Abstract
Background and Purpose:
The prostamides (prostaglandin-ethanolamides) and prostaglandin (PG) glyceryl esters are biosynthesized by COX-2 from the respective endocannabinoids anandamide and 2-arachidonyl glycerol. Agonist studies suggest that their pharmacologies are unique and unrelated to prostanoid receptors. This concept was further investigated using antagonists.
Experimental Approach:
The isolated feline iris was used as a key preparation, where prostanoid FP receptors and prostamide activity co-exist. Activity at human recombinant FP and other prostanoid receptors was determined using stable transfectants.
Key Results:
In the feline iris, AGN 204396 produced a rightward shift of the dose-response curves for prostamide F2α and the prostamide F2α analog bimatoprost but did not block the effects of PGF2α and synthetic FP receptor agonists. Studies on human recombinant prostanoid receptors confirmed that AGN 204396 did not behave as a prostanoid FP receptor antagonist. AGN 204396 exhibited no antagonism at DP and EP1-4, but was a highly effective TP receptor antagonist. Contrary to expectation, the FP receptor antagonist AL-8810 efficaciously contracted the cat iris. AGN 204396 did not affect AL-8810 induced contractions, demonstrating that AL-8810 and AGN 204396 are pharmacologically distinct. Unlike AL-8810, the ethylamide derivate of AL-8810 was not an agonist. Al-8810 did not block prostamide F2α activity. Finally, AGN 204396 did not block PGE2-glyceryl ester activity.
Conclusions and Implications:
The ability of AGN 204396 to selectively block prostamide responses suggests the existence of prostamide sensitive receptors as entities distinct from receptors recognizing PGF2α and PGE2-glyceryl ester.
Keywords: prostaglandins, prostglandin ethanolamides, FP receptor, iris, prostaglandin-glyceryl ester
Introduction
The endocannabinoids arachidonyl ethanolamide (anandamide) and 2-arachidonyl glyceryl ester (2-AG) are substrates for cyclooxygenase-2 (COX-2), with resultant conversion to the corresponding prostaglandin (PG) ethanolamides and glyceryl esters. This COX-2-specific biosynthetic pathway leads to the formation of a spectrum of PG ethanolamides (prostamides) and glyceryl esters that closely approache the diversity of the prostanoids (Yu et al., 1997; Burstein et al., 2000; Kozak et al., 2002; Koda et al., 2003). The biological significance of this pathway remains to be elucidated, but initial studies using recombinant enzymes have transitioned into cell (Kozak et al., 2002; Glass et al., 2005) and living animal studies (Weber et al., 2004).
A number of suggestions have been advanced and investigated to account for the biological significance of the PG ethanolamides (prostamides) and glyceryl esters. These include cannabimimetics (Berglund et al., 1999), vanilloid receptor agonists (Matias et al., 2004), prostaglandin mimetics (Sharif et al., 2001), fatty acid amide hydrolase (FAAH) substrates that indirectly reduce anandamide metabolism (Matias et al., 2004) and activators of peroxisome proliferation-activated receptor γ (PPARγ) (Rockwell and Kaminski, 2004). The greatest emphasis has been placed on comparative pharmacological studies with PGs, which suggest that the PG ethanolamides and glyceryl esters are pharmacologically unique. Thus, the effects of PGE2 ethanolamide in the guinea-pig trachea could not be readily explained by interaction with prostanoid EP receptors (Ross et al., 2002). Similarly, the Ca2+ signal and induction of protein kinase C (PKC) activity produced by PGE2 glyceryl ester were independent of conversion to PGE2 and did not appear to involve PGE2-sensitive receptors (Nirodi et al., 2004). The most extensive pharmacological studies to date have been performed on PGF2α-ethanolamide (prostamide F2α) and its structural analogue bimatoprost. As in the case of PGE2-glyceryl ester, the effects prostamide F2α and bimatoprost appear unrelated to PG formation and PG receptor stimulation and the existence of a population of receptors that preferentially recognize these molecules has been proposed (Woodward et al., 2001, 2003; Liang et al., 2003; Matias et al., 2004; Chen et al., 2005; Spada et al., 2005).
By virtue of its clinical status, bimatoprost is the most studied prostamide at this point in time. Bimatoprost behaves as a prostamide mimetic and is also the most efficacious antiglaucoma agent reported from patient studies to date (Dubiner et al., 2001; Higginbotham et al., 2002; Noecker et al., 2003; Parrish et al., 2003; Woodward et al., 2004). Moreover, patients refractory to latanoprost therapy are successfully treated with bimatoprost (Gandolfi and Cimino, 2003), suggesting a pharmacological distinction at the clinical level between the prostamide analog bimatoprost and the prostanoid FP receptor agonist prodrug latanoprost. Bimatoprost exhibits potent inherent pharmacological activity in certain in vitro pharmacological systems. These preparations include contraction of the cat lung (Woodward et al., 2003), cat iris (Woodward et al., 2001) and rabbit uterus (Chen et al., 2005), and upregulation of cysteine-rich angiogenic protein (Cyr 61) in human ciliary smooth muscle cells (Liang et al., 2003). Like the prostamides, bimatoprost activity at wild-type and recombinant FP and other prostanoid receptors is residual and occurs only at concentrations above 10−6 M (Sharif et al., 2001; Kelly et al., 2003; Woodward et al., 2003; Matias et al., 2004; Chen et al., 2005).
To date, pharmacological characterization of the prostamides has relied on agonist studies. The main focus has been comparing the activities of prostamide F2α and bimatoprost with those of PGF2α and selective prostanoid FP receptor agonists in a variety of prostamide-sensitive and-insensitive preparations. In all but one of these previous studies (Spada et al., 2005), it was not entirely clear if prostamide-sensitive tissues expressed a receptor subpopulation that preferentially recognized prostamides or an FP receptor subtype that equally recognized prostamides, PGF2α and FP receptor selective PG analogs. Resolution of this issue and further elucidation of prostamide receptor pharmacology demand the identification of an antagonist. This would allow the existing prostamide receptor hypotheses to be refuted or supported. With this aim, the effects of a recently discovered compound that blocked prostamide activity were compared to those of the FP receptor antagonist AL-8810 (Griffin et al., 1999) and its ethylamide derivative in the isolated feline iris. These studies suggest that there is a pharmacological distinction between prostamide and prostanoid FP receptor responses.
Methods
Test systems used
The test systems employed included the isolated feline iris as a key preparation as both prostamides (prostaglandin ethanolamides) and prostanoid FP receptor agonists potently elicit a contractile response in this tissue (Woodward et al., 2003; Matias et al., 2004). Activity at human prostanoid receptors was determined using recombinant receptors stably transfected into HEK-293 EBNA cells, using chimeric G proteins to enable Ca2+ signal responses to all receptor subtypes, as described previously (Woodward et al., 2003; Matias et al., 2004).
Measurements
Feline iris
Class A laboratory bred cats were housed communally in United States Department of Agriculture (USDA) and Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC) approved facilities, with standards that exceeded those for enrichment and group housing. Water was available ad libitum and food was standard cat nutritional diet. They were kept on a 12-h light–dark cycle. They (96) were euthanized by intravenous (i.v.) overdose of sodium pentobarbital (Anthony, Arcadia, CA, USA). The eyes were enucleated immediately thereafter and placed on ice. Two eyes provided a total of four iridial preparations. The iris sphincter was mounted vertically under 50–100 mg tension in a jacketed 10 ml organ bath. Smooth muscle tension of the isolated iris sphincter was measured isometrically with force displacement transducers (Grass FT-03) and recorded on a Grass polygraph (Model 7). The organ baths contained Krebs' solution maintained at 37°C by a heat exchanger and circulating pump. The Krebs' solution was gassed with 95% O2, 5% CO2 to give a pH of 7.4, and had the following composition: 118.0 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.9 mM CaCl2, 1.18 mM MgSO4, 25.0 mM NaHCO3, 11.7 mM glucose and 0.001 mM indomethacin. A 60-min stabilization period was provided before commencing each experiment. Activity was manifest as contractile responses and measured as such. These investigations were as humane as possible and adhered to the ‘Association for Research in Vision and Ophthalmology (ARVO) resolution on the Use of Animals in Research'.
Ca2+ signal studies on human recombinant prostanoid receptors
The use of chimeric G protein cDNAs allowed responses to Gs-and Gi-coupled prostanoid receptors to be measured as a Ca2+ signal, as described previously (Woodward et al., 2003; Matias et al., 2004). Prostanoid DP, EP2 and EP4 receptor cDNAs were co-transfected with chimeric Gqs cDNA containing a hemagglutanin (HA) epitope. The prostanoid EP3 receptor was co-transfected into HEK-293 EBNA cells, using pCEP4 as a vector, with chimeric Gqi-HA. Gqs and Gqi chimeric cDNAs (Molecular Devices, Sunnyvale, CA, USA) were cloned into a pCEP4 vector and also selected by using a hygromycin B selection marker. Transfection into HEK-293 EBNA cells was achieved by the FuGENE 6 method. Because Gqs and Gqi contained an HA epitope, protein expression was detected by Western blotting analysis using anti-mouse HA monoclonal antibody and horseradish peroxidase (HRP)-conjugated secondary antibody. For human recombinant EP1, FP, IP and TP receptors, stable transfectants were obtained as described previously (Woodward et al., 2003; Matias et al., 2004). Briefly, pCEP4 was used as the expression vector and transfection into HEK-293-EBNA cells was performed with FuGENE 6. Stable transfectants were again selected according to hygromycin resistance.
Ca2+ signaling studies were performed using a fluorimetric imaging plate reader (FLIPR) instrument. Cells were seeded at a density of 5 × 104 cells/well in Biocoat poly-D-lysine-coated, black wall, clear bottom 96-well plates (BD Biosciences, Franklin Lakes, NJ, USA) and allowed to attach overnight in an incubator at 37°C. The cells were then washed twice with Hanks' balanced salt solution (HBSS)–N-[2-hydroxyethyl]piperazine-N-[2-ethanesulphonic acid] (HEPES) buffer (Hanks' balanced salt solution without bicarbonate and phenol red, 20 mM HEPES, pH 7.4) using a Denley Cellwash plate washer (Labsystems, Franklin, MA, USA). After 45–60 min of dye loading in the dark using the Ca2+-sensitive dye Fluo-4AM, at a final concentration of 2 × 10−6 M, the plates were washed four times with HBSS–HEPES buffer to remove excess dye and leaving 100 μl of buffer in each well. The plates were then placed in the FLIPR instrument and allowed to equilibrate at 37°C. Compound solutions were added in a 50-μl volume to each well to give the desired final concentration. Cells were excited with an argon laser at 488 nm and emission was measured through a 510–570 nm bandwidth emission filter (FLIPR, Molecular Devices, Sunnyvale, CA, USA). The peak increase in fluorescence intensity was recorded for each well.
Experimental design
The feline iris experiments were designed so that a direct, four-way comparison for antagonist vs prostamide, vehicle vs prostamide, antagonist vs corresponding PG, and vehicle vs corresponding PG was provided in tissue preparations obtained from a single animal. One cumulative dose–response curve to agonist was obtained in each tissue. Vehicle (ethanol) and antagonist (AGN 204396) were given 30 min before the agonist dose–response curves were constructed. The response to PGF2α 10−7 M was determined at the beginning and end of each dose–response curve, with appropriate washout, and responses were calculated as % of this reference contraction.
The experimental design for the FLIPR studies was as follows. On each plate, four wells each served as negative (HBSS–HEPES buffer) and positive controls (standard agonist: DP=BW 245C, EP1–EP4=PGE2, FP=PGF2α, IP=carbaprostacyclin, TP=U-46619). The peak fluorescence change in each well containing drug was expressed relative to the controls. To obtain concentration–response curves, compounds were tested in duplicate in each plate over the desired concentration range. Each compound was tested on at least three separate plates using cells from different passages to give n=3.
Data analysis and statistical procedures
In order to calculate the pA2 value for AGN 204396 in the feline iris preparation, the mean concentration–response curve was plotted as log concentration–response −1 (CR−1) vs log antagonist concentration according to the method of Arunlakshana and Schild (1959), using GraphPad Prism 4 software. As sequential use of graded doses of AGN 204396 with washout in a single tissue was impossible (AGN 204396 is lipophilic and very difficult to washout), an adaptation of the prostamide F2α concentration–response curve in the presence of vehicle was required for analysis. Thus, for analysis, each point on the prostamide F2α vs vehicle concentration-response curve represents the mean of each of the vehicle vs prostamide F2α responses from the six separate antagonist experiments. As the slope of the CR−1 vs log[Antagonist] plot did not significantly differ from unity, the slope was constrained to 1. The effects of antagonists for all other experiments were statistically analyzed by comparing the midpoints of the curves (log EC50's) for the agonist concentration–response curves in the presence or absence of antagonist on the basis of an F-test. Null or alternative hypotheses were rejected at a minimum 0.05 level. All data were analyzed using GraphPad Prism 4.
Materials
AGN 204396, 3-(2-{(1R,2R,3S,4R)-3-[4-(4-cyclohexyl-butylcarbamoyl)-oxazol-2-yl]-7-oxa-bicyclo[2.2.1] hept–2-ylmethyl)-4-fluoro-phenyl)-propyl ethylamide, was synthesized at Allergan, Inc. (Irvine, CA, USA) Prostaglandins D2, E2 and F2α, U-46619, BW245C, AL-8810 were purchased from Cayman Chemical (Ann Arbor, MI, USA). AL-8810 ethylamide was synthesized by Selcia Ltd (Ongar, UK). Prostaglandin D2, E2 and F2α were synthesized at Allergan, Inc. or purchased from Cayman Chemical (Ann Arbor, MI, USA). Prostaglandin E2 1-glyceryl ester was a gift from LJ Marnett (Vanderbilt University, Nashville, TN, USA). All stock solutions were prepared in ethanol.
Results
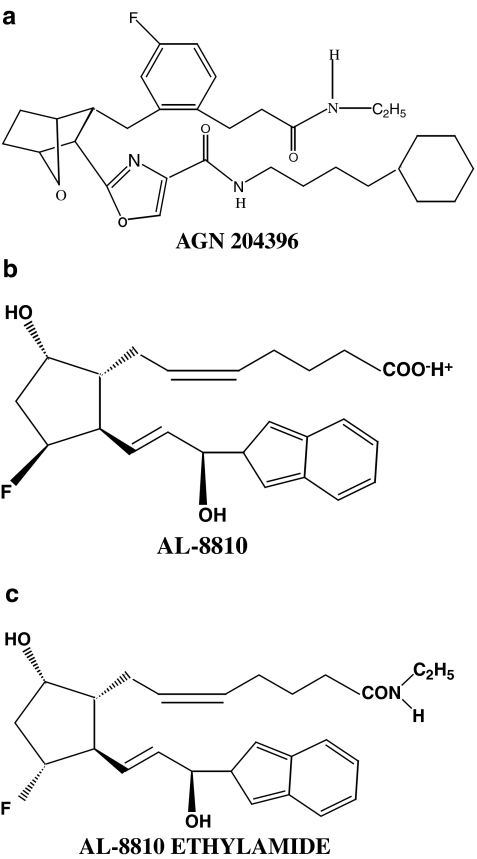
The structure of the prostamide antagonist AGN 204396, and AL-8810 and its ethanolamide derivative are depicted in Figure 1.
Figure 1.
Structures of (a) AGN 204396, (b) AL-8810 and (c) AL-8810 ethylamide.
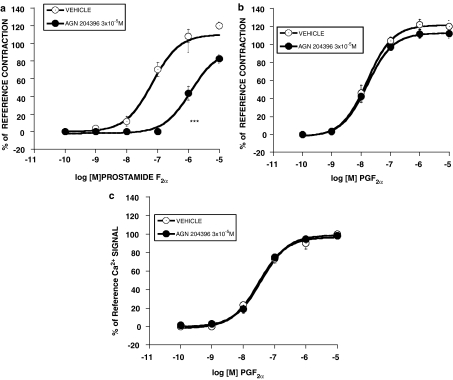
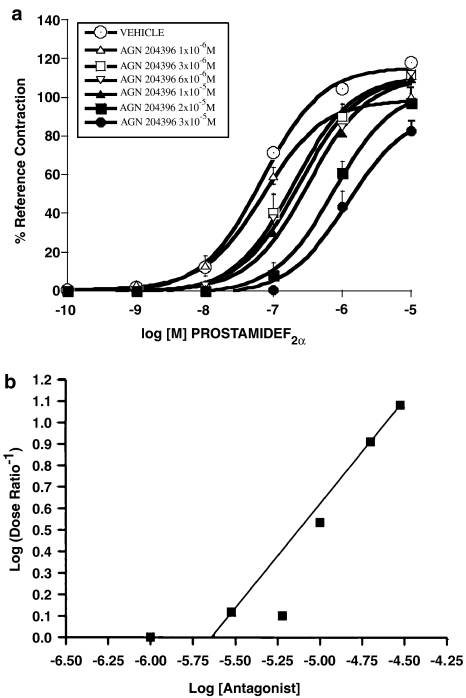
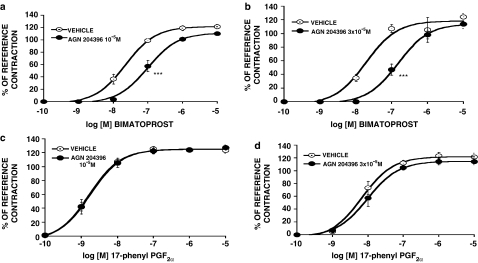
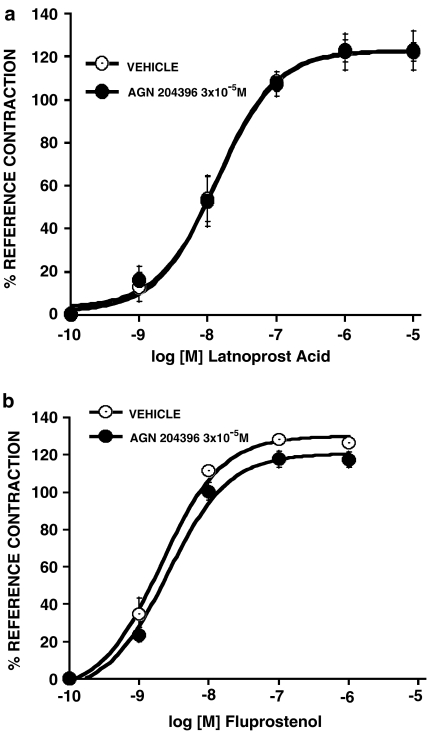
The effects of AGN 204396, 3 × 10−5 M, on contractions produced by prostamide F2α and PGF2α are shown in Figure 2. AGN 204396 produced a rightward shift of the prostamide F2α concentration–response curve (Figure 2a) but no meaningful displacement of the PGF2α concentration–response curve in the feline iris (Figure 2b) or the PGF2α concentration–response curve for Ca2+ signaling in human recombinant FP receptors (Figure 2c) was obtained. Comparing the mid-points (log EC50's) of the concentration–response curves at the maximum responses attained in the presence or absence of AGN 204396, the prostamide F2α response was antagonized at the P<0.0001 significance level but PGF2α responses were not significantly altered. The effects of graded concentrations of AGN 204396 on prostamide F2α-induced iridial contraction are shown in Figure 3a. A concentration-dependent rightward displacement was apparent, with the 10−6 M concentration being essentially ineffective. A Schild analysis is provided in Figure 3b. The points were distributed within the linear range of slope=1. The pA2 was 5.64. It was noticeable that the effects of AGN 204396 were never totally surmounted by 10 μM prostamide F2α. This may be due to residual activity at FP receptors (Matias et al., 2004), which are present in the feline iris (Coleman et al., 1984; Spada et al., 2005). The effects of AGN 204396 as an antagonist on bimatoprost-evoked iridial contraction is depicted in Figure 4a, both 10−5 and 3 × 10−5 M concentrations were effective. Although AGN 204396 blocked feline iridial contraction produced by the prostamide analog bimatoprost at the P<0.0001 significance level, it did not significantly affect the response to 17-phenyl PGF2α (Figure 4b). The effects of AGN 204396 on iridial contraction produced by latanoprost free acid and fluprostenol are shown in Figure 5, no significant antagonism was apparent.
Figure 2.
Effects of AGN 204396 (3 × 10−5 M) on contraction of the feline iris produced by (a) prostamide F2α (b) PGF2α and on (c) PGF2α induced Ca2+ signaling in stable transfectants expressing human recombinant FP receptors. Values are mean±s.e.m.; (a), (b) n=6 and (c) n=3 of duplicate determinations. ***P<0.0001 comparing EC50 values.
Figure 3.
Effects of graded doses of AGN 204396 on contraction of the feline iris produced by prostamide F2α (a). Points for the vehicle-treated iris represent the mean±s.e.m. of the mean values for each of the six individual antagonist experiments. Values are mean±s.e.m.; n=6 for each concentration of AGN 204396. A Schild plot of these data is depicted in (b).
Figure 4.
Effects of AGN 204396 on contraction of the feline iris produced by bimatoprost and 17-phenyl PGF2α. The effects of AGN 204396 at 10−5 and 3 × 10−5 M on bimatoprost-induced contractions are shown (a) and (b), respectively. The effects of AGN 204396 at 10−5 and 3 × 10−5 M on 17-phenyl PGF2α-induced contraction are shown (c) and (d), respectively. Values are mean±s.e.m.; n=6 for 3 × 10−5 M AGN 204396, n=12 for 10−5 M AGN 204396. ***P<0.0001 comparing EC50 values.
Figure 5.
Effects of AGN 204396 (3 × 10−5 M) on feline iridial contraction produced by (a) latanoprost free acid and (b) fluprostenol. Values are mean±s.e.m.; n=6.
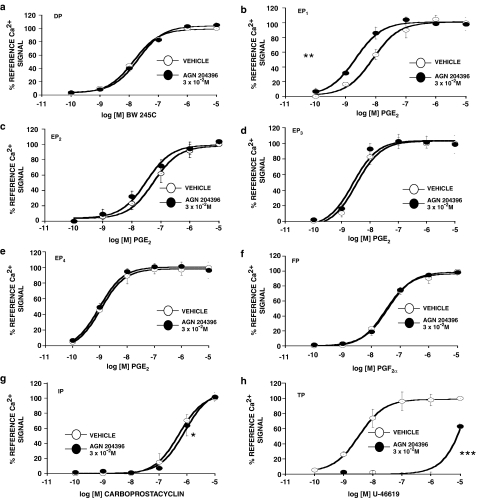
The effects of a 3 × 10−5 M concentration of AGN 204396 on Ca2+ signals associated with human recombinant prostanoid receptor stimulation are depicted in Figure 6. No antagonism was apparent at DP, EP1–4 or FP receptors, but AGN 204396 was a highly efficacious TP antagonist (Figure 6), with a kB value of 1.49 × 10−8 M. It was also a very weak IP receptor antagonist and appeared to potentiate PGE2 activity at the EP1 receptor, which was quite unexpected.
Figure 6.
Effects of AGN 204396 (3 × 10−5 M) on Ca2+ signals associated with human recombinant prostanoid receptors stably transfected in HEK-293 cells. Antagonism at (a) DP, (b) EP1, (c) EP2 (d) EP3, (e) EP4, (f) FP, (g) IP and (h) TP receptors was evaluated. Values are mean±s.e.m.; n=3 of duplicate determinations. *P<0.05, **P<0.01 ***P<0.001 comparing EC50 values.
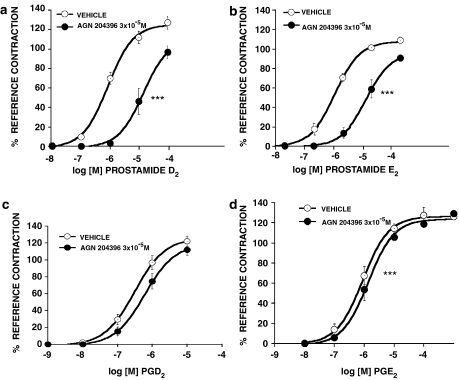
The effects of AGN 204396 on iridial contraction produced by prostamide D2 and E2 and the corresponding free acids PGD2 and PGE2 are graphically depicted in Figure 7. AGN 204396 exhibited clear and significant antagonistic activity vs prostamide D2 (Figure 7a) and prostamide E2 (Figure 7b). This was similar to activity recorded vs prostamide F2α. AGN 204396 was much less active against PGD2 (Figure 7c) and PGE2-induced (Figure 7d) iridial contraction, but there was a modest and statistically significant shift in the PGE2 concentration–response curve.
Figure 7.
Effects of AGN 204396 (3 × 10−5 M) on contraction of the feline iris in response to (a) prostamide D2 (b) prostamide E2 (c) PGD2 and (d) PGE2. Values are mean±s.e.m.; n=6. ***P<0.0001 comparing EC50 values.
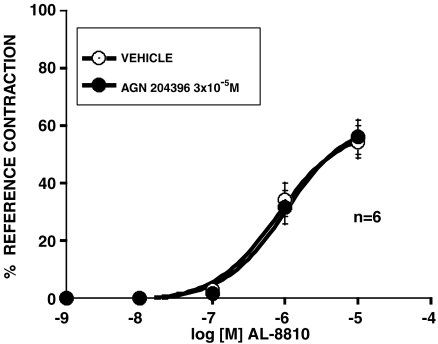
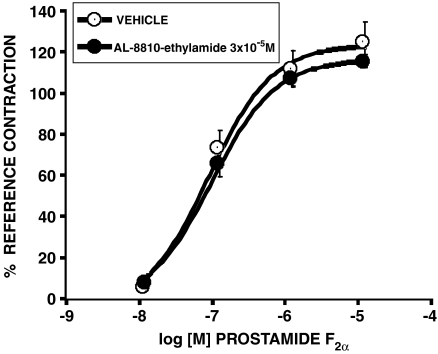
An attempt to use AL-8810 as an FP receptor antagonist (Griffin et al., 1999) was made in the feline iris. Contrary to expectation, AL-8810 appeared to behave as a weak, but full agonist in the feline iris preparation. To provide further pharmacological elucidation, a decision was made to attempt to block the effects of AL-8810 with AGN 204396. The iridial contraction produced by AL-8810 was not affected by AGN 204396 (Figure 8). This result indicates that AL-8810 and AGN 204396 are pharmacologically distinct. The ethylamide derivative of AL-8810 was also synthesized and tested. This did not contract the cat iris at concentrations up to 10−4 M. Moreover, AL-8810 ethylamide did not significantly antagonize prostamide F2α effects (Figure 9).
Figure 8.
Effect on AGN 204396 (3 × 10−5 M) on contraction of the feline iris produced by AL-8810. Values are mean±s.e.m.; n=6.
Figure 9.
Effect of AL-8810 ethylamide (3 × 10−5 M) on contraction of the feline iris produced by prostamide F2α. Values are mean±s.e.m.; n=6.
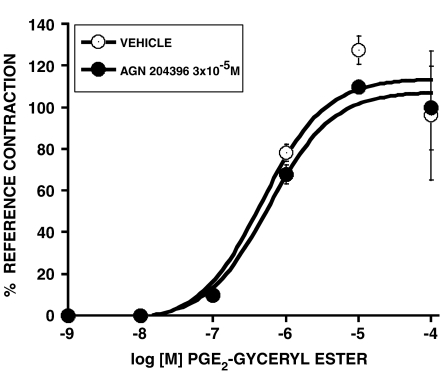
PGE2 1-glyceryl ester has recently emerged as a unique and interesting biologically active substance (Nirodi et al., 2004). AGN 204396 did not block the effect of PGE2 1-glyceryl ester in the feline iris, suggesting that prostamide and PGE2 1-glyceryl esters effects are distinct. These data are shown in Figure 10. Statistical comparisons of the effects of both AGN 204396 and AL-8810 ethylamide on contraction of the feline iris produced by various PGs, prostamides, and their analogs are provided in Table 1. An identical comparison for the antagonist effects of AGN 204396 3 × 10−5 M at human recombinant prostanoid receptors is given in Table 2.
Figure 10.
Effect of AGN 204396 (3 × 10−5 M) on contraction of the feline iris in response to PGE2 1-glyceryl ester. Values are mean±s.e.m.; n=6.
Table 1.
Antagonist activity of AGN 204396 at various concentrations and AL-8810 ethylamide at 3 × 10−5 M in the feline iris
| Agonist vs antagonist identity | Agonist log EC50±s.d. in the presence of vehicle | Agonist log EC50±s.d. in the presence of antagonist | P-Value (comparing agonist EC50 in the presence and absence of antagonist) |
|---|---|---|---|
| AGN 204396 1 × 10−6 M vs prostamide F2α | −7.365±0.066 | −7.150±0.063 | 0.224 NS |
| AGN 204396 3 × 10−6 M vs prostamide F2α | −7.065±0.039 | −6.713±0.068 | <0.0001*** |
| AGN 204396 6 × 10−6 M vs prostamide F2α | −7. 220±0.050 | −6.622±0.112 | <0.0001*** |
| AGN 204396 1 × 10−5 M vs prostamide F2α | −7.096±0.042 | −6.429±0.054 | <0.0001*** |
| AGN 204396 2 × 10−5 M vs prostamide F2α | −7.237±0.068 | −6.210±0.050 | <0.0001*** |
| AGN 204396 3 × 10−5 M vs prostamide F2α | −7.116±0.059 | −6.068±0.055 | <0.0001*** |
| AGN 204396 3 × 10−5 M vs PGF2α | −7.780±0.026 | −7.780±0.080 | 0.8722 NS |
| AGN 204396 1 × 10−5 M vs bimatoprost | −7.819±0.057 | −7.084±0.037 | <0.0001*** |
| AGN 204396 3 × 10−5 M vs bimatoprost | −7.654±0.052 | −6.828±0.065 | <0.0001*** |
| AGN 204396 1 × 10−5 M vs 17-phenyl PGF2α | −8.699±0.053 | −8.616±0.080 | 0.3962 NS |
| AGN 204396 3 × 10−5 M vs 17-phenyl PGF2α | −8.127±0.068 | −8.055±0.063 | 0.4390 NS |
| AGN 204396 3 × 10−5 M vs fluprostenol | −8.658±0.049 | −8.562±0.049 | 0.1727 NS |
| AGN 204396 3 × 10−5 M vs latanoprost free acid | −7.900±0.092 | −7.905±0.087 | 0.9711 NS |
| AGN 204396 3 × 10−5 M vs prostamide D2 | −6.259±0.046 | −5.302±0.070 | <0.001** |
| AGN 204396 3 × 10−5 M vs PGD2 | −7.042±0.069 | −6.979±0.042 | 0.4376 NS |
| AGN 204396 3 × 10−5 M vs Prostamide E2 | −6.049±0.049 | −4.999±0.060 | <0.0001*** |
| AGN 204396 3 × 10−5 M vs PGE2 | −6.500±0.056 | −6.126±0.059 | <0.0001*** |
| AGN 204396 3 × 10−5 M vs PGE2 - glyceryl ester | −6.155±0.037 | −6.174±0.033 | 0.7161 NS |
| AGN 204396 3 × 10−5 M vs AL-8810 | −5.167±0.079 | −5.079±0.069 | 0.4175 NS |
| AL-8810 Ethylamide 3 × 10−5 M vs Prostamide F2α | −7.100±0.072 | −7.093±0.047 | 0.9350 NS |
Abbreviation: NS=non significant.
Values and log EC50±s.d.. n=6 for all studies;
P<0.01
P<0.0001.
Table 2.
Antagonist activity of AGN 204396 3 × 10−5 M at human recombinant prostanoid receptor subtypes
| Human prostanoid receptor | Agonist log EC50±s.d. in the presence of vehicle | Agonist log EC50±s.d. in the presence of AGN 204396 3 × 10−5 M | P Value (comparing agonist EC50 in the presence and absence of AGN 204396 at 3 × 10−5 M) |
|---|---|---|---|
| DP | PGD2: −8.676±0.097 | PGD2: −8.706±0.091 | 0.8274 NS |
| EP1 | PGE2: −9.074±0.094 | PGE2: −9.630±0.093 | 0.0002** |
| EP2 | PGE2: −8.300±0.111 | PGE2: −8.493±0.110 | 0.2280 NS |
| EP3 | PGE2: −9.403±0.078 | PGE2: −9.580±0.116 | 0.2082 NS |
| EP4 | PGE2: −9.903±0.065 | PGE2: −9.979±0.061 | 0.4015 NS |
| FP | PGF2α: −8.442±0.055 | PGF2α: −8.409±0.062 | 0.6931 NS |
| IP | Carbocyclin: −7.347±0.077 | Carbocyclin: −7.094±0.078 | 0.0273* |
| TP | U-46619: −9.494±0.101 | U-46619: −6.175±0.057 | <0.0001*** |
Abbreviation: NS=non significant.
Values are log EC50±s.d.. n=3 of experiments performed in triplicate,
P<0.05,
P<0.001,
P<0.0001.
Discussion and conclusions
Previous studies on PG-ethanolamides (prostamides) have suggested that they may produce their effects by interacting with receptive targets that are distinct from PG receptors (Woodward et al., 2001; Ross et al., 2002; Matias et al., 2004). These present studies were performed to extend earlier investigations and employed a compound that selectively attenuated prostamide activity. The isolated feline iris sphincter was selected for these studies as it is very responsive to both prostamide F2α and prostanoid FP receptor agonists (Coleman et al., 1984; Matias et al., 2004), thereby providing an exacting test of the prostamide receptor hypothesis. The feline iris is also advantageous in that Ca2+ signals in response to the prostamide analog bimatoprost occur in a different cell population to those cells that respond to PGF2α and the FP receptor agonist 17-phenyl PGF2α (Spada et al., 2005). This offered an encouraging basis for the possible pharmacological separation of prostamide and FP receptor-mediated activities in the feline iris by intervention with putative antagonists. The antagonists selected for study were the FP receptor antagonist AL-8810 (Griffin et al., 1999), the ethylamide derivative of AL-8810 and a novel compound that appeared to block prostamide effects, designated AGN 204396.
AGN 204396 selectively antagonized the effects of prostamide F2α and its analog bimatoprost in the feline iris but did not similarly affect responses to PGF2α and prostanoid FP receptor selective agonists. AGN 204396 also antagonized the effects of prostamide D2 and prostamide E2 but was much less active in inhibiting the activities of the corresponding PGs, namely PGD2 and PGE2. Consistent with its lack of meaningful antagonist activity on PG-induced iridial contraction, AGN 204396 did not exhibit antagonist activity at human recombinant DP, EP1, EP2, EP3, EP4 or FP receptors. AGN 204396 was a potent TP receptor blocker but this activity does not interfere with analysis of the feline iris experiments, as this preparation does not contain functional TP receptors (Coleman et al., 1984) and the prostamides are not TP agonists (Matias et al., 2004). Similar reasoning applies to the very weak effect to AGN 204396 vs prostanoid IP receptors. From these data, AGN 204396 appears to behave as a compound uniquely capable of selectively blocking prostamide activity. Taken together with previous data obtained with prostamide F2α and its structural analog bimatoprost (Liang et al., 2003; Woodward et al., 2003; Matias et al., 2004; Chen et al., 2005; Spada et al., 2005), the results obtained with AGN 204396 are consistent with the hypothesis that prostamides may interact with a population of target receptors that is distinct in some way from known prostanoid receptors.
In addition to the prostamide antagonist studies, we also attempted ‘mirror-image' studies to block PGF2α and prostamide F2α effects with the prostanoid FP receptor antagonist AL-8810 (Griffin et al., 1999). Contrary to expectation, AL-8810 exhibited efficacious myotropic activity in the feline iris sphincter. Such activity was not anticipated as agonist effects of AL-8810 have been found to be minimal or absent in previous cell and isolated tissue studies (Griffin et al., 1999; Hutchinson et al., 2003; Liang et al., 2004). Nevertheless, residual FP receptor stimulation is apparent for Al-8810 in A7r5 cells (Griffin et al., 1999), which suggests partial agonism . It seems likely that AL-8810 is stimulating FP receptors in the feline iris given the following: (a) it can weakly agonize the FP receptor (Griffin et al., 1999; Hutchinson et al., 2003), (b) Al-8810 is a close structural analog of PGF2α, (c) An FP receptor has been functionally identified in the cat iris by the present study and the original prostanoid receptor classification (Coleman et al., 1984). As AL-8810 was unsuitable for use as an FP receptor antagonist in the feline iris, alternative experiments to those originally envisaged were undertaken. Thus, it was decided to examine the effect of AGN 204396 on AL-8810-induced iridial contraction to reveal any pharmacological interaction. Responses to AL-8810 were unaffected by AGN 204396 pretreatment, further indicating that the activity of AGN 204396 is not directly related to prostanoid FP receptors. Synthetic conversion of AL-8810 to its ethylamide derivative produced a compound that did not contract the feline iris at concentrations up to 10−4 M and did not block the effects of prostamide F2α. This result indicates that the structural requirements for FP receptor and prostamide receptor antagonism are not identical, thereby providing a further point of differentiation for prostanoid and prostamide pharmacology. Finally, the absent biological activity of AL-8810 ethylamide indicates that conversion to the biologically active free acid (AL-8810) does not occur. This is consistent with studies demonstrating that prostamide F2α and bimatoprost exert their activities as the intact molecules, according to direct analytical and bioassay-based metabolism studies (Woodward et al., 2003; Matias et al., 2004; Chen et al., 2005).
In addition to anandamide, a further endocannabinoid 2-AG is a substrate for COX-2, with the resultant biosynthesis of PG-glyceryl esters (Kozak et al., 2001, 2002). PGE2 1-glyceryl ester was reported to mobilize intracellular Ca2+ and activate PKC by a mechanism that did not involve PGE2 formation or PGE2-sensitive receptors (Nirodi et al., 2004). As PGE2 1-glyceryl ester also appears to be pharmacologically unique, we elected to test its activity in the feline iris preparation and its susceptibility to AGN 204396 pretreatment. Although PGE2 1-glyceryl ester produced a contraction, it was much less potent in the feline iris than in eliciting a Ca2+ signal in RAW 264.7 cells (Nirodi et al., 2004). This casts doubt on whether receptors recognizing PGE2 1-glyceryl ester in the feline iris and RAW 264.7 cells are identical. Nevertheless, a shared identity cannot be totally excluded as, for example, PGF2α is more than 10 times more potent as an FP receptor agonist in the rat colon compared to the mouse ileum (Woodward et al., 2003). What can be concluded with greater certainty is that PGE2 1-glyceryl ester does not cause contraction of the feline iris by stimulating prostamide-sensitive receptors, as its effects are not blocked by AGN 204396.
The biological significance of a COX-2-dependent pathway for the conversion of endocannabinoids/endovanilloids to electrochemically neutral PGs remains to be elucidated. The ability of COX-2 to recognize anandamide and 2-AG may ultimately transpire to be one of its most important enzymatic functions not shared by COX-1. It has recently been demonstrated that COX-2 induction by IL-1β results in preferential and copious prostamide biosynthesis from anandamide (Glass et al., 2005). Moreover, prostamides show 100% crossreactivity in ELISA assays, suggesting that their presence may be widespread (Glass et al., 2005). The prostamides and PGE2 1-glycerl ester appear to be pharmacologically distinct from the prostanoids (Matias et al., 2004; Nirodi et al., 2004; Chen et al., 2005), but at present, it is uncertain as to what their role in inflammation may be. Replacement of the charged carboxylate group of the PGs with a neutral amido or ester moiety not only alters the pharmacology but also metabolic inactivation. The stearic hindrance afforded by an ethanolamide or glyceryl ester functionality at position C1 decreases oxidation by 15-OH prostaglandin dehydrogenase (15-OH PGDH) (Kozak et al., 2001). This suggests that PG-ethanolamides and glyceryl esters may be longer lasting in vivo than PGs and may, perhaps exert their actions in tissues remote from their site of generation (Kozak et al., 2001). The stability of PGE2-ethanolamide and glyceryl ester in human plasma supports this notion (Kozak et al., 2001). In rat plasma, PGE2 glyceryl ester was rapidly hydrolyzed whereas prostamide E2 was detectable in rat plasma up to 2 h after dosing, suggesting that it is significantly longer acting than PGE2 (Kozak et al., 2001). The metabolic stability of endocannabinoid-derived neutral PGs opens up the possibility of additional biologically active substances with a range that extends beyond that of locally acting PGs biosynthesized from arachidonic acid. The availability of a drug, (AGN 204396), that can antagonize prostamide effects is a step forward in elucidating their biological significance.
Acknowledgments
We like to express their appreciation to Lisa L Rubin for preparation of the manuscript.
Abbreviations
- AAALAC
Association of Assessment and Accreditation of Laboratory Animal Care,International
- 2-AG
2-arachidonyl glycerol
- ARVO
Association for Research in Vision and Ophthalmology
- COX-1
cyclooxygenase 1
- COX-2
cyclooxygenase 2
- CR
concentration–response
- Cyr 61
cysteine-rich angiogenic protein
- EBNA
Epstein–Barr nuclear antigen
- ELISA
enzyme-linked immunosorbent assay
- FAAH
fatty acid amide hydrolase
- FLIPR
fluorimetric imaging plate reader
- HA
hemagglutanin
- HBSS
Hanks' balanced salt solution
- HEK
human embryonic kidney
- HEPES
N-[2-hydroxyethyl] piperazine-N-[2-ethanesulphonic acid]
- HRP
horseradish peroxidase
- PG
prostaglandin
- 15-OH PGDH
15-OH prostaglandin-dehydrogenase
- PKC
prostein kinase C
- PPARγ
peroxisome proliferation-activated receptor γ
- USDA
United States Department of Agriculture
Conflict of interest
The authors state no conflict of interest.
References
- Arunlakshana O, Schild HO. Some quantitative use of drug antagonists. Br J Pharmacol Chemother. 1959;257:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund BA, Boring DL, Howlett AC. Investigation of structural analogs of prostaglandin amides for binding to and activation of CB and CB2 cannabinoid receptors in rat brain and human tonsils. Adv Exp Med Biol. 1999;469:527–533. doi: 10.1007/978-1-4615-4793-8_77. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. PGs. Other Lipid Med. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Senior J, Marshall K, Abbas F, Dinh H, Dinh T, et al. Studies using isolated uterine and other preparations show bimatoprost and prostanoid FP agonists have different activity profiles. Br J Pharmcol. 2005;144:493–501. doi: 10.1038/sj.bjp.0706044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Humphrey PPA, Kennedy I, Lumley P. Prostanoid receptors: the development of a working classification. Trends Pharmacol Sci. 1984;5:303–306. [Google Scholar]
- Dubiner H, Cooke D, Dirks M, Stewart WC, Vandenburgh AM, Felix C. Efficacy and safety of bimatoprost in patients with elevated intraocular pressure. Surv Opthalmol. 2001;45:5353–5360. doi: 10.1016/s0039-6257(01)00212-0. [DOI] [PubMed] [Google Scholar]
- Gandolfi SA, Cimino L. Effect of bimatoprost on patients with primary open-angle glaucoma on ocular hypertension who are nonresponders to latanoprost. Ophthalmol. 2003;110:609–614. doi: 10.1016/S0161-6420(02)01891-2. [DOI] [PubMed] [Google Scholar]
- Glass M, Hong J, Sato TA, Mitchell MD. Misidentification of prostamides as prostaglandins. J Lipid Res. 2005;46:1364–1368. doi: 10.1194/jlr.C500006-JLR200. [DOI] [PubMed] [Google Scholar]
- Griffin BW, Klimko P, Crider JY, Sharif NA. AL-8810: a novel prostaglandin F2α analog with selective antagonist effects at the prostaglandin F2α (FP) receptor. J Pharmacol Exp Ther. 1999;290:1278–1284. [PubMed] [Google Scholar]
- Higginbotham EJ, Schuman JS, Goldberg I, Gross RL, Vandenburgh AM, Chen K, et al. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286–1293. doi: 10.1001/archopht.120.10.1286. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Marshall K, Senior J. Preliminary studies using a putative FP-receptor antagonist, AL-8810, on isolated mouse uterus. PA2. 2003;1:038P. [Google Scholar]
- Kelly CR, Williams GW, Sharif NA. Real-time intracellular Ca2+ mobilization by travoprost acid, bimatoprost, unoprostone, and other analogs via endogenous mouse, rat, and cloned human FP prostaglandin receptors. J Pharmacol Exp Ther. 2003;304:238–245. doi: 10.1124/jpet.102.042556. [DOI] [PubMed] [Google Scholar]
- Koda N, Tsutsui Y, Niwa H, Ito S, Woodward DF, Watanabe K. Synthesis of prostaglandin F ethanolamide by prostaglandin F synthase and identification of bimatoprost as a potent inhibitor of the enzyme: new enzyme method by LC/GSI/MS. Arch Biochem Biophys. 2003;424:128–136. doi: 10.1016/j.abb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Ray JL, Tai HH, Morrow JD, Marnett LJ. Metabolism of prostaglandin glyceryl esters and prostaglandin ethanolamides in vitro and in vivo. J Biol Chem. 2001;276:36993–36998. doi: 10.1074/jbc.M105854200. [DOI] [PubMed] [Google Scholar]
- Liang Y, Li C, Guzman VM, Chang WW, Evinger AJ, Pablo JV, et al. Upregulation of orphan nuclear receptor Nur 77 following PGF2α, bimatoprost and butaprost treatments. Essential role of a protein kinase C pathway involved in the EP2 receptor activated Nur 77 gene transcription. Br J Pharmacol. 2004;142:737–748. doi: 10.1038/sj.bjp.0705829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Li C, Guzman VM, Evinger AJ, Protzman CE, Krauss AH, et al. Comparison of PGF2α, bimatoprost (prostamide) and butaprost (EP2 agonist) on Cyr 61 and CTGF gene expression. J Biol Chem. 2003;278:27267–27277. doi: 10.1074/jbc.M301009200. [DOI] [PubMed] [Google Scholar]
- Matias I, Chen J, De Petrocellis L, Bisogno T, Ligresti A, Fezza F, et al. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J Pharmacol Exp Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW 264.7 cells. Proc Nat Acad Sci. 2004;101:1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noecker RS, Dirks MS, Choplin NT, Bernstein P, Batoosingh AL, Whitcup SM. A six-month randomized clinical trail comparing the intraocular pressure lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003;135:55–63. doi: 10.1016/s0002-9394(02)01827-5. [DOI] [PubMed] [Google Scholar]
- Parrish RK, Palmberg P, Sheu WP, for the XLT Study Group A comparison of latanoprost, bimatoprost, and travaprost in patients with elevated intraocular pressure: a 12-week randomized, masked evaluation multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther. 2004;34:683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, et al. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Williams GW, Kelly CB. Bimatoprost and its free acid are prostaglandin FP receptor agonists. Eur J Pharmacol. 2001;432:211–213. doi: 10.1016/s0014-2999(01)01486-8. [DOI] [PubMed] [Google Scholar]
- Spada CS, Krauss AH, Woodward DF, Chen J, Protzman CE, Nieves AL, et al. Bimatoprost and prostaglandin F2α selectively stimulate intracellular calcium signaling in different cat iris sphincter cells. Exp Eye Res. 2005;80:135–145. doi: 10.1016/j.exer.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Weber A, Ni J, Ling KH, Acheampong A, Tang-Liu DD, Cravatt BF, et al. Formation of prostaglandin 1-ethanolamides (prostamides) from anandamide in fatty acid amide hydrolose knockout (FAAH −/−) mice analyzed by high performance liquid chromatography with tandem mass spectrometry. J Lipid Res. 2004;45:757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH, Chen J, Lai RK, Spada CS, Burk RM, et al. The pharmacology of bimatoprost (Lumigan™) Surv Ophthalmol. 2001;45:5337–5345. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH, Chen J, Liang Y, Li C, Protzman CE, et al. Pharmacological characterizations of a novel antiglaucoma agent, bimatoprost (AGN 192024) J Pharmacol Exp Ther. 2003;305:772–785. doi: 10.1124/jpet.102.047837. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Phelps RL, Krauss AH, Weber A, Short B, Chen J, et al. Bimatoprost: a novel anti-glaucoma agent. Cardiovasc Drug Rev. 2004;22:103–120. doi: 10.1111/j.1527-3466.2004.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]