Abstract
Background and purpose:
The aim of the present study was to determine whether binding of [35S]t-butylbicyclophosphorothionate ([35S]TBPS) to the convulsant binding site of GABAA receptors in human postmortem brain samples can be used as an in vitro index of the functional activation of these receptors.
Experimental approach:
Postmortem stability of [35S]TBPS binding was assessed in rat brain samples harvested at various times after death and the binding properties of [35S]TBPS binding (KD and Bmax) were determined in human postmortem brain using radioligand binding studies. In addition, the ability of human brain [35S]TBPS binding to be allosterically modulated by compounds that bind at recognition sites distinct from the convulsant binding site was measured.
Key results:
Whereas binding of [3H]Ro 15-1788 to the benzodiazepine binding site and [3H]muscimol to the agonist (GABA) binding site were retained over a 20 h postmortem interval, there was a significant decrease in the affinity and number of [35S]TBPS binding sites. Nevertheless, [35S]TBPS binding in human brain could be inhibited by TBPS, picrotoxin, loreclezole and pentobarbital and modulated by GABA with potencies comparable to those observed in rats. In addition, the GABA-induced reduction in human brain [35S]TBPS binding could be modulated by benzodiazepine site ligands in a manner that reflected their intrinsic efficacies.
Conclusions and implications:
These results suggest that allosteric coupling between the [35S]TBPS, GABA and benzodiazepine binding sites is preserved in postmortem human brain and that [35S]TBPS binding in this tissue may be used to study functional characteristics of native human GABAA receptors.
Keywords: [35S]TBPS, GABAA receptor, convulsant binding site, postmortem human brain, allosteric modulation, benzodiazepine
Introduction
GABAA (γ-aminobutyric acid type A) receptors, the principal mediators of fast synaptic GABAergic inhibitory transmission in the central nervous system, are hetero-oligomeric ligand-gated chloride channels with a pentameric structure derived from the assembly of gene products that constitute the GABAA receptor subunit family (α1–6, β1–β3, γ1–γ3, δ, ɛ and θ; Barnard et al., 1998; Simon et al., 2004). Of the many theoretical pentameric combinations of these subunits, relatively few are actually expressed in native brain tissue (McKernan and Whiting, 1996), with the majority possessing a subunit stoichiometry of two α-, two β- and one γ-subunits (Sieghart and Sperk, 2002).
The GABAA receptor has a rich pharmacology, containing binding sites for not only the agonist (GABA) but also modulators such as benzodiazepines, barbiturates, neurosteroids, ethanol and certain volatile anaesthetics. All these compounds elicit their pharmacological effects as sedatives, anxiolytics and anticonvulsants by binding to their specific sites and either directly activating GABAA receptors or enhancing the effects of GABA (Sieghart, 1995). On the other hand, a variety of non-competitive GABAA receptor antagonists block the chloride channel. Such compounds include picrotoxinin (the active component of picrotoxin), pentylenetetrazol, certain polychlorocycloalkanes, two types of trioxabicyclo[2.2.2]octanes, namely the bicycloesters and the bicyclo-orthocarboxylates, as well as a number of insecticides (including the cyclodiene, dieldrine), all of which bind to the so-called convulsant binding site with an affinity that is proportional to their toxicity as judged by their mouse LD50 (Casida, 1993). This binding site can be labeled with either the picrotoxinin analogue [3H]dihydropicrotoxinin (Ticku and Olsen, 1979), the bicyclo-orthocarboxylates [3H]t-butylbicyclo orthobenzoate ([3H]TBOB; Lawrence et al., 1985) and [3H]ethynylpropylbicyclo-orthobenzoate ([3H]EBOB; Cole and Casida, 1992), or the bicyclophosphorus ester [35S]t-butylbicyclo phosphorothionate ([35S]TBPS; Squires et al., 1983), although [35S]TBPS offers the advantage of low levels of nonspecific binding. The location of the convulsant binding site within the ion channel of the GABAA receptor is implied by the fact that the ability of anions to enhance [35S]TBPS binding is correlated with the permeability of these same ions through the GABAA ion channel (Havoundjian et al., 1986).
The binding of [35S]TBPS to the convulsant binding site can be allosterically modulated by compounds acting not only at the agonist binding site but also at the benzodiazepine, barbiturate, loreclezole, anaesthetic and neurosteroid recognition sites (Supavilai and Karobath, 1983; Trifiletti et al., 1984; Gee et al., 1986; Huidobro-Toro et al., 1987; Concas et al., 1988; Im and Blakeman, 1991; Hawkinson et al., 1996; Xue et al., 1996). Consequently, the modulation of [35S]TBPS binding has proved a useful in vitro indicator of efficacy and potency of compounds acting at the GABAA receptor.
Given the utility of [35S]TBPS as an index of the functional state of the GABAA receptor, in the present study we have characterized the binding of this radioligand to GABAA receptors in post-mortem human brain. Despite a loss of [35S]TBPS binding sites in post-mortem human brain, the binding that remained was displaceable by picrotoxin and t-butylbicyclophosphorothionate (TBPS) with affinities similar to those in rat tissue. Moreover, human brain [35S]TBPS binding could be modulated by GABA and benzodiazepine site ligands, such as the non-selective full-agonist diazepam and the non-selective full-inverse agonist methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM; Dawson et al., 2006) with potency and kinetic values which suggested that the allosteric coupling between the [35S]TBPS and GABA and benzodiazepine binding sites was maintained post mortem.
Materials and methods
Membrane preparation
Male Sprague–Dawley rats (250–300 g; B&K Universal, Hull, UK) were sacrificed by stunning and decapitation, brains removed, and the cerebral cortex and cerebellum were dissected over ice. Each region was homogenized in 10 volumes of 0.32 M sucrose using a Teflon-glass homogenizer for 10 strokes at 500 r.p.m. Homogenates were centrifuged at 3000 g for 10 min at 4°C, after which the supernatant (S1) was retained and the pellet resuspended in 10 volumes of 0.32 M sucrose, homogenized and centrifuged again. The supernatant, S1′, was combined with the original S1 supernatant and centrifuged at 48 000 g for 20 min at 4°C. The resulting P2 pellet was resuspended in Tris/ethylenediaminetetraacetic acid (EDTA) (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) and centrifuged at 48 000 g for 20 min at 4°C. This P2 pellet was washed a further four times with Tris/EDTA. The final washed P2 pellet was resuspended in 0.2 M Tris-HCl, 0.1 M NaCl, 7.5 pH, containing 10% glycerol and stored at −80°C. On the day of the assay, aliquots of P2 membranes were thawed on ice and washed twice with Tris/EDTA. The fact that these extensive washing procedures had removed endogenous GABA was demonstrated indirectly by the fact that the GABA site competitive antagonist bicuculine (10 μM) did not alter [35S]TBPS binding (data not shown).
To examine the effect of post-mortem delay on GABAA receptor binding, rats were sacrificed and kept at room temperature for 8 h following which they were placed in a padded insulated box and placed in a cold room to mimic the refrigeration of the human cadaver. At various times during this process, the brains were removed, frozen at −80°C and membranes prepared as described above.
Post-mortem human brains were obtained with informed consent from relatives and under the appropriate ethical considerations from three males and one female with the average age of 63 years (ranging between 59 and 66) and a mean post-mortem interval of 10 h (ranging between 6 and 14). None of the subjects had a history of neurological or neuropsychiatric disorders. Brains were sectioned down the midline and hemispheres were sliced coronally in 1–2 cm thick slabs, frozen and stored at −80°C. From the coronal slabs, samples of frontal cortex and cerebellum were dissected and membranes were prepared as described above.
Radioligand binding assays
Resuspended P2 membranes containing appropriately 50 μg of protein were incubated in Tris/NaBr buffer (50 mM Tris-citrate, 0.2 M NaBr, pH 7.4) plus [35S]TBPS in a final assay volume of 0.5 ml. Nonspecific binding was defined using either picrotoxin (10 μM) or TBPS (5 μM). Competition and saturation binding analyses were performed using an incubation period of 2 h and respective [35S]TBPS concentrations of 8 nM and a range of 150–200 nM.
Kinetic (association and dissociation) experiments were performed using 8 nM [35S]TBPS in the absence and presence of 0.3, 0.6 or 1.0 μM GABA. Association assays were terminated by filtration between 1 and 120 min after initiation of incubations, whereas dissociation assays were performed after a 2-h incubation by filtration at various times (1–90 min) after addition of 5 μM TBPS. The effects of GABA (1 nM–10 μM) on the binding of [35S]TBPS were studied at a ligand concentration of 8 nM using an incubation time of 2 h.
Binding to the benzodiazepine and GABA binding sites of the GABAA receptor were performed using [3H]Ro15-1788 and [3H]muscimol, respectively, as described in more detail elsewhere (Quirk et al., 1994). In brief, 50 μg P2 membrane protein were incubated for 60 min in Tris buffer (50 mM Tris-HCl, pH 7.5) in a total volume of 0.5 ml containing either 0.5–20 nM [3H]Ro15-1788 or 2–50 nM [3H]muscimol with nonspecific binding defined using 10 μM flunitrazepam or 100 μM GABA, respectively.
All assays, which were performed at room temperature and in triplicate, were terminated by filtration over Whatman GF/B glass fiber filters followed by washing in ice-cold assay buffer (10 ml). Filter discs were placed into scintillation vials, 10 ml of scintillation fluid added and radioactivity was counted using a Beckman LS 6500 liquid scintillation counter. Protein concentrations in the P2 membranes were determined using the method of Lowry (Lowry et al., 1951).
Data analysis
Data were analysed using Prism data analysis software (GraphPad Software Inc., San Diego, CA, USA). More specifically, KD and Bmax values were calculated by nonlinear regression to a one-site hyperbolic function and IC50 values were calculated using nonlinear regression with data fitted to a four-parameter sigmoidal concentration–response model with a variable slope factor (Hill slope) and maximum and minimum response values constrained to 100 and 0%, respectively. For kinetic analyses (Bennett and Yamamura, 1985), the association binding was transformed to ln(Be/Be–Bt) and plotted as a function of time of association, where Be is binding at equilibrium calculated by nonlinear regression using a single-site, hyperbolic function and Bt is binding at each of the association times. In the dissociation study, on the other hand, ln(Bt/B0) was plotted as a function of dissociation time, where Bt is binding at each of the dissociation times and B0 is binding at the initiation of dissociation. The slopes of these linear regressions gave the Kob and Koff values, which represented the rates of association and dissociation of [35S]TBPS.
In the rat post-mortem delay study, the effect of post-mortem interval on KD and Bmax parameters for each radioligand ([35S]TBPS, [3H]Ro15-1788 or [3H]muscimol) were evaluated with one-way analysis of variance followed by Dunnett's post hoc t-tests as were the effects of diazepam, DMCM and Ro15-1788 on the GABA EC50. The KD and Bmax values for [35S]TBPS binding were compared between human and rat cortex or cerebellum samples using two-tailed unpaired t-tests. Similarly, the differences between the IC50 values for TBPS, picrotoxin, GABA, loreclezole and pentobarbital in human and rat samples were estimated using two-tailed unpaired t-test comparing corresponding human and rat brain regions (i.e., human vs rat cortex and human vs rat cerebellum).
Materials
Picrotoxin, GABA, DMCM and TBPS were purchased from Sigma-Aldrich (GIllingham, UK). GABA was dissolved in water whereas both TBPS and picrotoxin were dissolved in dimethylsulphoxide (DMSO). TBPS was made up at a stock concentration of 10 mM and was used within 2 days. Final concentrations of DMSO in the assay did not exceed 1%, a concentration at which the binding of [35S]TBPS to P2 membranes of either human or rat brain was not affected (data not shown). All radioligands included in the present study, [35S]TBPS (>2.22 TBq mmol−1), [3H]Ro15-1788 (2.59–3.22 TBq mmol−1) and [3H]muscimol (0.19–1.11 TBq mmol−1), were purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA, USA).
Results
Post-mortem stability of rat brain GABAA receptor binding sites
When rat brains were left for various post-mortem intervals, there was a significant decrease in both the affinity and number of [35S]TBPS binding sites (Figure 1a, Table 1). Thus, after a post-mortem interval of 20 h, the affinity for [35S]TBPS binding fell, whereas the number of binding sites was reduced by 49%. On the other hand, there were no significant differences in either the KD or Bmax for [3H]Ro15-1788 and [3H]muscimol binding sites (Figure 1b and c, Table 1). It should be emphasized that the rat cortex and cerebellum results described in the following sections all refer to brain samples obtained and frozen immediately after sacrificing (i.e., with no post-mortem delay).
Figure 1.
Saturation isotherms of (a) [35S]TBPS; (b) [3H]Ro15-1788; and (c) [3H]muscimol binding to P2 membranes prepared from whole-rat brains obtained either immediately (t=0) or 5, 10 or 20 h after sacrificing. Values shown are mean±s.e.m. of 3–4 separate animals.
Table 1.
Effect of postmortem delay on the characteristics of [35S]TBPS, [3H]Ro15-1788 and [3H]muscimol binding to rat whole-brain membranes
| PM delay (h) |
[35S]TBPS |
[3H]Ro15-1788 |
[3H]Muscimol |
|||
|---|---|---|---|---|---|---|
| KD (nM) | Bmax (pmol mg protein−1) | KD (nM) | Bmax (pmol mg protein−1) | KD (nM) | Bmax (pmol mg protein−1) | |
| 0 | 27±1 | 1.61±0.21 | 1.6±0.13 | 1.03±0.09 | 20±0.9 | 1.45±0.26 |
| 5 | 32±1* | 1.22±0.05* | 1.6±0.17 | 1.00±0.12 | 20±1.1 | 1.37±0.16 |
| 10 | 40±3* | 0.99±0.18* | 1.3±0.11 | 1.19±0.21 | 19±0.5 | 1.44±0.21 |
| 20 | 49±5* | 0.82±0.12* | 1.5±0.13 | 1.17±0.16 | 18±0.6 | 1.50±0.17 |
Abbreviations: ANOVA, analysis of variance; [35S]TBPS, [35S]t-butylbicyclophosphorothionate.
Data shown are mean±s.e.m. of values obtained from 3 to 4 separate animals from each time point. Statistical differences between groups were evaluated using a one-way ANOVA with post hoc Dunnett's multiple comparison tests for each radioligand. (*P<0.05).
Comparison of human and rat brain [35S]TBPS binding
Figure 2 shows a comparison of the binding of [35S]TBPS to P2 membranes derived from rat and post-mortem human cortex and cerebellum with the parameters derived from these data (KD and Bmax) being summarized in Table 2. Consistent with the data from the study on the effects of post-mortem interval (Figure 1 and Table 1), the affinity and number of [35S]TBPS binding sites were lower in post-mortem human compared to rat brain tissue. Thus the KD values for [35S]TBPS binding in human cerebral cortex and cerebellum were about twice those in the corresponding regions of rat brain and Bmax values in post-mortem human brain about half those in rat brain (Table 2).
Figure 2.
Saturation isotherms for [35S]TBPS binding to rat and human cortex (left) and cerebellum (right). Values shown are mean±s.e.m. (n=3–4).The KD and Bmax values derived from these data are shown in Table 2.
Table 2.
Comparison of characteristics of [35S]TBPS binding in rat and human postmortem cortex and cerebellum membranes
| Brain region | KD (nM) | Bmax (pmol mg protein−1) |
|---|---|---|
| Human cortex | 37±1.3* | 0.79±0.13* |
| Human cerebellum | 36±4.9* | 0.74±0.09* |
| Rat cortex | 19±1 | 1.56±0.09 |
| Rat cerebellum | 18±2 | 1.25±0.12 |
Abbreviation: [35S]TBPS, [35S]t-butylbicyclophosphorothionate.
Data shown are mean±s.e.m. (n=3–4).
P<0.05; comparison with corresponding rat tissue using an unpaired t-tests.
Pharmacology of human and rat brain [35S]TBPS binding
The inhibition of [35S]TBPS binding in rat and human cortex and cerebellum membranes by TBPS, picrotoxin, GABA, loreclezole and pentobarbital is illustrated in Figure 3 with the IC50 values being presented in Table 3. In human cerebral cortex and cerebellum, the IC50 values for TBPS were 2–2.5-fold higher than the corresponding values in rat and the affinity for picrotoxin was also lower in human compared to rat brain.
Figure 3.
Inhibition of [35S]TBPS binding by TBPS, picrotoxin, GABA, loreclezole and pentobarbital in P2 membranes derived from (a) human cortex; (b) human cerebellum; (c) rat cortex; and (d) rat cerebellum. Values shown are mean±s.e.m. (n=3–4).
Table 3.
Inhibition of [35S]TBPS binding by TBPS, picrotoxin, GABA, loreclezole and pentobarbital in postmortem human and rat cortex and cerebellum membranes
|
Human brain |
Rat brain |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Cortex |
Cerebellum |
Cortex |
Cerebellum |
|||||
| IC50 (μM) | Hill slope | IC50 (μM) | Hill slope | IC50 (μM) | Hill slope | IC50 (μM) | Hill slope | |
| TBPS | 0.054±0.009* | 0.86±0.15 | 0.046±0.012* | 1.18±0.23 | 0.021±0.005 | 0.91±0.07 | 0.022±0.005 | 0.95±0.02 |
| Picrotoxin | 0.31±0.03* | 0.84±0.02 | 0.43±0.06* | 0.87±0.11 | 0.18±0.04 | 0.85±0.03 | 0.18±0.03 | 0.87±0.03 |
| GABA | 0.90±0.07 | 1.35±0.05 | 0.65±0.11 | 1.24±0.17 | 1.13±0.15 | 1.56±0.14 | 0.90±0.08 | 1.21±0.08 |
| Loreclezole | 21±7* | 0.88±0.09 | 12±4* | 0.90±0.11 | 8.4±1.8 | 0.83±0.06 | 5.0±0.9 | 0.99±0.08 |
| Pentobarbital | 177±42* | 1.11±0.13 | 110±25* | 1.08±0.05 | 64±9 | 1.14±0.05 | 58±10 | 1.11±0.06 |
Abbreviations: GABA, γ-aminobutyric acid; [35S]TBPS, [35S]t-butylbicyclophosphorothionate; TBPS, t-butylbicyclophosphorothionate.
Data shown are mean±s.e.m. (n=3–4).
P<0.05; comparison with corresponding rat tissue using an unpaired t-tests.
Similarly, the inhibition of binding of [35S]TBPS by loreclezole and pentobarbital in human brain was 2–3-fold lower affinity than the corresponding values in rat brain membranes. However, the modulation of [35S]TBPS binding by GABA was essentially the same in human and rat cortex and cerebellum. Interestingly, the slopes of the curves for the GABA-mediated inhibition of [35S]TBPS binding were significantly greater than unity for both human and rat brain, indicating a degree of cooperativity in the allosteric coupling between the GABA and [35S]TBPS binding sites.
Effect of GABA on the kinetics of human and rat brain [35S]TBPS binding
The modulation of [35S]TBPS binding was further studied by examining the effects of 0.3, 0.6 and 1 μM GABA on the association and dissociation rates of [35S]TBPS with representative data from experiments using human cortex membranes being presented in Figure 4. A comparison of the effects of GABA on the association and dissociation kinetic data for human and rat cortex and cerebellum is presented in Figure 5.
Figure 4.
The kinetics of (a) association and (b) dissociation of specific [35S]TBPS binding in P2 membrane of human cerebral cortex observed in the absence and presence of 0.3, 0.6 and 1 μM GABA. The association and dissociation data were transformed into Ln(Be/Be−B) and Ln(Bt/B0) functions, respectively (insets) and the kinetic constants determined by linear regression, where Be = binding at equilibrium; B = binding at any time point in the association phase; Bt = binding at any time point during dissociation and B0 = binding at t=0.
Figure 5.
In (a), the association (Kob) and in (b), the dissociation constants (Koff) for [35S]TBPS binding to human and rat cortex (Cx) and cerebellum (Cb) are plotted as a function of GABA concentration. Values shown are mean±s.e.m. (n=3–4).
In the absence of GABA, the rates of association (0.023–0.036 min−1) and dissociation (0.024–0.026 min−1) were comparable across species and between brain regions (Figure 5). The effect of GABA was to accelerate the rates of association and dissociation such that at a concentration of 1 μM, which is around the IC50 value for the GABA modulatory effect (Table 4), both the on- and off-rates were around 3–4-fold higher than those seen in the absence of GABA.
Table 4.
Effects of benzodiazepine site ligands of differing intrinsic efficacies on GABA-mediated modulation of [35S]TBPS binding in rat and human postmortem cortex and cerebellum membranes
|
IC50, μM |
||||
|---|---|---|---|---|
|
Human |
Rat |
|||
| Cortex | Cerebellum | Cortex | Cerebellum | |
| Control | 0.90±0.07 | 0.65±0.11 | 1.13±0.15 | 0.90±0.08 |
| Diazepam | 0.43±0.04* | 0.33±0.10* | 0.56±0.03* | 0.48±0.03* |
| Ro15-1788 | 0.74±0.05 | 0.65±0.06 | 1.05±0.01 | 0.88±0.08 |
| DMCM | 2.29±0.38* | 1.42±0.31* | 1.91±0.04* | 2.54±0.11* |
Abbreviations: ANOVA, analysis of variance; DMCM, methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate; GABA, γ-aminobutyric acid; [35S]TBPS, [35S]t-butylbicyclophosphorothionate.
Data shown are mean±s.e.m. (n=3–4). Statistical differences between groups were evaluated using a one-way ANOVA with post hoc Dunnett's multiple comparison tests for each radioligand. (*P<0.05).
Modulation of [35S]TBPS binding by benzodiazepine site ligands
The GABA-induced inhibition of [35S]TBPS binding in human cortex and cerebellum could be modulated by diazepam and DMCM in a manner consistent with the benzodiazepine site agonist diazepam increasing, and the inverse agonist DMCM decreasing, the apparent affinity of the GABAA receptor for GABA and was comparable to that observed in rat brain (Table 4). More specifically, in both cortex and cerebellum, and under equilibrium binding conditions (i.e., an incubation time of 2 h), diazepam produced a leftward shift in the GABA concentration–effect curve, whereas DMCM resulted in a rightward shift. In contrast, the benzodiazepine site antagonist Ro15-1788 had essentially no effect on the GABA concentration–effect curve. The nature of these effects in human brain membranes (i.e., left- and rightward shifts for benzodiazepine site agonists and inverse agonists, respectively) are comparable to those observed in rat brain. Moreover, the extent of the shift in IC50 values in human membranes (i.e., the IC50 in the presence of diazepam was around half than seen with GABA alone, whereas the IC50 plus DMCM was in the region of 2.5-fold higher) was very similar to those seen in rat brain membranes (Table 4). When performed under non-equilibrium conditions (incubation time =15 min; see Figure 4), the opposite effects were observed with DMCM decreasing and diazepam increasing the IC50 of the GABA concentration–effect curve (data not shown).
Discussion
Post-mortem stability of rat brain GABAA receptor binding sites
A major issue in the study of radioligand binding in post-mortem human brain samples is that of the stability of the receptor of interest during the interval between death and removal and processing of the brain. This can be addressed by measuring receptor binding in samples of human post-mortem brain derived from a number of individuals and then relating receptor expression to the post-mortem interval within the particular cohort. In such a study, the expression of [35S]TBPS binding sites has been reported previously to be not greatly influenced by the post-mortem interval (Lloyd et al., 1991) although in this particular cohort, the post-mortem interval was relatively short (0.67–3.75 h) and therefore the applicability of these data to the longer post-mortem intervals normally experienced with human tissue is uncertain. Alternatively, the stability of receptor binding parameters can be studied in rat brains left for various intervals post mortem (Whitehouse et al., 1984), with the implicit assumption that any changes occurring in the rat brain will also occur in the human brain. Using this latter approach, we were clearly able to demonstrate a significant loss of rat brain [35S]TBPS binding sites at 5 and 10 h post mortem, which by 20 h post mortem had resulted in a loss of approximately 50% of binding sites. In addition, there was a progressive reduction in the affinity of the remaining binding sites, with those remaining after 20 h having approximately twofold lower affinity (KD=49 nM) than brain samples collected immediately after death (27 nM). The KD and Bmax values in samples with no post-mortem interval (27 nM, 1.61 pmol mg protein−1, respectively) are similar to those reported previously in rat cortex (26–41 nM; 1.36–4.2 pmol mg protein−1), forebrain (16 nM) and cerebellum (33 nM; 1.26 pmol mg protein−1: Squires et al., 1983; Supavilai and Karobath, 1983; Ticku and Ramanjaneyulu, 1984; Corda et al., 1993).
The loss of [35S]TBPS binding sites during the post-mortem interval is not a consequence of a loss of GABAA receptors as the expression and affinity of [3H]Ro15-1788 (benzodiazepine) and [3H]muscimol (GABA) binding sites remains relatively constant, in agreement with previous observations using [3H]flunitrazepam to label the rat or human brain benzodiazepine site (Whitehouse et al., 1984; Benes et al., 1997) or [3H]GABA to label the agonist binding site in human post-mortem brain (Lloyd and Dreksler, 1979). Consequently, the post-mortem reduction in the number of rat brain [35S]TBPS binding sites must reflect a selective alteration (for example, proteolysis) at the convulsant, but not benzodiazepine or GABA binding site of the GABAA receptor. The lability of convulsant but not benzodiazepine or GABA binding sites is also observed with, for example, Triton X-100 pretreatment (Supavilai and Karobath, 1983), emphasizing the relative instability of the GABAA receptor convulsant binding site.
In rat tissue with a long post-mortem interval (and, by analogy, in human post-mortem tissue), it is not certain whether the reduction in [35S]TBPS binding affinity is due to alterations of the GABAA receptor itself (such as might be caused by proteolysis) or is due to a selective loss of binding to GABAA receptors with a higher [35S]TBPS affinity. Nevertheless, these data clearly highlight the need for further characterization of the pharmacology of human post-mortem brain [35S]TBPS binding sites to establish whether the binding sites that remain have altered properties (i.e., they have changed to a slightly lower affinity state) or represent a specific population of GABAA receptors that is resistant to post-mortem changes. In regard to the latter possibility, GABAA receptor subunit composition has an effect upon the pharmacology of [35S]TBPS binding (Korpi and Lüddens, 1993; Lüddens and Korpi, 1995; Korpi et al., 1996) and it is possible that the [35S]TBPS binding that remains is associated with a specific subpopulation of GABAA receptors. At the very least, and in the absence of an understanding of the molecular nature of the post-mortem change in [35S]TBPS affinity, these data demonstrate that any prediction of the efficacy of novel subtype-selective compounds based upon the modulation of [35S]TBPS binding in human brain should be made with caution.
Characteristics of [35S]TBPS binding in post-mortem human brain
Rat brain [35S]TBPS binding was inhibited by TBPS and picrotoxin with IC50 values (0.021–0.022 and 0.18 μM, respectively), which are consistent with values published previously (Squires et al., 1983; Cole et al., 1984; Ticku and Ramanjaneyulu, 1984). The lower affinity of human compared to rat [35S]TBPS binding sites is presumably due to the fact that in the present study, human tissue with a mean post-mortem interval of 10 h was used; a period which produces a significant reduction in affinity and expression of binding sites in rat brain (Figure 1 and Table 1). However, and most importantly, the sites that remain in the post-mortem human brain retain their pharmacology as judged by the inhibition of binding by TBPS (0.046–0.054 μM) and picrotoxin (IC50 values 0.31–0.43 μM). These values demonstrate a somewhat higher affinity of native compared to recombinant human receptors since α1β2γ2-receptors have IC50 values for TBPS and picrotoxin of 0.16 and 1.3 μM, respectively (Yagle et al., 2003).
The kinetics of dissociation and association in human cortex and cerebellum were monophasic and were comparable to those observed in rat (see kinetic data in the absence of GABA; Figure 5). Whereas there is agreement that the association kinetics of rat brain binding are monophasic (Squires et al., 1983; Maksay and Ticku, 1985), dissociation kinetics have been reported to be either polyphasic (Squires et al., 1983) or monophasic (Maksay and Ticku, 1985), a discrepancy attributed to the method of membrane preparation which presumably relates to the presence or absence of endogenous GABA and/or kinetic experiments performed in the presence of different monovalent ions (Maksay and Ticku, 1985).
The characteristics of [35S]TBPS binding (IC50 values for TBPS and picrotoxin, modulation by GABA, loreclezole and pentobarbital and kinetics of association and dissociation) were similar in cerebral cortex and cerebellum of human and rat. Although the regional similarity in the binding constants and IC50 values for picrotoxin is consistent with previous published data (Ticku and Ramanjaneyulu, 1984), in the same study, GABA was reported to be more potent at inhibiting cerebellar compared to cortical [35S]TBPS (respective IC50 values =0.12 and 0.77 μM; Ticku and Ramanjaneyulu, 1984).
Modulation of [35S]TBPS binding by GABA in human brain
The binding of [35S]TBPS to GABAA receptors in human post-mortem brain tissue could be allosterically modulated by binding at sites distinct from the convulsant binding sites. Hence, the binding of [35S]TBPS to human cortex and cerebellum was reduced in a concentration-dependent way by GABA, loreclezole and pentobarbital in a manner similar to that observed in rat brain (Figure 3 and Table 3). More specifically, with regard to GABA, the allosteric coupling between the agonist and the convulsant binding sites was retained as GABA was able to inhibit the binding of [35S]TBPS in post-mortem human cortex and cerebellum with IC50 values (0.90 and 0.65 μM, respectively), not too dissimilar to those in corresponding regions of rat brain (1.13 and 0.90 μM). The potency of this inhibition of binding by GABA was consistent with previous published data using either native or recombinant rat GABAA receptors (Squires et al., 1983; Korpi and Lüddens, 1993; Im et al., 1994). Furthermore, the effects of GABA on the association and dissociation kinetics of [35S]TBPS binding were comparable in human and rat brain, with GABA increasing the rate of both [35S]TBPS association and dissociation (Figures 4 and 5).
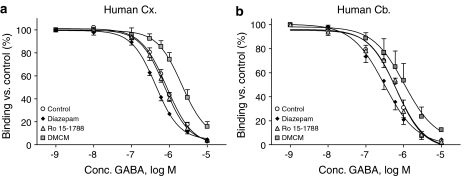
The fact that the allosteric coupling between the convulsant and other binding sites is maintained in human post-mortem GABAA receptors is further emphasized by the fact that benzodiazepine site ligands shift the GABA-mediated [35S]TBPS inhibition curve in a manner consistent with their intrinsic efficacies (Figure 6). Thus, the benzodiazepine site agonist diazepam, which increases the apparent affinity of GABAA receptors for GABA, produced a leftward shift in the GABA effect curve, whereas the inverse agonist DMCM, which reduced the apparent affinity of GABA, caused a rightward shift.
Figure 6.
Modulation of the GABA-mediated inhibition of [35S]TBPS binding to membranes of (a) human cortex (Cx) and (b) human cerebellum (Cb) by benzodiazepine site ligands with differing intrinsic efficacies, as measured under equilibrium binding conditions. The concentration–effect curve was essentially unaltered by the benzodiazepine site antagonist Ro15-1788 (100 nM) but shifted leftward by the agonist diazepam (1 μM) and shifted rightward by the inverse agonist DMCM (10 μM). Values shown are mean±s.e.m. (n=3–4).
In summary, although the binding of [35S]TBPS to the convulsant site of human GABAA receptors is relatively labile post mortem, the remaining sites demonstrate a pharmacological profile that is similar to native rat receptors. However, further studies are required to determine whether the post-mortem loss of binding sites is associated with a particular GABAA receptor subtype and until then the use of post-mortem human brain [35S]TBPS binding to predict the efficacy of novel compounds acting at the GABAA receptor should be interpreted with caution.
Abbreviations
- DMCM
methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate
- TBPS
t-butylbicyclophosphorothionate
Conflicts of interest
At the time this work was carried out, all authors were employees of the pharmaceutical company Merck Sharp & Dohme.
References
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Benes FM, Wickramasinghe R, Vincent SL, Khan Y, Todtenkopf M. Uncoupling of GABAA and benzodiazepine receptor binding activity in the hippocampal formation of schizophrenic brain. Brain Res. 1997;755:121–129. doi: 10.1016/s0006-8993(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Yamamura HI.Neurotransmitter, hormone, or drug receptor binding methods Neurotransmitter Receptor Binding 1985Raven Press: New York; 61–89.In: HI Yamamura, SJ Enna and MJ Kuhar (eds)2nd edn [Google Scholar]
- Casida JE. Insecticide action at the GABA-gated chloride channel: recognition, progress, and prospects. Arch Insect Biochem Physiol. 1993;22:13–23. doi: 10.1002/arch.940220104. [DOI] [PubMed] [Google Scholar]
- Cole LM, Casida JE. GABA-gated chloride channel: binding site for 4′-ethynyl-4-n-[2,3-3H2]propylbicycloorthobenzoate ([3H]EBOB) in vertebrate brain and insect head. Pestic Biochem Physiol. 1992;44:1–8. [Google Scholar]
- Cole LM, Lawrence LJ, Casida JE. Similar properties of [35S]t-butylbicyclophosphorothionate receptor and coupled components of the GABA receptor-ionophore complex in brains of human, cow, rat, chicken and fish. Life Sci. 1984;35:1755–1762. doi: 10.1016/0024-3205(84)90272-8. [DOI] [PubMed] [Google Scholar]
- Concas A, Serra M, Atsoggiu T, Biggio G. Foot-shock stress and anxiogenic β-carbolines increase t-[35S]butylbicyclophosphorothionate binding in the rat cerebral cortex, an effect opposite to anxiolytics and γ-aminobutyric acid mimetics. J Neurochem. 1988;51:1868–1876. doi: 10.1111/j.1471-4159.1988.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Corda MG, Cancedda E, Giorgi O. Modulation of 35S-TBPS binding by GABAergic drugs in the cerebral cortex of newborn and adult rats. Brain Res Bull. 1993;32:647–652. doi: 10.1016/0361-9230(93)90168-b. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, et al. An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Expt Therap. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Gee KW, Lawrence LJ, Yamamura HI. Modulation of the chloride ionophore by benzodiazepine receptor ligands: influence of γ-aminobutyric acid and ligand efficacy. Mol Pharmacol. 1986;30:218–225. [PubMed] [Google Scholar]
- Havoundjian H, Paul SM, Skolnick P. The permeability of γ-aminobutyric acid-gated chloride channels is described by the binding of a ‘cage' convulsant, t-butylbicyclophosphoro[35S]thionate. Proc Natl Acad Sci USA. 1986;83:9241–9244. doi: 10.1073/pnas.83.23.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkinson JE, Drewe JA, Kimbrough CL, Chen JS, Hogenkamp DJ, Lan NC, et al. 3α-hydroxy-3β-trifluoromethyl-5α-pregnan-20-one (Co 2-1970): a partial agonist at the neuroactive steroid site of the γ-aminobutyric acidA receptor. Mol Pharmacol. 1996;49:897–906. [PubMed] [Google Scholar]
- Huidobro-Toro JP, Bleck V, Allan AM, Harris RA. Neurochemical actions of anesthetic drugs on the γ-aminobutyric acid receptor–chloride channel complex. J Pharmacol Exp Therap. 1987;242:963–969. [PubMed] [Google Scholar]
- Im WB, Blakeman DP. Correlation between γ-aminobutyric acidA receptor ligand-induced changes in t-butylbicyclophosphoro[35S]thionate binding and 36Cl− uptake in rat cerebrocortical membranes. Mol Pharmacol. 1991;39:394–398. [PubMed] [Google Scholar]
- Im WB, Pregenzer JF, Thomsen DR. Effects of GABA and various allosteric ligands on TBPS binding to cloned rat GABAA receptor subtypes. Br J Pharmacol. 1994;112:1025–1030. doi: 10.1111/j.1476-5381.1994.tb13185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Lüddens H. Regional γ-aminobutyric acid sensitivity of t-butylbicyclophosphoro[35S]thionate binding depends on γ-aminobutyric acidA receptor α subunit. Mol Pharmacol. 1993;44:87–92. [PubMed] [Google Scholar]
- Korpi ER, Seeburg PH, Lüddens H. Modulation of GABAA receptor tert-[35S]butylbicyclophosphorothionate binding by antagonists: relationship to patterns of subunit expression. J Neurochem. 1996;66:2179–2187. doi: 10.1046/j.1471-4159.1996.66052179.x. [DOI] [PubMed] [Google Scholar]
- Lawrence LJ, Palmer CJ, Gee KW, Wang X, Yamamura HI, Casida JE. t-[3H]Butylbicycloorthobenzoate: new radioligand probe for the γ -aminobutyric acid-regulated chloride ionophore. J Neurochem. 1985;45:798–804. doi: 10.1111/j.1471-4159.1985.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Lloyd GK, Lowenthal A, Javoy-Agid F, Constantidinis J. GABAA receptor complex function in frontal cortex membranes from control and neurological patients. Eur J Pharmacol. 1991;197:33–39. doi: 10.1016/0014-2999(91)90361-s. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Dreksler S. An analysis of [3H]gamma-aminobutyric acid (GABA) binding in the human brain. Brain Res. 1979;163:77–87. doi: 10.1016/0006-8993(79)90152-5. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lüddens H, Korpi ER. GABA antagonists differentiate between recombinant GABAA/benzodiazepine receptor subtypes. J Neurosci. 1995;15:6957–6962. doi: 10.1523/JNEUROSCI.15-10-06957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksay G, Ticku MK. GABA, depressants and chloride ions affect the rate of dissociation of 35S-t-butylbicyclophosphorothionate binding. Life Sci. 1985;37:2173–2180. doi: 10.1016/0024-3205(85)90568-5. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Quirk K, Gillard NP, Ragan CI, Whiting PJ, McKernan RM. Model of subunit composition of γ-aminobutyric acid A receptor subtypes expressed in rat cerebellum with respect to their α and γ/δ subunits. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABAA receptor genes in the human genome. J Biol Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- Squires RF, Casida JE, Richardson M, Saederup E. [35S]t-Butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- Supavilai P, Karobath M. Differential modulation of [35S]TBPS binding by the occupancy of benzodiazepine receptors with its ligands. Eur J Pharmacol. 1983;91:145–146. doi: 10.1016/0014-2999(83)90378-3. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Olsen RW. Cage convulsants inhibit picrotoxinin binding. Neuropharmacology. 1979;18:315–318. doi: 10.1016/0028-3908(79)90132-1. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Ramanjaneyulu R. Differential interactions of GABA agonists, depressant and convulsant drugs with [35S]-t-butylbicyclophosphorothionate binding sites in cortex and cerebellum. Pharmacol Biochem Behav. 1984;21:151–158. doi: 10.1016/0091-3057(84)90145-x. [DOI] [PubMed] [Google Scholar]
- Trifiletti RR, Snowman AM, Snyder SH. Anxiolytic cyclopyrrolone drugs allosterically modulate the binding of [35S]t-butylbicyclophosphorothionate to the benzodiazepine/γ-aminobutyric acid-A receptor/chloride anionophore complex. Mol Pharmacol. 1984;26:470–476. [PubMed] [Google Scholar]
- Whitehouse PJ, Lynch D, Kuhar MJ. Effects of postmortem delay and temperature on neurotransmitter receptor binding in a rat model of the human autopsy process. J Neurochem. 1984;43:553–559. doi: 10.1111/j.1471-4159.1984.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Xue BG, Friend JM, Gee KW. Loreclezole modulates [35S]t-butylbicyclophosphorothionate and [3H]flunitrazepam binding via a distinct site on the GABAA receptor complex. Eur J Pharmacol. 1996;300:125–130. doi: 10.1016/0014-2999(95)00856-x. [DOI] [PubMed] [Google Scholar]
- Yagle MA, Martin MW, de Fiebre CM, de Fiebre NC, Drewe JA, Dillon GH. [3H]Ethynylbicycloorthobenzoate ([3H]EBOB) binding in recombinant GABAA receptors. Neurotoxicology. 2003;24:817–824. doi: 10.1016/S0161-813X(03)00051-2. [DOI] [PubMed] [Google Scholar]