appeared to be unrelated to the age of the mice, which was 80 weeks in WT and 75 weeks in cav-1KO. The high-affinity (H) component of the relaxant effects of (−)-isoprenaline, resistant to blockade by the combination of CGP20712 and ICI118551, amounted to 23% (Table 2). The H component was suspected to be mediated through β3-adrenoceptors. As expected, the β3-selective antagonist L748337 (100 nM), in the presence of both CGP20712 and ICI118551, shifted the concentration-relaxation curve for (−)-isoprenaline further to the right, and the curve became monophasic (Table 2 shows mean Emax values from curves fitted to single experiments; Figure 8 shows average curve).
Figure 8.
Comparison of the effects of β-blocker combinations on (−)-isoprenaline-evoked relaxation in WT and cav-1KO arteries. For −log EC50M and Emax data, see Table 2.
BRL37344-evoked relaxation
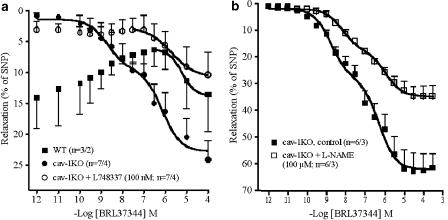
To investigate the involvement of β3-adrenoceptors further in the (−)-isoprenaline-evoked relaxation in cav-1KO arteries, the β3-selective agonist BRL37344 was used. In arteries from WT mice, BRL37344 only caused relaxation at concentrations greater than 1 μM, suggesting mediation through β1 and/or β2-adrenoceptors (Figure 9a). In arteries from cav-1KO mice, the concentration–relaxant curve for BRL37344 was biphasic with a high-affinity (H, nM) component and low-affinity component (L, μM) (Figure 9b, Table 2). L748337 (100 nM) blocked the H component, consistent with mediation through β3-adrenoceptors, but did not affect the L component (Table 2 shows mean Emax values from curves fitted to single experiments; Figure 9 shows average curve).
Figure 9.
Concentration-effects curves for the β3-adrenoceptor-selective agonist BRL37344. (a) Antagonism of BRL37344-evoked relaxations by L748337 in cav-1KO. (b) Partial reduction of the relaxant effects of BRL37344 by L-NAME. For −log EC50M and Emax data, see Table 2.
To determine whether the BRL37344-evoked relaxation was through β3-adrenoceptors located in endothelial cells, or smooth muscle cells we used the NO synthase inhibitor ΩL-nitroarginine (L-NAME). The concentration–effect curve for BRL37344 was not shifted by L-NAME (Figure 9b). The curves for BRL37344 were biphasic both in the absence and presence of L-NAME. The –log EC50M values for the H and L component of the BRL37344 response were not significantly changed by L-NAME (Table 2) but Emax was reduced significantly in the presence of L-NAME (Table 2). For unknown reasons, the experiments with BRL37344 in the absence of L-NAME (Figure 9b) showed stronger relaxation responses than the experiments depicted in Figure 9a, but their –log EC50M for the corresponding H and L components were not significantly different.
Discussion
The murine saphenous artery contracts in response to (−)-noradrenaline through activation of α1-adrenoceptors and relaxes in response to (−)-isoprenaline, mainly through activation of β2-adrenoceptors and to a moderate extent through β1-adrenoceptors. The absence of caveolae in arteries of cav-1KO mice does not reduce either α1-adrenoceptor-mediated contractions or β2-adrenoceptor-mediated relaxations, but abolishes β1-adrenoceptor-mediated relaxations and causes the appearance of β3-adrenoceptor-mediated relaxations. Thus, unlike β2-adrenoceptor-mediated relaxation, β1-adrenoceptor-mediated arterial relaxation requires intact caveolae.
Similar carbachol-induced relaxations in cav-1KO and WT
The interaction of the caveolin-1 scaffolding domain (CSD) and endothelial nitric oxide synthase (eNOS) inhibits eNOS activity (Ju et al., 1997). Injection of a peptide containing the CSD inhibits both acetylcholine-evoked vasodilatation of murine aortae and NO production (Bucci et al., 2000). As expected, NO production and acetylcholine-evoked relaxation of aortae are enhanced in cav-1KO mice (Drab et al., 2001; Razani et al., 2001). However, the carbachol-induced relaxation was not different in saphenous arteries of WT and cav-1KO mice, which does not accord with an enhanced release of NO. The reasons for this discrepancy between murine aorta and saphenous artery are not known.
(−)-Noradrenaline-evoked contractions are increased in cav-1KO
The walls of cav-1KO saphenous arteries showed smooth muscle hypertrophy compared with WT arteries. Wall hypertrophy could explain the greater maximal contractions produced by (−)-noradrenaline in cav-1KO than in WT arteries. However, for unknown reasons, 60 mM KCl contractures were decreased in cav-1KO, implying that (−)-noradrenaline and KCl increase vascular tension through different mechanisms. Prazosin caused concentration-dependent and similar antagonism of the effects of (−)-noradrenaline in cav-1KO and WT arteries. The steepening of the concentration–effect curves by prazosin suggests involvement of more than one α-adrenoceptor subtype and precluded a quantitative assessment of the affinity for the α1-adrenoceptors.
Our finding of enhanced noradrenaline-evoked contractions, mainly mediated through α1-adrenoceptors, of the saphenous artery in cav-1KO is at variance with previous work. Cav-1KO aortae have been found to exhibit blunted responses to phenylephrine (Drab et al., 2001; Razani et al., 2001). These authors attributed the blunted phenylephrine-induced contractions to an increase in constitutive activity of eNOS (and hence NO release), owing to the lack of constrain of this enzyme by caveolin-1 in cav-1KO. However, our results showing unaltered carbachol-induced relaxations in cav-1KO compared with WT suggest that this interpretation does not apply to the murine saphenous artery.
Cocaine-evoked contractions are increased in cav-1KO
The increased cocaine-evoked contractions in cav-1KO, compared with WT arteries, appear to be at least partially the result of vascular hypertrophy. Interestingly, the effect of cocaine could be washed out with PSS containing cocaine, making it unlikely that it has a direct interaction with the muscle cells. Noradrenaline may leak out from adventitial nerve terminals but partially be recaptured again by the nerves. The cocaine-evoked contractions could be the result of the prevention of neuronal reuptake, thereby making more (−)-noradrenaline available to the α1-adrenoceptors (De la Lande and Waterson, 1967). For unknown reasons, the postulated (−)-noradrenaline leakage could also be more pronounced in arteries from cav-1KO than from WT mice.
Abolished β1-adrenoceptor-mediated relaxation in cav-1KO
The relaxant potency of (−)-isoprenaline was slightly lower in cav-1KO than in WT, presumably owing to the loss of a β1-adrenoceptor-mediated component. The β1-adrenoceptor-selective antagonists CGP20712 reduced the Emax of (−)-isoprenaline-evoked relaxation in arteries from WT mice, but not from cav-1KO mice. For unknown reasons CGP20712 actually tended to enhance the relaxant responses to (−)-isoprenaline in cav-1KO. Nevertheless, the result suggests the existence of a β1-adrenoceptor component of (−)-isoprenaline-induced relaxation mediated through β1-adrenoceptors in WT but not in cav-1KO. Thus, caveolae appear to be essential for the manifestation of the β1-adrenoceptor component. This is a somewhat unexpected finding because, unlike cardiac β1-adrenoceptors, β1-adrenoceptors of rat aorta smooth muscle do not appear to be associated with caveolar membrane fractions (Ostrom et al., 2002). It seems that caveolae and caveolin-1 facilitate either the expression or the coupling of β1-adrenoceptors to the biochemical effectors leading to vascular relaxation. As β1-adrenoceptor mRNA levels were not different in arteries of WT and cav-1KO mice, it seems more likely that the absence of caveolae may prevent β1-adrenoceptor coupling through, as yet, unknown mechanisms.
G protein-coupled receptor kinases (GRKs) phosphorylate and desensitize G-protein coupled receptors, and it has been shown that GRK2-catalysed phosphorylation is inhibited by caveolin-1 (Carman et al., 1999). Conceivably, the lack of the caveolin-1 constraint in cav-1KO mice liberates GRK2 to phosphorylate β1-adrenoceptors of the murine saphenous artery more quickly, thereby partially preventing the (−)-isoprenaline-evoked relaxation, but this plausible mechanism requires further research.
Preserved function of vascular β2-adrenoceptors in cav-1KO mice
(−)-Isoprenaline relaxes the murine saphenous artery mainly through β2-adrenoceptors in both WT and cav-1KO mice. This finding is consistent with the lack of caveolar localization of β2-adrenoceptors in the smooth muscle of rat aorta (Ostrom et al., 2002). These results are also consistent with our data demonstrating similar β2-adrenoceptor mRNA levels in saphenous arteries from WT and cav-1KO mice.
β3-Adrenoceptors substitute for β1-adrenoceptors to mediate relaxation in cav-1KO mice
Both (−)-isoprenaline and the β3-adrenoceptor-selective agonist BRL37344 partially relaxed the saphenous artery in cav-1KO but not in WT mice. Their effects were antagonized by the β3-adrenoceptor-selective antagonist L748337, consistent with mediation through β3-adrenoceptors. Although real-time PCR did not reveal quantitative differences in mRNA expression of β3-adrenoceptors, the receptors only mediated relaxation in arteries from cav-1KO but not from WT mice. The two murine splice variants β3a and β3b, reported by Evans et al. (1993), were found apparently unaltered (Figure 2a). The unaltered mRNA levels suggest that the relaxant β3-adrenoceptor function is suppressed by caveolae through an unknown mechanism in WT arteries, either through lack of expression of sufficient β3-adrenoceptors or through uncoupling of these receptors or both.
The β3-adrenoceptors seem to replace the disappeared relaxant function of β1-adrenoceptors in saphenous arteries of cav-1KO mice. Both endothelial and smooth muscle β3-adrenoceptors can contribute to vascular relaxation. Endothelium-independent relaxations have been found in human placental arteries (Rouget et al., 2006) and corpus cavernosum (Cirino et al., 2003), canine pulmonary artery (Tamaoki et al., 1998), rat abdominal aortae (Matsushita et al., 2003) and carotid arteries (Oriowo, 1994). Endothelium-dependent relaxations appear to occur through β3-adrenoceptors in human mammary artery (Rozec et al., 2005), rat thoracic aorta (Trochu et al., 1999) and microvascular bed of human coronary artery (Dessy et al., 2005). In the latter system, (−)-isoprenaline relaxes the vessels through both the release of NO and a hyperpolarizing factor (Dessy et al., 2005).
Our results, showing that ΩL-nitroarginine reduces but does not abolish the H component of BRL37344-evoked relaxation, suggest that the β3-adrenoceptors that appeared in cav-1KO may be located on both endothelial cells and smooth muscle cells.
Unlike β1- and β2-adrenoceptors, the β3-adrenoceptor has fewer phosphorylation sites and is fairly resistent to desensitization (Liggett et al., 1993; Jockers et al., 1996). Therefore, prolonged agonist exposure, acting through β3-adrenoceptors of human myometrium, does not result in desensitization (Rouget et al., 2004). For the same reason, the β3-adrenoceptor-mediated relaxation of the cav-1KO saphenous artery remains apparent, unlike the β1-adrenoceptor-mediated relaxation, because this receptor could be phosphorylated by the unconstrained GRK2 activity, as discussed above.
Conclusions
We conclude that the function α1-adrenoceptors and β2-adrenoceptors is preserved in saphenous arteries from cav-1KO mice devoid of caveolae. However, the β1-adrenoceptor-mediated vascular relaxation, observed in WT mice, is abolished in cav-1KO mice, pointing to an essential role of caveolae. The disappearance of β1-adrenoceptor function is compensated by the appearance of functional β3-adrenoceptors, probably located on both endothelial cells and vascular smooth muscle cells in cav-1KO mice.
Abbreviations
- BRL37344
[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetate
- cav-1KO
caveolin-1 knockout
- CGP20712
2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl]amino]ethoxy]-benzamide
- ICI118551
1-[2,3-dihydro-7-methyl-1H-inden-4-yl)oxy-3-[(1-methylethyl)amino]-2-butanol
- L748337
(S)-N-[4-[2-[[3-[3-(acetoamidomethyl)phenoxy)-2-hydoxypropyl]-amino]-ethyl]-phenylbenzsulphonamide)
- WT
wild-type
Conflict of interest
The authors state no conflict of interest.
References
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- Bucci M, Roviezzo F, Brancaleone V, Lin MI, Di Lorenzo A, Cicala C, et al. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler Thromb Vasc Biol. 2004;24:721–726. doi: 10.1161/01.ATV.0000122362.44628.09. [DOI] [PubMed] [Google Scholar]
- Carman CV, Lisanti MP, Benovic JL. Regulation of G protein-coupled receptor kinases by caveolin. J Biol Chem. 1999;274:8858–8864. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- Chernogubova E, Hutchinson DS, Nedergaard J, Bengtsson T. Alpha1- and beta1-adrenoceptor signaling fully compensated for adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology. 2005;146:2271–2284. doi: 10.1210/en.2004-1104. [DOI] [PubMed] [Google Scholar]
- Cirino G, Sorrentino R, Di Villa BR, Popolo A, Palmieri A, Imbimbo C, et al. Involvement of β3-adrenergic receptor activation via cyclic GMP- but not NO-dependent mechanisms in human corpus cavernosum function. Proc Natl Acad Sci USA. 2003;100:5531–5536. doi: 10.1073/pnas.0931347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Lande IS, Waterson JG. Site of action of cocaine on the perfused artery. Nature. 1967;214:313–314. doi: 10.1038/214313a0. [DOI] [PubMed] [Google Scholar]
- Dessy C, Saliez J, Ghisdal P, Daneau G, Lobysheva II, Frerart F, et al. Endothelial β3-adrenoceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third generation β-blocker nevibolol. Circulation. 2005;112:1198–1205. doi: 10.1161/CIRCULATIONAHA.104.532960. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Evans BA, Papaionnanou M, Hamilton S, Summers RJ. Alternative splicing generates two isoforms of the beta3-adrenoceptor which are differently expressed in mouse tissues. Br J Pharmacol. 1993;127:1525–1531. doi: 10.1038/sj.bjp.0702688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Toya Y, Iwatsubo K, Onda T, Umemura S, Ishikawa Y. Accumulation of molecules involved in α1-adrenergic signal within caveolae: caveolin expression and the devolopment of cardiac hypertrophy. Cardiovasc Res. 2001;51:709–716. doi: 10.1016/s0008-6363(01)00348-0. [DOI] [PubMed] [Google Scholar]
- Insel PA, Head BP, Patel DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochemical Society Transactions. 2005;33:1131–1134. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- Jockers R, Da Silva A, Strosberg AD, Bouvier M, Marullo S. New molecular and structural determinants involved in beta 2-adrenergic receptor desensitization and sequestration. Delineation using chimeric beta 3/beta 2-adrenergic receptors. J Biol Chem. 1996;271:9355–9362. doi: 10.1074/jbc.271.16.9355. [DOI] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Freedman NJ, Schwinn DA, Lefkowitz RJ. Structural basis for receptor subtype-specific regulation revealed by a chimeric beta3/beta2-adrenergic receptor. Proc Natl Acad Sci USA. 1993;90:3665–3669. doi: 10.1073/pnas.90.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Horinouchi T, Tanaka Y, Tsuru H, Koike K. Characterization of β3-adrenoceptor-mediated relaxation in rat abdominal aorta smooth muscle. Eur J Pharmacol. 2003;482:235–244. doi: 10.1016/j.ejphar.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Oriowo MA. Atypical beta-adrenoceptors in rat isolated common carotid artery. Br J Pharmacol. 1994;113:699–702. doi: 10.1111/j.1476-5381.1994.tb17049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem. 2001;276:42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Liu X Head BP, Gregorian C, Seasholtz TM, Insel PA. Localization of adenylyl cyclase isoforms and G protein coupled receptors in vascular smooth muscle cells: expression in caveolin rich and noncaveolin domains. Mol Pharmacol. 2002;62:983–992. doi: 10.1124/mol.62.5.983. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Violin JD, Coleman S, Insel PA. Selective enhancement of beta adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol. 2000;57:1075–1079. [PubMed] [Google Scholar]
- Palade GE. Fine structure of blood capillaries. J App Physiol. 1953;24:1424–1436. [Google Scholar]
- Park DS, Cohen AW, Frank PG, Razani B, Lee H, Williams TM, et al. Caveolin-1 null mice show dramatic reductions in life span. Biochemistry. 2003;42:15124–15131. doi: 10.1021/bi0356348. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Rouget C, Barthez O, Goirand F, Leroy MJ, Breuiller-Fouche M, Rakotoniaina Z, et al. Stimulation of the ADRβ3 adrenergic receptor induces relaxation of human placental arteries: influence of preeclampsia. Biol Reprod. 2006;74:209–216. doi: 10.1095/biolreprod.105.043695. [DOI] [PubMed] [Google Scholar]
- Rouget C, Breuiller-Fouche M, Mercier FJ, Leroy MJ, Loustalot C, Naline E, et al. The human near-term myometrial beta 3-adrenoceptor but not the beta 2-adrenoceptor is resistant to desensitization after sustained agonist stimulation. Br J Pharmacol. 2004;141:831–841. doi: 10.1038/sj.bjp.0705616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozec B, Serpillon S, Toumaniantz G, Seze C, Rautureau Y, Baron O, et al. Characterization of beta3-adrenoceptors in human internal mammary artery and putative involvement in coronary artery bypass management. J Am Coll Cardiol. 2005;46:351–359. doi: 10.1016/j.jacc.2005.03.061. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenyl cyclase to cardiomycyte caveolae. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Schwencke C, Okumura S, Yamamoto M, Geng Y-J, Ishikawa Y. Colocalization of β-adrenergic receptors and caveolin within the plasma membrane. J Cell Biochem. 1999;75:64–72. doi: 10.1002/(sici)1097-4644(19991001)75:1<64::aid-jcb7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Tagaya E, Isono K, Nagai A. Atypical adrenoceptor-mediated relaxation of canine pulmonary artery through a cAMP-dependent pathway. Biochem Biophys Res Comm. 1998;248:722–727. doi: 10.1006/bbrc.1998.9047. [DOI] [PubMed] [Google Scholar]
- Trochu J-N, Leblais V, Rautureau Y, Beverelli F, Le Marec H, Berdeaux A, et al. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br J Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]