Abstract
Background and purpose:
An up-regulation of the sensory neural pathways in the lung has been implicated in asthma and chronic obstructive pulmonary disease (COPD) and is thought to contribute to mucus hypersecretion, an essential feature of both diseases. The aim of this study was to assess non-invasively the acute effects (up to 60 min) of sensory nerve stimulation by capsaicin in the lung, using magnetic resonance imaging (MRI).
Experimental approach:
Male Brown Norway rats were imaged prior to and 10, 30 and 60 min after intra-tracheal challenge with capsaicin (30 μgkg−1) or vehicle (0.5% ethanol solution). In subsequent studies, pre-treatment with the transient receptor potential vanilloid (TRPV)-1 antagonist, capsazepine; the dual neurokinin (NK) 1 and NK2 receptor antagonist, DNK333 and the mast cell stabilizer, di-sodium cromoglycate (DSCG) was used to modulate the effects of capsaicin.
Key results:
Diffuse fluid signals were detected by MRI in the lung as early as 10 min after capsaicin, remaining constant 30 and 60 min after treatment. Broncho-alveolar lavage (BAL) fluid analysis performed 60 min after capsaicin revealed increased mucin concentration. Capsazepine (3.5 mgkg−1), DNK333 (10 mgkg−1) but not DSCG (10 mgkg−1) administered prophylactically were able to block the effect of capsaicin in the airways.
Conclusions and implications:
These observations suggest that the fluid signals detected by MRI after capsaicin administration reflected predominantly the release of mucus following activation of sensory nerves. They point to the opportunity of non-invasively assessing with MRI the influence of neuronal mechanisms in animal models of asthma and COPD.
Keywords: sensory nerves, tachykinins, non-invasive, spontaneously breathing, TRPV-1, neurokinin
Introduction
Cough and sputum production are characteristic of asthma, a condition linked increased mucus production, goblet cell hyperplasia and submucosal gland hypertrophy (Liu et al., 1998; Rogers, 2001). Chronic mucus hypersecretion is indicative of poor asthma control and is closely associated with a decline in lung function, increasing the risk of morbidity and mortality (Rogers, 2004). Mucus hypersecretion and a reduced capacity to clear it are also important characteristics of chronic obstructive pulmonary disease (COPD) (Rogers, 2005), contributing to increased morbidity and mortality in COPD patients (Vestbo et al., 1996). Consequently, it is essential to understand the pathophysiology of mucus hypersecretion in asthma (Rogers, 2004) and the component of COPD attributable to impaired mucociliary clearance (Melton, 2002; Rogers, 2005).
An upregulation of sensory-efferent neural pathways is also implicated in COPD and asthma (Rogers, 1997). Airway sensory nerves release several neuropeptides of which the tachykinins (neurokinin A (NKA) and substance P (SP)) contribute most to the pathophysiology of asthma and COPD (Joos et al., 2003). These neuropeptides activate the NK receptors NK1, NK2 and NK3, thereby inducing a variety of biological effects (Patacchini and Maggi, 1995). In subjects with asthma, increased immunoreactivity to the tachykinin SP has been observed in plasma, induced sputum and nasal lavage. An increase in tachykininergic nerves and upregulation of a tachykinin receptor mRNA has also been observed (Mapp et al., 2000). In COPD, levels of SP are increased in sputum (Mapp et al., 2000). Tachykininergic nerve stimulation, or pollutant and irritant gases that activate sensory nerves (Germonpre et al., 1995), are implicated in the pathophysiology of COPD (Rogers, 2005) and have been shown experimentally to contribute to mucin upregulation (Rogers, 2001).
Activation of sensory nerves in the lung can be induced experimentally by a variety of insults such as electrical stimulation of the vagus nerve or by application of chemical and mechanical irritants, such as capsaicin, hypertonic saline and cigarette smoke (Germonpre et al., 1995). Activation of sensory nerves by capsaicin or other stimuli leads to the release of neuropeptides from nerve terminals either directly or through axonal reflexes (Reynolds et al., 1997). An increase in plasma extravasation from the rat trachea has been shown with capsaicin administered intratracheally (i.t.) at doses between 3 and 30 μg kg−1 (Fozard et al., 2001). Tachykinins may be involved in smooth muscle contraction, vasodilatation, edema, mucus secretion and inflammatory cell activation (Reynolds et al., 1997).
The measurement of fluid volumes from the lungs is important for the assessment of mucus plugging and inflammation, particularly in the peripheral compartment of the lung as the diagnostic techniques that are often used are more sensitive to changes in the central conducting airways than to those in the peripheral compartment of the lung (Mead, 1970). Through the assessment of lung fluid signal volumes, proton magnetic resonance imaging (MRI) techniques have been shown to be successful in detecting edema as a result of allergen challenge (Beckmann et al., 2001; 2002) or mucus hypersecretion following treatment with endotoxin.
Thus the aim of our study was to investigate non-invasively, by the use of MRI to determine lung fluid signal volumes, the acute effects induced in the lungs following the activation of airway sensory nerves by capsaicin in fully anaesthetized Brown Norway (BN) rats. The role of sensory nerves and tachykinins in mediating the effects of capsaicin in the airways was studied using the transient receptor potential vanilloid (TRPV)-1 antagonist capsazepine (3.5 mgkg−1 i.t.); and the dual NK1 and NK2 receptor antagonist N-[(R,R)-(E)-1-(3,4-dichlorobenzyl)-3-(2-oxoazepan-3-yl) carbamoyl]allyl-N-methyl-3,5-bis(trfluoromethy)benzamide (DNK333) (10 mg kg−1 i.p.). As TRPV-1 has also been observed in mast cells (Biro et al., 1998), we have also utilized the drug di-sodium cromoglycate (DSCG), which has been shown to inhibit mast cell activation in the rat (Norris, 1996).
Materials and methods
Experiments were performed with the approval of the Veterinary Authority of the City of Basel licence 567 (Kantonales Veterinaeramt, Basel-Stadt).
Animals
Fifty-three male BN rats, weighing 250–300 g, were supplied by IFFA CREDO (L' Arbresle Cedex, France). The rats were kept at an ambient temperature of 22±2°C under a 12 h normal phase light–dark cycle and fed NAFAG pellets (Nahr und Futtermittel AG, Gossau, Switzerland). Drinking water was freely available.
Administration of substances
Rats were kept anaesthetized with 2.5% isoflurane (Abbott, Cham, Switzerland) in a mixture of O2/N2O (1:2) administered via a face mask, throughout the whole experimental procedure. Animals were killed immediately at the end of each experiment. Following the baseline MRI signal acquisition (see below), animals were taken out of the magnet and a single dose of capsaicin (30 μg kg−1, n=12) or vehicle (0.5% alcohol, 0.2 ml, n=12) was administered i.t., at a level above the carina, without letting the animals wake up. The dose was chosen based on the experiments of Fozard et al. (2001), where doses ranging from 3 to 30 μg kg−1 of capsaicin were shown to cause plasma extravasation in the rat trachea. The rats were repositioned in the magnet immediately after capsaicin administration.
In subsequent studies animals were anesthetized and, following acquisition of baseline images, pretreated with (i) capsazepine (3.5 mg kg−1 i.t.; n=5) or its vehicle (0.2 ml of a 0.5% ethanol solution i.t.; n=4); (ii) DSCG (10 mg kg−1 i.t., n=5) or its vehicle (0.1 ml saline i.t., n=5); or (iii) DNK333 (10 mg kg−1 intraperitoneally (i.p.), n=5) or its vehicle (2 mg kg−1 of a 4% dimethyl sulphoxide (DMSO) solution in Neoral placebo (n=5)). Capsaicin (30 μg kg−1 i.t.) was administered i.t. thirty minutes after treatment with capsazepine, DNK333 or their respective vehicles, and immediately following DSCG or vehicle treatment. Following administration of capsaicin, animals were kept anaesthetized with 2.5% isoflurane and imaged 60 min after administration of capsaicin. The dose of capsazepine used was based on experiments by Lee and Lundberg (1994), where doses of 2 to 3.5 mg kg−1 i.v. inhibited chemoreflexes (apnea, bradycardia, and hypotension) induced by capsaicin. Oral administration of DNK333 (10 mg kg−1) has been shown to inhibit citric acid-induced cough by 82% and SP-induced bronchoconstriction when administered intraduodenally 30 min before endotoxin challenge in the guinea pig (Lewis et al., 2004). The affinity of DNK333 for the rat NK1 and NK2 receptors is of the same order as that for the guinea pig receptors (unpublished observations, Novartis Pharma Research). The dose of DSCG was based on experiments performed by Dixon and Barnes (1989), showing that inhaled DSCG (10 mg) was able to inhibit the effects of bradykinin-induced bronchoconstriction in mild asthmatics.
MRI
MRI acquisition protocol
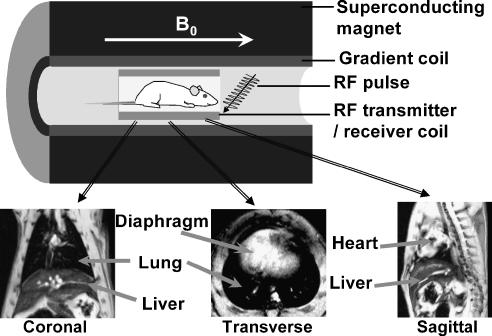
During MRI acquisitions rats were placed in a supine position in a cradle made of Plexiglas, in the centre of the magnet. Body temperature was maintained at 37±1°C using warm air, regulated by a rectal temperature probe (DM 852, Ellab, Copenhagen, Denmark). Rats were anaesthetized with 2.5% isoflurane, in a mixture of O2/N2O (1:2), administered via a facemask. All scans were performed on spontaneously breathing animals, and neither cardiac nor respiratory triggering was applied. Measurements were carried out with a Biospec 47/40 spectrometer (Bruker Medical Systems, Ettlingen, Germany) operating at 4.7 T, equipped with an actively shielded gradient system capable of generating a gradient of 200 m Tm−1 (Figure 1).
Figure 1.
Top: Diagrammatic representation of the components required for an MRI scan. B0 represents the homogenous magnetic field at 4.7 T. Bottom: Representative coronal, transverse and sagittal MR images of a rat showing the position of the lung, liver, diaphragm and heart in the different slices.
The operational software of the scanner was Paravision (version 3.1, Bruker). The parameters of a gradient-echo sequence were chosen with the aim of detecting fluid signals appearing in the lung as observed after an allergen or endotoxin challenge (Beckmann et al., 2001, 2002): repetition time, 5.6 ms; echo time, 2.7 ms; excitation pulse (Gaussian shape) length, 1 ms; flip angle of the excitation pulse approximately 15°; field-of-view, 6 × 6 cm2; matrix size, 256 × 128; slice thickness 1.5 mm. A single transverse slice image was obtained by computing the two-dimensional Fourier transform of the averaged signal from 45 individual image acquisitions and interpolating the data set to 256 × 256 pixels. There was an interval of 530 ms between individual image acquisitions, resulting in a total acquisition time of 56 s for a single slice. Twenty slices were acquired consecutively. Animals were subject to MRI scanning sessions at baseline (before capsaicin or vehicle administration) and at 10, 30 and 60 min after challenge. In experiments involving capsazepine, DSCG or DNK333, animals were imaged at baseline and 60 min after capsaicin (30 μg kg−1 i.t.) challenge.
MRI analysis
The volume of fluid signal was quantified using a semiautomatic segmentation procedure implemented in the Interactive Data Language Research Systems ((IDL) Boulder, CO, USA) environment (version 5.1) on a Linux platform. Images were first weakly lowpass filtered with a Gaussian profile filter and then transformed into a set of four gray level classes using adaptive Lloyd–Max histogram quantization. This method avoids operator bias owing to the arbitrary choice of threshold levels on each image (Beckmann et al., 2001). As the fluid comprised high signal intensities in the original images, it was represented by the highest gray level class in the transformed images. This class could be extracted interactively by use of a region grower. Because of the unknown extent of the fluid, no morphology parameters were incorporated into the region growing process. Instead, a contour serving as a growing border was drawn to control region growing manually. The segmentation parameters were the same for all the analyzed images, chosen to segment regions corresponding to high intensity signals. Twenty individual images covering the thorax were analyzed; the thickness of each individual image was 1.5 mm, thus permitting the software to determine the volume of signal present in each individual slice. The total volume corresponding to the sum of 20 consecutive slices per time point was calculated. Because the fluid signals and those from vessels were of comparable intensities, the volume corresponding to the vessels was assessed on baseline images and then subtracted from the total volume determined on post-treatment time points.
Post-mortem analyses
Following the MRI session (60 min after challenge) the animals were killed with an overdose of pentothal (250 mgkg−1 i.p.) for bronchoalveolar lavage (BAL) fluid (n=12; six capsaicin and six vehicle treated) and histological analysis (n=12; six capsaicin and six vehicle treated). In experiments involving capsazepine, DSCG and DNK333, only BAL fluid analysis was performed.
BAL fluid analysis
A detailed description of the procedure to obtain BAL fluid and the analysis of the parameters of inflammation herein has been provided earlier (Beckmann et al., 2002, Tigani et al., 2002). Briefly, after killing the animals with an overdose of pentothal, the lungs were lavaged three times with 4 ml of ethylenediaminetetraacetic acid+phosphate-buffered saline (PBS) (solution A) recovering between 10 and 11 ml of BAL fluid that was made up to a volume of 12 ml with solution A. For leukocyte numbers and cell differentiation, approximately 4 ml of BAL fluid was used by an automatic cell analyzing system used for the analysis (Cobas Helios 5Diff, Hoffmann-La Roche, Axon Laboratory, Switzerland). Determination of eosinophil peroxidase was based on the oxidation of o-phenylenediamine by eosinophil peroxidase in the presence of hydrogen peroxide. Myeloperoxidase activity was measured in a photometric assay based on the oxidation of O-dianiside dihydrochloride by myeloperoxidase in the presence of hydrogen peroxide. The level of protein in the BAL fluid supernatants was measured by a photometric assay, based on the reaction of protein with an alkaline copper tartrate solution and Folin reagent. Mucus in BAL fluid was assessed from 1 ml of BAL fluid per animal, retrieved before lavage for cell count and differentiation. The sandwich enzyme-linked lectin assay as described earlier (Beckmann et al., 2002) was used to determine the quantity of mucus by measuring the optical density of flat-bottomed Coastar plates coated with Ulex europaeus agglutinin-1 (Sigma) at a wavelength of 492 nm using a SpectraMax 250 plate reader (Molecular Devices, Surrey, UK).
Histological analysis
Lungs were inflated via a cannula inserted into the trachea with 5 ml of 10% PBS neutral formalin. The lungs were then removed from the thorax and immersed in formalin for at least 24 h but no longer than 72 h. The lung tissue was then sectioned transversally through the left lobe, the right apical, the right median and the right caudal lobes so as to include the main bronchi as well as the pulmonary alveoli. After processing to paraffin wax, sections were embedded in blocks. Slices of 3 μm thickness cut from these blocks were stained using Alcian blue-periodic acid Schiff (AB/PAS) for the detection of goblet cells acid and neutral mucus. Staining using Verhoeff's reaction was performed for the demonstration of elastic fibers and for the subsequent quantification of perivascular edema.
Quantification of goblet cells
Three to seven pictures of bronchi from the apical, median and caudal lobes of each lung were captured at × 10 magnification on AB/PAS-stained slides. Morphometric analyses were performed with the software ‘Image Access 4.0' (Imagic, Glattbrugg, Switzerland) connected to a video camera ‘Prog/Res/3008' (Jenoptic LOS, Eching, Germany). The circumference of each bronchus was manually delineated and the number of goblet cells was counted. Results were expressed as numbers of goblet cells per mm length bronchus. Only bronchi with a diameter between 800 and 2300 μm were measured. The total goblet cell numbers (average from the apical, median and caudal lobes) are presented.
Quantification of perivascular edema
Three to seven pictures of arterioles from each section of each lobe (apical, median and caudal) were captured at × 10 magnification on Verhoeff-stained slides. Morphometric analyses were performed with the software ‘Image Access 4.0' connected to a video camera ‘Prog/Res/3008'. As described by Quintana et al. (2006), the areas of external edema and external elastica lamina were manually circumscribed, and perivascular edema was calculated as a percentage of external elastica lamina area. Only vessels of diameter comprised between 20 and 200 μm were taken into account. Approximately 20 vessels were measured per animal. The total edema determined histologically (average of the edema in the apical, median and caudal lobes) is shown.
Statistics
A multiple comparisons analysis of variance (ANOVA) test (with the Holm–Sidak method) was applied for data relating to MRI fluid signal volume from vehicle- and capsaicin-treated rats imaged at baseline, 10, 30 and 60 min after treatment. Student's t-tests (two-tailed) were performed to assess differences in histological and BAL fluid parameters between capsaicin- and vehicle-treated animals and for MRI fluid signal volumes between vehicle- and drug-treated groups. The Mann–Whitney U-test was chosen for analysis of non-parametric histological data relating to goblet cell numbers. The significance levels are *P<0.05, **0.05<P<0.01 and ***P<0.001.
Materials
The suppliers for the drugs used in these experiments were as follows: capsaicin and capsazepine (Sigma-Aldrich, Buchs, Switzerland); di-sodium cromoglycate (DSCG) and DNK333 (Novartis Pharma, Basel, Switzerland) isoflurane (Abbott, Cham, Switzerland).
Results
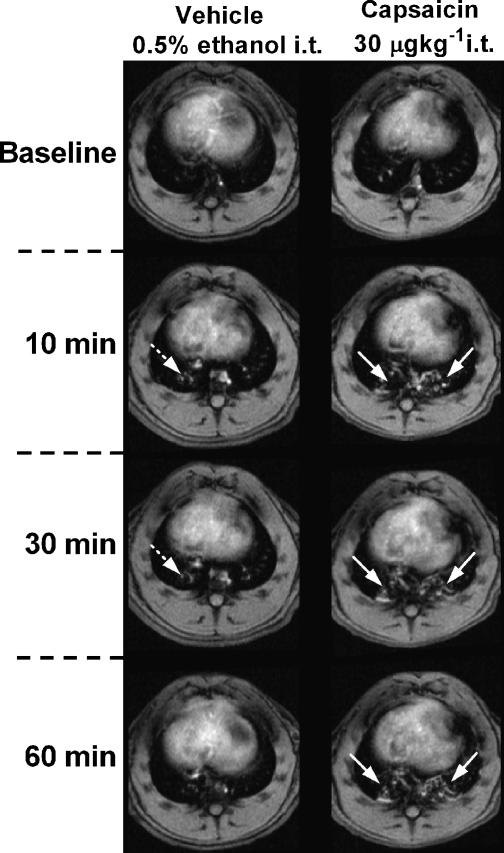
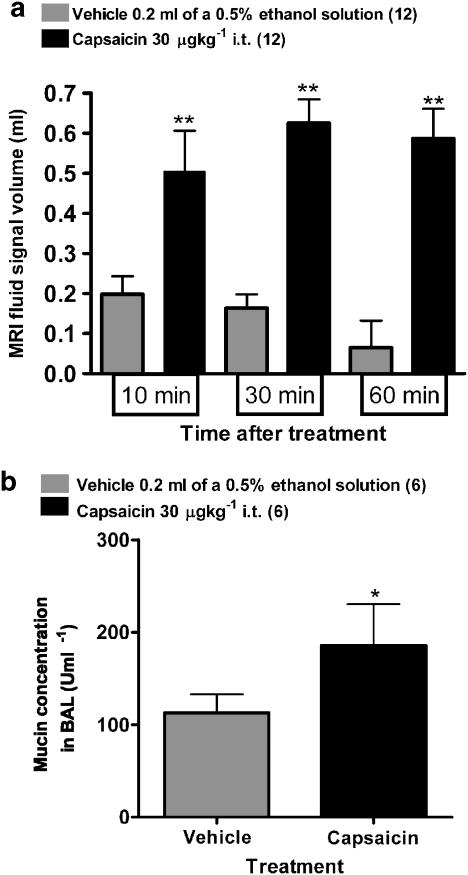
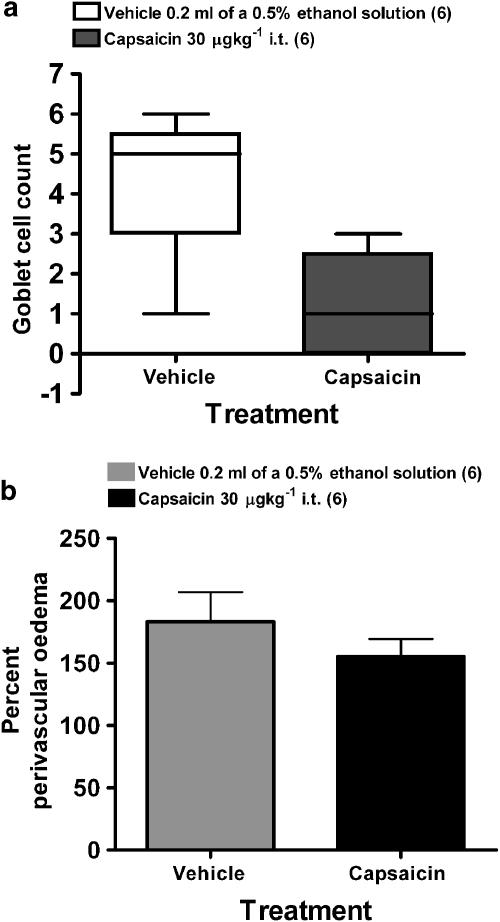
Instillation of capsaicin into the trachea resulted in the appearance of irregular fluid signals in MR images as early as 10 min after challenge, and which remained constant 30 and 60 min after treatment (Figures 2 and 3a). BAL fluid analysis performed 60 min after challenge revealed an increased mucin concentration in the fluid removed from capsaicin-treated animals (Figure 3b). Furthermore, an increase in the number of neutrophils, macrophages and myeloperoxidase (MPO) activity (indicating neutrophil activation) was observed in the BAL fluid (Table 1). Interestingly, no significant differences were observed by histology in goblet cell number between capsaicin- and vehicle-treated groups (Figure 4a). Also, no differences were found at this time point in protein concentration in the BAL fluid or in perivascular edema between capsaicin- and vehicle-treated groups (Table 1 and Figure 4b).
Figure 2.
Transverse sections through the thorax of rats treated with either vehicle (0.5% ethanol; 0.2 ml i.t.) or capsaicin (30 μg kg−1 i.t.). The time points are relative to the i.t. challenges. The white arrows denote diffuse signals related to capsaicin-induced mucus release as shown by post-mortem BAL fluid and histological analyses. The dotted white arrows show the appearance of a diffuse signal as a result of a volume of fluid being instilled in the lungs of the vehicle-treated animal.
Figure 3.
(a) Lung fluid signal volumes (means±s.e.m., n=12 per group) determined from MR images at baseline, 10, 30 and 60 min after challenge in rats treated with vehicle or capsaicin. The significance level **0.001<P<0.01 refers to ANOVAs (Holm–Sidak method) between capsaicin and vehicle treatment at each time point. (b) Mucin concentration (means±s.e.m., n=6 per group) in BAL removed 60 min after challenge with vehicle or capsaicin. Higher levels of mucin concentration were present in capsaicin-treated rats compared with vehicle-treated animals (*P<0.05; Student's t-test).
Table 1.
Inflammatory cell infiltration and activation (mean values ±s.e.m., n=6 animals per group) in BAL fluid of BN rats treated with vehicle (0.5% ethanol; 0.2 ml i.t.) or capsaicin (30 μgkg−1 i.t.)
| Vehicle (0.5% ethanol) 0.2 ml i.t. (n=6) | Capsaicin 30 μgkg−1 i.t. (n=6) | |
|---|---|---|
| Neutrophils × 106 | 0.18±0.03 | 0.29±0.02* |
| MPO (mU ml−1) | 52.1±10.0 | 94.9±10.3* |
| Eosinophils × 106 | 0.93±0.24 | 1.09±0.05 |
| EPO (mU ml−1) | 2.0±0.2 | 2.2±0.3 |
| Protein (μg ml−1) | 0.21±0.03 | 0.26±0.03 |
| Macrophages (× 106) | 1.99±0.25 | 4.14±0.33** |
| Lymphocytes (× 106) | 0.39±0.10 | 0.44±0.05 |
| Total cells (× 106) | 3.60±0.44 | 5.96±0.40** |
Abbreviations: BN, Brown Norway; EPO, eosinophil peroxidase; MPO, myeloperoxidase.
Animals were killed 60 min after challenge with either substance. The significance levels *P<0.05, and **0.001<P<0.01 refer to comparisons between vehicle- and capsaicin-treated animals.
Figure 4.
(a) Goblet cell number (means±s.e.m., n=6 rats per group) in individual bronchi determined from the apical, median and caudal lobes taken 60 min after challenge from rats treated with vehicle (0.5% ethanol; 0.2 ml i.t.) or capsaicin (30 μg kg−1 i.t.). The Mann–Whitney U-test revealed no difference between the treatment groups. (b) Perivascular edema assessed from apical, median and caudal lobes. Values (means±s.e.m. for six animals in each group) represent total edema values expressed as a percentage.
Intratracheal administration of the vehicle for capsaicin (0.2 ml of a 0.5% ethanol solution) resulted in the appearance of diffuse MRI fluid signals of lower magnitude than those following capsaicin. These signals were short-lived, lasting no more than 30 min and are assumed to reflect the introduction of a volume of fluid (0.2 ml) into the lungs of the rat.
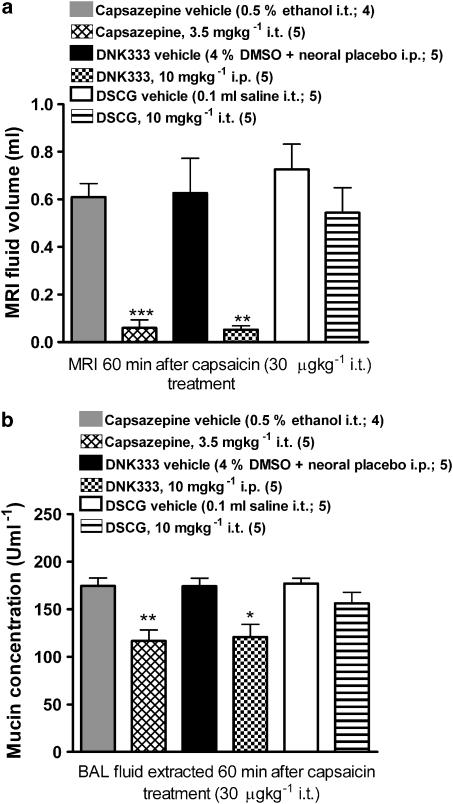
Both the TRPV-1 antagonist (capsazepine; 3.5 mgkg−1 i.t.) and the dual NK1 and NK2 receptor antagonist (DNK333; 10 mgkg−1 i.p.) were able to inhibit the response to capsaicin in the airways when compared with their respective vehicle groups, in terms of MRI fluid signal volumes (Figure 5a). Mucin concentrations in BAL fluid were also reduced in capsazepine- and DNK333-treated rats compared with their respective vehicle groups (Figure 5b). Treatment with DSCG (10 mg kg−1 i.t.) immediately before capsaicin challenge did not have any effect on MRI fluid signal volumes (Figure 5a or in mucin concentration in BAL fluid when compared with its vehicle group (Figure 5b). Pre-treatment with capsazepine, DNK333, or DSCG did not reduce the infiltration of inflammatory cells present in BAL fluid and did not inhibit MPO activity in BAL fluid (Table 2).
Figure 5.
(a) Lung fluid signal volumes (means±s.e.m., n=5 per group) determined from MR images at baseline, and 60 min after capsaicin challenge in rats pre-treated with (i) capsazepine (3.5 mg kg−1 i.t.) or its vehicle (0.2 ml of a 0.5% ethanol solution i.t.); (ii) DNK333 (10 mg kg−1 i.p.) or its vehicle (4% DMSO in Neoral placebo i.p.); or (iii) DSCG (10 mgkg−1 i.t.) or its vehicle (0.2 ml saline i.t.). Capsaicin was administered 30 min after capsazepine or DNK333 treatment, and immediately after DSCG. Significance levels **0.001<P<0.01 and ***P<0.001 refer to Student's t-test comparisons between each treatment regimen and its respective vehicle. (b) Mucin concentration (means±s.e.m.) in BAL fluid removed 60 min after capsaicin challenge, immediately following an MRI acquisition, from rats that were submitted to the treatment regimens specified in (a). Both capsazepine and DNK333 resulted in lower concentrations of mucin in BAL fluid when compared with their respective vehicle groups (*0.01<P<0.05 and **0.001<P<0.01; Student's t-tests).
Table 2.
Inflammatory cell infiltration and activation (mean values±s.e.m.) in BAL fluid of BN rats treated with (i) capsazepine (3.5 mgkg−1 i.t.; n=5) or its vehicle (0.2 ml of a 0.5% ethanol solution i.t.; n=4); (ii) DSCG (10 mgkg−1 i.t., n=5) or its vehicle (0.1 ml saline i.t., n=5); or (iii) DNK333 (10 mgkg−1 i.p., n=5) or its vehicle (2 mgkg−1 of a 4% DMSO solution in Neoral placebo, n=5). Capsaicin was administered 30 min after treatment with capsazepine, DNK333 or their respective vehicles, and immediately following administration of DSCG or its vehicle
| Vehicle (0.5% ethanol) | Capsazepine (3.5 mg kg−1 i.t.) | Vehicle (4% DMSO in Neoral placebo) | DNK333 (10 mg kg−1 i.p.) | Vehicle saline 0.2 ml i.t. | DSCG (10 mg kg−1 i.t.) | |
|---|---|---|---|---|---|---|
| Neutrophils (× 106) | 0.49±1.58 | 0.37±0.09 | 0.25±0.04 | 0.25±0.04 | 0.49±1.58 | 0.17±0.03 |
| MPO mU mL−1 | 46±3 | 35±8 | 43±5 | 40±2 | 46.8±4 | 41±1 |
| Eosinophils (× 106) | 1.22±0.32 | 0.80±0.1* | 0.94±0.31 | 0.83±0.39 | 1.17±0.12 | 0.92±0.16 |
| Macrophages (× 106) | 5.10±0.76 | 4.60±0.49 | 3.73±0.64 | 3.03±0.69 | 4.77±0.82 | 5.62±0.48 |
| Lymphocytes (× 106) | 1.30±0.56 | 0.83±0.06 | 0.68±0.30 | 0.26±0.06 | 1.28±0.37 | 0.28±0.05* |
| Total cells (× 106) | 8.10±1.66 | 6.10±0.63 | 5.81±0.91 | 4.37±1.12 | 7.70±1.39 | 6.98±0.48 |
Abbreviations: BAL, bronchoalveolar lavage; DMSO, dimethyl sulfoxide; DSCG, di-sodium cromoglycate; MPO, myeloperoxidase.
Animals were killed 60 min after capsaicin administration for BAL fluid analysis. Substances were administered i.t., with the exception of DNK-33 and its vehicle, which were applied i.p. The significance levels *P< 0.05 refer to comparisons between vehicle-treated and capsazepine or DSCG-treated animals.
Discussion and conclusions
The major finding of the present study was that local administration of capsaicin (30 μgkg−1 i.t.) resulted in the appearance of diffuse fluid signals in MR images of the lung at 10 min after treatment which were still present 30 and 60 min after administration of the pungent chili pepper extract, capsaicin. The changes correlated with an increase of mucin concentration in the BAL fluid, and a tendency towards a reduction of goblet cells in histological slides from capsaicin-treated, compared with vehicle-treated groups. These results suggest that mucus release from goblet cells induced by capsaicin was the principal contributor to the MRI signals. Pre-treatment with capsazepine, a TRPV-1 antagonist, or with DNK333, an NK1 and NK2 receptor antagonist, prevented the appearance of fluid signals in MR images acquired following capsaicin challenge. Consistent with these observations, a reduced level of mucin concentration was found in capsazepine- and DNK333-treated animals compared with their corresponding vehicle groups.
Capsaicin excites a subset of primary sensory neurons with somata in the dorsal root ganglia or the trigeminal ganglion (Szallasi and Blumberg, 1999). Airway sensory nerves effects are induced via the jugular ganglion of the vagus or the rostral portion of the nodose ganglion (McDonald et al., 1988). Capsaicin-induced effects are mediated through the activation of the vanilloid receptor TRPV-1, a heat-sensitive cation channel on nociceptor terminals (Caterina et al., 1997; Szallasi and Blumberg, 1999). In the present studies, capsazepine (3.5 mgkg−1 i.t.), a TRPV-1 antagonist (Bevan et al., 1992), administered 30 min before capsaicin was able to fully inhibit the effects of the chili pepper extract in the lungs as detected by MRI and BAL fluid analysis. This finding further supports a role for the vanilloid receptor, TRPV-1, in mediating capsaicin-induced mucus release in the lungs. However, expression of TRPV-1 is not confined to sensory nerves; evidence suggests that this receptor is also present in mast cells (Biro et al., 1998) and that in the presence of capsaicin, the level of intracellular calcium is raised (Biro et al., 1998). In the present investigation, inhibition of mast cell degranulation by DSCG did not inhibit mucus release induced by capsaicin. This supports the role of TRPV-1 expressed in sensory nerves and not mast cells in mediating the effects of capsaicin challenge in the airways.
Stimulation of sensory nerves by capsaicin results in the release of neuropeptides such as SP, calcitonin gene-related peptide (CGRP) and NKA (Szallasi and Blumberg, 1999). Among these sensory neuropeptides, release of SP shows the best correlation with activation of TRPV-1 (Buck and Burks, 1986; Holzer 1991). SP and NKA act mainly through NK1 receptors in secretory glands and in the respiratory epithelium mediating mucus hypersecretion, edema formation, vasodilatation, and release of prostaglandins and pro-inflammatory cytokines (Rogers, 1995; Reynolds et al., 1997). Activation of NK1 receptors is strongly linked to neurogenic airway secretor processes that comprise mucus secretion and ion transport (Rogers, 1995). In vitro studies showed that SP stimulates mucus secretion from submucosal glands in ferret and in human airways (Rogers et al., 1989; Meini et al., 1993; Ramnarine et al., 1994), as well as in the guinea pig trachea (Kuo et al., 1990). Further evidence of the role of sensory nerves in mucus secretion comes from studies where sensory nerves have been de-sensitized. De-sensitization of sensory nerves by sub-chronic administration of capsaicin either in neonatal animals (resulting in degeneration of nerve fibers) or in adult animals (resulting in depletion of sensory neuropeptides) leads to inhibition of goblet cell discharge and in a reduction of microvascular leakage (Solway and Leff, 1991; Barnes, 2001). In our study, pre-treatment with the dual NK1 and NK2 receptor antagonist DNK333 (Gerspacher et al., 2003) fully inhibited mucus release provoked by capsaicin. These results support a role for neuropeptide release following stimulation of sensory nerves leading to NK1 and/or NK2-mediated mucus release.
In addition to the increase in mucin concentration, raised levels of macrophages, neutrophils, and MPO concentration (closely linked to neutrophil activation) were found in BAL fluid 60 min following capsaicin-treatment (Table 1). Neutrophils and macrophages have been implicated in promoting mucus hypersecretion via the release of neutrophil elastase, a potent goblet cell degranulating agent, and through the release of tumor-necrosis factor-α (Delgado et al., 2003), leading to the expression and activation of epithelial growth factor receptors (Kim and Nadel, 2004). However, despite a reduction in MRI fluid signals and a decline in mucin concentration in BAL fluid following therapy with capsazepine or DNK333, we observed no concomitant changes in MPO activity or in macrophage and neutrophil influx. Taken together, these findings suggest that capsaicin challenge results in the activation of sensory nerves leading to the release of tachykinins that induce mucus secretion via direct activation of secretory glands, through activation of NK receptors.
It is interesting to note that changes in MRI fluid signal volume were much greater in magnitude than the changes in mucin levels in BAL fluid. An explanation for this may be that mucus is a visco-elastic fluid composed of glycoproteins, proteins (such as mucin), and lipids in a watery mixture (King, 2005). Assessment of mucin concentration in BAL fluid by the sandwich enzyme-linked lectin assay is likely to detect only a single component of mucus. However, MRI is able to detect the entire visco-elastic fluid, predominantly the water component of mucus. This together with the fact that the lung is practically devoid of signals (it appears black in MR images) provides a good contrast for the detection of mucus (water is rich in protons and thus will lead to a signal of high intensity) that could explain the increased sensitivity of the MRI technique compared with assays of mucin or protein from the BAL fluid. Despite the fact that there is an increase in mucin (and other mucus-related proteins) following capsaicin treatment, these are not detected by the Folin protein assay. However, it is important to consider that there are numerous proteins in BAL fluid that would be measured by a non-specific protein assay. This could mask a small change in mucus-related proteins, which would be detected with a more selective assay such as the enzyme-linked lectin assay for mucin.
It is important to emphasize that neither determination of protein concentration in BAL fluid nor histological assessment of perivascular edema revealed significant differences between capsaicin- and vehicle-treated groups. Activation of sensory nerves by capsaicin is known to cause plasma protein extravasation (Szallasi and Blumberg, 1999). Thus we suppose that the extent of edema was too low to be detected by the Folin protein assay and by histological assessment. A more sensitive method such as tagging plasma albumin with Evan's Blue may have demonstrated plasma protein extravasation in the airways. It is plausible that a small amount of edema could have been detected by MRI following capsaicin administration, but was not detected by the Folin protein assay or by histology. Nevertheless; the fact that (i) increased mucin content was observed in the BAL fluid of capsaicin-treated animals and (ii) the number of goblet cells detected by histology appeared to decrease following capsaicin-treatment compared with vehicle-challenged rats supports the view of mucus release as the primary contributor to MRI signals detected after capsaicin challenge.
Fozard et al. (2001) demonstrated an increased extravasation of plasma protein tagged with Evans Blue in the rat trachea (from the larynx to the bifurcation) 10 min after i.t. administration of capsaicin (3–30 μg kg−1). In our studies, MRI signals following capsaicin administration were observed in lower parts of the lung that are associated with an increase of mucin concentration in BAL fluid. An explanation for the discrepancy between the current results and those of Fozard et al. (2001) could be the greater density of sensory nerves in upper airways compared to lower ones as demonstrated by Larson et al. (2003). Thus it cannot be excluded that capsaicin induces different responses in the tracheal region and in more distal sections of the lung. In support of this argument, studies performed by McDonald et al. (1988) in rats demonstrated that electrical stimulation of the superior laryngeal nerves leads to an increase in permeability of venules in the trachea, but not the bronchi. Additionally, all nerves studied seemed to have bilateral effects in the trachea but vagus nerves appeared to have a unilateral effect in the bronchi (McDonald et al., 1988). Moreover, it has been shown that mast cells are present in close proximity to SP and CGRP containing sensory nerves in the trachea of mammals (Lundberg et al., 1984, Uddman et al., 1985). SP released by sensory nerves in the trachea activates local mast cells (Maggi, 1997), leading to plasma protein extravasation. Therefore, it is possible that activation of sensory nerves by capsaicin in the trachea and in the peripheral airways may lead to distinct outcomes within 60 min after administration of the compound.
DSCG has been shown to inhibit rat mast cell activation (Norris, 1996). However, DSCG also possesses an inhibitory effect upon sensory nerves as reviewed by Page (1994). Our observations support those of Ray et al. (1991) in the rat trachea where DSCG did not inhibit SP release induced by capsaicin. They are also in line with the fact that DSCG did not inhibit hypotension and bronchospasm induced by capsaicin administration to guinea pigs (Biggs and Goel, 1985) as well as with clinical studies performed by Collier and Fuller (1984) showing that DSCG pre-treatment did not alter cough induced by nebulized capsaicin. Taken together, these studies suggest that DSCG does not inhibit capsaicin-sensitive sensory nerve terminals and the responses that we observe by capsaicin are unlikely to be due to activation of mast cells.
In summary, our data indicate that stimulation of sensory nerves by capsaicin leads to a TRPV1-dependent response that promotes the release of neuropeptides resulting predominatly in the release of mucus through NK1 and/or NK2 receptors. The current study points to the opportunity of non-invasively assessing, with MRI, the influence of neuronal mechanisms in animal models of asthma and COPD.
Acknowledgments
NB acknowledges the 3R Research Foundation, Muesingen, Switzerland for supporting this work (Project 82/02).
Abbreviations
- BAL
bronchoalveolar lavage
- CGRP
calcitonin gene related peptide
- DMSO
dimethylsulphoxide
- DNK333, N-[(R,R)-(E)-1-(3,4-dichlorobenzyl)-3-(2-oxoazepan-3-yl) carbamoyl]allyl-N-methyl-3
5-bis(trifluoromethy)benzamide
- DSCG
di-sodium-cromoglycate
- EPO
eosinophil peroxidase
- i.p.
intraperitoneally
- i.t.
intratracheally
- MPO
myeloperoxidase
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- NKA
neurokinin A
- s.e.m.
standard error of the mean
- SP
substance P
- TRPV1
transient potential receptor vanilloid (subtype)-1
Conflict of interest
The authors state no conflict of interest.
References
- Barnes PJ. Related Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154. doi: 10.1016/s0034-5687(00)00210-3. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Tigani B, Ekatodramis D, Borer R, Mazzoni L, Fozard JR. Pulmonary edema induced by allergen challenge in the rat: noninvasive assessment by magnetic resonance imaging. Magn Reson Med. 2001;45:88–95. doi: 10.1002/1522-2594(200101)45:1<88::aid-mrm1013>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Tigani B, Sugar R, Jackson AD, Jones G, Mazzoni L, Fozard JR. Noninvasive detection of endotoxin-induced mucus hypersecretion in rat lung by MRI. Am J Physiol Lung Cell Mol Physiol. 2002;283:L22–L30. doi: 10.1152/ajplung.00373.2001. [DOI] [PubMed] [Google Scholar]
- Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs DF, Goel V. Mechanisms of action of sodium cromoglycate. Can J Physiol Pharmacol. 1985;63:760–765. doi: 10.1139/y85-126. [DOI] [PubMed] [Google Scholar]
- Biro T, Maurer M, Modarres S, Lewin NE, Brodie C, Acs G, Acs P, Paus R, Blumberg PM. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–1340. [PubMed] [Google Scholar]
- Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Collier JG, Fuller RW. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol. 1984;81:113–117. doi: 10.1111/j.1476-5381.1984.tb10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003;37:355–361. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dixon CM, Barnes PJ. Bradykinin-induced bronchoconstriction: inhibition by nedocromil sodium and sodium cromoglycate. Br J Clin Pharmacol. 1989;27:831–836. doi: 10.1111/j.1365-2125.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard JR, Baur F, Wolber C, Collingwood SP. Inhibition by viozan of extravasation induced in rat trachea by capsaicin is mediated exclusively by beta 2-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:570–572. doi: 10.1007/s00210-001-0488-8. [DOI] [PubMed] [Google Scholar]
- Germonpre PR, Joos GF, Everaert E, Kips JC, Pauwels RA. Characterization of neurogenic inflammation in the airways of two highly inbred rat strains. Am J Respir Crit Care Med. 1995;152:1796–1804. doi: 10.1164/ajrccm.152.6.8520739. [DOI] [PubMed] [Google Scholar]
- Gerspacher M, Lewis C, Ball HA, Howes C, Subramanian N, Ryffel K, Fozard JR. Stereoselective preparation of N-[(R,R)-(E)-1-(3,4-dichlorobenzyl)-3- (2-oxoazepan-3-yl)carbamoyl]allyl-N-methyl-3,5 bis(trifluoromethyl)benzamide, a potent and orally active dual neurokinin NK(1)/NK(2) receptor antagonist. J Med Chem. 2003;46:3508–3513. doi: 10.1021/jm030786m. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Joos GF, De Swert KO, Schelfhout V, Pauwels RA. The role of neural inflammation in asthma and chronic obstructive pulmonary disease. Ann N Y Acad Sci. 2003;992:218–230. doi: 10.1111/j.1749-6632.2003.tb03152.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Nadel JA. Role of neutrophils in mucus hypersecretion in COPD and implications for therapy. Treat Respir Med. 2004;3:147–159. doi: 10.2165/00151829-200403030-00003. [DOI] [PubMed] [Google Scholar]
- King M.Mucus and its role in airway clearance and cytoprotection Physiologic Basis of Respiratory Disease 2005BC Decker Inc: Hamilton; 409–416.In: Qutayba H, Shannon J, Martin JG (eds) [Google Scholar]
- Kuo H, Rhode JAL, Tokuyama K, Barnes PJ, Rogers DF. Capsaicin and sensory neuropeptide stimulation of goblet cell secretion in guinea pig trachea. J Physiol (Lond) 1990;431:629–641. doi: 10.1113/jphysiol.1990.sp018351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SD, Schelegle ES, Hyde DM, Plopper CG. The three-dimensional distribution of nerves along the entire intrapulmonary airway tree of the adult rat and the anatomical relationship between nerves and neuroepithelial bodies. Am J Respir Cell Mol Biol. 2003;28:592–599. doi: 10.1165/rcmb.4889. [DOI] [PubMed] [Google Scholar]
- Lee LY, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J Appl Physiol. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- Lewis CA, El-Hashim AZ, Gerspacher M, Hoshiko K, Mazzoni L, Pfannkuche HJ, Subramanian N, Fozard JR. The airways pharmacology of DNK333, a potent, selective, non-peptide dual NK1/NK2 receptor antagonist. Drug Dev Res. 2004;63:161–173. [Google Scholar]
- Liu Y-C, Khawaja AM, Rogers DF.Pathophysiology of airway mucus secretion in asthma Basic Mechanisms and Clinical Managaement 1998Academic Press: San Diego; 205–227.In: Barnes PJ, Rodger IW, Thomson NC, (eds)., Third Edition [Google Scholar]
- Lundberg JM, Hokfelt T, Martling CR, Saria A, Cuello C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res. 1984;235:251–261. doi: 10.1007/BF00217848. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997;7:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Mapp CE, Miotto D, Braccioni F, Saetta M, Turato G, Maestrelli P, Krause JE, Karpitskiy V, Boyd N, Geppetti P, Fabbri LM. The distribution of neurokinin-1 and neurokinin-2 receptors in human central airways. Am J Respir Crit Care Med. 2000;161:207–215. doi: 10.1164/ajrccm.161.1.9903137. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Mitchell RA, Gabella G, Haskell A. Neurogenic inflammation in the rat trachea II. Identity and distribution of nerves mediating the increase in vascular permeability. J Neurocytol. 1988;17:605–628. doi: 10.1007/BF01260989. [DOI] [PubMed] [Google Scholar]
- Mead J. The lung's ‘quiet zone'. N Engl J Med. 1970;282:1318–1319. doi: 10.1056/NEJM197006042822311. [DOI] [PubMed] [Google Scholar]
- Meini S, Mak JCW, Rohde JAL, Rogers DF. Tachykinin control of ferret airways: Mucus secretion, bronchoconstriction and receptor mapping. Neuropeptides. 1993;24:81–89. doi: 10.1016/0143-4179(93)90025-6. [DOI] [PubMed] [Google Scholar]
- Melton L. Does mucus hypersecretion matter in airway disease. Lancet. 2002;359:1924. doi: 10.1016/S0140-6736(02)08788-3. [DOI] [PubMed] [Google Scholar]
- Norris AA. Pharmacology of sodium cromoglycate. Clin Exp Allergy. 1996;26:5–7. doi: 10.1111/j.1365-2222.1996.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Page C. Sodium cromoglycate, a tachykinin antagonist. Lancet. 1994;343 8889:70. doi: 10.1016/s0140-6736(94)90812-5. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Maggi CA. Tachykinin receptors and receptor subtypes. Arch Int Pharmacodyn Ther. 1995;329:161–184. [PubMed] [Google Scholar]
- Quintana HK, Cannet C, Schaeublin E, Zurbruegg S, Sugar R, Mazzoni L, Page CP, Fozard JR, Beckmann N. Identification with MRI of the pleura as a major site of the acute inflammatory effects induced by ovalbumin and endotoxin challenge in the airways of the rat. Am J Physiol Lung Cell Mol Physiol. 2006;291:L651–L657. doi: 10.1152/ajplung.00303.2005. [DOI] [PubMed] [Google Scholar]
- Ramnarine SI, Hirayama Y, Barnes PJ, Rogers F. Sensory-efferent neural control of mucus secretion: Characterization using tachykinin receptor antagonists in ferret trachea in vitro. Br J Pharmacol. 1994;113:1183–1190. doi: 10.1111/j.1476-5381.1994.tb17122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jones AJ, Keen P. Morphine, but not sodium cromoglycate, modulates the release of substance P from capsaicin-sensitive neurones in the rat trachea in vitro. Br J Pharmacol. 1991;102:797–800. doi: 10.1111/j.1476-5381.1991.tb12254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PN, Holmes MD, Scicchitano L. Role of tachykinins in bronchial hyper-responsiveness. Clin Exp Pharmacol Physiol. 1997;24:273–280. doi: 10.1111/j.1440-1681.1997.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Rogers DF, Aursudkij B, Barnes PJ. Effects of tachykinins on mucus secretion on human bronchi in vitro. Eur J Pharmacol. 1989;174:283–286. doi: 10.1016/0014-2999(89)90322-1. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Neurokinin receptors subserving airways secretion. Can J Physiol Pharmacol. 1995;73:932–939. doi: 10.1139/y95-129. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Neurogenic inflammation in lung disease: burn out. Inflammo Pharmacol. 1997;5:319–329. doi: 10.1007/s10787-997-0029-2. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Motor control of airway goblet cells and glands. Resp Physiol. 2001;125:129–144. doi: 10.1016/s0034-5687(00)00209-7. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology. Curr Opin Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Mucociliary dysfunction in COPD: effect of current pharmacotherapeutic options. Pulm Pharmacol Ther. 2005;18:1–8. doi: 10.1016/j.pupt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tigani B, Schaeublin E, Sugar R, Jackson AD, Fozard JR, Beckmann N. Pulmonary inflammation monitored noninvasively by MRI in freely breathing rats. Biochem Biophys Res Commun. 2002;292:216–221. doi: 10.1006/bbrc.2002.6633. [DOI] [PubMed] [Google Scholar]
- Uddman R, Luts A, Sundler F. Occurrence and distribution of calcitonin gene-related peptide in the mammalian respiratory tract and middle ear. Cell Tissue Res. 1985;241:551–555. doi: 10.1007/BF00214575. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]