Abstract
Background and purpose:
P-glycoprotein (P-gp) is an important efflux transporter that supports the barrier function of the gut against invading antigens and against administered drugs. Since glucocorticoids, such as budesonide, are frequently used during inflammatory bowel disease we investigated how budesonide influences P-gp expression in different intestinal cell lines.
Experimental approach:
LS180 and Caco-2 cells were incubated with budesonide and changes in P-gp expression were determined on mRNA, protein and functional level. The mRNA expression levels of glucocorticoid receptor (GR) and pregnane X receptor (PXR) were determined in these cell lines. PXR receptor was transiently transfected into Caco-2 cells.
Key results:
Budesonide showed an induction of P-gp in LS180 cells and a down-regulation in Caco-2 cells. Expression levels of nuclear receptors revealed high expression of PXR only in LS180 cells and exclusive expression of GR in Caco-2 cells. Mifepristone, an anti-glucocorticoid, could not reverse the down-regulation of P-gp by budesonide in Caco-2 cells. In PXR-transfected Caco-2 cells the budesonide-mediated down-regulation of P-gp was abolished. Furthermore the expression of cytochrome P450 3A4 (CYP3A4), another PXR target gene, was induced in PXR-transfected Caco-2 cells after budesonide treatment.
Conclusions and Implications:
Budesonide has the potential to influence MDR1 expression in vitro. In LS180 cells, the induction of MDR1 by budesonide probably is mediated via PXR. The mechanism of the down-regulation in Caco-2 cells still remains unclear, but GR does not seem to be involved. Further studies are required to evaluate how budesonide alters P-gp expression in vivo.
Keywords: P-glycoprotein; MDR1; ABCB1; budesonide; Caco-2, LS180; intestine; PXR; glucocorticoid receptor
Introduction
Glucocorticoids are an important therapeutic option in treating inflammatory disorders like asthma, rheumatoid arthritis or inflammatory bowel disease (IBD). Budesonide is a newer synthetic glucocorticoid that is increasingly used in IBD patients (Kane et al., 2002). If applied in a controlled release formulation, budesonide is, after oral administration, topically released in the distal small intestine and colon, the predominant sites of inflammation (Klotz and Schwab, 2005). There, it is well absorbed, but it is extensively pre-systemically metabolized (Schwab and Klotz, 2001). Consequently, upon oral administration budesonide exerts strong anti-inflammatory effects in intestinal tissue with minimal systemic side effects (Hofer, 2003).
The molecular mechanism of glucocorticoid action involves intracellular binding to the glucocorticoid receptor (GR), followed by a translocation of activated GR to the nucleus. There, it stimulates or inhibits gene expression by binding to glucocorticoid response elements on DNA (Wright et al., 1993). Moreover, ligand-bound GR can repress a number of pro-inflammatory genes by physically associating with transcription factors via direct protein–protein interactions. Examples are the repression of nuclear factor-κB (NF-κB) or activator protein-1 (AP-1) (Smoak and Cidlowski, 2004).
Glucocorticoids such as dexamethasone are further known to activate the pregnane X receptor (PXR) (Kliewer et al., 1998). PXR belongs to the group of orphan nuclear receptors that function as heterodimers with the retinoic X receptor (Chawla et al., 2001). They are important regulators of xenobiotic metabolism and upon activation they can induce the expression of metabolizing enzymes (e.g. cytochrome P450 3A4 (CYP3A4)) and drug transporters such as P-glycoprotein (P-gp) (Bertilsson et al., 1998; Geick et al., 2001).
P-gp is the gene product of the multidrug resistance gene 1 (MDR1, gene symbol: ABCB1). It is an adenosine triphosphate-dependent drug efflux pump with wide substrate specificity (Juliano and Ling, 1976). It has barrier function in tissues such as kidney, blood brain barrier and intestine (Cordon-Cardo et al., 1990). In the intestine, P-gp is localized in the apical membrane of epithelial cells and is continuously expressed along the intestinal tract (Thiebaut et al., 1987; Zimmermann et al., 2005). Therefore, P-gp can influence significantly the bioavailability of many drugs, including glucocorticoids, as it has been shown previously that budesonide, dexamethasone and prednisone are substrates of this transporter (Ueda et al., 1992; Fromm, 2003; Dilger et al., 2004). Besides the induction through PXR, the expression level of MDR1 is dependent on a complex transcriptional regulation with a redundancy of signalling pathways (Labialle et al., 2002).
Several publications demonstrated that dexamethasone induces MDR1 expression in the intestine of rats (Lin et al., 1999; Yumoto et al., 2001; Perloff et al., 2004). So far, there is no explanation of how glucocorticoids, in particular budesonide, influence MDR1 expression in human intestinal cells. Here, we have investigated how the glucocorticoid budesonide influences the expression of MDR1 in different human intestinal cell lines. We chose the Caco-2 cell line, as it shows transporter expressions comparable to those in the human jejunum (Taipalensuu et al., 2001). Furthermore, these cells are an established model for small intestinal transport (Hidalgo et al., 1989). Secondly, we used LS180 cells, which are a suitable model for intestinal gene induction studies (Bhat et al., 1995; Thummel et al., 2001; Zhou et al., 2004). Their expression level of efflux transporters is similar to human colonic tissue (Pfrunder et al., 2003). The findings of this study could be relevant in evaluating the involvement of P-gp in glucocorticoid action and glucocorticoid side effects.
Methods
Cell culture
The LS180 (used between passage 40 and 50) and Caco-2 cell lines (used between passage 54 and 70) were purchased from American Type Culture Collection (Manassas, NE, USA). LS180 and Caco-2 cells were cultured in Dulbecco's modified Eagle's medium with Glutamax-I, supplemented with 10% (v v−1) fetal bovine serum, 1% non-essential amino acids, 1% sodium pyruvate, 50 μg ml−1 gentamycin (Invitrogen AG, Basel, Switzerland). Cells were seeded into 12-well plastic culture dishes (3.8 cm2 per well, BD Falcon AG, Allschwil, Switzerland) and were maintained in a humidified 37°C incubator with a 5% carbon dioxide in air atmosphere. After Caco-2 cells had reached confluence, or near confluence for LS180, they were treated with the substances as indicated or with vehicle alone. Medium was changed every 24 h. Toxicity was tested in advance for all applied substances using sulforhodamine B staining (Sigma-Aldrich, St Louis, MO, USA) (Skehan et al., 1990).
Real-time RT-PCR
After the indicated incubation procedures LS180 and Caco-2 cells were disintegrated by adding lysis buffer RLT (Qiagen, Hilden, Germany) and homogenized by using QIAshredder columns (Qiagen). Total RNA was extracted from cell lysates using the RNeasy Mini Kit (Qiagen). RNA was quantified with a Nanodrop Spectrophotometer (Witeg AG, Littau-Luzern CH). The purity of the RNA preparations was high as demonstrated by the 260 nm over 280 nm ratio (range 1.8–2.1). After DNase I digestion (Gibco, Life Technologies, Basel, Switzerland) 0.75 μg of total RNA was reversed transcribed by Superscript II (Gibco) according to the manufacturer's protocol using random hexamers as primers (Applied Biosystems, Rotkreuz, Switzerland).
TaqMan analysis was carried out on a 7900HT Sequence Detection System (Applied Biosystems). Polymerase chain reaction (PCR) conditions were 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Each TaqMan reaction contained 10 ng of cDNA in a total volume of 10 μl. qPCR Mastermix Plus from Eurogentec (Seraing, Belgium) was used. Primers and probes were used at concentrations of 900 and 225 nM, respectively. They were synthesized by Invitrogen (Basel, Switzerland) and by Eurogentec (Seraing, Belgium), respectively. Primers and probes for MDR1 and PXR were designed according to the guidelines of Applied Biosystems with help of the Primer Express 2.0 software. Sequences for glucocorticoid receptor-α (GR-α) and GR-β were adopted from a previous paper (Pedersen and Vedeckis, 2003). Corresponding sequences of primers and probes for TaqMan analysis are shown in Table 1. All samples were run in triplicates and not reverse-transcribed RNA served as a negative control. For the quantification, the expression of the genes of interest was normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA expression.
Table 1.
Primers and probes for TaqMan analysis
| Gene | Probe |
|---|---|
| MDR1 | 5′-AAGCTGTCAAGGAAGCCAATGCCTATGACTT-3′ |
| GR-α | 5′-TTTCAACCACTTCATGCATAGAAT-3′ |
| GR-β | 5′-CATAACATTTTCATGCATAGAATCCAAGAGTTTTGTCA-3′ |
| PXR | 5′-AGCCCTTGCATCCTTCACATGTCATGA-3′ |
| CYP3A4 | 5′-TTCTCCTGGCTGTCAGCCTGGTGC-3′ |
| Gene | Forward primer |
| MDR1 | 5′-CTGTATTGTTTGCCACCACGA-3′ |
| GR-α | 5′-GGCAGCGGTTTTATCAACTGA-3′ |
| GR-β | 5′-AACTGGCAGCGGTTTTATCAA-3′ |
| PXR | 5′-GGCCACTGGCTATCACTTCAA-3′ |
| CYP3A4 | 5′-TCTCATCCCAGACTTGGCCA-3′ |
| Gene | Reverse primer |
| MDR1 | 5′-AGGGTGTCAAATTTATGAGGCAGT-3′ |
| GR-α | 5′-AATGTTTGGAAGCAATAGTTAAGGAGA-3′ |
| GR-β | 5′-TGTGAGATGTGCTTTCTGGTTTTAA-3′ |
| PXR | 5′-GTTTCATGGCCCTCCTGAAA-3′ |
| CYP3A4 | 5′-CATGTGAATGGGTTCCATATAGATAGA-3′ |
For absolute quantification, we used external standard curves. Standards were gene-specific cDNA fragments that cover the TaqMan primer/probe area and they were generated by PCR. Sequences of the corresponding primers are shown in Table 2. The PCR products were purified by running a 1.5% agarose gel and a subsequent gel extraction (gel extraction kit, Qiagen). The standards were quantified using the PicoGreen reagent (Molecular Probes, Eugene, OR, USA) and were checked by sequencing (Microsynth GmbH, Balgach, Switzerland).
Table 2.
Primers for cDNA standards
| Gene | Forward primer | Reverse primer |
|---|---|---|
| MDR1 | 5′-ACAGTCCAGCTGATGCAGAGG-3′ | 5′-CCTTATCCAGAGCCACCTGAAC-3′ |
| GR-α | 5′-TACCCTGCATGTACGACCAA-3′ | 5′-TTTTGGTATCTGATTGGTGATGA-3′ |
| GR-β | 5′-TACCCTGCATGTACGACCAA-3′ | 5′-TTGTCGATGAGCATCAGTTG-3′ |
| PXR | 5′-GCAGTCCAAGAGGCCCAGAA-3′ | 5′-CGTCGGACATGATCATCTCCTTC-3′ |
| CYP3A4 | 5′-TAGTGATGGCTCTCATCCCAGA-3′ | 5′-TGAAGGTTGGAGACAGCAATGA-3′ |
Western blot analysis
LS180 and Caco-2 cells were incubated for 72 h with 25 μM budesonide or with vehicle (0.25%. dimethylsulphoxide (DMSO)) as a negative control. LS180 cells additionally were incubated with 10 μM rifampicin. Then proteins were extracted with protein extraction buffer (20 mM Tris-HCl, 1% Igepal CA-630, 0.5 mM sodium orthovanadate), including 1 mM of the protease inhibitor phenylmethylsulfonyl fluoride (Sigma-Aldrich, St Louis, MO, USA) and protease inhibitor cocktail tablet, Complete Mini (Roche Diagnostics, Germany). The quantification of the protein content was performed with the bicinchoninic acid protein assay kit (Pierce Chemical, Rockford, IL, USA). Protein concentration was determined by measuring the absorbance at 562 nm with Spectra MAX 250 Microplate Spectrophotometer (Molecular Devices Corporation, California, USA).
For immunoblotting, 50 μg of total protein extract was mixed with Laemmli sample buffer (Bio Rad Laboratories, Reinach, Switzerland) and transferred to the polyacrylamide gel. Gel electrophoresis was performed with a Mini Protean 3 Electrophoresis Cell (Bio Rad) applying 80 V for 15 min for the stacking gel (4% acrylamide) and 120 V for 1 h for the separating gel (10% acrylamide). After electrophoresis, proteins were blotted to the nitrocellulose membrane (250 mA for 2.5 h) using a Mini Trans-Blot Cell (Bio Rad). Protein transfer was verified by Ponceau S staining. The membrane was blocked overnight at 4°C with phosphate-buffered saline (PBS) containing 5% milk powder and 0.05% Tween 20. After washing three times for 15 min (0.05% Tween in PBS), the membrane was incubated for 2 h at room temperature with the primary, mouse anti-human antibody C219 against P-gp, 0.1 mg ml−1 (Alexis Corporation, Lausen, Switzerland) diluted 1:100 in PBS containing 0.05% Tween and 1% milk powder. As loading control, β-actin mouse monoclonal antibody (abcam, Cambridge, UK) was used with the dilution of 1:1000. After the first incubation, the membrane was washed three times for 15 min and then incubated with the secondary, horseradish peroxidase-conjugated, rabbit anti-mouse immunoglobulin-G (Amersham, Buckinghamshire, UK) diluted 1:1000. Secondary antibody incubation was performed for 1 h at room temperature. Membranes were washed, and P-gp and β-actin detection was performed with the enhanced chemiluminescence system (ECL-Detection-Kit, Amersham). The molecular weight was identified by using Precision Plus Protein TM Standard Dual Color (Bio Rad).
Rhodamine 123 accumulation assay
LS180 and Caco-2 cells were incubated for 72 h either with medium containing 20 μM budesonide or vehicle only (0.2% DMSO). LS180 cells were additionally incubated with 10 μM rifampicin. Medium was changed every day. Following drug treatment, cells were washed with Hanks'-buffered saline solution (HBSS) (supplemented with 1 mM pyruvate throughout) and incubated for 30 min at 37°C with HBSS containing additionally 100 μM verapamil and 0.5 μM rhodamine 123 (R123) (Molecular Probes, Eugene, OR, USA). In this step, cells are loaded with R123, whereas P-gp function is blocked with verapamil. R123 uptake was stopped by transferring the cells to ice. They were washed three times with ice-cold HBSS in the presence or absence of 100 μM verapamil. R123 efflux was started by incubating the cells with or without 100 μM verapamil in HBSS at 37°C. After 60 min, cells were washed at 4°C and lysed in 400 μl 5% Triton X-100. Samples of the homogenized cell lysates (200 μl) were analysed with a HTS 7000 Plus Bio Assay Reader (Perkin Elmer Ltd, Buckinghamshire, UK) with 485 nm excitation and 535 nm emission filters. The ratio of intracellular R123 fluorescence in the absence and presence of verapamil is indicative of the activity of P-gp. This approach has been reported previously for the measurement of P-gp activity in LS180 cells (Collett et al., 2004).
mRNA decay measurement
The measurement of mRNA stability was performed in Caco-2 cells using actinomycin D as an inhibitor of transcription. Cells were preincubated for 1 h with budesonide 25 μM or vehicle. Actinomycin D (5 μg ml−1) was added and total RNA was extracted after different time points. The expression level of MDR1 mRNA after 0, 4, 8, 24 and 48 h was determined by real-time reverse transcription (RT)-PCR.
Transfections
The Full ORF Expression vector of PXR (IOH34726-pT-Rex-DEST30) was purchased from the Deutsches Ressourcenzentrum für Genomforschung GmbH. The empty vector was obtained by cutting the PXR plasmid with the restriction enzymes EcoRV and MluI followed by purification on a 0.5% agarose gel. The cut ends were blunt-ended with T4 DNA polymerase and self-circulated with T4 DNA ligase. Transfection was performed with Lipofectamine 2000 (Invitrogen) during 48 h following the manufacturer's protocol using 1.6 μg DNA and 4 μl Lipofectamine 2000. As negative control, the empty vector was used. After transfection Caco-2 cell were incubated with budesonide 25 μM and rifampicin 10 μM or vehicle only. Transfection was verified by measuring PXR mRNA expression using TaqMan analysis.
Statistics
Treatment groups were compared to control group by analysis of variance. If this analysis revealed significant differences and more than one treatment group was included in the analysis, pairwise comparisons of treatment groups with the control group was performed subsequently using Dunnett's two-sided multi-comparison test or multiple unpaired two-sided t-test with Bonferoni's correction, as appropriate, to account for the multiplicity of testing. All tests were performed using the SPSS for Windows software (version 14.0). The level of significance was P<0.05.
Materials
Budesonide (Sigma-Aldrich, St Louis, MO, USA), rifampicin (Fluka Chemie, Buchs SG, Switzerland) and actinomycin D (Sigma-Aldrich) were dissolved in DMSO. Mifepristone (Sigma-Aldrich) and R123 (Molecular Probes, Eugene, OR, USA) were dissolved in ethanol.
Results
MDR1 mRNA expression
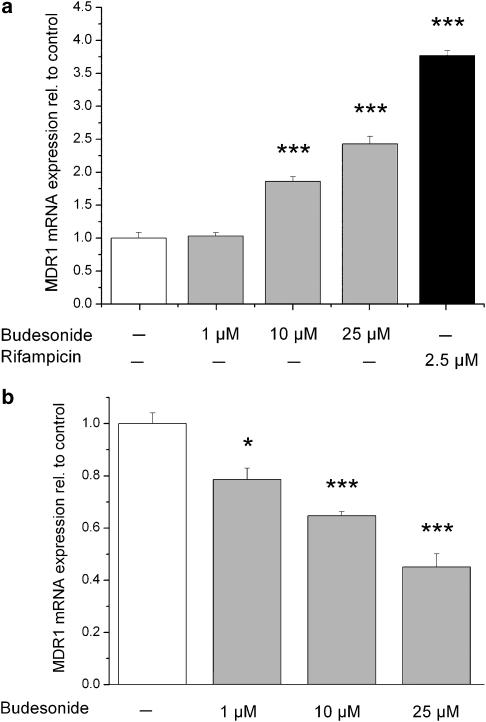
The basal MDR1 mRNA expression level was higher in Caco-2 cells compared to LS180 cells. The effect of budesonide on MDR1 mRNA expression was investigated in these two different intestinal cell lines (LS180 and Caco-2). Cells were incubated for 48 h with increasing budesonide concentrations and mRNA expression was analysed using real-time RT-PCR. In LS180 cells, we observed a dose-dependent induction of MDR1 mRNA expression by budesonide (Figure 1a). Rifampicin as a positive control also showed an induction. In Caco-2 cells, MDR1 mRNA expression decreased in a dose-dependent manner after budesonide treatment (Figure 1b).
Figure 1.
Effect of budesonide on MDR1 mRNA expression in LS180 (a) and Caco-2 (b) cell lines. Cells were incubated for 48 h and mRNA expression was determined by quantitative real-time PCR. Rifampicin was used as a positive control for MDR1 induction in LS180 cells. Results are normalized to control cells and expressed as mean±s.e.m. (n=4). *P<0.05, ***P<0.001 vs control cells.
MDR1 protein expression
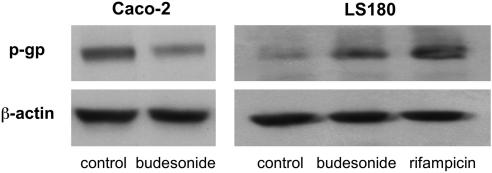
The observed changes in MDR1 mRNA expression in LS180 and Caco-2 cells were confirmed on protein level by Western blot analysis (Figure 2). In three independent assays, cells were incubated for 72 h with 25 μM budesonide and 10 μM rifampicin. Compared to control cells, MDR1 protein levels increased in LS180 cells and decreased in Caco-2 cells after budesonide treatment.
Figure 2.
Effect of budesonide on P-gp protein expression. LS180 and Caco-2 cells were incubated for 72 h with 25 μM budesonide or vehicle. Rifampicin was used as a positive control for MDR1 induction in LS180 cells. P-gp protein and β-actin were determined by Western blot analysis. β-Actin was used as loading control.
Measurement of P-gp activity
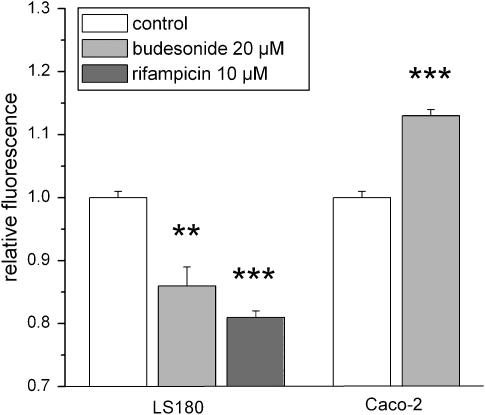
R123 is a fluorescent P-gp substrate that can be used for the determination of P-gp activity (Figure 3). After passive diffusion into the cells, it is actively transported out of the cell by P-gp. Measurement of cellular R123 accumulation revealed that the observed changes of MDR1 expression were reflected by changes in P-gp function. In LS180 cells, where MDR1 is induced by budesonide, relative R123 uptake was significantly lower compared to control cells. Similar effects were observed for rifampicin, a positive control for MDR1 induction in LS180 cells. In Caco-2 cells, the decreased MDR1 expression led to a significant accumulation of intracellular R123.
Figure 3.
P-gp activity was assessed using R123 accumulation in LS180 and Caco-2 cells. Cells were pretreated with 20 μM budesonide or vehicle for 72 h. Rifampicin was used as a positive control for P-gp induction. Data represent R123 fluorescence normalized to verapamil-treated cells. A decrease in intracellular fluorescence is indicative of an increase in P-gp activity and vice versa. Results are expressed as mean±s.e.m. (n=3–4). **P<0.01, ***P<0.001 vs control cells.
Expression of the nuclear receptors PXR and GR in intestinal cell lines
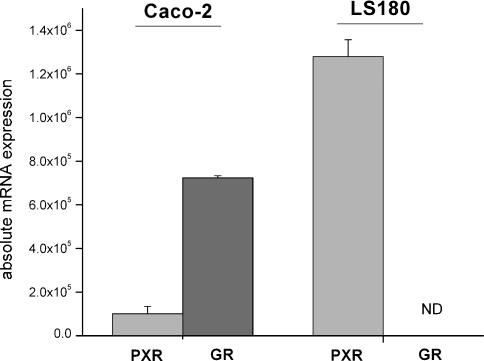
To elucidate the differential effects of budesonide on the expression of MDR1 in the cell lines studied, we determined whether this could be attributed to differential expression levels of nuclear receptors (Figure 4). Glucocorticoids exert their anti-inflammatory effects through the GR-α. However, the PXR can also be activated by glucocorticoids like dexamethasone. Real-time PCR analysis (n=4 for both cell lines) revealed low expression levels of PXR in Caco-2 cells and high expression in LS180 cells. GR-α, on the other hand, was exclusively expressed in Caco-2 cells. As the GR-β apparently exhibits inhibitory effects on the GR-α, we analysed also the expression rate of this receptor. However, no GR-β mRNA was detectable in either cell line. There was no effect of budesonide treatment on these receptors. In addition, basal MDR1 mRNA expression was not correlated to PXR expression level in both investigated cell lines.
Figure 4.
Expression of PXR and GR-α mRNA in the investigated intestinal cell lines Caco-2 and LS180. Expression was determined by quantitative real-time PCR. Results are expressed as mean±s.e.m. (n=4). ND=not detectable.
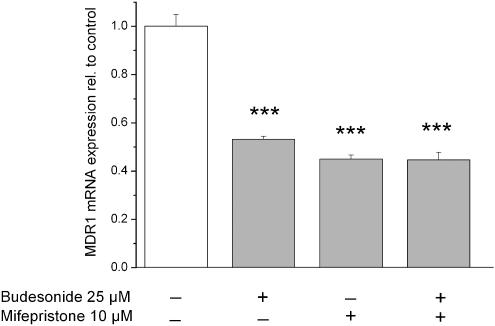
Effect of mifepristone on MDR1 mRNA expression in Caco-2 cells
To determine whether the GR plays a role in the budesonide-induced downregulation of MDR1 in Caco-2 cells, mifepristone (RU486) a known anti-glucocorticoid was used. Mifepristone could not reverse the effect of budesonide when co-applied; when the drug was given alone, it induced a similar downregulation of MDR1 (Figure 5).
Figure 5.
Effect of the anti-glucocorticoid mifepristone on MDR1 mRNA expression. Cells were incubated for 48 h and mRNA expression was determined by quantitative real-time PCR. Results are normalized to control cells and expressed as mean±s.e.m. (n=4). ***P<0.001 vs control cells.
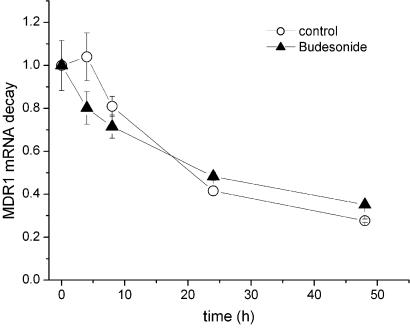
MDR1 mRNA stability
We have shown that budesonide decreases the expression of MDR1 in Caco-2 cells on the mRNA level. However, beside a transcriptional regulation, this result could also reflect a decrease in mRNA stability. Therefore, we determined the decay of MDR1 mRNA after the addition of the transcription inhibitor actinomycin D in Caco-2 cells (Figure 6). The stability of MDR1 mRNA was not different between control cells and cells treated with budesonide. For both treatments, the half-life of MDR1 mRNA in Caco-2 cells appeared to be about 20 h.
Figure 6.
Effect of budesonide on MDR1 mRNA stability in Caco-2 cells. Cells were treated with 25 μM budesonide or vehicle, whereas transcription was inhibited with actinomycin D (5 μg ml−1). mRNA expression was determined by quantitative real-time PCR after 0, 4, 8, 24 and 48 h incubation. Each data value is expressed relative to the expression at 0 h. Data represent mean±s.e.m. (n=3).
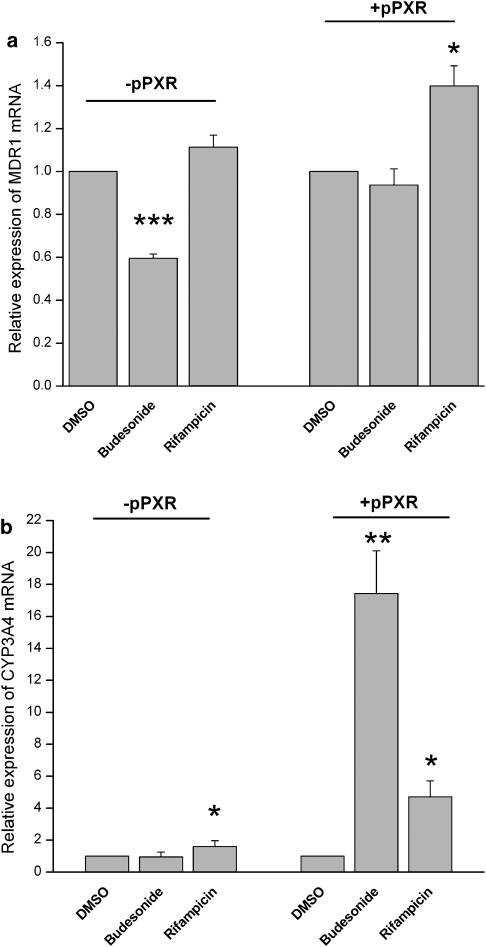
Transfection of PXR into Caco-2 cells
To investigate whether PXR is involved in the regulation of MDR1 by budesonide, PXR was transiently transfected into Caco-2 cells. Caco-2 cells express only low levels of endogenous PXR and known PXR activators such as rifampicin are not able to induce PXR target genes in Caco-2 cells (data not shown and Pfrunder et al., 2003). In a control experiment, cells transfected with the empty vector showed the expected downregulation of MDR1 mRNA after budesonide treatment and no changes after rifampicin treatment compared to control cells. On the other hand, PXR transfection could reverse the downregulation of MDR1 mRNA after budesonide treatment. Rifampicin, the positive control for PXR activation, induced MDR1 mRNA in PXR-transfected Caco-2 cells (Figure 7a).
Figure 7.
Expression of MDR1 (a) and CYP3A4 (b) in Caco-2 cells transfected with the empty vector (−pPXR) and PXR vector (+pPXR) after treatment with budesonide, rifampicin or vehicle only. In two independent experiments, cells were transfected during 48 h, followed by 48 h drug treatment. mRNA expression was determined by quantitative real-time PCR. Results are normalized to the respective control cells and expressed as mean±s.e.m. (n=5–6). *P<0.05, **P<0.01, ***P<0.001 vs control cells.
CYP3A4, another target gene of PXR, is only slightly expressed in Caco-2 cells. Nevertheless, in parallel to MDR1, we could see a significant induction of CYP3A4 in PXR-transfected Caco-2 cells after treatment with budesonide and rifampicin (Figure 7b).
Discussion
At present, there are no data on the intestinal regulation of MDR1 by budesonide. Given that this glucocorticoid is often used in the treatment of IBD, we investigated its effects on MDR1 expression in two different, frequently used intestinal cell lines. Our results indicate that the regulation of this efflux transporter by budesonide is complex. MDR1 expression was induced in LS180 cells; in contrast, it was downregulated in Caco-2 cells.
An altered intestinal expression of P-gp can have important clinical implications. On the one hand, an induction of MDR1 can lead to an increased efflux of P-gp substrates out of the enterocytes (Westphal et al., 2000). Ineffective therapy or even therapy resistance could be the outcome. On the other hand, a decreased expression can impair the barrier function of the intestinal epithelial cells. In this respect, it was shown that mice deficient for MDR1a developed an inflammation of the large intestine similar to IBD (Panwala et al., 1998). The inflammation was dependent on the presence of intestinal bacteria, suggesting a function of P-gp to protect the body from toxins produced by intestinal bacteria. This hypothesis is in concordance with data from patients with ulcerative colitis, where the expression of MDR1 and PXR was significantly reduced in mucosal biopsy specimens from non-affected regions of the colon and terminal ileum (Langmann et al., 2004). Here, we have shown that, in Caco-2 cells, MDR1 expression was repressed by budesonide in a dose-dependent manner. An enhanced mRNA decay is not responsible for the observed MDR1 downregulation (as shown in the experiments with actinomycin D), although increased MDR1 mRNA decay with dexamethasone has been reported in primary rat hepatocytes (Schuetz et al., 1995).
In contrast to LS180 cells, the GR was highly expressed in Caco-2 cells. This led us to assume that the GR could mediate the observed downregulation of MDR1 in this cell line. The downregulation by budesonide occurred, however, in the range of micromolar concentrations only, despite the fact that the GR receptor has a high affinity for glucocorticoids. Furthermore, the addition of mifepristone (RU486), an established antagonist at the GR, did not abolish this effect. In fact, mifepristone repressed MDR1 expression in the same way as budesonide. Therefore, the involvement of the GR can most likely be ruled out, but the mechanism behind the repression of MDR1 in Caco-2 cells remains still unclear. However, a not yet identified nonspecific effect appears to be most likely.
In contrast, when we used LS180 cells as an intestinal model, incubations with budesonide showed an increase in MDR1 expression. We assumed that budesonide might be able to activate PXR, leading to an induction of MDR1 and other PXR target genes.
An nonspecific induction of MDR1 through toxic drug effects could be ruled out by performing cytotoxicity assays before the incubation procedures. In our experiments, the induction occurred with budesonide concentrations starting from 10 μM. This is in concordance with the fact that PXR is a receptor with only low affinity for glucocorticoids, as the affinity of corticosterone for PXR was reported to be in the range of 10–30 μM (Sheppard, 2002). The applied budesonide concentrations up to 25 μM are high, but they could represent relevant local concentrations in the gut lumen after oral administration or when the drug is applied rectally as an enema. The induction of MDR1 in the intestine through glucocorticoids has been shown previously for dexamethasone in the rat (Lin et al., 1999; Yumoto et al., 2001; Perloff et al., 2004). The mechanism of this upregulation by glucocorticoids seems to involve PXR. Pascussi et al. (2001) demonstrated that dexamethasone activates the nuclear receptor PXR. Furthermore, it has been shown that PXR mediates MDR1 induction (Geick et al., 2001).
From our experiments, there are several lines of evidence to support the suggestion that budesonide induced MDR1 expression through activation of PXR. Firstly, PXR expression was high in LS180 cells, where we observed an induction of MDR1. Secondly, rifampicin, a known activator of PXR (Geick et al., 2001), induced MDR1 in parallel with budesonide in LS180 cells. Finally, the PXR target genes MDR1 and CYP3A4 were induced in PXR-transfected Caco-2 cells compared to cells transfected with the empty vector.
In PXR-transfected Caco-2 cells, the induction of MDR1 apparently resulted in a reversal of the previously observed downregulation. For CYP3A4, on the other hand, a clear induction could be seen. Surprisingly, this induction of CYP3A4 was even more prominent with budesonide compared to rifampicin.
PXR is known to be expressed in the human intestine. Therefore, budesonide treatment might lead to increased intestinal levels of P-gp and CYP3A4. Thus, potential drug–drug interactions have to be taken into account, when substrates of P-gp or CYP3A4 are co-administered. On the other hand, the intestinal expression level of PXR itself is a factor that can vary in diseases, such as ulcerative colitis, where PXR has been shown to be downregulated (Langmann et al., 2004). Consequently, results from in vivo studies in healthy subjects cannot directly be applied to the situation in patients with IBD.
In conclusion, we have shown in this study that budesonide has the potential to influence the expression of intestinal MDR1 in vitro. The investigated intestinal cell lines showed opposing regulatory effects of this transporter. Further studies have to be carried out to evaluate the impact of glucocorticoid treatment on intestinal P-gp expression in vivo.
Acknowledgments
We thank Ursula Behrens for excellent technical assistance.
Abbreviations
- AP-1
activator protein-1
- CYP3A4
cytochrome P450 3A4
- DMSO
dimethylsulphoxide
- GR
glucocorticoid receptor
- MDR
multidrug resistance
- NF-κB
nuclear factor-κB
- P-gp
P-glycoprotein
- PXR
pregnane X receptor
- R123
rhodamine 123
Conflict of interest
The authors state no conflict of interest.
References
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat UG, Winter MA, Pearce HL, Beck WT. A structure-function relationship among reserpine and yohimbine analogues in their ability to increase expression of mdr1 and P-glycoprotein in a human colon carcinoma cell line. Mol Pharmacol. 1995;48:682–689. [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Collett A, Tanianis-Hughes J, Warhurst G. Rapid induction of P-glycoprotein expression by high permeability compounds in colonic cells in vitro: a possible source of transporter mediated drug interactions. Biochem Pharmacol. 2004;68:783–790. doi: 10.1016/j.bcp.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Dilger K, Schwab M, Fromm MF. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P-glycoprotein. Inflamm Bowel Dis. 2004;10:578–583. doi: 10.1097/00054725-200409000-00012. [DOI] [PubMed] [Google Scholar]
- Fromm MF. Importance of P-glycoprotein for drug disposition in humans. Eur J Clin Invest. 2003;33 Suppl 2:6–9. doi: 10.1046/j.1365-2362.33.s2.4.x. [DOI] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- Hofer KN. Oral budesonide in the management of Crohn's disease. Ann Pharmacother. 2003;37:1457–1464. doi: 10.1345/aph.1D059. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kane SV, Schoenfeld P, Sandborn WJ, Tremaine W, Hofer T, Feagan BG. The effectiveness of budesonide therapy for Crohn's disease. Aliment Pharmacol Ther. 2002;16:1509–1517. doi: 10.1046/j.1365-2036.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Klotz U, Schwab M. Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57:267–279. doi: 10.1016/j.addr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Labialle S, Gayet L, Marthinet E, Rigal D, Baggetto LG. Transcriptional regulators of the human multidrug resistance 1 gene: recent views. Biochem Pharmacol. 2002;64:943–948. doi: 10.1016/s0006-2952(02)01156-5. [DOI] [PubMed] [Google Scholar]
- Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Lin JH, Chiba M, Chen IW, Nishime JA, deLuna FA, Yamazaki M, et al. Effect of dexamethasone on the intestinal first-pass metabolism of indinavir in rats: evidence of cytochrome P-450 A and p-glycoprotein induction. Drug Metab Dispos. 1999;27:1187–1193. [PubMed] [Google Scholar]
- Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem. 2001;268:6346–6358. doi: 10.1046/j.0014-2956.2001.02540.x. [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42:10978–10990. doi: 10.1021/bi034651u. [DOI] [PubMed] [Google Scholar]
- Perloff MD, Von Moltke LL, Greenblatt DJ. Ritonavir and dexamethasone induce expression of CYP3A and P-glycoprotein in rats. Xenobiotica. 2004;34:133–150. doi: 10.1080/00498250310001630215. [DOI] [PubMed] [Google Scholar]
- Pfrunder A, Gutmann H, Beglinger C, Drewe J. Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1-MRP5) and hPXR in three different human colon carcinoma cell lines. J Pharm Pharmacol. 2003;55:59–66. doi: 10.1111/j.2042-7158.2003.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Silverman JA, Thottassery JV, Furuya KN, Schuetz EG. Divergent regulation of the class II P-glycoprotein gene in primary cultures of hepatocytes versus H35 hepatoma by glucocorticoids. Cell Growth Differ. 1995;6:1321–1332. [PubMed] [Google Scholar]
- Schwab M, Klotz U. Pharmacokinetic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2001;40:723–751. doi: 10.2165/00003088-200140100-00003. [DOI] [PubMed] [Google Scholar]
- Sheppard KE. Nuclear receptors. II. Intestinal corticosteroid receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282:G742–G746. doi: 10.1152/ajpgi.00531.2001. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Tornblom H, Lindberg G, Einarsson C, Sjoqvist F, Melhus H, et al. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 2001;299:164–170. [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, et al. Transcriptional control of intestinal cytochrome P-4503A by 1á,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, et al. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248–24252. [PubMed] [Google Scholar]
- Westphal K, Weinbrenner A, Zschiesche M, Franke G, Knoke M, Oertel R, et al. Induction of P-glycoprotein by rifampin increases intestinal secretion of talinolol in human beings: a new type of drug/drug interaction. Clin Pharmacol Ther. 2000;68:345–355. doi: 10.1067/mcp.2000.109797. [DOI] [PubMed] [Google Scholar]
- Wright AP, Zilliacus J, McEwan IJ, Dahlman-Wright K, Almlof T, Carlstedt-Duke J, et al. Structure and function of the glucocorticoid receptor. J Steroid Biochem Mol Biol. 1993;47:11–19. doi: 10.1016/0960-0760(93)90052-x. [DOI] [PubMed] [Google Scholar]
- Yumoto R, Murakami T, Sanemasa M, Nasu R, Nagai J, Takano M. Pharmacokinetic interaction of cytochrome P450 3A-related compounds with rhodamine 123, a P-glycoprotein substrate, in rats pretreated with dexamethasone. Drug Metab Dispos. 2001;29:145–151. [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Sadatrafiei A, Grun F, Blumberg B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab Dispos. 2004;32:1075–1082. doi: 10.1124/dmd.104.000299. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Gutmann H, Hruz P, Gutzwiller JP, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos. 2005;33:219–224. doi: 10.1124/dmd.104.001354. [DOI] [PubMed] [Google Scholar]