Abstract
Background and purpose:
Extracellular nucleotides act as potent mitogens for renal mesangial cells (MC). In this study we determined whether extracellular nucleotides trigger additional responses in MCs and the mechanisms involved.
Experimental approach:
MC migration was measured after nucleotide stimulation in an adapted Boyden-chamber. Sphingosine kinase-1 (SK-1) protein expression was detected by Western blot analysis and mRNA expression quantified by real-time PCR. SK activity was measured by an in vitro kinase assay using sphingosine as substrate.
Key results:
Nucleotide stimulation caused biphasic activation of SK-1, but not SK-2. The first peak occurred after minutes of stimulation and was followed by a second delayed peak after 4–24 h of stimulation. The delayed activation of SK-1 is due to increased SK-1 mRNA steady-state levels and de novo synthesis of SK-1 protein, and depends on PKC and the classical MAPK cascade. To see whether nucleotide-stimulated cell responses require SK-1, we selectively depleted SK-1 from cells by using small-interference RNA (siRNA). MC migration is highly stimulated by ATP and UTP; this is mimicked by exogenously added S1P. Depletion of SK-1 by siRNA drastically reduced the effect of ATP and UTP on cell migration but not on cell proliferation. Furthermore, MCs isolated from SK-1-deficient mice were completely devoid of nucleotide-induced migration.
Conclusions and implications:
These data show that extracellular nucleotides besides being mitogenic also trigger MC migration and this cell response critically requires SK-1 activity. Thus, pharmacological intervention of SK-1 may have impacts on situations where MC migration is important such as during inflammatory kidney diseases.
Keywords: sphingosine kinase-1, sphingosine 1-phosphate, ATP, UTP, purinoceptors, migration, mesangial cells
Introduction
Extracellular nucleotides exert important and diverse effects on many biological processes including smooth muscle contraction, platelet aggregation, neurotransmission, exocrine and endocrine secretion, immune responses, inflammation and pain (for review, see Boarder and Hourani, 1998; Ralevic and Burnstock, 1998; Abbracchio et al., 2006). They mediate their effects by activation of distinct cell surface receptors, the purinoceptors, which are divided into two main classes, the P1 and the P2 receptors, of which the P1 receptors are selective adenosine receptors, whereas the P2 receptors bind various purines and pyrimidines and are further subdivided into the ionotropic P2X receptors, and the metabotropic G protein-coupled P2Y receptors. To date, seven P2X receptors (P2X1–7) and eight P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11–14) have been identified (Ralevic and Burnstock, 1998; Abbracchio et al., 2006).
In renal mesangial cells, extracellular nucleotides have been shown to stimulate the hydrolysis of phosphatidylinositol bisphosphate by a phospholipase C, leading to the generation of 1,2-diacylglycerol and inositol 1,4,5-trisphosphate (Pfeilschifter, 1990a, 1990b) with subsequent mobilization of intracellular calcium (Pavenstädt et al., 1993). Furthermore, they activate protein kinase C (PKC) isoenzymes (Pfeilschifter and Huwiler, 1996; Huwiler et al., 1997a), the classical mitogen-activated protein kinases (MAPKs) (Huwiler and Pfeilschifter, 1994), the stress-activated protein kinase and c-Jun N-terminal protein kinase (Huwiler et al., 1997b), the p38-MAPK (Huwiler et al., 2000a) and the protein kinase B/Akt (Huwiler et al., 2002). Functionally, extracellular nucleotides lead to an increased formation of prostaglandin E2 owing to cytosolic phospholipase A2 activation (Pfeilschifter, 1990a), to highly increased proliferation of mesangial cells (Schulze-Lohoff et al., 1992; Huwiler and Pfeilschifter, 1994), and to protection of mesangial cells from stress-induced apoptosis (Huwiler et al., 2002). Most of these nucleotide-triggered effects in mesangial cells are mediated by the P2Y2 receptor, although other purinoceptors such as the P2Y1, P2Y4, P2Y6, P2X2, P2X4 and P2X7 have also been identified in the kidney (Bailey et al., 2000; Schwiebert and Kishore, 2001; Leipziger, 2003).
Sphingosine 1-phosphate (S1P) has gained a lot of attention in the last few years owing to its potential involvement in cell growth and survival (Olivera and Spiegel, 1993; for review, see Huwiler et al., 2000b; Spiegel and Milstien, 2003a). It was shown that S1P binds to and activates cell surface receptors, the S1P receptors (formerly denoted as Edg receptors), which belong to the class of seven transmembrane spanning G protein-coupled receptors (for review, see Sanchez and Hla, 2004; Rosen and Goetzl, 2005) and couple to various signalling cascades including the classical MAPK/extracellular signal-regulated kinase (ERK) and protein kinase B/Akt. Besides this extracellular way of action, an intracellular effect of S1P has also been proposed (for review, see Spiegel and Milstien, 2003b), although the direct intracellular targets of S1P remain unknown.
S1P is generated by the action of sphingosine kinases (SKs) of which two subtypes have been identified, SK-1 and SK-2 (Kohama et al., 1998; Liu et al., 2000). Furthermore, it was reported that SK-1 exists as at least eight alternative transcripts, denoted as sphk1a to −1 h, that are time- and tissue-specifically expressed. All eight transcripts can be found in the kidney (Imamura et al., 2001, 2004).
SKs are activated by a variety of stimuli including growth factors such as platelet-derived growth factor (Olivera and Spiegel, 1993), epidermal growth factor (Meyer zu Heringdorf et al., 1999; Hait et al., 2005; Döll et al., 2005) and nerve growth factor (Edsall et al., 1997), but also by differentiation factors such as phorbol ester (Buehrer et al., 1996; Huwiler et al., 2006) and vitamin D3 (Kleuser et al., 1998), or even by the proinflammatory cytokine tumour necrosis factor α (Xia et al., 1999; Osawa et al., 2001; Billich et al., 2005). It was proposed that the rapid activation of SK-1 by these factors requires direct phosphorylation by the classical MAPK; this is followed by translocation of the enzyme to the membrane (Pitson et al., 2003, 2005).
Interestingly, SK-1 has been mapped to chromosome 17q25, which contains loci related to diseases such as sclerosis, psoriasis and epidermodysplasia (Tomfohrde et al., 1994; Kuokkanen et al., 1997; Nair et al., 1997; Becker et al., 1998; Ramoz et al., 1999). The rat sphk1 gene is localized at a telomeric region of chromosome 10, which was identified as a susceptible region related to inflammatory autoimmune diseases and rheumatoid arthritis (Remmers et al., 1996).Thus, it may be speculated that SK-1 plays a role in the development of these diseases.
In the present study, we showed that in renal mesangial cells extracellular nucleotides activate SK-1 in a biphasic manner. The delayed second phase of activation occurring after 4–24 h of stimulation correlated with a transcriptional upregulation of SK-1 messenger RNA (mRNA) followed by increased de novo synthesis of SK-1 protein. Furthermore, this nucleotide-induced SK-1 activation is critically involved in the process of nucleotide-stimulated cell migration, but not in nucleotide-stimulated cell proliferation of mesangial cells.
Methods
Cell culturing
Rat renal mesangial cells were cultivated and characterized as described previously (Pfeilschifter, 1990a). In a second step, single cells were cloned by limited dilution on 96-microwell plates. Clones with mesangial cell morphology were characterized as described previously (Pfeilschifter, 1990a). For the experiments in this study passages 7–25 were used. Mouse renal mesangial cells were isolated from either a SK-1 knockout mouse (generated by Genoway, Lyon, France) or a SK-1 (+/+) control littermate. In brief, the kidneys were collected and, after removal of the capsule, pressed through three subsequent filters of 108 μM, 180 μM and 53 μM. Glomeruli attached to the 53 μM filter were collected and kept in culture. Outgrown mesangial cells were subcultured and further used up to passage 8. Cells were characterized by positive staining for α-smooth muscle actin and negative staining for cytokeratin (to exclude epithelial cell contamination).
Peptide synthesis and generation of antibodies
For rat SK-1 two synthetic peptides based on the sequences (amino acids 6–19: CPRGLLPRPCRVLV and amino acids 174–188: VADVDLESEKYRSLG) were synthesized, coupled to keyhole-limpet haemocyanin, and used to immunize rabbits.
Cell stimulation and Western blot analysis
Confluent mesangial cells in 60-mm-diameter dishes were stimulated with the indicated substances in Dulbecco's modified Eagle medium (DMEM) containing 0.1 mg ml−1 of fatty acid-free bovine serum albumin (BSA). Following stimulation the medium was withdrawn and the cells were washed once with phosphate-buffered saline (PBS) solution. Thereafter, cells were scraped into ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM ethylenediaminetetraacetic acid (EDTA), 2 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N',-tetraacetic acid, 40 mM β-glycerophosphate, 50 mM sodiumfluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin A, 1 mM phenylmethyl sulphonyl fluoride) and homogenized by 10 passes through a 26G-needle fitted to a 1 ml syringe. The samples were then centrifuged for 10 min at 13 000 g and the supernatant was taken for protein determination and Western blot analysis. Cell lysates containing equal amounts of protein (50–100 μg) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide gel) and transferred to a nitrocellulose membrane and subjected to Western blot analysis as described previously (Huwiler et al., 1995).
In vitro SK activity assay
The SK-1 activity assay was performed exactly as described previously (Döll et al., 2005). In brief, 50 μg of total protein were dissolved in buffer (containing 20 mM Tris-HCl, pH 7.4, 20% (v/v) glycerol, 1 mM β-mercaptoethanol, 1 mM EDTA, 1 mM sodium orthovanadate, 40 mM β-glycerophosphate, 15 mM NaF, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 1 mM phenylmethyl sulphonyl fluoride). For SK-2 activity assay, the same buffer including 1 M KCl was used (Shu et al., 2002). Thereafter, 10 μl of 1 mM sphingosine (dissolved in 4 mg ml−1 BSA in PBS), 10 μl MgCl2 (200 mM) and 10 μCi 20 mM−1 γ-[32P]ATP (adenosine triphosphate) were added. The reaction was terminated after 15 min at 37°C by addition of 20 μl HCl (1N), 800 μl of CHCl3/MeOH/HCl (100:200:1, v/v), 240 μl CHCl3 and 240 μl KCl (2 M). After phase separation, 50 μl of the lower organic phase were separated by thin-layer chromatography with 1-butanol/EtOH/acetic acid/water (80:20:10:20, v/v). Radioactive spots corresponding to S1P were analysed by an Imaging system (Fujifilm Europe GMBH, Düsseldorf, Germany).
Quantitative real-time PCR (TAQMAN)
Two micrograms of total RNA isolated with TRIZOL reagent was used for reverse transcriptase-polymerase chain reaction (RT-PCR) (First-strand synthesis kit, MBI) utilizing a hexanucleotide primer for amplification. The real-time PCR reactions were carried out in ABgene plates. Probes, primers of rat SK-1 and 18sRNA, and the reporter dyes 6-fluorochrome 6-carboxyl-fluorescein and VIC were from Applied Biosystems (Germany). The PCR buffer used in the experiments was from ABgene. After complementary DNA synthesis one microlitre of PCR product was used for further analysis. The run was performed on the Applied Biosystems 7700 HT sequence detection system. The cycling conditions were as following: 95°C for 15 min (one cycle), 95°C for 15 s and 60°C for 1 min (40 cycles). SDS version 1.9.1 software was used to analyse real-time and end point fluorescence.
siRNA transfections
Cells were plated in six-well-plates at a density of 1 × 105 cells per well and cultured overnight. The transfection was carried out with Oligofectamine Reagent (Invitrogen, Karlsruhe, Germany) following the manufacturer's instructions. Small-interfering RNA (siRNA) of rat SK-1 (5′-CAG CUU CUU UGA ACU ACU ATT-3′) was used at 100 nM concentration. After transfection, medium was exchanged for normal growth medium and cells were cultured for 8 h before a starvation period of 12 h followed by stimulation in serum-free DMEM as indicated in the figure legends.
Cell migration assay
The effect of extracellular nucleotides on mesangial cell migration was measured as the ability of cells to migrate through a Transwell filter (6.5 mm diameter, 8 μM pore size). After serum starvation for 24 h, cells were detached by trypsinization and seeded into transwell filters at 1 × 105 cells in 100 μl starvation medium with or without the indicated stimuli; 500 μl of starvation medium were placed in the lower compartment, and the cells were left to migrate for the indicated time periods. After treatment, the medium was removed and the non-migrating cells were removed from the filter by wiping the filter with a cotton pad. The filters were washed twice with PBS, fixed in 4% formaldehyde in PBS for 30 min at room temperature and again washed in water and methanol. Thereafter, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1 μg ml−1) for 15 min at 37°C, washed with methanol and dried. Cells that had migrated into the pores of the filters were counted using fluorescence microscopy. Five random areas were examined per sample and the experiments were repeated twice in triplicate. Data are expressed as (%) of control migrated cells per standard microscopic field.
[3H]Thymidine incorporation
Transfected cells in 24-well-plates were rendered serum free for 1 day in DMEM including 0.1 mg ml−1 BSA before stimulation with the indicated concentrations of ATP or uridine triphosphate (UTP) in the presence of 1 μCi ml−1 of [3H]methyl-thymidine. After 24 h of stimulation, cells were washed twice with PBS and incubated in 5% (w/v) trichloroacetic acid at 4°C for 30 min, and the DNA was dissolved in 0.5 mol l−1 NaOH for 30 min at 37°C. Finally, [3H]thymidine incorporation was determined in a β-counter.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance. For multiple comparisons with the same control group, the limit of significance was divided by the number of comparisons according to Bonferroni.
Chemicals
All nucleotides and DAPI were obtained from Sigma Aldrich Fine Chemicals, Deisenhofen, Germany or Fluka, Bucks, Switzerland; γ-[32P]ATP (specific activity: >5000 Ci mmol−1), anti-rabbit horseradish peroxidase-coupled IgG and Hyperfilm were purchased from GE Health Care Systems, Freiburg, Germany; 12-O-tetradecanoylphorbol 13-acetate (TPA), 1,4-diamino-2,3-dicyano-1,4[2-aminophenylthio]butadiene (U0126), bisindolylmaleimide IX (Ro 31-8220) and SB203580 (4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole) were obtained from Merck Biosciences, Schwalbach, Germany; all cell culture nutrients were from Life Technologies, Karlsruhe, Germany.
Results
Extracellular nucleotides induce a biphasic activation of SK-1, but not of SK-2
As extracellular nucleotides like ATP and UTP have turned out to be potent mitogens for mesangial cells (Schulze-Lohoff et al., 1992; Huwiler and Pfeilschifter, 1994), we investigated here whether these nucleotides induce additional mitogenic factors that could act synergistically to increase mesangial cell proliferation.
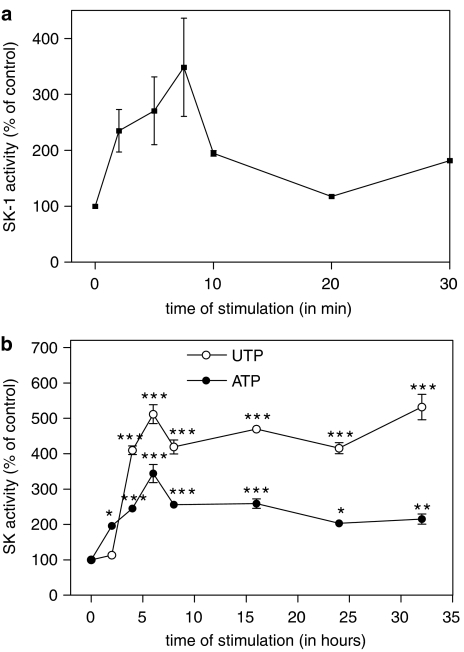
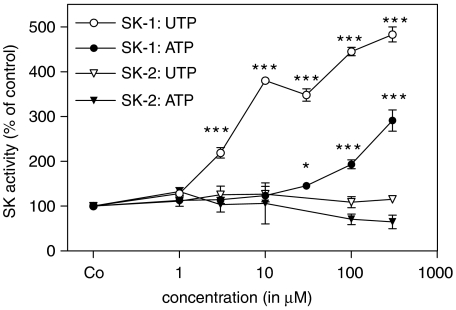
Stimulation of rat renal mesangial cells with an extracellular nucleotide such as UTP induced a rapid increase of SK-1 activity, as measured in an in vitro kinase assay using sphingosine as a substrate. A maximal peak of SK-1 activity occurred after 5–7 min of stimulation with UTP (Figure 1a). Interestingly, exposure of cells to extracellular nucleotides for longer time periods resulted in a second more delayed and sustained peak of SK-1 activation with maximal values at 6–24 h for ATP and UTP (Figure 1b). This delayed activation of SK-1 was concentration-dependent (Figure 2). After 24 h of stimulation with UTP significantly enhanced SK-1 activity was seen at 3 μM and a maximal effect at 300 μM, whereas ATP was less potent and 100 μM of ATP was needed to see any significant effects (Figure 2). To see whether SK-2 activity is also increase after nucleotide stimulation, the same samples were subjected to an in vitro kinase assay in the presence of 1 M KCl, which inhibits SK-1 but does not affect SK-2 activity (Shu et al., 2002). However, under these conditions no increased SK-2 activity was detected after either ATP or UTP (Figure 2).
Figure 1.
Effect of extracellular nucleotides on short-term (a) and long-term (b) activation of SK-1 in renal mesangial cells. (a) Quiescent mesangial cells were stimulated with either vehicle (2 min) or UTP (300 μM) for the indicated time periods (in minutes). (b) Cells were stimulated for the indicated time periods (in h) with either vehicle (24 h), ATP (100 μM) or UTP (100 μM). Thereafter, cell lysates containing 50 μg of protein were taken for an in vitro SK-1 activity assay as described in the Methods. Data are expressed as % of control values and are means with vertical lines showing s.d. (n=6) *P<0.05, **P<0.01, ***P<0.001 considered statistically significant when compared to the unstimulated control value.
Figure 2.
Concentration-dependent effects of extracellular nucleotides on long-term SK-1 activation in renal mesangial cells. Quiescent mesangial cells were stimulated for 24 h with either vehicle (Co), or the indicated concentrations of ATP or UTP (all in μM). Thereafter, cell lysates containing 50 μg of protein were taken for in vitro SK-1 or SK-2 activity assays as described in the Methods. Data are expressed as % of control values and are means with vertical lines showing s.d. (n=6) *P<0.05, ***P<0.001 considered statistically significant when compared to the unstimulated control values.
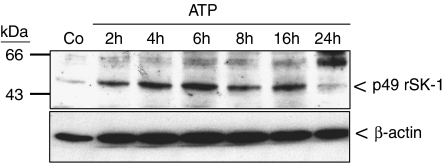
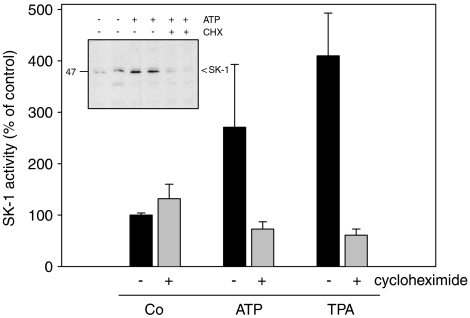
In a next step, Western blot analyses were performed to see whether SK-1 protein expression is altered by stimulation with nucleotides. For this a specific anti-rat SK-1 antibody was generated and characterized. As seen in Figure 3, upper panel, lysates of nucleotide-stimulated mesangial cells show a time-dependent increase of the p49 SK-1 protein expression, which is most prominent after 6 h of ATP stimulation and thereafter slightly declines. Interestingly, not only the 49 kDa enzyme is enhanced but also the 67 kDa band which may represent a modified form of SK-1. So far, no splice variants have been reported for the rat SK-1, thus the identity of the 67 kDa band remains unknown. During this long-term stimulation regimen the expression of the housekeeping enzyme β-actin was not altered (Figure 3, lower panel). The increased protein expression of SK-1 upon nucleotide stimulation is due to increased protein synthesis, as cycloheximide (CHX), which is an inhibitor of the translation machinery of the cell, is able to abolish the delayed ATP- and TPA-induced SK-1 activity (Figure 4). In parallel, nucleotide-upregulated protein expression of SK-1 was also blocked by CHX (Figure 4 inset).
Figure 3.
Effect of ATP and UTP on SK-1 protein expression in renal mesangial cells. Quiescent mesangial cells were stimulated for 20 h with either vehicle (Co) or with 100 μM of either ATP for the indicated time periods (in h). Thereafter, cell lysates containing 100 μg of protein were separated on SDS-PAGE, transferred to nitrocellulose and subjected to a Western blot analysis using antibodies against either SK-1 (upper panel) or β-actin (lower panel) at dilutions of 1:1500 and 1:10 000, respectively. Bands were visualized by the enhanced chemiluminescences method according to the manufacturer's instructions. Data are representative of six independent experiments giving similar results.
Figure 4.
Effect of CHX on ATP- and UTP-induced SK-1 protein expression and activity in renal mesangial cells. Quiescent mesangial cells were stimulated for 8 h with either vehicle (Co), ATP (100 μM) or TPA (100 nM) in the absence (−) or presence (+) of CHX (100 ng ml−1, pretreated for 30 min before stimulation). Thereafter, cell lysates containing 50 μg of protein were taken for an SK-1 activity assay as described in the Methods section. Data are means and vertical lines show s.d. (n=3). The inset shows SK-1 protein expression from two representative samples detected by Western blot analysis as described in the Methods.
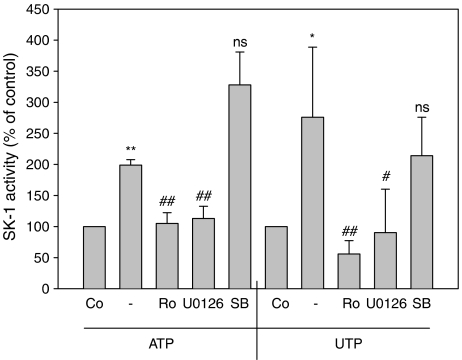
Furthermore, the upregulation of SK-1 expression also involves activation of PKC and the classical MAPK/ERK cascade, as the PKC inhibitor Ro 31-8220 and the MAPK/ERK kinase (MEK) inhibitor U0126 are able to reduce ATP- and UTP-induced SK-1 protein expression (data not shown) and, in parallel, inhibit ATP- and UTP-stimulated delayed SK-1 activity (Figure 5). In contrast, the p38-MAPK inhibitor SB203580 was not able to block SK-1 activity (Figure 5). Inhibition of MEK by U0126 also blocked the rapid nucleotide-induced SK-1 activity (data not shown), thus, confirming the critical involvement of the classical MEK/ERK cascade in the acute SK-1 activation observed previously by others (Pitson et al., 2003, 2005).
Figure 5.
Effect of MEK, p38-MAPK and PKC inhibitors on ATP-induced SK-1 activation in renal mesangial cells. Quiescent mesangial cells were preincubated for 20 min with the indicated inhibitors (Ro 31-8220 at 10 μM; U0126 at 20 μM; SB203580 at 10 μM) before stimulation for 6 h with either vehicle (Co), ATP or UTP (each 100 μM). Thereafter, cell lysates containing 50 μg of protein were taken for an in vitro SK-1 activity assay as described in the Methods section. Data are expressed as % of control values and are means with vertical lines showing s.d. (n=4) *P<0.05, **P<0.01 considered statistically significant when compared to the unstimulated control values; #P<0.05, ##P<0.01 considered statistically significant when compared to the ATP- or UTP-stimulated values, respectively; NS, not significant.
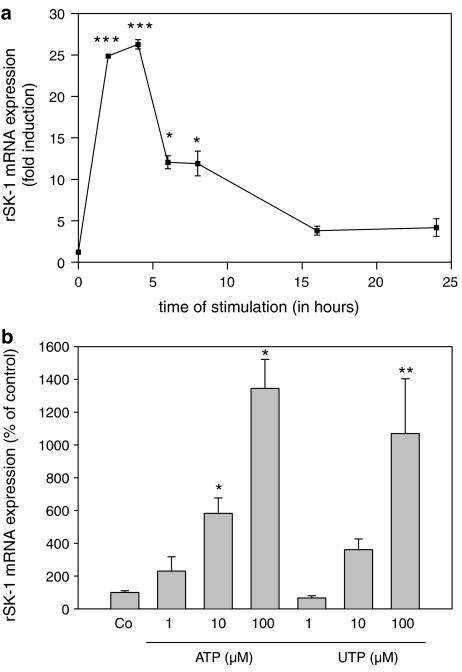
The increased protein synthesis of SK-1 upon nucleotide stimulation is preceded by increased expression of SK-1 mRNA. To this end, we performed quantitative real-time PCR experiments. UTP time-dependently upregulated SK-1 mRNA levels with maximal values at 2–4 h of stimulation which thereafter constantly declined over the next 20 h (Figure 6a). The upregulation of SK-1 mRNA expression also occurred in a concentration-dependent manner, as seen after 6 h of stimulation with ATP and UTP (Figure 6b).
Figure 6.
Effect of ATP and UTP on SK-1 mRNA expression in renal mesangial cells. (a) Quiescent rat mesangial cells were stimulated with vehicle (control, 2 h) or with UTP (100 μM) for the indicated time periods (in hours). (b) Cells were stimulated with either vehicle (Co) or with the indicated concentrations of ATP and UTP for 6 h. Thereafter, RNA was extracted and taken for real-time PCR using primers for rat SK-1 and 18S-RNA. The ΔΔCt values were calculated and were taken as a measure of SK-1 mRNA expression compared to 18S-RNA expression. Results are expressed as % of control values and are means with vertical lines showing s.d. (n=3), *P<0.05, #P<0.05, **P<0.01, ##P<0.01, ***P<0.001, ###P<0.001 considered statistically significant compared to the corresponding control values.
Extracellular nucleotides stimulate migration of mesangial cells in a SK-1-dependent manner
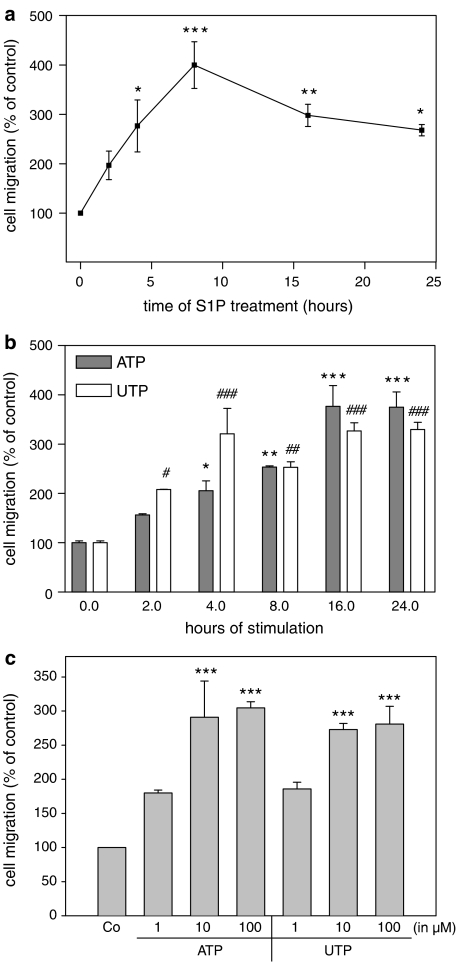
As S1P has been shown to affect the migration of a variety of cell types, we investigated whether mesangial cell migration is modulated by S1P. Stimulation of cells with exogenous S1P leads to an enhancement of mesangial cell migration as measured in a modified Boyden chamber. A significant effect is seen as early as 4 h after S1P stimulation and shows a maximal migratory effect after 8 h of stimulation (Figure 7a). A similar time-dependent (Figure 7b) and dose-dependent (Figure 7c) increase of mesangial cell migration was also seen with ATP and UTP.
Figure 7.
Effect of S1P and extracellular nucleotides on mesangial cell migration. Quiescent mesangial cells (1 × 105) were used for a migration assay employing a modified Boyden chamber assay system as described in the Methods section. Cells were treated for 24 h with either vehicle (control), or with 1 μM of S1P (a), 100 μM of ATP (b) and 100 μM of UTP (b), at the time periods indicated in h, or cells were treated for 24 h with the indicated concentrations (in μM) of ATP (c) or UTP (c). Data are expressed as % of control values and are means with vertical lines showing s.d. (n=3–6), *P<0.05, #P<0.05, **P<0.01, ##P<0.01, ***P<0.001, ###P<0.001 considered statistically significant compared to the corresponding control values.
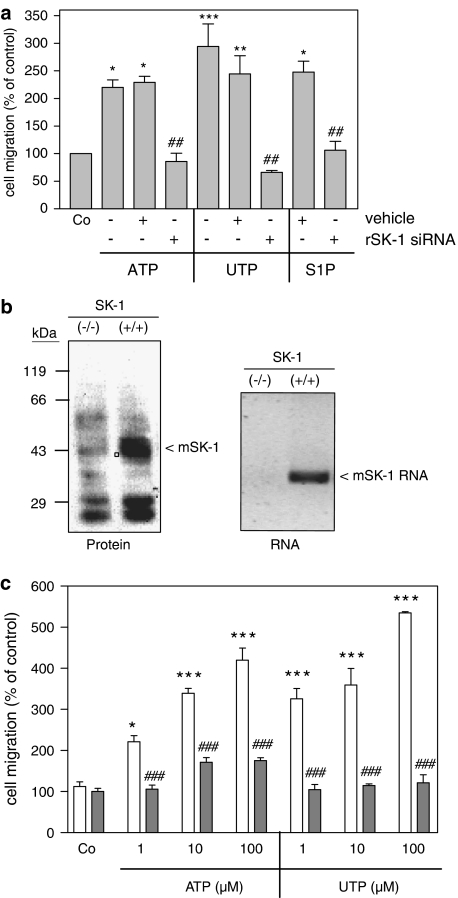
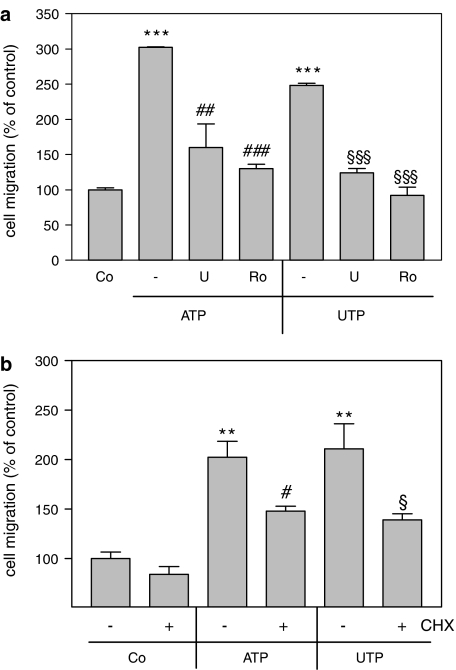
Interestingly, depletion of SK-1 from mesangial cells by siRNA transfection (Döll et al., 2005; Huwiler et al., 2006), completely abolished ATP-, UTP- and also S1P-triggered cell migration (Figure 8a). In an additional approach, mesangial cells were isolated from either a SK-1 (−/−) mouse or a SK-1 (+/+) control littermate. The lack of SK-1 in these mice was verified by Western blot analysis of kidney protein extracts (Figure 8b, left panel) and by RT-PCR of kidney RNA extracts (Figure 8b, right panel). Whereas SK-1 (+/+) control cells respond well to ATP and UTP by increased migration (Figure 8c), SK-1 (−/−) cells were completely devoid of nucleotide-induced cell migration (Figure 8c), although the basal migration rates of wild-type and knockout cells were not significantly different. Furthermore, in the presence of either the MEK inhibitor U0126 or the PKC inhibitor Ro 318220, ATP- and UTP-induced rat mesangial cell migration was attenuated (Figure 9a). In the presence of CHX nucleotide-induced migration was blocked by approximately 50% (Figure 9b). All these data suggest that SK-1 is a critical mediator of extracellular nucleotide-mediated mesangial cell migration and that SK-1 activation is especially involved in the delayed phase of the migratory mechanism.
Figure 8.
Effect of SK-1 depletion on extracellular nucleotide-induced migration of mesangial cells. (a) Rat mesangial cells were either used untransfected or transfected with specific SK-1 siRNA or vehicle as indicated; 48 h after transfection, cells were taken for a migration assay as described in the Methods section. They were stimulated for 24 h with either vehicle (Co), ATP (100 μM), UTP (100 μM) or S1P (1 μM). (b) Mouse kidneys from either SK-1 knockout mice (−/−) or control littermates (+/+) were analysed for SK-1 protein expression by Western blot analysis (left panel) or RNA expression by RT-PCR (right panel) to confirm SK-1 depletion. (c) Mouse mesangial cells isolated from either SK-1 knockout mice (−/−) or control littermates (+/+) were taken for a migration assay by stimulating with either vehicle (Co), ATP (100 μM) or UTP (100 μM). Migrated cells were determined as described in the Methods section. Data are expressed as (%) of control values and are means with vertical lines showing s.d. (n=4) *P<0.05, ***P<0.001 considered statistically significant when compared to the unstimulated control values; ###P<0.001 considered statistically significant when compared to the respective stimulations in SK-1 (+/+) control mice.
Figure 9.
Effect of PKC and MEK inhibitors and CHX on extracellular nucleotide-induced mesangial cell migration. Quiescent mesangial cells (1 × 105) were used for a migration assay employing a modified Boyden chamber assay system as described in the Methods section. (a) Cells were pretreated for 30 min with either vehicle (−), U0126 (U, 20 μM; a), Ro 318220 (Ro, 10 μM; a) or CHX (100 ng ml−1; b) before stimulation for 24 h with either ATP (100 μM) or UTP (100 μM). Data are expressed as % of control values and are means, with vertical lines showing s.d. (n=3), **P<0.01, ***P<0.001 considered statistically significant compared to unstimulated control values; #P<0.05, ##P<0.01, ###P<0.001 considered statistically significant compared to the ATP-stimulated values; §P<0.05, §§§P<0.001 considered statistically significant compared to the UTP-stimulated values.
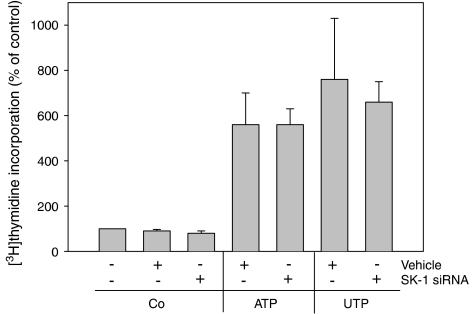
Finally, as extracellular nucleotides are also well known to induce proliferation of mesangial cells (Schulze-Lohoff et al., 1992; Huwiler and Pfeilschifter, 1994), we also investigated whether SK-1 is involved in this cell response. However, as seen in Figure 10, depletion of SK-1 had no significant effect on either ATP- or UTP-triggered [3H]thymidine incorporation, suggesting that this nucleotide-mediated response is not dependent on SK-1 and S1P production.
Figure 10.
Effect of SK-1 depletion on extracellular nucleotide-induced mesangial proliferation. Mesangial cells used were either untransfected or transfected with specific SK-1 siRNA or vehicle as indicated. Thereafter, cell proliferation was measured by [3H]thymidine incorporation into DNA as described in the Methods section. Cells were stimulated with either vehicle (Co), ATP or UTP (both at 100 μM). Data are expressed as (%) of control values and are means, with vertical lines showing s.d. (n=6).
Discussion
In the last few years it has become more and more apparent that ATP not only functions as an intracellular energy source, but may also acts as a signalling molecule with the ability to bind to and activate cell surface receptors, the P2 purinoceptors and thereby start signal transduction finally leading to various cell responses including cell growth (Schulze-Lohoff et al., 1992; Huwiler and Pfeilschifter, 1994; Ralevic and Burnstock, 1998) and cell survival (Huwiler et al., 2002; Ahmad et al., 2005). In addition, ATP also plays a role in chemotaxis and cell migration. Thus, immune cells including microglias (Honda et al., 2001), oligodendrocytes (Agresti et al., 2005) and immature dendritic cells (Idzko et al., 2002), display an increased migratory response upon ATP exposure. Furthermore, endothelial cells and epithelial cells stimulated by ATP also show enhanced migration, which is considered an important event in wound repair and tissue remodelling (Ehring et al., 2000; Honda et al., 2001; Klepeis et al., 2004).
In this study we show for the first time that renal mesangial cells also respond to ATP and UTP with increased cell migration. Migration of mesangial cells may be an important event in the repair mechanism during inflammatory kidney diseases. Often, these diseases are characterized by an initial acute phase of mesangial cell apoptosis which needs to be compensated for by increased migration of mesangial cells into the intercapillary tuft, and is subsequently followed by increased proliferation of the cells. ATP may be released into the extracellular space during the first phase of mesangial cell apoptosis and may then act in an autocrine manner to activate purinoceptors and trigger mesangial cell migration and proliferation and thereby may contribute to the repair processes of such kidney diseases. The involvement and importance of P2Y receptors in inflammatory diseases was also reported by Rost et al. (2002); they demonstrated that the P2Y receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) is able to attenuate anti-Thy1-induced glomerulonephritis in rats.
Our data further reveal that SK-1 activation is an important event in extracellular nucleotide-induced signal transduction. The activation of SK-1 by nucleotides occurs in a biphasic manner. An initial rapid activation takes place after minutes of stimulation and involves post-translational mechanisms such as phosphorylation reactions. In this context, it has been shown that human SK-1 is directly phosphorylated by the classical MAPK/ERK on Ser225, which leads to a translocation of the enzyme to the plasma membrane and increased activity (Pitson et al., 2003, 2005). The second peak of SK-1 activation by ATP and UTP is delayed and involves enhanced SK-1 mRNA expression followed by increased de novo synthesis of SK-1 protein. Based on a recent publication (Nakade et al., 2003) it is tempting to speculate that the increased mRNA expression derives from a transcriptional activation of the SK-1 gene owing to stimulated promoter activity. The promoter of SK-1 has recently been cloned from human leukaemia cells MEG (Nakade et al., 2003) and shows a multitude of putative transcription factor binding sites that could be stimulated by growth factors. In fact, the direct PKC activator phorbol ester (Nakade et al., 2003; Huwiler et al., 2006) and the pathophysiologically important PKC activator histamine (Huwiler et al., 2006) have been shown to be potent inducers of SK-1 promoter activity. Within the promoter, several potential binding sites for Sp1, activator protein (AP)-1, AP-2, AP-4, signal transducers and activators of transcription and nuclear factor kappa B were identified. Clearly, more detailed studies, including deletion and point mutation promoter studies, will be needed to identify the transcription factors involved in the nucleotide-induced upregulation of SK-1 mRNA expression.
A biphasic regulation of SK-1 by nucleotides, as described here, may guarantee a rapid adaptation by ‘fine-tuning' of SK-1 activity through phosphorylation steps. Also it would allow a long-term adaptation to environmental changes by altered gene transcription of SK-1 that may be necessary for tissue repair and remodelling after injury. Interestingly, SK-1 seems to be a critical mediator and also a switch point in the cellular responses triggered by extracellular nucleotides. Thus, when SK-1 is depleted either by siRNA transfection or by using mouse mesangial cells isolated from SK-1 knockout mice, the nucleotides are no longer able to induce cell migration (Figure 8a and c). Furthermore, as the protein synthesis inhibitor CHX is able to block the delayed phase of SK-1 activation (Figure 4) without affecting the acute SK-1 activation, and additionally also inhibits, at least partially, the nucleotide-induced migration (Figure 9b), it is tempting to speculate that the delayed activation of SK-1 is involved in the migratory mechanism. However, as it is not, at present, possible to deplete the first acute phase of SK-1 activation without altering the second delayed phase of SK-1 activation, we cannot exclude the possibility that the acute activation is necessary for the chronic activation phase. Thus, inhibition of PKC and MEK not only blocked the acute activation of SK-1 (data not shown; and Pitson et al., 2003) but also the delayed SK-1 activation.
Interestingly, another prominent cell response triggered by nucleotides, that is, proliferation, seems not to involve SK-1, as depletion of SK-1 had no effect on nucleotide-triggered cell proliferation (Figure 10). This differs from other cell types where SK-1 has been shown to be involved in both cell responses, migration and proliferation. In the human breast cancer cell line MCF7, depletion of SK-1 abolishes both migration and proliferation of tumour cells (Döll et al., 2005). Furthermore, it has recently been shown that chemotherapeutic agents like doxorubicin and etoposide downregulate the expression of SK-1 protein in tumour cells (Taha et al., 2004). These authors suggested a correlation between the loss of SK-1 expression and increased apoptosis of tumour cells by chemotherapy. In addition, various solid tumour tissues show an increased mRNA expression of SK-1 (French et al., 2003; Kawamori et al., 2006).
All these data support the hypothesis that SK-1 is positively involved in tumour growth and hence, the downregulation of SK-1 may have favourable effects in tumour therapy. On the other hand, cell proliferation and angiogenesis are also important processes in tissue repair. Hence, reduced expression of SK-1 may correlate with an adverse effect on wound healing.
The involvement of S1P in cell migration is well established. Depending on the cell type S1P can either stimulate or inhibit cell migration. This seemingly paradox behaviour is explained by the presence of different S1P receptor subtypes which initiate opposing signalling pathways. In smooth muscle cells, which prominently express the S1P2 receptor, migration is inhibited by S1P (Ryu et al., 2002). Furthermore, when using a S1P2 receptor antagonist, JTE-013, the inhibitory effect of S1P on smooth muscle cell migration could be reversed (Osada et al., 2002). In contrast, S1P stimulates migration of endothelial cells through activation of either S1P1 or S1P3 receptors, both predominant subtypes in these cells. Mesangial cells have been shown to express several S1P receptor subtypes, including S1P1, S1P2, S1P3, S1P4 and S1P5 (formerly known as Edg-1, -5, -3, -6 and -8 receptors) (Gennero et al., 2002; Katsuma et al., 2002), of which S1P2 and S1P3 have been particularly proposed to be involved in the mitogenic function of S1P (Katsuma et al., 2002). Whether a particular S1P receptor, or all of them, is involved in the ATP-induced migration of mesangial cells is still not known, as not all of the S1P-triggered cell responses necessarily involve S1P receptors. Previously, it has been suggested that intracellularly generated S1P may also act as a second messenger activating intracellular targets. However, these targets have not been molecularly defined. In mesangial cells, S1P is mainly generated inside the cell as SK-1 is not significantly secreted (data not shown). This contrasts with the situation described for endothelial cells where SK-1 was found to be constitutively secreted (Ancellin et al., 2002) and consequently may generate S1P extracellularly.
Clearly, our experiments show that the SK-1 is involved in the mechanism of nucleotide and also exogenous S1P-induced migration of mesangial cells. However, we were not able to detect increases in cellular S1P levels upon nucleotide stimulation. This may be due to a very rapid conversion of S1P into other sphingolipid species or degradation products. Moreover, the issue of whether cell surface S1P receptors are involved in the ATP-triggered cell migration or whether an intracellular mechanism is involved still needs to be elucidated. Nevertheless, our data reveal that one of the S1P receptors does contribute to cell migration when extracellular S1P is present. Clearly, further experiments are required to address this issue in more detail.
In summary, our data show that extracellular nucleotides not only trigger cell proliferation but also stimulate mesangial cell migration. This latter cellular response critically involves the activation of SK-1. Thus, SK-1 may represent a promising target for inhibiting cell movement in inflammatory kidney diseases.
Acknowledgments
SK was supported by the University Hospital, Frankfurt am Main (Nachwuchsstipendium). This work was financially supported by the Swiss National Foundation, the German Research Foundation (GRK757/2, FOG784), the European Community (FP6: LSHM-CT-2004-005033), the Wilhelm Sander-Stiftung and the Novartis Foundation. We gratefully acknowledge the excellent technical assistence of Mrs Luise Reinsberg, Isolde Römer, Svetlana Bubnova and Simone Hildbrand.
Abbreviations
- CHX
cycloheximide
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco's modified Eagle medium
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- S1P
sphingosine 1-phosphate
- siRNA
small-interfering RNA
- SK
sphingosine kinase
Conflict of interest
The authors state no conflict of interest.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonte C, Aloisi F, et al. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Rev. 2005;48:157–165. doi: 10.1016/j.brainresrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Ahmad A, McConville G, Schneider BK, Allen CB, Manzer R, et al. Lung epithelial cells release ATP during ozone exposure: signaling for cell survival. Free Radic Biol Med. 2005;39:213–226. doi: 10.1016/j.freeradbiomed.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, et al. Extracellular export of sphingosine kinase-1 enzyme Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. J Auton Nerv Syst. 2000;81:264–270. doi: 10.1016/s0165-1838(00)00125-9. [DOI] [PubMed] [Google Scholar]
- Becker KG, Simon RM, Bailey-Wilson JE, Freidlin B, Biddison WE, McFarland HF, et al. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc Natl Acad Sci USA. 1998;95:9979–9984. doi: 10.1073/pnas.95.17.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1β and TNF-α induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Buehrer BM, Bardes ES, Bell RM. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim Biophys Acta. 1996;1303:233–242. doi: 10.1016/0005-2760(96)00092-6. [DOI] [PubMed] [Google Scholar]
- Döll F, Pfeilschifter J, Huwiler A. The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7. Biochim Biophys Acta. 2005;1738:72–81. doi: 10.1016/j.bbalip.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Piirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring GR, Szabo IL, Jones MK, Sarfeh IJ, Tarnawski AS. ATP-induced Ca2+ -signaling enhances rat gastric microvascular endothelial cell migration. J Physiol Pharmacol. 2000;51:799–811. [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- Gennero I, Fauvel J, Nieto M, Cariven C, Gaits F, Briand-Mesange F, et al. Apoptotic effect of sphingosine 1-phosphate and increased sphingosine 1-phosphate hydrolysis on mesangial cells cultured at low cell density. J Biol Chem. 2002;277:12724–12734. doi: 10.1074/jbc.M108933200. [DOI] [PubMed] [Google Scholar]
- Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, et al. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Briner VA, Fabbro D, Pfeilschifter J. Feedback regulation of extracellular ATP-stimulated phosphoinosititde hydrolysis by protein kinase C-α in bovine glomerular endothelial cells. Kidney Int. 1997a;52:329–337. doi: 10.1038/ki.1997.338. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Döll F, Ren S, Klawitter S, Greening A, Römer I, et al. Histamine increases sphignosine kinase-1 expression and activity in EA.hy 926 cells by a PKC-α-dependent mechanism. Biochim Biophys Acta. 2006;1761:367–376. doi: 10.1016/j.bbalip.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000b;1485:63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br J Pharmacol. 1994;113:1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Rölz W, Dorsch S, Ren S, Pfeilschifter J. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y2 purinoceptor in renal mesangial cells. Br J Pharmacol. 2002;136:520–529. doi: 10.1038/sj.bjp.0704748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Stabel S, Fabbro D, Pfeilschifter J. Platelet-derived growth factor and angiotensin II stimulate the mitogen-activated protein kinase cascade in renal mesangial cells: comparison of hypertrophic and hyperplastic agonists. Biochem J. 1995;305:777–784. doi: 10.1042/bj3050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, van Rossum G, Wartmann M, Pfeilschifter J. Stimulation by extracellular ATP and UTP of the stress-activated protein kinase cascade in rat renal mesangial cells. Br J Pharmacol. 1997b;120:807–812. doi: 10.1038/sj.bjp.0700979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Wartmann M, van den Bosch H, Pfeilschifter J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br J Pharmacol. 2000a;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Dichmann S, Ferrari D, Di Virgilio F, La Sala A, Girolomoni G, et al. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2Y receptors. Blood. 2002;100:925–932. doi: 10.1182/blood.v100.3.925. [DOI] [PubMed] [Google Scholar]
- Imamura T, Miyauchi-Senda N, Tanaka S, Shiota K. Identification of genetic and epigenetic similarities of SPHK1/Sphk1 in mammals. J Vet Med Sci. 2004;66:1387–1393. doi: 10.1292/jvms.66.1387. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ohgane J, Ito S, Ogawa T, Hattori N, Tanaka S, et al. CpG island of rat sphingosine kinase-1 gene: tissue-dependent DNA methylation status and multiple alternative first exons. Genomics. 2001;76:117–125. doi: 10.1006/geno.2001.6607. [DOI] [PubMed] [Google Scholar]
- Katsuma S, Hada Y, Ueda T, Shiojima S, Hirasawa A, Tanoue A, et al. Signalling mechanisms in sphingosine 1-phosphate-promoted mesangial cell proliferation. Genes Cells. 2002;7:1217–1230. doi: 10.1046/j.1365-2443.2002.00594.x. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V. P2Y receptors play a critical role in epithelial cell communication and migration. J Cell Biochem. 2004;93:1115–1133. doi: 10.1002/jcb.20258. [DOI] [PubMed] [Google Scholar]
- Kleuser B, Cuvillier O, Spiegel S. 1α,25-Dihydroxyvitamin D3 inhibits programmed cell death in HL-60 cells by activation of sphingosine kinase. Cancer Res. 1998;58:1817–1824. [PubMed] [Google Scholar]
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Kuokkanen S, Gschwend M, Rioux JD, Daly MJ, Terwilliger JD, Tienari PJ, et al. Genomewide scan of multiple sclerosis in Finnish multiplex families. Am J Hum Genet. 1997;61:1379–1387. doi: 10.1086/301637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- Meyer Zu Heringdorf D, Lass H, Kuchar I, Alemany R, Guo Y, Schmidt M, et al. Role of sphingosine kinase in Ca(2+) signalling by epidermal growth factor receptor. FEBS Lett. 1999;461:217–222. doi: 10.1016/s0014-5793(99)01463-5. [DOI] [PubMed] [Google Scholar]
- Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W, et al. Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) b genome-wide scan. Hum Mol Genet. 1997;6:1349–1356. doi: 10.1093/hmg/6.8.1349. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Banno Y, T-Koizumi K, Hagiwara K, Sobue S, Koda M, et al. Regulation of sphingosine kinase 1 gene expression by protein kinase C in a human leukemia cell line, MEG-O1. Biochim Biophys Acta. 2003;1635:104–116. doi: 10.1016/j.bbalip.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine 1 phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun. 2002;299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Banno Y, Nagaki M, Brenner DA, Naiki T, Nozawa Y, et al. TNF-α-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J Immunol. 2001;167:173–180. doi: 10.4049/jimmunol.167.1.173. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H, Gloy J, Leipziger J, Klaer B, Pfeilschifter J, Schollmeyer P, et al. Effect of extracellular ATP on contraction, cytosolic calcium activity, membrane voltage and ion currents of rat mesangial cells in primary culture. Br J Pharmacol. 1993;109:953–959. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J. Extracellular ATP stimulates polyphosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Involvement of pertussis toxin-sensitive guanine nucleotide binding protein and feedback inhibition by protein kinase C. Cell Signal. 1990a;2:129–138. doi: 10.1016/0898-6568(90)90016-4. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. No indications for the involvement of separate purino- and pyrimidino-ceptors. Biochem J. 1990b;272:469–472. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Huwiler A. Regulatory functions of protein kinase C isoenzymes in purinoceptor signalling in mesangial cells. J Auton Pharmacol. 1996;16:315–318. doi: 10.1111/j.1474-8673.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ramoz N, Rueda LA, Bouadjar B, Favre M, Orth G. A susceptibility locus for epidermodysplasia verruciformis, an abnormal predisposition to infection with the oncogenic human papillomavirus type 5, maps to chromosome 17qter in a region containing a psoriasis locus. J Invest Dermatol. 1999;112:259–263. doi: 10.1046/j.1523-1747.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- Remmers EF, Longman RE, Du Y, O'Hare A, Cannon GW, Griffiths MM, et al. A genome scan localizes five non-MHC loci controllino collagen-induced arthritis in rats. Nat Genet. 1996;14:82–85. doi: 10.1038/ng0996-82. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Rost S, Daniel C, Schulze-Lohoff E, Baumert HG, Lambrecht G, Hugo C. P2 receptor antagonist PPADS inhibits mesangial cell proliferation in experimental mesangial proliferative glomerulonephritis. Kidney Int. 2002;62:1659–1671. doi: 10.1046/j.1523-1755.2002.00621.x. [DOI] [PubMed] [Google Scholar]
- Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, et al. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB. Extracellular ATP stimulates proliferation of cultured mesangial cells via P2-purinergic receptors. Am J Physiol. 1992;263:F374–F383. doi: 10.1152/ajprenal.1992.263.3.F374. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol. 2001;280:F945–F963. doi: 10.1152/ajprenal.2001.280.6.F945. [DOI] [PubMed] [Google Scholar]
- Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003a;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003b;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, et al. Down-regulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53. J Biol Chem. 2004;279:20546–20554. doi: 10.1074/jbc.M401259200. [DOI] [PubMed] [Google Scholar]
- Tomfohrde J, Silverman A, Barnes R, Fernendez-Vina MA, Young M, Lory D, et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science. 1994;264:1141–1145. doi: 10.1126/science.8178173. [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]