Abstract
Background and purpose:
Superoxide anions produced during vascular disease scavenge nitric oxide (NO), thereby reducing its biological activity. The aim of the present study was to investigate whether reactive oxygen species (ROS) have a direct effect on soluble guanylyl cyclase (sGC) subunit levels and function and to ascertain the mechanism(s) involved.
Experimental approach:
Rat aortic smooth muscle cells (RASM) or freshly isolated vessels were exposed to reactive oxygen species (ROS)-generating agents and sGC subunit expression was determined at the mRNA and/or protein level. cGMP accumulation was also determined in RASM exposed to ROS.
Key results:
Incubation of smooth muscle cells with H2O2, xanthine/xanthine oxidase (X/XO) or menadione sodium bisulphite (MSB) significantly decreased protein levels of α1 and β1 subunits of sGC and reduced SNP-induced cGMP formation. Similarly, sGC expression was reduced in freshly isolated vessels exposed to ROS-generating agents. The ROS-triggered inhibition of α1 and β1 levels was not blocked by proteasome inhibitors, suggesting that decreased sGC protein was not due to protein degradation through this pathway. Real time RT-PCR analysis demonstrated a 68% reduction in steady state mRNA levels for the α1 subunit following exposure to H2O2. In addition, α1 promoter-driven luciferase activity in RASM decreased by 60% after H2O2 treatment.
Conclusion and implications:
We conclude that oxidative stress triggers a decrease in sGC expression and activity that results from reduced sGC steady state mRNA levels. Altered sGC expression is expected to contribute to the changes in vascular tone and remodeling observed in diseases associated with ROS overproduction.
Keywords: cGMP, soluble guanylyl cyclase, reactive oxygen species, nitric oxide
Introduction
Soluble guanylyl cyclase (sGC) is a heterodimeric enzyme composed of one α1 and one β1 subunit that share a molecule of heme (Hobbs, 1997; Pyriochou and Papapetropoulos, 2005). The presence of heme is critical for the expression of enzymatic activity and renders sGC sensitive to nitric oxide (NO); sGC activity has been shown to increase up to 400-fold in the presence of NO (Waldman and Murad, 1987; Friebe and Koesling, 2003). In many instances, activation of sGC and generation of cyclic guanosine monophosphate (cGMP) mediates the effects of NO. In the vasculature, NO-induced relaxation and inhibition of platelet aggregation occur through cGMP (Lucas et al., 2000). Although the main endogenous activator of sGC is NO acute exposure of purified enzyme, cells or tissues to carbon monoxide or reactive species (hydroxyl radicals, peroxynitrite or hydrogen peroxide) can also increase cGMP accumulation and vasorelaxation, but to a lesser extent than NO (Waldman and Murad, 1987; Tarpey et al., 1995; Hobbs, 1997; Wolin, 2000). Moreover, subacute exposure of cells or tissues to NO-generating compounds leads to sGC desensitization without altering sGC subunit expression, whereas more prolonged incubation with NO donors reduces mRNA levels for α1 and β1 (Waldman and Murad, 1987; Moncada et al., 1991; Papapetropoulos et al., 1996b; Filippov et al., 1997). The NO-triggered downregulation of sGC subunit mRNA is mediated by a depletion in HuR, an elav-like 34-kDa protein that binds to AU-rich elements in the 3′ untranslated region (UTR) of the sGC α1 and β1 mRNAs and protects them from degradation (Kloss et al., 2003, 2004).
Reactive oxygen species (ROS) affect many smooth muscle cell functions, including growth, differentiation, apoptosis and tone (Irani, 2000; Su et al., 2001). In addition, many of the actions of vasoactive substances such as angiotensin II, platelet-derived growth factor and thrombin are mediated through the generation of H2O2 and superoxide anions (O2−) following binding of these ligands to their cognate receptors (Thannickal and Fanburg, 2000). Increased production of ROS has been documented in many pathophysiological conditions associated with vascular dysfunction, including atherosclerosis, hypertension, diabetes, sepsis, ischemia/reperfusion and cigarette smoking (Harrison, 1997; Maytin et al., 1999). Although scavenging of NO by O2− could explain the reduced vasodilation observed in many vascular diseases, evidence from genetic models of hypertension associated with enhanced ROS generation indicates that dysfunction of the NO/cGMP pathway might also be attributed to a reduction in sGC expression (Bauersachs et al., 1998). The aim of the present study was to examine the effects of ROS producing agents on sGC subunit protein levels and function and to investigate the mechanism(s) underlying the changes observed.
Methods
Cell culture
Rat aortic smooth muscle (RASM) cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% FCS, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 2 mM L-glutamine at 37°C in a humidified atmosphere with 5% CO2 to 95% air. For the present study, cells between passages 2 and 7 were used.
Cell treatments
Before treatment, RASM cells were serum-starved for 4 h using fresh DMEM supplemented with L-glutamine, antibiotics and 1% BSA. In all subsequent steps, cells were maintained in this serum-free medium. To induce oxidative stress, cells were exposed for 24 h to varying concentrations of hydrogen peroxide (H2O2; 150 or 500 μM), X/XO (75/12.5 or 300/50 μM/μU) and menadione sodium bisulfite (MSB; 10 or 15 μM). When proteasome inhibition was desired cells were pretreated with MG-132 (10 μM) or lactacystine (10 μM) for 2 h and then incubated with either H2O2 (500 μM) or MSB (10 μM) for an additional 24 h. For c-Jun N-terminal kinase (JNK) inhibition, cells were pretreated with anthra[1,9-cd]pyrazol-6(2H)-one (SP600125) (10 μM) for 1 h and then incubated with H2O2 (500 μM) for an additional 24 h. Viability of cells after exposure to ROS was determined by the trypan blue exclusion assay.
Rat aortic strips
In some experiments, freshly isolated rat aortic tissue was used. Rats (12–14 weeks of age) were anesthetized, killed by exsanguination and thoracic aortas collected; the endothelium was removed by gentle rubbing and vessels were cut longitudinally; strips from each animal were placed in tissue culture plates and treated with ROS-generating agents for 24 h in serum-free medium.
Cell-lysate preparation and Western blotting
Following treatments, cells were lysed and processed as described (Zhou et al., 2004). The antibody dilutions used were as follows: anti-α1, 1:5000; anti-1, 1:2000; anti-actin, 1:5000; anti-rabbit horseradish peroxidase (HRP), 1:5000; anti-mouse HRP, 1:2000. Western blots were quantified using the Gel-Pro Analyzer software version 4.0 (Media Cybernetics Inc., Silver Spring, MD, USA); values obtained for the α1 and β1 sGC subunit were normalized for actin and presented as % of control.
cGMP enzyme immunoassay
RASM cells were grown in 24 multiwell plates until confluent, serum-starved for 4 h as described above and then treated with the indicated ROS concentration for 24 h. For proteasome inhibition, cells were pretreated with MG-132 (10 μM) for 2 h and then incubated with either H2O2 (500 μM) or MSB (10 μM) for an additional 24 h. Following treatments, cells were washed with Hank's balanced salt solution (HBSS) and incubated in the presence of 1 mM of the phosphodiesterase inhibitor, isobutylmethylxanthine (IBMX). After 10 min, cells in half of the wells were stimulated with 10 μM of sodium nitroprusside (SNP) for 15 min. At the end of the 15 min period, media were aspirated and 300 μl of 0.1 N HCl added to all wells to extract cGMP. After 30 min, HCl extracts were collected and analyzed for cGMP using the cGMP enzyme immunoassay kit according to the manufacturer's instructions. Values are presented as cGMP per well; typically, the amount of protein per C-24 well was 100–150 μg.
Total RNA isolation, semiquantitative reverse transcriptase-polymerase chain reaction and quantitative real-time polymerase chain reaction
RASM cells were grown until confluent, serum-starved for 4 h and then treated with H2O2 (500 μM) for 12 and 24 h. Total RNA was isolated using Trizol. After photometric quantitation at 260 nm, only RNA samples with ratio of 1.85 or higher were used for further analysis. For reverse transcriptase-polymerase chain reaction (RT-PCR), reverse transcription was performed using 3 μg of total RNA per sample and oligo dT15 according to the manufacturer's protocol (Superscript; Invitrogen, Paisley, UK). The complementary DNA (cDNA) produced was then diluted 1:5 with water and amplified using primers for sGC α1 and GADPH (sense for α1: CACCATGTTCTGCAGGAAGTTCAAA; antisense for α1: ATCTACCCCTGATGCTTTGCCTA). Reactions were carried out by mixing 5 or 0.5 μl of cDNA (for α1 and GADPH amplification, respectively), 5 μl of 10 × PCR buffer, 1 μl of 10 mM dNTPs, 0.5 μl of 100 mM α1 or GADPH gene primer pair and 0.2 μl of 5 U μl−1 platinum Taq DNA polymerase. Amplification was carried out for 26, 28, 30 and 32 cycles. Each cycle consisted of denaturing for 1 min at 94°C, annealing for 1.5 min at 56°C and extension for 3 min at 72°C. PCR products were electrophorised on 1% agarose gels, stained with ethidium bromide and photographed under UV light. For quantitative-PCR, 2 μg of each RNA sample was reversely transcribed into cDNA as described above; cDNA was again diluted 1:5 with water and amplified using specific primers for sGC α1 and 18S (sense for α1: CGGAAAATCAATGTCAGCCC, antisense for α1: AGGGAAGTTTGGTGGAAGTC; sense for 18S: TCAAGAACGAAAGTCGGAGGTT, antisense for 18S: GGACATCTAAGGGCATCACAG). Each PCR included 1 × PCR buffer with 2.5 mM MgCl2, 80 nN dNTPs, 0.3 μM of each primer, 2.5 U of platinum Taq polymerase and 1 × SYBR Green I. The cycling parameters included: 95°C for 5 min, 95°C for 30 s and 60°C for 30 s for 40 cycles. The amplifications were carried out in a PTC-200 Peltier Thermal Cycler with a CHROMO 4 Detector (BioRad, Hercules, CA, USA) and analyzed with the Opticon Monitor software version 2.03. The relative quantity of the target mRNA (sGC α1) was calculated using the ΔΔCT method and 18S rRNA as a control for normalization.
Transient transfections of RASM cells and luciferase assays
Cells were plated in six-multiwell plates at a density of 2 × 105 cells per well and allowed to grow overnight. They were then transfected with the appropriate plasmid using the jetPEI transfection reagent according to the manufacturer's instructions, applying a total of 4 μg of DNA and 4.8 μl of jetPEI per well. Two different plasmids were used, one that contained the luciferase gene and, upstream, the 1.45 kb sGC α1 promoter region with the full-length 5′UTR region and another construct that contained no 5′UTR region. Twenty-four hours after transfection, cells were serum-starved for 4 h and incubated with H2O2 (500 μM) for an additional 24 h. At the end of the incubation time, cells were lysed and luciferase activity was measured by the luciferase reporter gene assay kit and according to the manufacturer's instructions. Protein concentration of the lysates was also determined using the Bio-Rad protein assay kit.
Statistical analysis
Data are expressed as means±s.e.m. of the mean of the indicated number of observations. Statistical comparisons between groups were performed using one-way analysis of variance. Differences among means were considered significant with *P<0.05.
Materials
DMEM, FCS, trypsin and penicillin/streptomycin were obtained from GIBCO-BRL (Paisley, UK). Hydrogen peroxide was obtained from Applichem (Darmstadt, Germany). SP600125 was obtained from Tocris Cookson (Avonmouth, UK). Trizol, Superscript First-Strand Synthesis System for reverse transcription and platinum Taq polymerase were purchased from Invitrogen (Paisley, UK); dNTPS and Taq DNA polymerase from Fermentas (St Leon-Rot, Germany) and NucleoSpin Plasmid kits from Macherey-Nagel (Düren, Germany). The mouse α1 promoter plasmids were a generous gift from Roberto Vazquez-Padron. jetPEI transfection reagent was obtained from Polyplus-transfection (Illkirch, France); cGMP enzyme immunoassay kits from R&D Systems (Minneapolis, MN, USA) and luciferase reporter gene assay kit from Boehringer-Mannheim (Mannheim, Germany). DC protein assay kit, Tween-20 and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) reagents were obtained from Bio-Rad (Munich, Germany) and nitrocellulose membrane Hybond ECL from Amersham Biosciences (Vienna, Austria). Polyclonal antibodies (pAb) for α1 and β-actin were obtained from Sigma (St Louis, MO, USA). pAb to β1 was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Secondary anti-rabbit and anti-mouse HRP-labeled antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and NEN Life Science Products Inc. (Boston, MA, USA), respectively. SuperSignal West Pico chemiluminescent substrate was purchased from Pierce (Rockford, IL, USA) and X-ray film from Eastman Kodak (Rochester, NY, USA). All other chemicals were purchased from Sigma (St Louis, MO, USA).
Results
ROS reduce protein levels of both α1 and β1 sGC subunits and SNP-induced cGMP accumulation
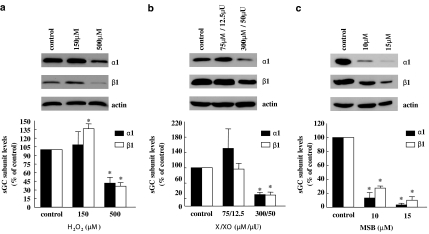
To study the effects of oxidative stress on the abundance of sGC, RASM cells were exposed for 24 h to varying concentrations of hydrogen peroxide (H2O2–150, 500 μM), X/XO (75/12.5 and 300/50 μM/μU) or MSB (10, 15 μM). Following treatment with the lower H2O2 concentration, the protein levels of the α1 subunit remained unaltered. Interestingly, β1 levels showed a small, but significant increase. Following treatment with the higher concentration of H2O2, protein levels of both subunits decreased, with the maximal reduction reaching 58% for α1 and 64% for β1 (Figure 1a). Exposure to X/XO resulted in a 70% decrease in protein levels for both subunits at the maximum concentration used (Figure 1b). In addition, RASM treated with MSB, an agent that releases superoxide anions, exhibited drastic reductions in sGC protein levels (Figure 1c). It should be noted that sGC levels in RASM were also reduced, although to a smaller extent, when ROS-generating agents were applied for 12 h (data not shown). Exposure of cells to the ROS-generating agents for 24 h did not result in overt toxicity based on (a) measurements of protein content per monolayer (data not shown), (b) actin levels and (c) cell viability estimated by trypan blue exclusion (81.8%±3.2 for control; 74.7% ±4.0 for 500 μM H2O2 and 78.4% ±1.7 for 10 μM MSB-treated cells).
Figure 1.
ROS-generating agents reduce the protein levels of both sGC subunits in cultured cells. RASM cells were incubated for 24 h with hydrogen peroxide (H2O2) (150 and 500 μM; (a)) X/XO (75/12.5 and 300/50 μM/μU; (b)) MSB (10 and 15 μM; (c)) or the corresponding vehicle. Cell lysates were subjected to SDS-PAGE and Western blot analysis using sGC α1- and β1-specific antibodies. The upper panels show blots of representative experiments. The bar graphs represent the mean±s.e.m. obtained from densitometric analysis of blots from three independent experiments and show percentages of α1 and β1 proteins, normalized against actin levels. *P<0.05 versus control.
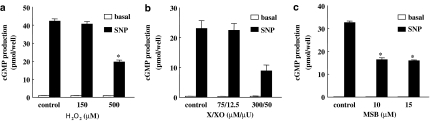
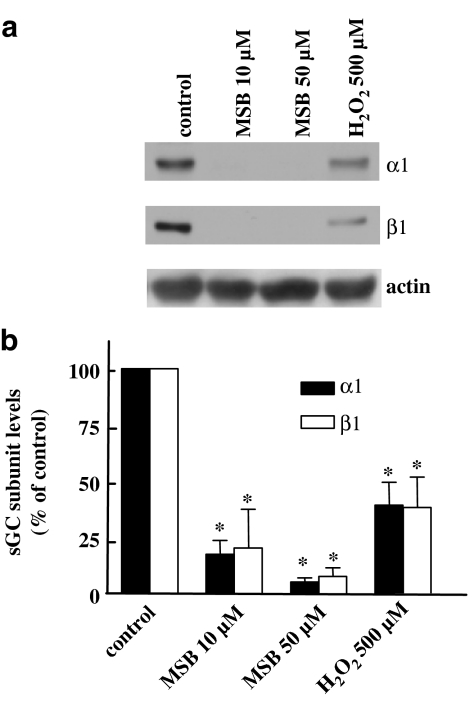
To demonstrate changes in the properties of cells exposed to ROS-regenerating agents, we measured cGMP accumulation in RASM stimulated with SNP. Following SNP treatment, cGMP levels increased 25–40-fold in control cells. As illustrated in Figure 2a–c, cells treated with H2O2, X/XO or MSB displayed reduced cGMP levels after stimulation with the NO donor, in a manner that correlated with the reduction in subunit levels. To determine whether ROS have the ability to downregulate sGC expression in contractile smooth muscle cells, rat aortic strips were incubated with vehicle, MSB or H2O2. As observed with cultured cells, both α1 and β1 levels were reduced after a 24 h incubation with ROS-generating agents (Figure 3a and b).
Figure 2.
ROS-generating agents inhibit SNP-induced cGMP-accumulation. RASM cells were treated as in Figure 1. After 24 h, cells were washed with HBBS and incubated in the presence of the phosphodiesterase inhibitor IBMX (1 mM) with or without the NO donor SNP for 15 min. The intracellular cGMP was then extracted with 0.1 N HCl and quantified by enzyme immunoassay. Values are means±s.e.m. (n=4); *P<0.05 versus control SNP.
Figure 3.
ROS-generating agents reduce the protein levels of both sGC subunits in freshly isolated arteries. Rat aortic strips were incubated for 24 h with hydrogen peroxide (H2O2; 500 μM), MSB (10 and 50 μM) or the corresponding vehicle in serum-free medium. Cell lysates were subjected to SDS–PAGE and Western blot analysis using sGC α1- and β1-specific antibodies. The bar graphs in (b) represent the mean±s.e.m. obtained from densitometric analysis of blots from four different animals and show percentages of α1 and β1 proteins, normalized against actin levels. *P<0.05 versus control. (a) shows blots from a representative experiment.
Mechanisms of ROS-induced downregulation of sGC
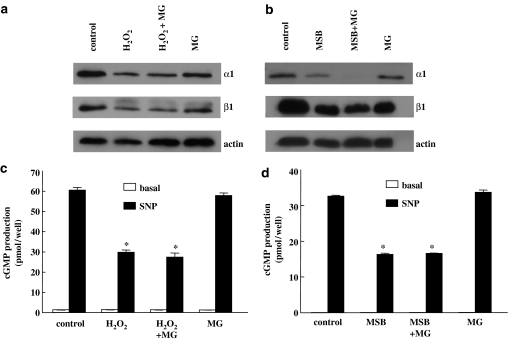
To identify the mechanisms responsible for the downregulation of sGC by ROS, we initially tested whether reduced sGC levels were the result of proteolytic degradation through the proteasome pathway. Therefore, RASM cells were exposed for 2 h with the proteasome inhibitor MG-132 before H2O2 or MSB treatment. sGC subunit protein levels and cGMP accumulation assessed 24 h later were comparable irrespective of the presence or not of the proteasome inhibitor (Figure 4a–d). Similar results were also obtained with another proteasome inhibitor, lactasystin (data not shown).
Figure 4.
The proteasome inhibitor MG-132 does not block ROS-induced downregulation of sGC. Cells were pretreated for 2 h with either vehicle or 10 μM of the proteasome inhibitor MG-132. After 2 h, cells were incubated for 24 h with H2O2 (500 μM; a and c) or MSB (10 μM; b and d). In a and b, cell lysates were prepared and blotted for sGC α1 and β1. Equal protein loading was verified by immunostaining of actin. Blots are representative of experiments repeated twice with similar results. In c and d, after 24 h, cells were washed with HBBS and cGMP accumulation was measured in the absence (basal) or presence of the NO donor SNP (10 μM) using the cGMP (low pH) immunoassay kit according to the manufacturer's instructions. Values are means±s.e.m. (n=4), *P<0.05 versus control SNP.
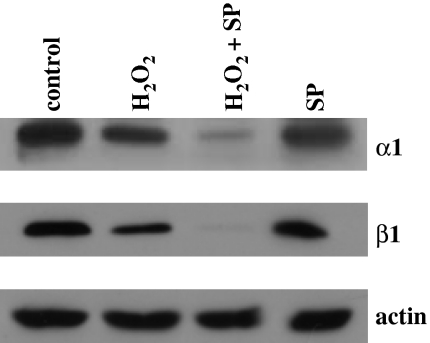
Recent evidence in the literature supports the notion that JNK activation reduces sGC expression (Krumenacker et al., 2005). To investigate the contribution of this pathway in the ROS-induced downregulation of sGC, we used the JNK inhibitor SP600125. As illustrated in Figure 5, exposure of unstimulated cells to SP600125 reduced α1 protein levels, suggesting that sGC expression requires basal JNK activity; moreover, pretreatment with SP600125 potentiated the H2O2-induced downregulation of sGC subunits.
Figure 5.
The JNK inhibitor SP600125 does not block H2O2-induced reduction of sGC subunit protein levels. RASM were serum-starved for 4 h and then pretreated for 1 h with either vehicle or 10 μM of the JNK inhibitor SP600125. After 1 h, cells were exposed to 500 μM of H2O2 for an additional 24 h. Cell lysates were subjected to SDS-PAGE and Western blot analysis using sGC α1- and β1-specific antibodies. Equal protein loading was verified by immunostaining of actin. The blots shown are representative of experiments repeated three times with similar results.
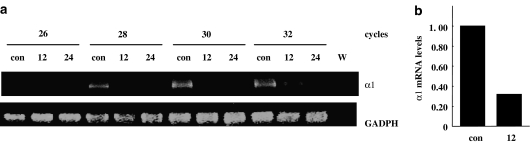
We next set out to investigate whether the reduction in protein levels observed was preceded by a decline in steady-state mRNA levels. We isolated total RNA from RASM cells exposed to H2O2 for 12 and 24 h and α1 mRNA levels were assessed using semiquantitative RT-PCR and quantitative real-time PCR. As illustrated in Figure 6a, high levels of α1 mRNA are present in the control cells, whereas H2O2 treatment for 12–24 h decreases expression of this subunit. Steady-state levels of α1 mRNA after 12 h of treatment with H2O2 were reduced by 68%, as determined by real-time PCR (Figure 6b).
Figure 6.
H2O2 reduces steady-state α1 mRNA levels. RASM were incubated for 12 and 24 h with 500 μM of H2O2 (12 and 24) or the corresponding vehicle (con). Total RNA was isolated, and cDNA was prepared as described in the Methods. α1 mRNA levels determined by semiquantitative RT-PCR (a) and quantitative real-time PCR (b). (a) Photograph depicts an ethidium bromide-stained agarose gel with the RT-PCR products for sGC α1 and GADPH. W=water, used as control in the PCR. (b) The bar graph shows the relative decrease of α1 mRNA levels in cells treated with H2O2 for 12 h compared to control in quantitative real-time RT-PCR.
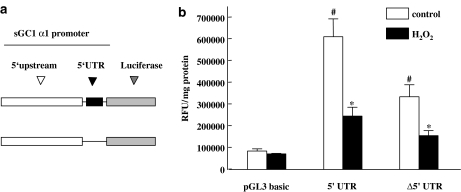
To examine the possible mechanisms implicated in downregulation of α1 steady-state mRNA levels observed, we tested the effect of H2O2 on the activity of α1 promoter-driven luciferase activity. RASM cells were transfected with the promoter constructs (Vazquez-Padron et al., 2004), exposed to 500 μM H2O2 for 24 h and luciferase activity was measured. As illustrated in Figure 7b, following H2O2 treatment promoter activity of the 1.45 kb fragment flanking the α1 gene decreased by 60%. Deletion of the UTR from the promoter sequence resulted in lower levels of transcriptional activity that was also inhibited by stimulation with H2O2. The above-mentioned observations taken together are consistent with the hypothesis that reduced steady-state α1 mRNA levels brought about by H2O2 are responsible for downregulation of sGC expression; the effects of H2O2 could be caused by reduced transcription and/or accelerated mRNA decay.
Figure 7.
H2O2 reduces α1 promoter-driven luciferase activity. (a) Schematic diagram of the promoter constructs used. (b) RASM cells were transiently transfected with the sGC α1 promoter constructs. Twenty-four hours after transfection, cells were serum-starved for 4 h and then incubated for an additional 24 h with 500 μM of H2O2. After 24 h cell, lysates were prepared and luciferase activity was measured. Values are means±s.e.m. (n=4), #P<0.05 versus empty vector (pGL3basic); *P<0.05 versus control.
Discussion
Overproduction of ROS is a common finding in cardiovascular disease and is accompanied by reduced endothelium-derived NO responses (Harrison, 1997; Maytin et al., 1999; Cai and Harrison, 2000). In some experimental models, however, decreased responsiveness of blood vessels to endothelium-independent vasorelaxing agents such as nitroglycerin and other NO donors is also observed, whereas dilation in response to cell-permeable cGMP analogs remains unaffected (Kagota et al., 2001). Taken together, these observations suggest that, in addition to reduced NO bioavailability, impaired sGC function might contribute to the increased tone seen in pathophysiological conditions associated with chronic overproduction of ROS. Despite these findings, however, no direct link between enhanced ROS and decreased sGC expression has been established.
To test directly the effect of oxidative stress, smooth muscle cells were exposed to H2O2 or X/XO and sGC subunit protein levels were determined. Exposure of RASM cells or freshly isolated aortic strips to ROS-generating agents resulted in a downregulation of both the α1 and β1 subunit, as well as sGC activity. In agreement with our observations, exposure of pulmonary artery smooth muscle cells to a H2O2-generating system led to a decrease in α1 protein levels and sGC activity (Wedgwood et al., 2005). In contrast, Weber et al. (2001) reported no change in the levels of β1 in rat aortic rings incubated with X/XO. The discrepancy between our data and that of Weber et al. (2001) might be explained by the shorter incubation time of the tissues (4 h) used in the latter study.
The concentrations of ROS-generating agents used in the present study are well within the range of concentrations commonly used in the literature. On the basis of the observations that actin levels remain unaltered following treatments with ROS-generating agents for 24 h and that protein content and cell viability are not affected, we can rule out the possibility that the observed effects on sGC levels and activity result from toxicity. It should be noted that endogenously produced ROS in response to receptor activation might mimic the effects of exogenously applied ROS-generating agents. We and others have shown that exposure of RASM to lipopolysaccharide (LPS), an agent that stimulates the production of ROS in mammalian cells, leads to a reduction in α1 and β1 mRNA and/or protein levels, as well as impaired relaxant responses to nitrovasodilators (Papapetropoulos et al., 1996a; Hoque et al., 1998; Takata et al., 2001). Although uncontrolled production of NO in these cells has been implicated in the downregulation of sGC levels, the observation that LPS reduces sGC expression in cells isolated from iNOS−/− mice (Takata et al., 2001) suggests that ROS might contribute to the effects of LPS on sGC subunit levels. Several additional observations made by a number of different laboratories support the notion that endogenously produced ROS reduce sGC expression. Spontaneously hypertensive rats exhibiting increased production of O2− present a deficit in sGC expression and in vascular relaxation in response to nitrovasodilators (Bauersachs et al., 1998; Ruetten et al., 1999; Kloss et al., 2000); under these conditions eNOS levels remained unchanged. Reduced β1 levels have also been reported in the aortas of rats rendered hypertensive by high salt intake (Kagota et al., 2001). Moreover, ageing, a condition characterized by higher than normal ROS levels (Finkel and Holbrook, 2000), has been linked to decreased α1 and β1 levels in normal and hypertensive animals (Ruetten et al., 1999; Kloss et al., 2000). Finally, reduction in the levels of β1 in animals that developed hypertension following lead administration is prevented by treatment with the antioxidant vitamin C (Marques et al., 2001). In contrast, Mulsch et al. (2001) observed an increase in sGC expression in nitroglycerin-tolerant vessels, in spite of the accompanying overproduction of superoxide.
ROS are known to activate multiple signaling pathways in mammalian cells including members of the mitogen-activated protein kinase (MAPK) family of proteins (Thannickal and Fanburg, 2000). Recently, JNK activation was found to mediate the decrease in α1 expression brought about by nerve growth factor and a tumor necrosis factor-α/interleukin-1β mixture (Krumenacker et al., 2005). To test the involvement of JNK in H2O2-induced reduction of sGC protein levels, cells were pretreated with the JNK inhibitor, SP600125. JNK inhibition downregulated sGC subunit protein levels in smooth muscle cells; in addition, the H2O2-triggered reduction in sGC levels was exacerbated in cells treated with SP600125 suggesting that the effects of H2O2 on sGC levels are JNK-independent. However, results obtained with this particular pharmacological inhibitor should be interpreted with caution, as it is known to inhibit other kinases in addition to JNK.
Mammalian cells have limited protein repair mechanisms and oxidatively damaged proteins are frequently targeted for destruction by proteolytic enzymes. Ample evidence suggests that many proteins in cells exposed to oxidative stress are degraded by the proteasome pathway (Davies, 2001). Pretreatment of cells with proteasome inhibitors did not prevent the H2O2-induced reduction of sGC protein, suggesting that ROS do not inhibit sGC expression by promoting proteasomal degradation of the enzyme subunits. To investigate if the reduced sGC protein levels could be because of reduced transcription and/or mRNA stability of the sGC subunits, we ascertained steady-state mRNA levels following treatment with H2O2. Indeed, such treatment caused a drastic decline in α1 mRNA levels that was accompanied by reduced α1 promoter activity. Treatment of cells with H2O2 might inhibit the binding or the function of transcription factors required for basal sGC α1 expression or promote the interaction of transcriptional repressors with the α1 promoter sequences. Alternatively, exposure of cells to H2O2 might inhibit the levels of the sGC mRNA stabilizing protein HuR; preliminary observations indicate that smooth muscle cells exposed to ROS exhibit a transient decrease in HuR protein (Gerassimou and Papapetropoulos; unpublished data).
In summary, exposure of smooth muscle cells to exogenously applied ROS-generating agents causes a decrease in α1 and β1 sGC subunit levels and attenuates cGMP formation induced by SNP. Moreover, ROS downregulate α1 steady-state mRNA levels; this decline in α1 expression is accompanied by a decrease in α1 promoter activity. As cGMP has been implicated in pathways regulating smooth muscle cell proliferation as well as vessel tone, inhibition of sGC expression by ROS in pathophysiological states would be expected to modulate vascular remodeling and tone.
Acknowledgments
This study was supported by a grant from the Greek Secretariat of Research and Technology and by the Thorax Foundation (Athens, Greece).
Abbreviations
- HBSS
Hank's balanced salt solution
- IBMX
isobutylmethylxanthine
- JNK
c-Jun N-terminal kinase
- MSB
menadione sodium bisulphite
- RASM
rat aortic smooth muscle cells
- ROS
reactive oxygen species
- RT-PCR
reverse transcriptase–polymerase chain reaction
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- X/XO
xanthine/xanthine oxidase
- SP600125
anthra[1,9-cd]pyrazol-6(2H)-one
Conflicts of interest
The authors have no conflict of interest.
References
- Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- Filippov G, Bloch DB, Bloch KD. Nitric oxide decreases stability of mRNAs encoding soluble guanylate cyclase subunits in rat pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:942–948. doi: 10.1172/JCI119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Harrison D. Endothelial function and oxidant stress. Clin Cardiol. 1997;20:II-11–II-17. [PubMed] [Google Scholar]
- Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci. 1997;18:484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- Hoque AM, Papapetropoulos A, Venema RC, Catravas JD, Fuchs LC. Effects of antisense oligonucleotide to iNOS on hemodynamic and vascular changes induced by LPS. Am J Physiol Heart Circ Physiol. 1998;275:H1078–H1083. doi: 10.1152/ajpheart.1998.275.3.H1078. [DOI] [PubMed] [Google Scholar]
- Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- Kagota S, Tamashiro A, Yamaguchi Y, Sugiura R, Kuno T, Nakamura K, et al. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br J Pharmacol. 2001;134:737–744. doi: 10.1038/sj.bjp.0704300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35:43–47. [PubMed] [Google Scholar]
- Kloss S, Furneaux H, Mulsch A. Post-transcriptional regulation of soluble guanylyl cyclase expression in rat aorta. J Biol Chem. 2003;278:2377–2383. doi: 10.1074/jbc.M206453200. [DOI] [PubMed] [Google Scholar]
- Kloss S, Srivastava R, Mulsch A. Down-regulation of soluble guanylyl cyclase expression by cyclic AMP Is mediated by mRNA-stabilizing protein HuR. Mol Pharmacol. 2004;65:1440–1451. doi: 10.1124/mol.65.6.1440. [DOI] [PubMed] [Google Scholar]
- Krumenacker JS, Kots A, Murad F. Effects of the JNK inhibitor anthra[1,9-cd]pyrazol-6(2H)-one (SP600125) on soluble guanylyl cyclase {alpha}1 gene regulation and cGMP synthesis. Am J Physiol Cell Physiol. 2005;289:C778–C784. doi: 10.1152/ajpcell.00057.2005. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- Marques M, Millas I, Jimenez A, Garcia-Colis E, Rodriguez-Feo JA, Velasco S, et al. Alteration of the soluble guanylate cyclase system in the vascular wall of lead-induced hypertension in rats. J Am Soc Nephrol. 2001;12:2594–2600. doi: 10.1681/ASN.V12122594. [DOI] [PubMed] [Google Scholar]
- Maytin M, Leopold J, Loscalzo J. Oxidant stress in the vasculature. Curr Atheroscler Rep. 1999;1:156–164. doi: 10.1007/s11883-999-0012-z. [DOI] [PubMed] [Google Scholar]
- Moncada S, Rees D, Schulz R, Palmer R. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. PNAS. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsch A, Oelze M, Kloss S, Mollnau H, Topfer A, Smolenski A, et al. Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in aorta. Circulation. 2001;103:2188–2194. doi: 10.1161/01.cir.103.17.2188. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Abou-Mohamed G, Marczin N, Murad F, Caldwell R, Catravas J. Downregulation of nitrovasodilator-induced cyclic GMP accumulation in cells exposed to endotoxin or interleukin-1 beta. Br J Pharmacol. 1996a;118:1359–1366. doi: 10.1111/j.1476-5381.1996.tb15545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A, Go C, Murad F, Catravas J. Mechanisms of tolerance to sodium nitroprusside in rat cultured aortic smooth muscle cells. Br J Pharmacol. 1996b;117:147–155. doi: 10.1111/j.1476-5381.1996.tb15167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyriochou A, Papapetropoulos A. Soluble guanylyl cyclase: more secrets revealed. Cell Signal. 2005;17:407–413. doi: 10.1016/j.cellsig.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Ruetten H, Zabel U, Linz W, Schmidt HHHW. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res. 1999;85:534–541. doi: 10.1161/01.res.85.6.534. [DOI] [PubMed] [Google Scholar]
- Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, et al. Redox regulation of vascular smooth muscle cell differentiation. Circ Res. 2001;89:39–46. doi: 10.1161/hh1301.093615. [DOI] [PubMed] [Google Scholar]
- Takata M, Filippov G, Liu H, Ichinose F, Janssens S, Bloch DB, et al. Cytokines decrease sGC in pulmonary artery smooth muscle cells via NO-dependent and NO-independent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;280:L272–L278. doi: 10.1152/ajplung.2001.280.2.L272. [DOI] [PubMed] [Google Scholar]
- Tarpey MM, Beckman JS, Ischiropoulos H, Gore JZ, Brock TA. Peroxynitrite stimulates vascular smooth muscle cell cyclic GMP synthesis. FEBS Letters. 1995;364:314–318. doi: 10.1016/0014-5793(95)00413-4. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Vazquez-Padron RI, Pham SM, Pang M, Li S, Aitouche A. Molecular dissection of mouse soluble guanylyl cyclase alpha1 promoter. Biochem Biophys Res Commun. 2004;314:208–214. doi: 10.1016/j.bbrc.2003.12.078. [DOI] [PubMed] [Google Scholar]
- Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- Weber M, Lauer N, Mulsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Rad Biol Med. 2001;31:1360–1367. doi: 10.1016/s0891-5849(01)00706-7. [DOI] [PubMed] [Google Scholar]
- Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, et al. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2005;289:L660–L666. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gross S, Roussos C, Meurer S, Müller-Esterl W, Papapetropoulos A. Structural and functional characterization of the dimerization region of soluble guanylyl cyclase. J Biol Chem. 2004;279:24935–24943. doi: 10.1074/jbc.M402105200. [DOI] [PubMed] [Google Scholar]