Abstract
Background and Purpose:
Recent reports suggest that n-3 (omega-3) polyunsaturated fatty acids (PUFAs) may reduce atrial fibrillation (AF). Reduction of the atrial effective refractory period (ERP) is believed to be an important early remodeling event that favors the development and perpetuation of AF. We hypothesized that n-3 PUFAs would attenuate early atrial electrophysiolgical remodeling in a canine model of acute atrial tachypacing.
Experimental Approach:
Adult dogs of either sex received n-3 PUFAs (n=6), n-6 PUFAs (n=6), or saline (n=6) infused over 1 h. After a stable ERP was established, treatment was initiated concurrently with 6 h of rapid atrial pacing (400 b.p.m.). Serial right atrial ERPs were measured during rapid atrial pacing, and induction of atrial tachyarrhythmias was attempted at the conclusion of each study.
Key results:
There was no change in P wave duration or in the PQ, QRS, QT or QTc intervals in any of the treatment groups. N-3 PUFA treatment significantly reduced the shortening of atrial ERP, compared to both control groups (P<0.05). In separate experiments, the same n-3 PUFA infusion was given to dogs remaining in normal sinus rhythm. During sinus rhythm, n-3 PUFA infusion did not alter any electrocardiogram (ECG) parameter or the atrial ERP.
Conclusions and Implications:
We conclude that acute n-3 PUFA treatment prevents acute atrial electrophysiological remodeling during high rate activity, which may minimize the self-perpetuation of AF.
Keywords: antiarrhythmic agents, atrial function, ECG, atrial fibrillation, n-3 polyunsaturated fatty acids, n-6 polyunsaturated fatty acids
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. In a seminal clinical study, a loss of normal rate-related adaptation of the atrial effective refractory period (ERP) was reported in patients with increased vulnerability to AF (Attuel et al., 1982). This clinical observation was confirmed by experimental models of AF or rapid pacing, which are characterized by shortening and a loss of rate-adaptation of the atrial ERP (Morillo et al., 1995; Wijffels et al., 1995). This early reduction in ERP is believed to be an initiating factor in the self-perpetuation of AF, whereby ‘AF begets AF' (Wijffels et al., 1995). Recent evidence suggests that oxidative stress contributes to the pathology of AF (Carnes et al., 2001; Mihm et al., 2001).
The two essential classes of polyunsaturated fatty acids (PUFAs), n-3 (omega-3) and n-6 (omega-6), are obtained through dietary intake (Leaf et al., 2003). Fish are the primary source of n-3 PUFAs, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), whereas n-6 PUFAs are primarily obtained from vegetable oil sources (parent compound, linoleic acid). Clinical observations and intervention studies have reported variable efficacy of n-3 PUFAs in reducing the frequency of life-threatening ventricular tachyarrhythmias (Marchioli et al., 2002; Albert et al., 2005; Christensen et al., 2005; Leaf et al., 2005; Hooper et al., 2006). Acute intravenous (i.v.) administration of n-3 PUFAs can prevent ventricular arrhythmias in a canine model of high-risk post-myocardial infarction (Billman et al., 1994, 1999).
PUFA administration has been shown to reduce the incidence of AF after coronary bypass grafting, where both systemic inflammation and oxidative stress have been implicated as contributing to post-operative AF (Calo et al., 2005). In addition, recurrences of paroxysmal AF and incident AF are also reduced by n-3 PUFAs (Biscione et al., 2004; Mozaffarian et al., 2004), although this inverse relationship between n-3 PUFA intake and AF has not been consistently observed (Frost and Vestergaard, 2005; Brouwer et al., 2006).
We hypothesized that n-3 PUFA treatment would attenuate acute atrial electrophysiological remodeling. We used a canine model of atrial tachypacing to evaluate the acute effects of n-3 PUFA treatment on atrial electrophysiology during high rate electrical activity.
Methods
Test systems used
All animal procedures were approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Adult mongrel dogs (n=18, 20–27 kg) of either sex were anaesthetized and anaesthesia maintained with isoflurane, which was kept at the minimum alveolar concentration (1–2.5%) to eliminate movement throughout the study. The dogs were kept on a heating pad in order to maintain stable body temperature throughout the study. The femoral artery was cannulated to measure systemic arterial blood pressure (Model SPC-350; Millar instruments, Inc., Houston, TX, USA) to assess the stability of the preparation.
Experimental design
Right atrial tachypacing and ERP determinations were performed as described previously (Carnes et al., 2001). Dogs were randomly assigned to one of three i.v. treatment groups (n=6 per group): (1) n-3 PUFAs (EPA 1.25–2.82 g 100 ml−1, DHA 1.44–3.09 g 100 ml−1): 20 ml over 5 min followed by 80 mls over 60 min, (2) matched volumes of a 10% n-6 PUFA control, or (3) matched volumes of normal saline.
In a separate set of experiments, dogs (n=5) were instrumented as described above, but remained in normal sinus rhythm. The effects of n-3 PUFA treatment on atrial electrophysiology during normal sinus rhythm were evaluated by serial measurements of the atrial ERP. The electrocardiogram (ECG) was also measured serially during these experiments, at the same times used in the dogs subjected to atrial tachypacing.
Measurements
Atrial tachypacing (400 b.p.m.) was initiated after a stable baseline ERP was obtained, and pacing was terminated briefly (<8 min) for serial ERP measurements and electrocardiograms. Electrocardiographic intervals (Biopac Systems, Inc., Goleta, CA, USA) were measured from three consecutive beats at baseline and serially. All ECG measurements were made by a single observer with appropriate internal controls. After the 6 h of ERP and ECG measurements, three attempts were made to induce atrial tachyarrhythmias (burst pacing at 10 Hz for 10 s) at 5 min intervals at the end of each study.
Data analysis and statistical procedures
The effects of n-3 PUFA treatment during normal sinus rhythm were evaluated using repeated-measures analysis of variance. When appropriate, post hoc testing was performed using Tukey's test. For the atrial tachypacing experiments, two-way analysis of variance with repeated measures was used to evaluate time and treatment effects, after determining the absence of statistical interaction between time and treatment. When appropriate, post hoc testing was performed using the Student-Newman-Keuls test (SAS for Windows, v.8.2, SAS Institute, Cary, NC, USA). A P<0.05 was the criterion for statistical significance. All data are presented as mean±s.e.
Drugs, chemicals, reagents, and other materials
n-3 PUFAs (EPA 1.25–2.82 g 100 ml−1, DHA 1.44–3.09 g 100 ml−1; Omegaven) were provided by Fresenius-Kabi (Homberg, Germany). The 10% n-6 PUFA control (Intralipid) and the matched volumes of normal saline were manufactured by Baxter Healthcare Corp. (Round Lake, IL, USA).
Results
Effects of n-3 PUFA treatment in normal sinus rhythm
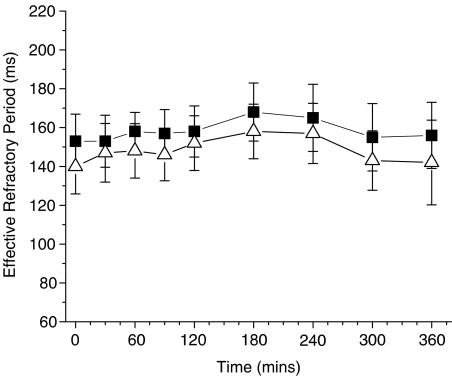
Treatment with n-3 PUFAs, in the absence of atrial tachypacing, did not statistically alter the atrial ERP at any of the cycle lengths. Data are shown (Figure 1) at cycle lengths of 300 and 200 ms, and the results did not differ at the other cycle length (250 ms) tested (data not shown). Similarly, there were no effects of n-3 PUFA infusion on the RR interval, P wave duration, PQ interval, the QRS interval, or the QT or QTc interval during 6 h of observation.
Figure 1.
Atrial ERP period is not altered by n-3 PUFA infusion during normal sinus rhythm. Data presented are for cycle lengths of 300 (closed squares) and 200 (open triangle) ms. No statistically significant time-dependent changes were observed.
Effects of n-3 PUFA treatment during rapid atrial stimulation
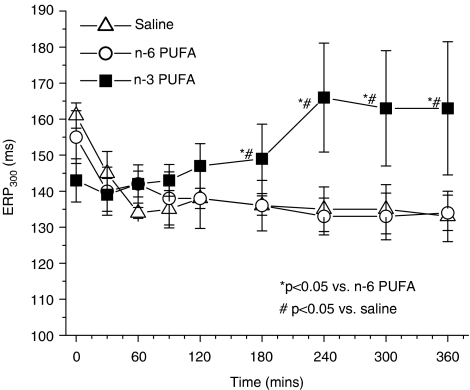
There were no differences between groups in the baseline ERPs at any cycle length. The combined baseline refractory periods were 161±4, 154±4 and 145±4 ms at cycle lengths of 300, 250 and 200 ms, respectively.
After initiation of rapid atrial pacing, the ERP was significantly longer at all cycle lengths in the n-3 PUFA-treated group, compared to controls beginning at 3 h of pacing. This effect persisted throughout the observation period in the n-3 PUFA-treated group. Data at a cycle length of 300 ms is shown in Figure 2. In the control group, and the omega-6 control group there was the expected shortening of ERP. There was no difference between these two control groups, consistent with the absence of efficacy of omega-6 PUFAs. At cycle lengths of 250 and 200 ms, we made observations similar to those at 300 ms (data not shown), where pacing-induced reductions in the ERP were attenuated between 3 h of pacing and the conclusion of the experiments.
Figure 2.
n-3 PUFA treatment prevents shortening of the atrial ERP during rapid atrial stimulation. Data presented are at a basic cycle length of 300 ms.
There were no time-dependent changes in any ECG parameter (P wave duration, PQ, QRS, RR or QTc intervals) in any of the treatment groups (data not shown). AF or other tachyarrhythmias were not induced at the conclusion of any experiment. We did observe brief episodes of AF in two dogs in the saline control group during ERP determinations, but not in response to burst pacing.
Discussion and conclusions
AF is a progressive arrhythmia and is associated with pathological electrophysiological remodeling of the atria (Attuel et al., 1982; Wijffels et al., 1995). High-rate activity of the atria (lone AF models) results in a significant reduction in the duration of the atrial ERP, which is associated with action potential duration (Attuel et al., 1982). Shortening the atrial ERP creates a positive feedback loop by which ‘AF begets AF,' and ERP shortening is considered to be the ‘first factor' in the perpetuation of AF (Wijffels et al., 1995; Allessie et al., 2002). The shortened atrial refractory periods result in a reduction in wavelength and an increased stability of the arrhythmia (Morillo et al., 1995; Gaspo et al., 1997; Allessie et al., 2002).
We found that n-3 PUFA administration prevented acute atrial electrophysiological remodeling during high-rate activity in a canine model. In vitro studies have shown that acute exposure to DHA and EPA reduces sarcolemmal sodium, calcium and potassium (transient outward and delayed rectifier) currents (Xiao et al., 1995, 1997, 1998, 2002). However, we did not observe any time-dependent changes in the ECG in either the non-paced or atrial tachypaced groups receiving n-3 PUFA infusions. Furthermore, in the present study, the lack of prolongation of the atrial ERP, in the absence of atrial tachypacing, is also consistent with a lack of a direct effect of n-3 PUFAs on atrial electrophysiology.
In a chronic feeding study in pigs, Verkerk and colleagues found that after 9 weeks of n-3 PUFA supplementation, there was a significant enrichment of n-3 PUFA content in the ventricular sarcolemma accompanied by a shortened ventricular action potential duration (Verkerk et al., 2006). This was accompanied by significant reductions in both sarcolemmal calcium current and the sodium–calcium exchanger. In contrast, the slow component of the delayed rectifier K+ current and the inward rectifier K+ current were increased, whereas sodium current and the rapid component of the delayed rectifier K+ current were unchanged. It is apparent that the acute application of DHA and/or EPA does not necessarily result in the same electrophysiological effects observed after chronic feeding. One potential explanation is that incorporation of sufficient quantities into the sarcolemmal membrane is necessary to exhibit a ‘chronic' response to n-3 PUFA treatment.
Alternatively, it is possible that an alternative mechanism (e.g. anti-inflammatory or antioxidant) (Mori et al., 2000; Mori and Beilin, 2004; Korantzopoulos et al., 2005) contributes to the variable time-dependent electrophysiological effects observed with n-3 PUFA treatment or supplementation. Further investigation is required to delineate the mechanism of the causes of the differential responses between acute and chronic treatment with n-3 PUFAs.
Atrial electrophysiological remodeling is associated with the development of oxidative stress (Carnes et al., 2001; Mihm et al., 2001; Dudley Jr et al., 2005). Anti-inflammatory and antioxidant therapies have shown efficacy in reducing atrial electrophysiological remodeling (Carnes et al., 2001; Shiroshita-Takeshita et al., 2006). As we did not observe any significant alterations in the refractory period with n-3 PUFA treatment during normal sinus rhythm, we suggest that effects through one of these alternative pathways may have contributed to the attenuation in atrial electrophysiological remodeling observed in our study.
An n-6 PUFA treatment containing soybean and safflower oils as the fat source was used to control nonspecific membrane effects. The n-6 PUFA treatment did not prevent atrial electrophysiological remodeling, or affect the ERP relative to the saline control group. Interestingly, in a clinical observational study, fatty, but not fried fish, reduced the risk of the incidence of AF (Mozaffarian et al., 2004). Frying is known to increase the n-6 PUFA content, an expected result, as vegetable oils are a significant source of linoleic acid (Candela et al., 1998). It appears that fried fish consumption does not provide the same cardiovascular outcomes as consumption of fish with a high content of n-3 PUFA.
To date, three observational studies have examined the relationship between dietary fish intake and AF. In one report, dietary intake of fatty fish was found to reduce the risk of AF and the frequency of paroxysmal AF (Mozaffarian et al., 2004). However, an analysis from the Danish Diet, Cancer, and Health Study did not find any association between dietary n-3 fatty acid intake and a reduced risk of AF (Frost and Vestergaard, 2005). A recent report from The Rotterdam Study also describes a lack of association between dietary n-3 fatty acid intake from fish sources and AF (Brouwer et al., 2006). These conflicting results may be due to the use of dietary intake questionnaires to estimate dietary n-3 fatty acid content, the differences in ages of the study populations, or variability in the detection of AF, an arrhythmia which is often asymptomatic (Miyasaka et al., 2006). Mechanistic support for the concept that n-3 PUFAs provide protection from atrial arrhythmias comes from a recent investigation in a rabbit model where stretch-induced atrial arrhythmias were reduced by chronic feeding of a fish-oil supplement (Ninio et al., 2005). Additional studies are required to address the clinical efficacy of fish oil on AF further.
Limitations
The dose used for this study has previously shown efficacy in reducing ischaemia-induced ventricular fibrillation when used in a canine model of post-myocardial infarction (in dogs of similar size to those used in the present study). We did not evaluate the efficacy of other doses in this model.
We did not observe any inducible AF at the conclusion of any study. This is not unexpected after this duration of rapid atrial pacing, based on previous findings (Morillo et al., 1995; Wijffels et al., 1995). Although we did not expect the occurrence of AF, testing arrhythmia inducibility did allow evaluation of any adverse proarrhythmic effect.
It has been demonstrated that chronic n-3 PUFA intake may have a parasympathomimetic effect (Christensen et al., 1999; Christensen and Schmidt, 2001), but we did not observe any significant changes in heart rate in either of the n-3 PUFA-treated groups. Increased vagal tone is associated with an increased risk of AF, but we did not observe any increase in inducibility of AF in the n-3 PUFA-treated group, suggesting a lack of increased vagal tone during our experimental conditions. However, these experiments do not rule out changes in other markers of autonomic tone (e.g. heart rate variability), or a long-term effect of n-3 PUFA treatment on autonomic regulation.
Conclusions
In summary, we provide direct evidence that acute administration of n-3 PUFAs can minimize the early electrophysiological changes that are believed to initiate the self-perpetuation of AF. As our observation period was relatively short, the duration of this beneficial effect is undetermined. Further experimentation will be required to define the efficacy of this treatment approach in patients.
Acknowledgments
This study was supported by NIH Grant HL68609. Pacemakers and leads were provided by Medtronic, Inc.
Abbreviations
- AF
atrial fibrillation
- ERP
effective refractory period
- PUFAs
polyunsaturated fatty acids
Conflict of interest
The authors state no conflict of interest.
References
- Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–3238. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- Attuel P, Childers R, Cauchemez B, Poveda J, Mugica J, Coumel P. Failure in the rate adaptation of the atrial refractory period: its relationship to vulnerability. Int J Cardiol. 1982;2:179–197. doi: 10.1016/0167-5273(82)90032-8. [DOI] [PubMed] [Google Scholar]
- Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- Biscione F, Totteri A, De Vita A, Lo Bianco F, Altamura G. Effect of omega-3 fatty acids on recurrences of paroxismal atrial arrhythmias (abstract) Heart Rhythm. 2004;2:S191. [Google Scholar]
- Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006;151:857–862. doi: 10.1016/j.ahj.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- Candela M, Astiasaran I, Bello J. Deep-fat frying modifies high-fat fish lipid fraction. J Agric Food Chem. 1998;46:2793–2796. [Google Scholar]
- Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Schmidt EB. n-3 fatty acids and the risk of sudden cardiac death. Lipids. 2001;36 Suppl:S115–S118. doi: 10.1007/s11745-001-0693-9. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999;70:331–337. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Riahi S, Schmidt EB, Molgaard H, Kirstein PA, Heath F, et al. n-3 Fatty acids and ventricular arrhythmias in patients with ischaemic heart disease and implantable cardioverter defibrillators. Europace. 2005;7:338–344. doi: 10.1016/j.eupc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:50–54. doi: 10.1093/ajcn/81.1.50. [DOI] [PubMed] [Google Scholar]
- Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–4035. doi: 10.1161/01.cir.96.11.4027. [DOI] [PubMed] [Google Scholar]
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korantzopoulos P, Kolettis TM, Goudevenos JA. The anti-inflammatory and antioxidant effects of long-chain n-3 fatty acids or oil-rich fish may favorably affect atrial remodeling in atrial fibrillation. Med Hypotheses. 2005;64:1245–1246. doi: 10.1016/j.mehy.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- Mori TA, Puddey IB, Burke V, Croft KD, Dunstan DW, Rivera JH, et al. Effect of omega 3 fatty acids on oxidative stress in humans: GC–MS measurement of urinary F2-isoprostane excretion. Redox Rep. 2000;5:45–46. doi: 10.1179/rer.2000.5.1.45. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio DM, Murphy KJ, Howe PR, Saint DA. Dietary fish oil protects against stretch-induced vulnerability to atrial fibrillation in a rabbit model. J Cardiovasc Electrophysiol. 2005;16:1189–1194. doi: 10.1111/j.1540-8167.2005.50007.x. [DOI] [PubMed] [Google Scholar]
- Shiroshita-Takeshita A, Brundel BJ, Lavoie J, Nattel S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc Res. 2006;69:865–875. doi: 10.1016/j.cardiores.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Verkerk AO, Van Ginneken AC, Berecki G, Den Ruijter HM, Schumacher CA, Veldkamp MW, et al. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res. 2006;70:509–520. doi: 10.1016/j.cardiores.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci USA. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Kang JX, Morgan JP, Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci USA. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Morgan JP, Leaf A. Effects of polyunsaturated fatty acids on cardiac voltage-activated K(+) currents in adult ferret cardiomyocytes. Sheng Li Xue Bao. 2002;54:271–281. [PubMed] [Google Scholar]
- Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proc Natl Acad Sci USA. 1998;95:2680–2685. doi: 10.1073/pnas.95.5.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]