Abstract
Background and purpose:
The aim was to characterize the recently discovered non-peptide antagonist MEN16132 at the mouse B2 receptor, relative to other antagonists.
Experimental approach:
[3H]-BK binding experiments used mouse lung and ileum tissue membranes and antagonist potency was measured in the isolated ileum contractility assay.
Key results:
Two BK binding sites resulted from saturation and homologous competition experiments. A role for the B1 receptor was excluded because of the poor affinity of B1 receptor ligands (pIC50 <5). MEN16132, and the other reference antagonists, inhibited only one portion of BK specific binding, and the rank order of potency was (pIC50): Icatibant (lung 10.7; ileum 10.2)=MEN11270 (lung 10.4; ileum 9.9)=MEN16132 (lung 10.5; ileum 9.9). > LF16-0687 (lung 8.9; ileum 8.8) > FR173657 (lung 8.6; ileum 8.2). BK homologous curves performed with lung membranes after treatment with the antagonist MEN16132 or Icatibant (10 nM) displayed only the low affinity site. The functional antagonism by MEN16132 (pA2 9.4) and Icatibant (pA2 9.1), towards BK (control EC50 6.1 nM) induced ileum contractions, was concentration-dependent and surmountable, but the Schild plot slope was less than unity.
Conclusions and Implications:
In mouse tissue, radiolabelled BK recognizes two binding sites and B2 receptor antagonists can compete only for the higher affinity one. The pharmacological profile of the novel non-peptide antagonist MEN16132 indicates that it exhibits subnanomolar affinity and potency for the mouse B2 receptor and is suitable for further characterization in in vivo pathophysiological models.
Keywords: airways, FR173657, ileum, Icatibant, lung, MEN11270, MEN16132, LF16-0687, peptide, non-peptide
Introduction
Kinins exert their biological effects through the activation of two pharmacologically distinct receptor subtypes, namely B1 and B2, belonging to the family of transmembrane G-protein-coupled receptors. Both receptors have been sequenced from different mammalian species and the mechanisms involved after agonist activation have been investigated (Leeb-Lundberg et al., 2005, for a recent review). Among kinins, bradykinin (BK: H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH) preferentially acts at the B2 receptor subtype, which is ubiquitously expressed, and involved in mediating the cell-specific inflammatory responses. The murine species has been largely used to elucidate the role of BK in different pathological models and to prove the efficacy of BK B2 receptor antagonists (carrageenan-induced pleurisy, Saleh et al., 1998; dextran sulfate-induced colitis, Arai et al., 1999; nociception Heapy et al., 1993, Corrêa et al. (1996); cerebral ischemia Ding-Zhou et al., 2003). Alternatively, the targeted disruption of the BK B2 receptor gene in mice has become a further tool to investigate the physiological role of this receptor (Borkowski et al., 1995; Alfie et al., 1996; Madeddu et al., 1999).
With respect to pharmacological characterization in vitro, few reports have described the potency of B2 receptor selective antagonists towards smooth muscle contractility induced by BK (Nsa Allogho et al., 1997; Zhang et al., 2004), and, to the best of our knowledge, no characterization has been presented with mouse tissue and radiolabelled BK after the cloning of the mouse receptor (McIntyre et al., 1993; Hess et al., 1994).
With the aim of evaluating the affinity of the novel non-peptide B2 receptor antagonist MEN16132 (Cucchi et al., 2005; Valenti et al., 2005) at the mouse B2 receptor we performed radioligand binding experiments, using tritiated BK and membranes prepared from lung and ileum tissues. MEN16132 pharmacology was compared to that of other B2 receptor antagonists, both of peptidic (Icatibant and MEN11270, Hock et al., 1991; Meini et al., 1999) and non-peptidic (FR173657 and LF16-0687, Asano et al., 1997; Pruneau et al., 1999) structure. The antagonist potency of Icatibant and MEN16132 towards the BK contractile responses produced in the isolated ileum smooth muscle is also described.
Methods
Animals
Adult male mice (ICR/CD-1 strain, 25–30 g) were obtained from Harlan Italy (Udine, Italy), and were maintained in a light (12 h dark/12 h light), temperature (22±1°C) and humidity (55±10%) controlled environment with free access to food and water. Animals were killed by cervical dislocation. Animal care and killing were approved by the local Ethics Committee.
Tissue membrane preparation
Each preparation was derived from 12–15 animals. Lung (including trachea and bronchi) and ileum tissues were placed in N-tris[hydroxymethyl]methyl-2-aminoethanesulphonic acid (TES, 10 mM, pH 7.4, at 4°C) and a cocktail of peptidase inhibitors added: 1,10 phenanthroline (1 mM), ethylene glycol bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (1 mM), captopril, leupeptin, soy bean trypsin inhibitor, DL-2-mercaptomethyl-3-guanidoethylthiopropanoic acid (1 μM each), chymostatin (3.3 μM), phenylmethyl-sulphonyl fluoride (0.1 mM) and bacitracin (140 μg ml−1). The tissues were minced and homogenized with a Polytron (PT 3000, Kinematica), set at 15 000 r.p.m., for 30 s in 10 ml g−1 of the above buffer. The homogenate was centrifuged at 2500 g for 10 min to remove cellular debris. The supernatant was homogenized as above and centrifuged at 45 000 g (4°C) for 30 min. The pellet was resuspended in binding buffer and frozen immediately in 2 ml aliquots by immersion in liquid nitrogen, and there stored until use. The protein concentration was determined by the method of Bradford (1976). Immediately before use, frozen membrane aliquots were thawed in binding buffer (see below) and mixed to give a homogeneous membrane suspension.
Radioligand binding experiments
The buffer used for all binding experiments was TES (10 mM, pH 7.4) containing 1,10-phenanthroline (1 mM), bacitracin (140 μg ml−1) and bovine serum albumin (1 g l−1). Binding assays were performed at room temperature in a final volume of 0.5 ml, and an incubation time of 60 min was selected on the basis of preliminary time course association experiments that indicated a steady state of [3H]-BK-specific binding (data not shown). Preliminary experiments were performed also to choose the membrane protein concentration: specific binding of [3H]-BK at 0.15 nM was directly proportional to membrane concentration (data not shown), and 300 or 450 μg ml−1 for lung or ileum tissue, respectively, were chosen to obtain a good signal-to-noise ratio. Nonspecific binding was defined as the amount of bound radioligand in the presence of 1 μM of cold BK. Competing ligands were tested in a wide range of concentrations (1 pM–1 μM). All incubations were terminated by rapid filtration through UniFilter-96 plates GF/B (Perkin Elmer, Shelton, CT, USA), pre-soaked for at least 2 h in polyethylenimine 0.6%, and using a MicroMate 96 Cell Harvester (Perkin Elmer). The tubes and filters were then washed five times with 0.5 ml aliquots of Tris buffer (50 mM, pH 7.4, 4°C). Filters were dried and soaked in Microscint 40 (50 μl in each well, Perkin Elmer), and bound radioactivity was counted by a TopCount Microplate Scintillation Counter (Packard Instrument Company). Each experiment was performed in duplicate and with a different membrane preparation.
Smooth muscle contractility assay
The abdomen was opened and a segment of ileum (8–10 cm proximal to the caecum) was removed, placed in oxygenated (95% O2 and 5% CO2) Krebs–Henseleit solution (KH, mM composition: NaCl 119; KCl 4.7; MgSO4 1.5; NaHCO3 25; KH2PO4 1.2, CaCl2 2.5, glucose 11), and the content was gently flushed out with KH solution.
From each segment three or four preparations (about 20 mm in length) were excised and suspended longitudinally in 5 ml organ baths at 37°C, containing oxygenated (95% O2 and 5% CO2) KH solution, containing atropine (1 μM), guanethidine (3 μM), chlorpheniramine (1 μM) and captopril (1 μM). The tissues were attached to isometric force transducers (Ugo Basile, Comerio VA, Italy) under an initial tension of 5 mN. Mechanical activity was digitally recorded by using a PowerLab/8sp hardware system and analyzed using the Chart 4.2 software (AD Instruments, Castle Hill, Australia). After 1 h equilibration period, KCl (80 mM) was administered to evaluate the maximal contractile response of each preparation. After the recovery of basal tone a cumulative concentration–response curve to BK (1 nM–10 μM) was constructed, each agonist concentration being added when the effect of the previous one had reached a steady state. At the end of each curve, KCl (80 mM) was again administered to check the contractility of the smooth muscle. Antagonists or vehicle (dimethylsulphoxide (DMSO)) were preincubated 60 min before the construction of the agonist cumulative concentration–response curve. Preliminary experiments indicated that no differences were observed with a shorter (15 min) period of antagonist incubation. Concentration–response curves to BK in control (vehicle-treated) and antagonist-treated preparations were performed in parallel on different specimens belonging to the same animal. The final concentration of DMSO in the bath solution was 0.1%, which per se did not affect the basal activity or the agonist-induced contraction of the smooth muscle preparation.
Analysis of data
Each value in the text is mean±s.e.m. or the mean and 95% confidence limits (c.l.) in parentheses, of the indicated number of experiments (n).
Binding data were fitted by the appropriate nonlinear regression using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA), in order to determine (i) the maximum binding site density (Bmax) and the equilibrium dissociation constant (Kd) from saturation experiments and (ii) the ligand concentration inhibiting the radioligand binding of the 50% (IC50) from heterologous competition experiments. In order to better visualize the two sites suggested from saturation data, the Scatchard plot was constructed by using dpm data.
Functional data (concentration–response curves) were fitted by sigmoidal nonlinear regression (GraphPad Prism 4.0) to determine the agonist concentration producing the 50% (EC50) of the maximal response (Emax).
The nature of the antagonist interaction with the receptor was checked by the Schild regression as follows: antagonist-induced parallel shifts of concentration–response curves to the agonist were calculated as the ratio (concentration-ratio, CR) of equieffective concentrations of agonist (EC50) obtained in the presence and in the absence of antagonist. Estimates of log[CR–1] were plotted against log[antagonist concentration]. The slopes of linear regression lines were calculated to ascertain the competitive antagonist behaviour. The antagonist potency was expressed in terms of pA2 calculated from the equation: pA2=log [CR–1]–log[antagonist concentration] (Kenakin, 1997) from each experiment.
Materials
[3H]-BK (specific activity 62–64 Ci mmol−1) was provided by Amersham Biosciences UK Ltd (GE Healthcare, Chalfont St Giles, UK). BK, [desArg9]-BK and [desArg9-Leu8]-BK were obtained from Neosystem (Strasbourg, France), and bestatin from Peninsula (Peninsula Laboratories Europe, Cheshire, UK). Thiorphan was from Bachem (Essex, UK), captopril from Sigma (Dorset, UK). All salts used were purchased from Merck (Darmstadt, Germany). All B2 receptor antagonists used in this work were synthesized in Menarini Ricerche (Chemistry Departments of Florence and Pomezia, Italy). MEN11270 (H-DArg-Arg-Pro-Hyp-Gly-Thi-c(Dab-DTic-Oic-Arg)c(7γ-10α)) and Icatibant (H-DArg-Arg-Pro-Hyp-Gly-Thi-Ser-DTic-Oic-Arg-OH) were dissolved in H2O, whereas MEN16132 (4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulphonamido]-tetrahydro-2H-4-pyranylcarbonyl}piperazino)-5-oxopentyl](trimethyl)ammonium chloride hydrochloride), FR173657 ((E)-3-(6-acetamido-3-pyridyl)-N-[N-[2,4-dichloro-3-[(2-methyl-8-quinolinyl) oxymethyl] phenyl]-N-methylaminocarbonylmethyl]acrylamide), and LF16-0687 (1-[[2,4-dichloro-3-[(2,4-dimethylquinolin-8-yl)oxy]methyl]-phenyl]sulphonyl]-N-[3-[[4-(aminoiminomethyl)-phenyl]carbonylamino]propyl]-2(S)-pyrrolidinecarboxamide) were dissolved in DMSO at 1 mM concentration and stored at −25°C.
Results
BK binding affinity by means of saturation and homologous binding curves
[3H]-BK binding was characterized in mouse lung and ileum membranes by means of saturation studies. Binding saturation isotherms performed with lung tissue membranes (Figure 1a) fitted a two-site binding model, and the calculated affinity constant values were Kd1 44 pM and Kd2 7.3 nM (6.0–8.5, 95% c.l.). The higher affinity site was scarcely represented (3%) compared to the lower affinity site (Bmax2 814±21 fmol mg−1 proteins), as can be seen in the Scatchard plot (inset in Figure 1a) (n=4).
Figure 1.
Isotherms of [3H]-BK binding to mouse membranes prepared from lung (a) and ileum (b) tissues. Increasing concentration of [3H]-BK (0.01–17 nM, incubated with membranes for 60 min at room temperature) are plotted against the specific binding (fmol mg−1 of proteins) determined in the presence of unlabelled BK (1 μM). Insets show the Scatchard plots constructed by using dpm data. Kd and Bmax values were calculated from saturation binding curves and are described in the text. Data shown were pooled and analysed from four independent experiments, each one performed in triplicate on a different membrane preparation.
When the [3H]-BK saturation isotherms were carried out with the ileum tissue membranes (Figure 1b) again, the analysis indicated a two-site binding model as appropriate: [3H]-BK bound with higher affinity (Kd1 34 pM, 5–63, 95% c.l.) to 13% of receptors (Bmax1 15±2 fmol mg−1 proteins) and with nM affinity (Kd210.7 nM, 6.5–15, 95% c.l.) to the second site (Bmax2 115±10 fmol mg−1 proteins) (n=4).
Homologous displacements curves were carried out at the [3H]-BK binding site with lung membranes, and revealed a different binding behaviour depending on the radioligand concentration used. [3H]-BK was used at 0.05, 0.15 or 1 nM, and the specific binding was 18±2, 34±3 or 118±6 fmol mg−1 of membrane proteins (n=3). Data obtained at each radioligand concentration were expressed as a percentage of the appropriate specific binding and are shown in Figure 2. When the lower radioligand concentration (0.05 nM) was used, data were best fitted by a two-site competition model (R2 0.9776) and the resulting IC50 values were 65 pM (44–97, 95% c.l.) and 23 nM (12–42, 95% c.l.), the amount for the high-affinity site being 66±3% of the total specific binding. At the intermediate radioligand concentration (0.15 nM, this concentration was the same used for heterologous binding competition experiments described in the following section) data were again best fitted by a two-site competition model (R2 0.9653) indicating IC50 values of 42 pM (27–65, 95% c.l.) and 17 nM (9–31, 95% c.l.), the portion of the high-affinity site being 55±6% of the total specific bound at this radioligand concentration. On the contrary, when BK competition curves were performed at a higher radioligand concentration (1 nM) data were best fitted to a one-site binding model (R2 0.9755) and the IC50 was 10 nM (8–12, 95% c.l.).
Figure 2.
BK homologous inhibition curves were carried out in mouse lung membranes at three different [3H]-BK concentrations as shown. The log concentration of unlabelled BK (x-axis) is plotted against the percentage of radioligand specific binding (y-axis) determined in each condition (as detailed in the Results section). Data are expressed as mean±s.e.m. of three independent experiments, each one performed in duplicate.
A different pattern was obtained when the same protocol was applied to ileum membranes. The lower [3H]-BK concentration used with lung membranes (0.05 nM) was excluded because of the poor specific binding observed in the ileum preparation. At 0.15 and 1 nM radioligand concentration, the specific binding was 17±2 and 24±3 fmol mg−1 proteins, respectively, and homologous inhibition curves to BK were best fitted to the one-site binding model notwithstanding the radioligand concentration used (0.15–1 nM). The estimated IC50 values were 0.65 nM (0.51–0.81, 95% c.l.) and 1.6 nM (0.95–2.7, 95% c.l.) respectively (data not shown), and Hill slope values were not significantly different from unity.
Heterologous radioligand binding experiments
The affinity of the B2 receptor antagonists (MEN16132, Icatibant, MEN11270, LF16-0687 and FR173657) was evaluated by means of inhibition curves at the [3H]-BK (0.15 nM) binding site.
All antagonists inhibited the [3H]-BK binding in a concentration-dependent (1 pM–1 μM) manner when tested in lung membranes, but all of them only inhibited about 50% of the specifically bound radioligand. In Table 1, we show the pIC50 values deriving from competition analysis and the proportion of specific binding not inhibited at 1 μM of competing ligands. The inhibition curves of the peptide antagonist Icatibant and the novel non-peptide antagonist MEN16132 are shown in Figure 3a. Inhibition curves (1 pM–10 μM) with the selective B1 agonist and antagonist ligands, [desArg9]-BK and [desArg9,Leu8]-BK, respectively, were carried out under the same experimental conditions. Both B1 receptor ligands did not affect [3H]-BK binding up to 1 μM concentration, and at 10 μM the inhibition was of 40±5 and 36±4% for [desArg9]-BK and [desArg9,Leu8]-BK, respectively (n=3).
Table 1.
Affinity of a panel of B2 receptor antagonists in inhibiting tritiated BK bound to lung and ileum tissue membranes
|
[3H]-BK binding |
||||
|---|---|---|---|---|
|
Lung |
Ileum |
|||
| Antagonist | pIC50 (95% c.l.) | % of not inhibited specific binding | pIC50 (95% c.l.) | % of not inhibited specific binding |
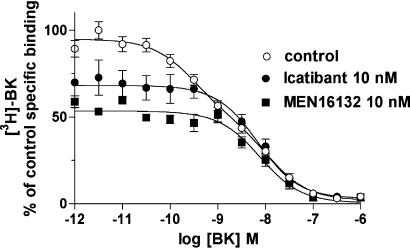
| Icatibant | 10.7 (10.4–10.9) | 55±2 | 10.2 (10.1–10.4) | 7±4 |
| MEN11270 | 10.4 (10.2–10.7) | 57±4 | 9.9 (9.8–10.1) | 11±4 |
| MEN16132 | 10.5 (10.3–10.8) | 53±3 | 9.9 (9.7–10.1) | 12±4 |
| LF16-0687 | 8.9 (8.7–9.1) | 50±5 | 8.8 (8.6–9.0) | 11±6 |
| FR173657 | 8.6 (8.4–8.8) | 52±2 | 8.2 (8.0–8.4) | 10±5 |
Abbreviations: BK, bradykinin; c.l., confidence limit.
Antagonists inhibition curves were performed with [3H]-BK (0.15 nM) binding in mouse lung and ileum membranes. Values of pIC50 and the percentage of radioligand specific binding which was not inhibited by ligands at 1 μM concentration are indicated. Data are from four independent experiments each one performed in duplicate. Inhibition curves obtained with Icatibant and MEN16132 are shown in Figure 3.
Figure 3.
Icatibant and MEN16132 inhibition curves at the [3H]-BK (0.15 nM) binding compared to BK in mouse lung (a) and ileum (b) membranes. Experimental conditions are described in the Methods section. The calculated IC50 values are reported in Table 1. Data points represent the mean±s.e.m. of four independent experiments, each one performed in duplicate.
Heterologous competition experiments were performed also with ileum membranes, and the resulting rank order of potency of the tested antagonists was similar to that measured in the lung membranes assay, although in the ileum membranes, a greater proportion of [3H]-BK specific binding was inhibited by the antagonists (Figure 3b, Table 1). However, no differences were observed among antagonists in their maximal inhibition of BK specific binding both in lungs and ileum, as observed at 1 μM concentration (Table 1).
BK homologous inhibition curves carried out with membranes pretreated with B2 receptor antagonists
In order to distinguish the [3H]-BK binding site displaced by antagonists in the lung receptor preparation, in a further set of experiments BK homologous binding curves were compared by using control membranes, and membranes pre-incubated (30 min, room temperature) with 10 nM concentration of antagonist before being added to the binding plate. Results obtained with Icatibant and MEN16132-treated membranes are shown in Figure 4. Data are normalized according to the specific binding measured in control conditions in each experimental session (n=3). As reported above (Figure 2) in control membranes, BK inhibition curves were best fitted to a two-site binding model (IC50 values 0.30 nM, 0.11–0.7, 95% c.l. and 11.5 nM, 4.5–29.6, 95% c.l.). On the contrary, BK homologous curves performed with membranes pretreated with Icatibant or MEN16132 were better fitted to the one-site binding model and the IC50 values were in the nM range: 7 (3.8–12.4, 95% c.l.) and 8.7 nM (5.8–12.9, 95% c.l.), respectively (Figure 4).
Figure 4.
Homologous inhibition curves of BK were performed with control or antagonists (10 nM, 30 min at room temperature) treated mouse lung membranes. [3H]-BK (0.15 nM) and membranes were then incubated for 60 min at room temperature. Data are expressed as percentage of specific binding obtained with control membranes, and represent the mean±s.e.m. of three independent experiments, each one performed in duplicate.
BK-induced mouse ileum contraction and antagonism by Icatibant and MEN16132
BK (0.1 nM–1 μM)-induced concentration-dependent contractions of the mouse ileum smooth muscle: the Emax was 5.1±0.4 mN, corresponding to 62±3% of tissue contraction to KCl (80 mM). The EC50 value calculated from control (vehicle treated) concentration–response curves to BK was 6.1 nM (4.4–8.3, 95% c.l.) (n=20). The functional selectivity of BK was validated by using B1 receptor antagonist [desArg9Leu8]-BK, which at 10 μM did not affect the BK concentration–response curve (EC50 4 nM, 1–14, 95% c.l., n=4). MEN16132 and Icatibant antagonist potency was evaluated against the contractile effect of BK. Both B2 receptor antagonists were devoid of any effect on the resting tension of ileum preparation at the concentrations used, and did not alter the maximal contraction induced by KCl.
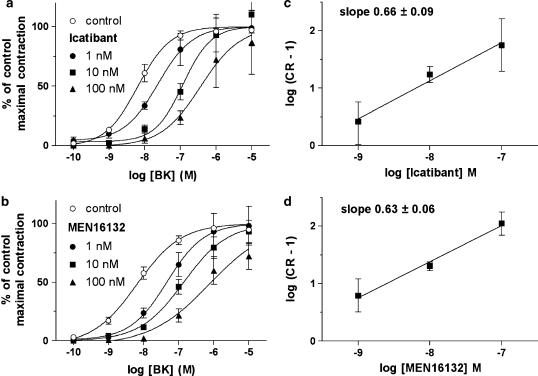
Icatibant and MEN16132 shifted the BK concentration–response curve to the right and their effect was concentration-dependent (1–10–100 nM, Figure 5a and b). Data were analyzed by means of the Schild analysis and the slope values of linear regression were significantly lower than unity (Figure 5c and d). The antagonists potency calculated as pA2 value from the concentration-ratio of single experiments, was 9.1 (8.8–9.4, 95% c.l.) and 9.4 (9.2–9.6, 95% c.l.) for Icatibant and MEN16132, respectively.
Figure 5.
Antagonism of Icatibant and MEN16132 towards the contractile responses induced by BK in the isolated mouse ileum smooth muscle. (a and b) Concentration–response curves to BK were obtained as described in Methods in vehicle (control) and antagonist (pre-incubated 60 min before agonist) treated preparations. Data are expressed as percentage of control maximal responses and represent the mean±s.e.m. of six experiments. (c and d) Schild plots were constructed as described in Methods and the slope values of linear regression are indicated.
Discussion
The present study describes the affinity and potency of the novel B2 receptor non-peptide antagonist MEN16132 in mouse preparations, and compares its pharmacological outline with that of other antagonists. Owing to the lack of reports providing radioligand binding characterization in mouse tissue, we first analyzed the molecular properties of radiolabelled BK. Our data indicate that BK binds to membranes prepared from mouse lung and ileum tissue with pM and nM affinity values, here referred to high and low-affinity binding sites, respectively. Although the two BK affinity values are in the same order of magnitude as those previously observed at the recombinant receptor (37 pM and 5–10 nM, McIntyre et al., 1993), our data on native receptor membrane preparations exclude a participation of B1 receptors in [3H]-BK binding, as the B1 receptor selective ligands ([desArg9]-BK and [desArg9Leu8]-BK) were unable to produce inhibition at concentrations less than μM, in agreement with data reported by Hess et al. (1994). When we tested the affinity of B2 receptor antagonists in inhibiting the BK binding, we found that MEN16132 (pIC50 values 10.5 and 9.9 in lung and ileum, respectively) was as potent as Icatibant (pIC50 values 10.7 and 10.2), and its C-terminal constrained analogue MEN11270, but more potent (by 10- to 80-fold) than the two non-peptide antagonists, LF16-0687 or FR173657. In addition, all the B2 receptor antagonists tested inhibited only a part of the specifically bound BK. Overall from these data it can be assumed that BK binds to a site that is not recognized by all the available B2 antagonists, independently of their rank order of potency or their different structural features (peptide versus non-peptide). The analysis of BK homologous competitive binding curves performed with lung membranes preincubated with antagonists (at a concentration which reached a plateau in inhibiting the BK binding), indicated that antagonists could prevent the higher affinity BK interaction, leaving unaltered the affinity of BK for the residual specific binding. These results strongly suggest that the BK binding site displaced by the antagonists is the higher affinity one and that the two sites are independently recognized by BK (De Lean et al., 1980; Leeb-Lundberg et al., 1994). Moreover, present saturation and homologous competition experiments indicate that a greater proportion of lower affinity sites is present in the lung, compared with the ileum membrane preparation. Indeed, the portion of BK-specific binding not inhibited by antagonists is greater in the lung (about 50%) than in the ileum (about 10%) preparation. The greater presence of the lower affinity BK binding site in the lung could be related to the tissue heterogeneity of this preparation, that is, the bulky presence of vascular tissue, neuronal projections and/or the presence of different receptors recognized from BK, but not from other selective B2 receptor ligands (Meini et al., 2004).
Previous reports indicated that the disruption of the gene encoding the B2 receptor in mice abolished the [3H]-BK binding in mouse ileum membranes (Borkowski et al., 1995). Thus, it might be postulated that the two sites recognized by BK in the present radioligand binding experiments might be coded by a single gene for the B2 receptor in the mouse species, but this aspect deserves further appropriate investigation.
Although there is evidence of species-related differences in the pharmacology of B2 receptor ligands (Regoli et al., 2001), the rank order of potency deriving from the present experiments is quite similar to that we previously measured at the human or guinea-pig B2 receptor (Cucchi et al., 2005). On the other hand, the ability of all antagonists to inhibit only partially the BK-specific binding seems to be related to the mouse species, as all the antagonists used in the current study could completely inhibit the binding of radiolabelled BK, under the same experimental conditions, to guinea-pig ileum or airway membrane preparations (Meini et al., 2000; Cucchi et al., 2005).
The functional antagonist potency of MEN16132 was evaluated against the BK-induced contractions in the mouse isolated ileum and compared with that of Icatibant. Both antagonists produced a concentration-dependent shift of the agonist effect without significantly affecting the Emax. Notwithstanding, the Schild plot analysis indicated a ‘not competitive' behaviour, as the slope of linear regression was significantly less than unity. This behaviour is in agreement with previous data obtained with Icatibant in the mouse isolated trachea, stomach fundus and urinary bladder preparations (Nsa Allogho et al., 1997; Zhang et al., 2004). Although there were some differences in the calculated potency (pKB values 9.7, 8.3 and 8.6, respectively), all reports measured lower concentration-ratio values when higher concentrations of Icatibant were used, thus leading to shallow Schild plot regressions. To overcome non-equilibrium conditions that may be responsible for this feature of the antagonism (Nsa Allogho et al., 1997), a contact time of 60 min was used in the present experiments, but preliminary data obtained with a shorter time period of incubation with antagonists (15 min) indicated similar results (data not shown), suggesting that non-equilibrium conditions were not responsible for this antagonist behaviour. Another possible cause of Schild plots with slopes less than unity is the presence of heterogeneous receptor populations (Kenakin, 1997), but the B1 receptor type does not appear to be involved in the present assays. In fact, in agreement with the binding affinity data presented here, the B1 receptor antagonist [desArg9Leu8]-BK, at a quite high concentration (10 μM), did not modify the BK-induced concentration–response curve. Moreover, [desArg9Leu8]-BK also lacked any residual agonist activity in the isolated ileum preparation (α=0), contrary to what was shown in the mouse stomach fundus smooth muscle, which has been described a mixed B1-B2 receptor bioassay (α=0.64, Nsa Allogho et al., 1997), or in the rat isolated ileum, a B1 receptor bioassay (α=1, Meini et al., 1996).
The possibility that the two BK affinity states, highlighted in the present study, are related to differently activated signalling pathways, leading to the described contraction (indomethacin-resistant) or relaxation (indomethacin-sensitive) of the mouse isolated trachea, is ruled out by the ability of the B2 receptor antagonist Icatibant to block both responses (van Heuven-Nolsen et al., 1997; Zhang et al., 2004), but the involvement of other receptor conformations linked to further transduction mechanisms cannot be excluded a priori.
In summary, this study reports for the first time the characterization of BK binding to B2 receptors expressed in mouse tissue and presents data indicating that BK binds with pM and nM affinity both to mouse lung and ileum membrane preparations (the nM affinity site being present to a greater extent in the lung) but the B1 receptor does not appear to be involved. On the other hand, an unusual pharmacological profile has emerged with a panel of B2 receptor antagonists that can compete for a portion of BK binding; that portion being the one recognized with high affinity by BK. Finally, evidence presented here indicates that the novel non-peptide MEN16132 possesses a pharmacological profile that resembles that of the peptide Icatibant, having high affinity (pIC50 10.5 and 9.9) and antagonist potency (pA2 9.4) for the mouse receptor, thus being suitable for further in vivo characterization.
Acknowledgments
This work was supported by grants from Italian Ministry of University and Research (RIF.506/DSPAR 98).
Abbreviations
- BK
bradykinin
- DMSO
dimethylsulphoxide
- TES
N-tris[hydroxymethyl]methyl-2-aminoethanesulphonic acid
Conflict of interest
The authors state no conflict of interest.
References
- Alfie ME, Yang XP, Hess F, Carretero OA. Salt-sensitive hypertension in bradykinin B2 receptor knockout mice. Biochem Biophys Res Commun. 1996;224:625–630. doi: 10.1006/bbrc.1996.1076. [DOI] [PubMed] [Google Scholar]
- Arai Y, Takanashi H, Kitagawa H, Wirth KJ, Okayasu I. Effect of icatibant, a bradykinin B2 receptor antagonist, on the development of experimental ulcerative colitis in mice. Dig Dis Sci. 1999;44:845–851. doi: 10.1023/a:1026694732602. [DOI] [PubMed] [Google Scholar]
- Asano M, Inamura N, Hatori C, Sawai H, Fujiwara T, Katayama A, et al. The identification of an orally active, nonpeptide bradykinin B2 receptor antagonist, FR173657. Br J Pharmacol. 1997;120:617–624. doi: 10.1038/sj.bjp.0700955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski JA, Ransom RW, Seabrook GR, Trumbauer M, Chen H, Hill RG, et al. Targeted disruption of a B2 bradykinin receptor gene in mice eliminates bradykinin action in smooth muscle and neurons. J Biol Chem. 1995;270:13706–13710. doi: 10.1074/jbc.270.23.13706. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;75:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corrêa CR, Kyle DJ, Chakraverty S, Calixto JB. Antinociceptive profile of the pseudopeptide B2 bradykinin receptor antagonist NPC 18688 in mice. Br J Pharmacol. 1996;117:552–558. doi: 10.1111/j.1476-5381.1996.tb15226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchi P, Meini S, Bressan A, Catalani C, Bellucci F, Santicioli P, et al. MEN16132 a novel potent and selective non-peptide antagonist for the human bradykinin B2 receptor. In vitro pharmacology and molecular characterization. Eur J Pharmacol. 2005;528:7–16. doi: 10.1016/j.ejphar.2005.10.014. [DOI] [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- Ding-Zhou L, Margaill I, Palmier B, Pruneau D, Plotkine M, Marchand-Verrecchia C. LF 16-0687 Ms, a bradykinin B2 receptor antagonist, reduces ischemic brain injury in a murine model of transient focal cerebral ischemia. Br J Pharmacol. 2003;139:1539–1547. doi: 10.1038/sj.bjp.0705385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heapy CG, Shaw JS, Farmer SC. Differential sensitivity of antinociceptive assays to the bradykinin antagonist Hoe 140. Br J Pharmacol. 1993;108:209–213. doi: 10.1111/j.1476-5381.1993.tb13464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JF, Borkowski JA, MacNeil T, Sonesifer GY, Fraher J, Strader CD, et al. Differential pharmacology of cloned human and mouse B2 bradykinin receptors. Mol Pharmacol. 1994;45:1–8. [PubMed] [Google Scholar]
- Hock FJ, Wirth K, Albus U, Linz W, Gerhards HJ, Wiemer G, et al. Icatibant a new potent and long acting bradykinin antagonist: in vitro studies. Br J Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T.Competitive antagonism Pharmacologic Analysis of Drug–receptor Interaction 1997Lippincott-Raven Press: Philadelphia; 331–373.3rd edn. [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Mathis SA, Herzig MCS. Antagonists of bradykinin that stabilize a G-protein-uncoupled state of the B2 receptor act as inverse agonists in rat myometrial cells. J Biol Chem. 1994;269:25970–25973. [PubMed] [Google Scholar]
- Madeddu P, Emanueli C, Gaspa L, Salis B, Milia AF, Chao L, et al. Role of the bradykinin B2 receptor in the maturation of blood pressure phenotype: lesson from transgenic and knockout mice. Immunopharmacol. 1999;44:9–13. doi: 10.1016/s0162-3109(99)00105-8. [DOI] [PubMed] [Google Scholar]
- McIntyre P, Phillips E, Skidmore E, Brown M, Webb M. Cloned murine bradykinin receptor exhibits a mixed B1 and B2 pharmacological selectivity. Mol Pharmacol. 1993;44:346–355. [PubMed] [Google Scholar]
- Meini S, Bellucci F, Cucchi P, Giuliani S, Quartara L, Giolitti A, et al. Bradykinin B2 and GPR100 receptors: a paradigm for receptor signal transduction pharmacology. Br J Pharmacol. 2004;143:938–941. doi: 10.1038/sj.bjp.0706025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S, Lecci A, Maggi CA. The longitudinal muscle of rat ileum as a sensitive monoreceptor assay for bradykinin B1 receptors. Br J Pharmacol. 1996;17:1619–1624. doi: 10.1111/j.1476-5381.1996.tb15331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S, Quartara L, Rizzi A, Patacchini R, Cucchi P, Giolitti A, et al. MEN11270, a novel selective constrained peptide antagonist with high affinity at the human B2 kinin receptor. J Pharmacol Exp Ther. 1999;289:1250–1256. [PubMed] [Google Scholar]
- Meini S, Patacchini R, Lecci A, Quartara L, Maggi CA. Peptide and non-peptide bradykinin B2 receptor agonists and antagonists: a reappraisal of their pharmacology in the guinea-pig ileum. Eur J Pharmacol. 2000;409:185–194. doi: 10.1016/s0014-2999(00)00850-5. [DOI] [PubMed] [Google Scholar]
- Nsa Allogho S, Gobeil F, Pheng LH, Nguyen-Le XK, Neugebauer W, Regoli D. Antagonists for kinin B1 and B2 receptors in the mouse. Can J Physiol Pharmacol. 1997;75:558–562. doi: 10.1139/cjpp-75-6-558. [DOI] [PubMed] [Google Scholar]
- Pruneau D, Paquet J-L, Luccarini J-M, Defrêne E, Fouchet C, Franck R-M, et al. Pharmacological profile of LF16-0687, a new potent non-peptide bradykinin B2 receptor antagonist. Immunopharmacol. 1999;43:187–194. doi: 10.1016/s0162-3109(99)00128-9. [DOI] [PubMed] [Google Scholar]
- Regoli D, Rizzi A, Perron SI, Gobeil F. Classification of kinin receptors. Biol Chem. 2001;382:31–35. doi: 10.1515/BC.2001.005. [DOI] [PubMed] [Google Scholar]
- Saleh TS, Vianna RM, Creczynski-Pasa TB, Chakravarty S, Mavunkel BJ, Kyle DJ, et al. Oral anti-inflammatory action of NPC 18884, a novel bradykinin B2 receptor antagonist. Eur J Pharmacol. 1998;363:179–187. doi: 10.1016/s0014-2999(98)00778-x. [DOI] [PubMed] [Google Scholar]
- Valenti C, Cialdai C, Giuliani S, Lecci A, Tramontana M, Meini S, et al. MEN16132, a novel potent and selective nonpeptide kinin B2 receptor antagonist: in vivo activity on bradykinin-induced bronchoconstriction and nasal mucosa microvascular leakage in anesthetized guinea pigs. J Pharm Exp Ther. 2005;315:616–623. doi: 10.1124/jpet.105.088252. [DOI] [PubMed] [Google Scholar]
- van Heuven-Nolsen D, Westra-De Vlieger JF, Muis T, Denee JH, Rivas TO, Nijkamp FP. Pharmacology and mode of action of bradykinin on mouse-isolated trachea. Naunyn Schmiedeberg's Arch Pharmacol. 1997;356:134–138. doi: 10.1007/pl00005020. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adner M, Cardell L-O. Up-regulation of bradykinin receptors in a murine in-vitro model of chronic lung inflammation. Eur J Pharmacol. 2004;489:117–126. doi: 10.1016/j.ejphar.2004.02.033. [DOI] [PubMed] [Google Scholar]