Abstract
Background and purpose:
Two mechanisms have been proposed to explain the insulin-sensitising properties of metformin in peripheral tissues: (a) inhibition of electron transport chain complex I, and (b) activation of the AMP activated protein kinase (AMPK). However the relationship between these mechanisms and their contribution to β-cell death and dysfunction in vitro, are currently unclear.
Experimental approach:
The effects of biguanides (metformin and phenformin) were tested on MIN6 β-cells and primary FACS-purified rat β-cells. Cell metabolism was assessed biochemically and by FACS analysis, and correlated with AMPK phosphorylation state and cell viability, with or without fuel substrates.
Key results:
In MIN6 cells, metformin reduced mitochondrial complex I activity by up to 44% and a 25% net reduction in mitochondrial reducing potential. In rat β-cells, metformin caused NAD(P)H accumulation above maximal glucose-inducible levels, mimicking the effect of rotenone. Drug exposure caused phosphorylation of AMPK on Thr172 in MIN6 cell extracts, indicative of kinase activation. Methyl succinate, a complex II substrate, appeared to bypass metformin blockade of complex I. This resulted in reduced phosphorylation of AMPK, establishing a link between biguanide-induced mitochondrial inhibition and AMPK activation. Corresponding assessment of cell death indicated that methyl succinate decreased biguanide toxicity to β-cells in vitro.
Conclusions and implications:
AMPK activation can partly be attributed to metformin's inhibitory action on mitochondrial complex I. Anaplerotic fuel metabolism via complex II rescued β-cells from metformin-associated toxicity. We propose that utilisation of anaplerotic nutrients may reconcile in vitro and in vivo effects of metformin on the pancreatic β-cell.
Keywords: insulin, diabetes mellitus, islets of Langerhans, apoptosis, LKB1, metformin, AMP activated protein kinase, anaplerosis
Introduction
Metformin (N′,N′-dimethylbiguanide) is the most commonly prescribed oral medication for treatment of type II diabetes mellitus (T2DM). Plant-derived biguanide alkaloids from Galega officinalis were used as an early treatment for metabolic disturbances, but pharmacological application of metformin for T2DM started only in the late 1950s in Europe and in the mid-1990s in North America (Klepser and Kelly, 1997; Krentz and Bailey, 2005). Despite decades of extensive use and study, there are still uncertainties regarding the mechanism of action of biguanides. Metformin is generally considered to have an insulin sensitising effect on peripheral tissues, with little or no effect on insulin secretion per se. Insulin target tissues exhibit diminished gluconeogenesis and enhanced glucose uptake and utilisation in treated patients; this improves glucose tolerance and reduces hyperglycemic markers (glycated haemoglobin, fructosamine), therefore diminishing the risk of diabetic complications (Klepser and Kelly, 1997; Krentz and Bailey, 2005).
Two key observations regarding the potential mechanism of action of metformin have been described recently. First, the compound is able to inhibit partially respiratory complex I (NADH:ubiquinone oxidoreductase) activity in liver and muscle (El-Mir et al., 2000; Owen et al., 2000; Brunmair et al., 2004). This property appears to be a class action of all biguanides. Inhibition of the electron-transport chain by phenformin (N′N′-phenylethylbiguanide) was demonstrated over four decades ago (Steiner and Williams, 1958; Davidoff, 1971), and more recently for the antimalarial biguanide, proguanil (Srivastava and Vaidya, 1999). Second, also in hepatocytes and myocytes – key insulin target tissues – metformin activates AMP-activated protein kinase (AMPK) (Zhou et al., 2001; Musi et al., 2002). AMPK is a heterotrimeric protein kinase, comprising of regulatory (γ) and catalytic (α) domains separated by a scaffolding subunit (β), and is activated not only by an increase in the AMP/ATP ratio, but also by elevated NAD:NADH ratios and reactive oxygen species (ROS) (Rutter et al., 2003; Rafaeloff-Phail et al., 2004; Kahn et al., 2005). The pre-requisite for AMPK activity is phosphorylation on Thr172 by an upstream kinase, LKB1 (AMPK kinase), which is also sensitive to cellular energy status. Biochemical studies of AMPK activation have concluded that this enzyme acts as a sensor to modulate appropriately cellular metabolism according to nutrient availability. Substrates for AMPK include acetyl-CoA carboxylase and HMG-CoA reductase, mediating its acute effects on lipid and steroid metabolism and, in addition, AMPK appears to alter tissue hexose transport via GLUT isoform levels and subcellular location via pleiotropic signalling pathways (Kurth-Kraczek et al., 1999; Abbud et al., 2000; Rutter et al., 2003; Cidad et al., 2004; Kahn et al., 2005). Long-term cellular changes result from AMPK-mediated alteration of specific transcription factors and modulation of the translational machinery (Bolster et al., 2002; Horman et al., 2002; Kimura et al., 2003; Rutter et al., 2003; Kahn et al., 2005).
The pancreatic islets of Langerhans control whole body glucose homeostasis via the appropriate secretion of two opposing hormones, insulin and glucagon. The insulin secreting β-cell is exquisitely sensitive to nutrient status, rapidly sensing increases in blood sugar, causing metabolic acceleration which is coupled to insulin exocytosis (Hinke et al., 2004). Thus, several lines of research examined the potential role of AMPK in β-cell function (Salt et al., 1998; da Silva Xavier et al., 2000, 2003; Leclerc et al., 2004). As early studies have shown glucose deprivation of β-cells to induce the apoptotic cascade (Hoorens et al., 1996; Van de Casteele et al., 2003) and nutrient restriction activates AMPK, experiments were performed to establish if AMPK and its downstream signalling cascade play a role in the cell death observed. Indeed, AMPK activation in vitro by low glucose, AICAR (an AMP precursor), metformin, or adenoviral overexpression of constitutively active AMPK, initiated the caspase-dependent apoptotic program in MIN6 cells and primary rat β-cells (Kefas et al., 2003a, 2003b, 2004; Richards et al., 2005). Metformin-stimulated β-cell death in vitro is controversial, as metformin is generally considered to have only peripheral effects in vivo but none on insulin secretion, and the drug is well tolerated in T2DM patients.
The current investigation sought to clarify the mechanism of action of metformin in the β-cell and to examine a possible mechanism by which the in vitro metformin toxicity could be metabolically circumvented in treated patients. Biochemical approaches were used to demonstrate that mitochondrial inhibition by metformin in β-cells leads to AMPK activation and cell death, specifically via inhibition of complex I (NADH:Ubiquinone oxidoreductase) activity. Furthermore, the mitochondrial substrate methyl succinate, electrons of which can enter the respiratory chain via complex II (succinate dehydrogenase), was able to prevent partially the cell death induced by metformin in vitro.
Methods
Cell culture
MIN6 cells (passages 18–35) were cultured as described previously (Kefas et al., 2003a) in a humidified (37°C) atmosphere of air and regulated CO2. Cells were routinely passaged by 1:5 dilution in growth media (Dulbecco's modified Eagle's medium (DMEM), 15% foetal calf serum (FCS), 50 μM β-mercaptoethanol, antibiotics) and seeded into 75 cm2 T-flasks treated for adherent cell types (Falcon, Becton-Dickinson, Erembodegem-Aalst, Belgium). Cells were plated into 6-, 24- or 96-well plates (Falcon) for use in biochemical assays, protein studies or viability assays. Standard concentrations of metformin were used (250 μM–2 mM), consistent with prior in vitro studies (Kefas et al., 2004; Leclerc et al., 2004); in vivo, metformin slowly accumulates in tissues and thermodynamic estimates suggest it concentrates in the mitochondrial matrix by up to 1000-fold of its circulating concentration (Owen et al., 2000). Hence, higher concentrations are normally used in vitro for shorter periods of time, however, lower concentrations can be used over longer periods showing the same results (El-Mir et al., 2000; Owen et al., 2000; Rutter et al., 2003; Kefas et al., 2004). The related lipophilic biguanide, phenformin, permeates biological membranes more rapidly (Davidoff, 1971; Owen et al., 2000), and was used over a concentration range of 10 μM–2 mM for selected experiments. Cell viability was determined by Trypan blue exclusion cytometry and a Bürker cell counting chamber with an inverted microscope. Toxicity indices (TI) were calculated using the following equation:
 |
Experimental support of biochemical data observed in MIN6 insulin secreting cells has been provided using primary flow-sorted rat β-cells (Stangé et al., 2003). Use of this model for biochemistry was precluded by scarcity of cells; however, FACS-based metabolic redox experiments and cell viability assays were performed. Islets from male Wistar rats (250–300 g; Elevage Janvier, Le Genest St-Isle, France) were hand-picked following collagenase digestion and dispersed into single islet cells; sorting of primary β-cells was accomplished by flow cytometry (FACStar Plus; Becton Dickinson) using endogenous FAD autofluorescence and size discrimination (forward scatter), as described in detail elsewhere (Stangé et al., 2003). Rat β-cells (>90% insulin-positive) were then cultured in Ham's F10 media supplemented with 0.5% bovine serum albumin (Cohn Analogue), 2 mM glutamine, antibiotics (penicillin and streptomycin) and 10 mM glucose. Metformin and metabolic substrates were added as indicated, and cells were assayed for metabolic redox state or viability according to previously published methods (Martens et al., 2005). Steady-state autofluorescent NAD(P)H levels were monitored by FACS (argon laser 351–363 nm excitation/400–470 nm emission) following 1.5 or 3 h culture in metformin or phenformin and indicated metabolic substrates, by population analysis of 10 000 propidium iodide negative cells. The effect of these compounds on cellular viability of primary rat β-cells was evaluated by staining cells adhered to polylysine coated multiwell dishes with propidium iodide and Hoescht 33342 (Martens et al., 2005) and calculating the toxicity index as above.
Complex I activity
NADH:Ubiquinone oxidoreductase activity was measured using the quartz cuvette spectrophotometric technique described by Jewess and Devonshire (1999), and is similar to the methodologies previously applied to metformin inhibition of complex I (El-Mir et al., 2000; Owen et al., 2000; Brunmair et al., 2004). Briefly, MIN6 cells cultured in the presence or absence of biguanides were sonicated for 20 s on ice in IME buffer (50 mM imidazole, 2 mM MgCl2, 1 mM ethylenediamine tetra-acetic acid (EDTA), with protease inhibitors), protein content was measured by the BCA method and aliquots were stored at −80°C until assayed. Complex I activity was measured kinetically by the consumption of NADH at 340 nM at 30°C using a Shimadzu UV-Vis spectrophotometer. Reaction components were 150 μM NADH, 100 μM coenzyme Q1, 1 mM EDTA, 50 mM KCl, 1 mM KCN, 1.2 μM antimycin A, 10 mM Tris–HCl (pH 7.4), 80 μg MIN6 cell extract, with or without 2.5 μM rotenone. Resultant activity was determined from the slope of NADH consumption per mg protein, subtracting the NADH consumption which could not be inhibited by rotenone.
Citrate synthase assay
To confirm specificity of biguanide effects on complex I, the activity of an alternate mitochondrial enzyme was measured. Citrate synthase can also be used to confirm that mitochondrial mass has not changed as a result of experimental conditions. Cells were similarly grown in the presence or absence of biguanides and harvested in CS sample buffer (40 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 1 mM EDTA, 2 mM MgCl2 and 1% Triton X-100, pH 7.4) by brief sonication and incubation on ice for 15 min, before clearing insoluble material by centrifugation at 4°C. Citrate synthase activity was measured in cell extracts in assay buffer containing 0.4 mM acetyl-CoA, 0.25 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 40 mM HEPES (pH 7.4) and the presence or absence of 0.5 mM oxaloacetic acid, and monitoring the production of the coloured product (λ=412 nm) (Matsuoka and Srere, 1973). Rates of product formation in the absence of oxaloacetate were subtracted from those in the presence of substrate to give specific catalytic activity per mg of protein in the cell extract. Purified porcine heart citrate synthase was employed as a positive control and to assess interassay variability (8.1%).
Lactate/lactate dehydrogenase (LDH) assay
L-(+)-lactate was measured in the supernatant media of cells cultured with or without metformin using the classical method of Gutmann and Wahlefeld (1974), and a standard curve of known lactate concentrations prepared in the same media. Standards and samples were deproteinated using 10% TCA and refrigerated centrifugation; samples were neutralized with K2HPO4 and 10 μl were combined with 80 μl 0.5 M glycine/0.4 M hydrazine (pH 9) and 10 μl 40 mM NAD+ stock. The reaction was started by the addition of 50 μl of pig heart L-LDH (1:50 dilution from 10 mg ml−1 commercial preparation). The commercial preparation of pig heart LDH used in the current study for lactate measurement had a specific activity of 33.4 U mg−1 protein. The reaction was incubated at 37°C for 30 min, and steady-state NADH produced was detected at λ=340 nm. Lactate concentrations were calculated from the linear standard curve.
MIN6 and mouse tissue lactate dehydrogenase activities were measured in sonicated cell fractions according to the technique of Bergmeyer and Bernt (1974). Extracts were incubated in potassium phosphate-buffered (50 mM, pH 7.5) pyruvate solution (1 mM) with 360 μM NADH-Na2, and monitored against time at λ=340 nm. Specific activity was calculated from the molar extinction coefficient of NADH and normalized to the protein content of the extracts.
Mitochondrial activity
Reduction of MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) to insoluble formazan by mitochondrial dehydrogenases can be used to measure mitochondrial nutrient metabolism in many cell types and correlates well with glucose oxidation in pancreatic β-cells (Janjic and Wollheim, 1992; Segu et al., 1998). The MTT reduction assay was used to examine the acute (3 h) oxidative metabolism of various nutrients in HEPES-buffered Krebs solution (KRBH; mM: HEPES 25, pH 7.4, NaCl 125, KCl 4.74, KH2PO4 1.2, MgSO4 1.2, NaHCO3 5, CaCl2 1 and BSA 0.1%), as well as the long-term metabolic effects of nutrient deprivation and metformin in MIN6 culture media. Briefly, cells were seeded at 25 000 cells per well into 96-well plates and grown for 3 days. In the case of acute experiments, media was replaced with 100 μl KRBH with varied nutrient concentrations (0–25 mM) at 37°C; 1 h later, 10 μl of MTT (5 mg ml−1 in PBS, filter sterilized) was added and the incubation was allowed to proceed for an additional 2 h. Published methodologies for the dissolution and measurement of formazan were applied to MIN6 cells; centrifugation and solubilization of crystals in DMSO (Mena et al., 2003), yielded the greatest signal to noise ratio and did not suffer from the high background derived from phenol red. Insoluble precipitated formazan produced was dissolved in 100 μl DMSO following 10 min centrifugation (3000 r.p.m.) of the microtitre plate at 4°C and removal of the supernatant. Absorbance was measured on a Wallac Victor2 micro plate reader at 490 nm. Time course experiments (3–24 h) were conducted in MIN6 culture media, with MTT added 2 h before the end point, and treated in a similar manner; t=0 was defined as the MTT-formazan production from untreated cells cultured for the same duration. Data are presented as absorbance (A490) values or normalized to the parallel control response of cells incubated for the identical time period in 25 mM glucose.
AMPK activity
Immunoblotting was performed according to routine molecular biology methods. MIN6 cellular protein was extracted following culture in the presence or absence of glucose, succinate and metformin using lysis buffer containing phosphatase inhibitors (1% Triton X-100, 60 mM β-glycerophosphate, 20 mM MOPS pH 7.2, 5 mM EDTA, 5 mM EGTA, 1 mM Na3VO4, 20 mM NaF, 140 μg ml−1 aprotinin and 1 mM PMSF). Cells were sonicated and lysates were cleared by centrifugation. Protein concentration was determined and cell extracts were stored at −80°C. Samples containing 25 μg of protein were electrophoresed against Benchmark pre-stained protein ladder (Bio-Rad, Nazareth Eke, Belgium) on either a 7.5 or 12% polyacrylimide gel under denaturing conditions, followed by transfer to nitrocellulose. Membranes were blocked with 5% milk solids in TBST; primary (3 h) and secondary (1 h) antibody incubations were performed in the same buffer, with three 10 min washes in TBST intervening. Polyclonal antibodies used in the current study have been published previously (Kefas et al., 2003a, 2003b, 2004), and all give rise to single protein bands of the expected molecular weights (rabbit anti-actin, 1:1000, Santa Cruz Biotechnology; rabbit anti-(P)Thr-172-AMPK and -total AMPK, both 1:1000, Cell Signaling Technology). Horseradish peroxidase conjugated secondary anti-rabbit IgG antibodies (1:1000; Amersham) and ECL (enhanced chemiluminescence) reagent were used to detect proteins upon exposure of X-ray film. Protein bands were subjected to densitometric analysis using open-source ImageJ software (v1.33u, NIH, USA).
The SAMS assay and phosphoserine79-ACC immunoblotting were performed as described previously (Meisse et al., 2002; Kefas et al., 2003a) to confirm that the observed changes in AMPK phosphorylation status conferred parallel changes in AMPK activity.
Statistical methods
The data are expressed as mean±s.e.m., with the number of independent experiments (n) indicated in the figure legends and text. Differences were considered statistically significant if calculated P-values were less than 0.05 using t-tests or ANOVA and the Newmann–Keuls post hoc test, where appropriate.
Materials
Cell culture materials were from Invitrogen (Merelbeke, Belgium; DMEM), Perbio Science (Erembodegem-Aalst, Belgium; Hyclone Foetal Calf Serum), Bio-Rad (β-mercaptoethanol), Sigma-Aldrich (Bornem, Belgium; penicillin/streptomycin, Trypan blue and trypsin/EDTA solutions) and BD Bioscience (Erembodegem-Aalst, Belgium; culture flasks and serological pipettes). Pierce BCA protein determination kits were from Perbio Science. β-NAD, β-NADH, coenzyme Q1, EDTA, EGTA, rotenone, L-(+)-lactate, pyruvate, trichloroacetic acid, hydrazine, MTT, DTNB, oxaloacetate, acetyl-CoA, purified porcine citrate synthase, DTT, BSA (RIA grade, fraction V and Cohn Analog) and DMSO were from Sigma-Aldrich or Merck (Darmstadt, Germany). HEPES, glycine, acrylamide and bis-acrylamide, SDS and Tris–HCl were from Invitrogen; APS and TEMED were from Bio-Rad. Pig heart LDH was purchased from Roche (Vilvoorde, Belgium).
Results
Metformin inhibits complex I mitochondrial activity and increases lactate accumulation
MIN6 cells were cultured in normal growth media with or without metformin or phenformin for 12 h, before assay of complex I activity in cell lysates. Under the given reaction conditions for complex I activity measurement, consumption of NADH was explicitly dependent upon the presence of coenzyme Q1, >75% of rotenone-inhibitable complex I activity could be recovered in the pellet fraction following centrifugation, and rotenone was able to block between 40–48% of the total NADH consumption in control cells (Finsi, 2004). Inhibition of complex I in cell homogenates or isolated mitochondria by metformin is relatively weak and thought to result from partial loss of energetic properties. However, metformin treated cultured cells demonstrate more profound time-dependent, yet self-limiting inhibition of NADH:ubiquinone oxidoreductase (Owen et al., 2000). Direct incubation of MIN6 cell homogenates with 2 mM metformin inhibited complex I activity by only 7–8% (Finsi, 2004), whereas 12 h culture of the cells with 0.5 or 2 mM metformin resulted in loss of complex I activities by 25 and 44%, respectively (Figure 1a). As reported previously in other cell types, more potent inhibition of complex I activity was observed with phenformin (Figure 1a). In contrast, no significant effect of biguanides was observed on mitochondrial citrate synthase activity under the same conditions, indicating mitochondrial mass was constant in this experiment, and that biguanide inhibition of complex I was specific (Figure 1a).
Figure 1.
Effects of metformin on β-cell oxidative metabolism. (a) Direct measurements of complex I (NADH:Ubiquinone oxidoreductase) activity of the electron transport chain (black bars) or mitochondrial citrate synthase (cross-hatched bars) were made in extracts of MIN6 cells, cultured for 12 h in the presence or absence of metformin or phenformin. Citrate synthase assays were performed to confirm the specificity of the biguanide inhibition and to eliminate the possibility of change in mitochondrial mass. (b) Lactate accumulation in MIN6 cell media as a function of metformin concentration and duration of exposure. (c) LDH activity measured in MIN6 extracts cultured in the presence or absence of metformin. (d) LDH activity in MIN6 cells was 8–17 times lower than that measured in control mouse tissues (heart, kidney and liver). Data represent mean±s.e.m. of at least three independent experiments. *P<0.05; ‡P<0.05 vs 0.6G alone.
Culture medium of MIN6 cells was analysed for lactate accumulation after 24 or 48 h incubation with or without metformin (1–2 mM), and cell pellets were analysed for LDH activity. Lactate accumulation in basal culture medium containing 25 mM glucose was more than doubled after 48 h of culture (Figure 1b). Removal of added glucose to the culture media resulted in low levels of lactate after 24 h and, by 48 h, lactate dropped further to levels close to the assay's limit of detection. Metformin dose-dependently increased the lactate accumulation in media of MIN6 cells cultured in 25 mM glucose, at both 24 h and 48 h time points (Figure 1b). This effect on lactate production was also observed in media with low glucose (0.6 mM; Figure 1b).
β-cell lactate dehydrogenase enzyme activity was low and not influenced by these culture conditions (Figure 1c). The average LDH specific activity from mouse heart, kidney and liver was 818±99 mU mg−1 protein (Figure 1d; n=3; range 650–1000 mU mg−1 protein), one order of magnitude greater than the LDH activity measured in MIN6 cells. Low LDH levels is a well characterised feature of β-cells (Ainscow et al., 2000).
Mitochondrial MTT reducing activity in MIN6 cells
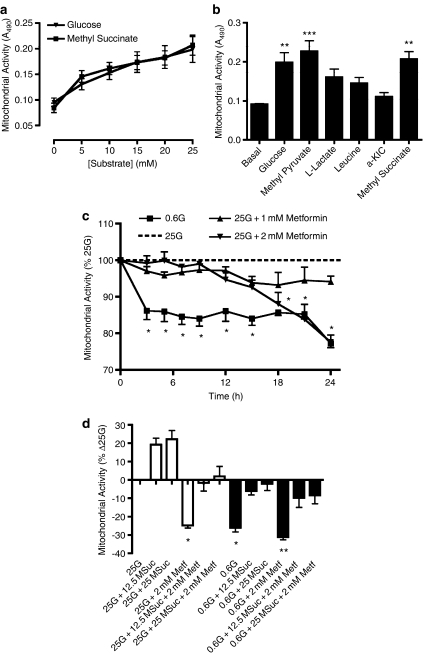
Although MTT is most commonly used as a toxicity/viability screening tool, its action is to detect net cellular mitochondrial metabolism (reducing potential). It has been applied previously to rat islets and insulinoma cells, correlating well with glucose oxidation (Janjic and Wollheim, 1992; Segu et al., 1998). We first established the acute concentration dependency of MTT reduction by various nutrients in KRBH (Figure 2a and b). Of the substrates tested, only glucose and succinate (methyl ester) demonstrated potent and concentration-dependent effects (EC50 values: ∼5.8 and 6.8 mM, respectively; Figure 2a). Pyruvate (methyl ester) also potently stimulated MTT reduction (Figure 2b), however, this effect was not concentration-dependent (range: 5–25 mM). L-lactate, leucine and α-ketoisocaproic acid (α-KIC) produced only small increases in MTT reduction (P>0.05, Figure 2b). Similar results were obtained in HEPES-buffered DMEM, with the exception that maximal responses to methylpyruvate and α-KIC were slightly enhanced, whereas that of lactate was somewhat reduced (data not shown).
Figure 2.
MIN6 cell mitochondrial activity measured using the MTT reduction assay. (a) Acute concentration response curves for glucose and methyl succinate stimulating mitochondrial activity in MIN6 cells. (b) Acute stimulation of mitochondrial activity by 25 mM of various fuels in MIN6 cells. (c) Time dependent inhibition of MIN6 cell mitochondrial activity by glucose deprivation or addition of metformin. (d) Stimulation of mitochondrial activity by methyl succinate (12.5 or 25 mM) in the presence or absence of glucose and/or metformin (1 or 2 mM) during 24 h exposure of MIN6 cells. In all experiments, MTT was added to cell culture wells 2 h before the end point, and treated as per described in the Methods section. Data are mean±s.e.m. (n⩾4); *P<0.05, **P<0.01, ***P<0.001.
The MTT assay was used to detect changes in mitochondrial metabolism in MIN6 cells cultured in 25 mM glucose with 1 or 2 mM metformin, or in low glucose (Figure 2c). MTT-reducing activity was decreased by ⩾15% in MIN6 cells exposed to 0.6 mM glucose (3–24 h), as compared to MIN6 cells cultured in 25 mM glucose. When MIN6 cells were cultured in 25 mM glucose in presence of metformin, a time-dependent inhibition of MTT-formazan production was observed, and the extent of this inhibition was dependent on metformin concentration (Figure 2c). These data supported the notion that metformin inhibited mitochondrial activity, as shown by complex I measurements. The effect of combining methyl succinate with low glucose or metformin was therefore examined at 24 h (Figure 2d). This showed that (i) both low glucose or 2 mM metformin decreased mitochondrial function equally (Figure 2d) as compared to control cells, and (ii) the combination of 2 mM metformin and low glucose resulted in a greater loss of mitochondrial activity; (iii) methyl succinate (25 mM) completely restored mitochondrial activity of MIN6 cells cultured either in 0.6 mM glucose or in presence of 2 mM metformin, although it could partially restore the mitochondrial activity of MIN6 cells cultured in 0.6 mM glucose combined with 2 mM metformin (Figure 2d).
AMPK phosphorylation status and activity
Activation of AMPK in MIN6 cells was monitored by immunoblotting using an antibody directed against [(P)Thr172]AMPK. Densitometric analysis was performed on 5–7 immunoblots derived from independent experiments on MIN6 cells cultured for 24 h in the presence or absence of glucose, methyl succinate and/or metformin (Figures 3a and 4a). The results indicated that metformin increased AMPK phosphorylation: 2.1±0.3-fold at 1 mM and 3.5±0.4-fold at 2 mM, as compared to control cells cultured in 25 mM glucose (P<0.05; Figure 3b). As reported previously (Kefas et al., 2003a, 2003b), 24 h culture in low glucose did not significantly increase AMPK phosphorylation (1.6-fold that in 25 mM glucose, P>0.05; Figure 4b). In 0.6 mM glucose, addition of 1 mM or 2 mM metformin further increased AMPK phosphorylation 1.7-fold and 3-fold, respectively (Figure 4b), indicating an additive effect between low glucose and metformin on AMPK activation. The reduction of basal AMPK phosphorylation in control media (25 mM glucose) and the suppression of low glucose induced AMPK phosphorylation, induced by inclusion of 25 mM methyl succinate were not significant (both P>0.05; Figures 3b and 4b). However, 25 mM methyl succinate clearly decreased the phosphorylation of AMPK induced by 2 mM metformin (∼30%) though incompletely, at either control (25 mM) or low glucose concentrations (P<0.01; Figures 3b and 4b). Cellular expression of total AMPK was not influenced by glucose, metformin or methyl succinate (Figures 3b and 4b; P>0.05).
Figure 3.
Concentration-dependent induction of MIN6 AMPK phosphorylation state by metformin, in the presence and absence of methyl succinate, cultured in media containing 25 mM glucose. (a) Representative Western blots from protein extracts (25 μg per lane) taken from MIN6 cells cultured 24 h under the given conditions. Immunoblots were performed under standard conditions as described in the Methods section. (b) Compiled densitometric analysis of Western blots ((P)Thr172AMPK, clear bars; total AMPK, black bars). Data are the mean±s.e.m. of pixel density scans from 5–7 bands for each condition, from independent protein extracts. §P<0.05 vs 25G alone; *P<0.01 comparing 25G+2 mM metformin to the same condition with 25 mM methyl succinate.
Figure 4.
Concentration-dependent induction of MIN6 AMPK phosphorylation state by metformin, in the presence and absence of methyl succinate, cultured in media containing 0.6 mM glucose. (a) Representative Western blots from protein extracts (25 μg per lane) taken from MIN6 cells cultured 24 h under the given conditions. Immunoblots were performed under standard conditions as described in the Methods section. (b) Compiled densitometric analysis of Western blots ((P)Thr172AMPK, clear bars; total AMPK, black bars). Data are the mean±s.e.m. of pixel density scans from 5–6 bands for each condition, from independent protein extracts. §P<0.05 vs 25G alone; *P<0.01 comparing 0.6G+2 mM metformin to the same condition supplemented with 25 mM methyl succinate.
MIN6 cell viability
Cell viability was measured by Trypan blue exclusion. Under standard culture conditions in 25 mM glucose, MIN6 cell viability was 87.1±0.7% (n=7). In low glucose media (0.6 mM) in the continued presence of FCS, cell viability declined to 81.5±1.6% after 24 h, giving a calculated toxicity index of 6.5±1.8% (P<0.01; n=7; Figure 5b). In the presence of 25 mM glucose, metformin concentration-dependently reduced MIN6 viability at 24 h, hence increasing the toxicity indices (Figure 5a), confirming earlier results that long-term in vitro exposure to biguanide compounds is cytotoxic to β-cells (Kefas et al., 2004). In low glucose, long-term exposure to 2 mM metformin further decreased cell viability and increased toxicity index (Figure 5b), probably as the result of further inhibition of cellular metabolism. Importantly, addition of 25 mM methyl succinate was able to reduce the toxicity of 2 mM metformin, regardless of the glucose concentration (Figure 5a and b). A small increase (∼7%) in toxicity was caused by 25 mM methyl succinate alone when cells were stressed in low glucose (Figure 5b). However, in the context of the extensive toxicity caused by 2 mM metformin, the anaplerotic substrate methyl succinate was clearly protective, reducing the toxicity index by ∼30% (Figure 5a and b).
Figure 5.
MIN6 cell viability by Trypan blue exclusion cytometry following 24 h culture under the given conditions. (a) Toxicity index of 1 or 2 mM metformin, for MIN6 cells cultured in the presence of 25 mM glucose and/or methyl succinate. (b) Toxicity index as in (a), but with MIN6 cells cultured in media without added glucose (0.6 mM). Toxicity index was calculated as described in the text. Data are mean±s.e.m. for seven independent trials. §P<0.01 vs control 25G, **P<0.01, ***P<0.001.
Influence of biguanides on primary rat β-cell redox state and viability
To evaluate acute or direct effects of biguanides on cellular metabolism of primary β-cells, time- and concentration-dependent effects of metformin and phenformin on redox status of FACS purified rat β-cells were examined. We used flow cytometry to measure steady-state total cellular NAD(P)H (Figure 6a and b) and mitochondrial reduced riboflavin levels (not shown) in large populations of freshly isolated, living (propidium iodide negative) β-cells exposed for either 1.5 or 3 h to increasing concentrations of metformin (0.25–2 mM) and the more lipophilic biguanide compound, phenformin (10 μM–2 mM) in the presence of 10 mM glucose. The metabolic glucose responsiveness of healthy β-cells is reflected by a glucose concentration-dependent increase in NAD(P)H, with an EC50 value approximating the K1/2 of glucokinase (∼7.5 mM); raising ambient glucose levels from 0 to 20 mM approximately doubles cellular NAD(P)H fluorescence, with the greatest effect observed between 5 and 10 mM glucose (Bennett et al., 1996; Martens et al., 2005). Further elevating the glucose milieu did not result in significantly higher NAD(P)H (not shown), whereas addition of 100 nM rotenone dramatically (+50%, P<0.005) increased NAD(P)H levels within seconds, reflecting rotenone-induced inhibition of NADH consumption by electron transport complex I (Figure 6a and b). Metformin similarly increased NAD(P)H autofluorescence in 10 mM glucose-cultured β-cells, in a time- and concentration-dependent manner (Figure 6a): after 1.5 h, 2 mM metformin significantly increased NAD(P)H in the primary cells (P<0.01, n=5), to the same levels measured in drug-free 20-mM-glucose-exposed cells. After a 3 h incubation, NAD(P)H accumulation was also detected with 0.5 mM metformin (25% increase, P<0.001), and 1 or 2 mM metformin-induced NAD(P)H reached levels comparable to those in rotenone-treated cells (Figure 6a), well above levels that can be obtained by maximal glucose stimulation. Phenformin also caused time- and concentration-dependent increases in NAD(P)H (P<0.01, Figure 6b), with a 10–20-fold greater potency than metformin, in accordance with previously reported values (Owen et al., 2000). Biguanide-induced NAD(P)H accumulation was rapidly reversible upon addition of the respiratory uncoupler, CCCP, (10 μM, 30–50% decrease after 5 min, not shown). As uncouplers are known to stimulate respiration, this suggested that biguanides caused a reversible inhibition rather than blockade of the respiratory chain. Metformin-induced NAD(P)H accumulation was also observed in the murine β-cell-line MIN6, in a time-and concentration-dependent manner. At 2 mM, metformin caused 30% increase MIN6 NAD(P)H level (P<0.001, n=7) and this increase was sustained at 24 h (data not shown). These results suggested a direct action of biguanides on the mitochondrial respiratory complex I in β-cells.
Figure 6.
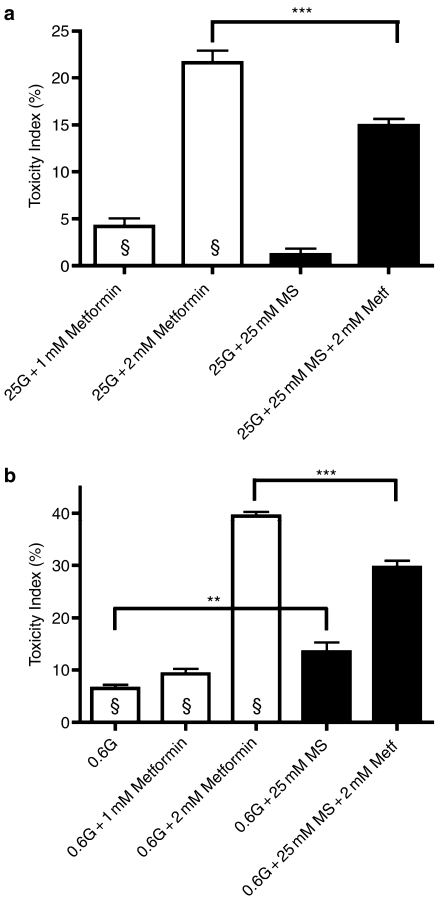
Metabolic redox state and viability of primary rat β-cells when exposed to biguanides. (a) Effect of metformin (Metf) or (b) phenformin (Phenf) on cellular NAD(P)H in 10 mM glucose-stimulated rat β-cells. Cells were incubated for 1.5 h (clear bars) or 3 h (black bars) under the indicated conditions as specified in Methods section, followed by FACS-measurement of total cellular NAD(P)H in intact (propidium iodide-negative) cells. Rotenone (100 nM) was added 5 min before FACS analysis. Changes in β-cell NAD(P)H induced by 0–20 mM glucose in the absence of biguanides are also shown (a); data in (b) were collected in 10 mM glucose-exposed β-cells. Data represent mean fluorescence intensities (MFI, mean±s.e.m., n=7), expressed as %MFI measured in 10 mM glucose-stimulated, untreated cells; * indicates significant (P<0.01) effect of biguanides relative to control cells. (c) Rat β-cells were exposed for 72 h to phenformin or metformin, both in the presence of 10 mM glucose and their survival compared to cells that were maintained in low glucose (Glc, 3 mM), in the absence of biguanides. These incubations were carried out in standard culture media (black bars), or in media supplemented with 10 mM methyl pyruvate (MPyr, 10 mM, cross-hatched bars) or 10 mM methyl succinate (MSuc, 10 mM, clear bars). Data represent mean±s.e.m. (n=5) Toxicity Indices, reflecting the amount of apoptotic beta cells under the indicated condition, *indicates significant (P<0.01) effect of MSuc or MPyr as compared to cells cultured in standard medium (black bars).
Biguanide-induced NAD(P)H accumulation in β-cells is not caused by accelerated metabolic rate or activation of AMPK
To exclude the possibility that the biguanide-induced NAD(P)H accumulation in primary β-cells was caused by an acceleration of mitochondrial NADH-formation by AMPK, we compared the biguanide effects with those of another established activator of AMPK, AICA-riboside (AICAR) (Kefas et al., 2004). Exposure of rat β-cells for 3 h to 1 mM AICAR increased AMPK phosphorylation on Thr172 and thus kinase activity (Kefas et al., 2003b), however, this was not associated with a changed NAD(P)H level (Supplementary Figure 1, n=5). Additionally, it was possible to measure the biguanide-induced NAD(P)H accumulation in the absence of extracellular glucose (+28%, P<0.01), or in the presence of 10 mM glucose when β-cell glycolysis was inhibited with 20 mM mannoheptulose (+48%, P<0.01; Supplementary Figure 1). The metabolic effects of biguanide are consistent with a rotenone-like inhibition of mitochondrial NADH consumption. However, although the rotenone effect is an all-or-none phenomenon, the biguanide-induced inhibition appears to be more subtle in terms of its concentration dependence and time course.
Methyl succinate protects primary rat β-cells from biguanide-induced apoptosis
Finally, we examined if the protective effect of methyl succinate on metformin-induced MIN6 cell death also occurred in primary β-cells isolated from rats. The studied cell preparations were first cultured overnight in 10 mM glucose, and then exposed for 72 h to 10 mM glucose (control) with or without 1 mM metformin or 50 μM phenformin, in the presence or absence of methyl succinate or methyl pyruvate (Figure 6c). Under these conditions, both biguanides induce apoptosis (nuclear blebs and fragmentation) in a subset (30–40%) of β-cells ((Kefas et al., 2004) and Figure 6c), and thereby mimic the degree of apoptosis observed in glucose deprived β-cells (3 mM glucose, ∼20% apoptosis). Cell death in glucose-deprived β-cells was partially prevented by addition of 10 mM methyl pyruvate (35% reduction, P<0.01, Figure 6c) and near-completely suppressed by complex II substrate methyl succinate (92% reduction, P<0.001), indicating that both compounds can be metabolized by β-cells and at least partially substitute for glucose. In contrast, methyl pyruvate was unable to prevent apoptosis in biguanide-treated β-cells. Methyl succinate addition, however, markedly prevented cell death both in phenformin (−50%, P<0.01) and metformin (−35%, P<0.05) treated primary rat β-cells (Figure 6c).
Discussion
The beneficial effects of biguanide derivatives in the treatment of human T2DM are evident from the widespread use of this compound and the favourable clinical reports (Klepser and Kelly, 1997; Krentz and Bailey, 2005). These compounds improve insulin sensitivity in multiple tissues, yet their precise molecular and cellular mechanisms have been elusive and many potential pathways have been proposed. Although metformin remains the most prescribed anti-diabetic medication, predecessors such as phenformin were removed from clinical use owing to the side effect of lactic acidosis (Klepser and Kelly, 1997; Krentz and Bailey, 2005). This was previously attributed to phenformin's potential to inhibit complex I (NADH:Ubquinone oxidoreductase) of the respiratory chain (Steiner and Williams, 1958; Davidoff, 1971), and in agreement with reported biological effects of other biguanide-based compounds (Srivastava and Vaidya, 1999), including metformin (El-Mir et al., 2000; Owen et al., 2000).
The activation of AMPK by metformin and other biguanides is consistent with the inhibition of mitochondrial respiration, as AMPK is activated by a number of stimuli which would be expected under this condition: a reduction in ATP:AMP ratio and increased ROS formation (Zhou et al., 2001; Musi et al., 2002; Rutter et al., 2003; Kahn et al., 2005). Parallel studies showed the beneficial effects of AICAR on insulin sensitivity in diabetic models, and the role of AMPK as a mediator. Subsequent reports revealed this kinase as the probable mediator of metformin's beneficial action on peripheral tissues in human diabetes (Rutter et al., 2003; Kahn et al., 2005). To date, the relation between metformin's ability to inhibit mitochondrial respiration and its AMPK-activating effect has not been established. Metformin was proposed previously to activate AMPK without causing detectable changes in cellular AMP:ATP ratio (Hawley et al., 2002). The same group first proposed that biguanides, in immortalized fibroblasts and HeLa cells, activated AMPK via a poorly defined, energy status-independent activation of LKB1 (Hawley et al., 2003). However, in subsequent studies of more physiologically relevant conditions (contracting muscle), LKB1 was not required for biguanide-induced AMPK activation (Sakamoto et al., 2004).
Our data clearly show for the first time that metformin activates AMPK, at least in part, via its inhibition of complex I-driven respiration. Bypassing complex I with methyl succinate was able to prevent metformin's inhibitory effects on mitochondrial reducing potential (Figure 2a), and was able to rescue a significant percentage of β-cells from biguanide-induced cell death (Figures 5a, b and 6c), and this was associated with a decreased activated state of AMPK (Figures 3b and 4b). In vivo, it is likely that metabolic fuels that bypass complex I are potentially derived from leucine, isoleucine and valine metabolism (either from nutrient ingestion or tissue catabolism), would provide sufficient substrates utilising the complex II pathway to prevent metformin toxicity. It was also noted that methyl succinate was unable to reverse completely AMPK activation by metformin. This may provide an explanation as to how metformin's beneficial effects mediated by AMPK are not completely abolished in vivo. It may therefore be interesting to test whether methyl succinate is capable of diminishing the insulin-sensitising effects of metformin on peripheral tissues.
The present data are also compatible with a potential tumour-suppressor role of AMPK in patients treated with AMPK-activating compounds. The tumour suppressor LKB1 was identified as an upstream AMPK-activating kinase (AMPKK), and loss of function mutations in humans lead to benign gastrointestinal polyps typified by the autosomal-dominant Peutz-Jeghers syndrome and are also frequently observed in sporadic lung adenocarcinomas (Tiainen et al., 2002; Jimenez et al., 2003; Carretero et al., 2004). Indeed, activation of AMPK by AICAR in hepatoma cells in vitro induced tumour suppressors p53 and p21, thus initiating the apoptotic cascade in these cells (Imamura et al., 2001; Meisse et al., 2002). Similarly, AICAR was capable of suppressing tumour growth of various prostate cancer cells in vitro via AMPK activation (Xiang et al., 2004). Consistent with these findings were the results indicating that AMPK activation via nutrient deprivation, or pharmacological stimulation with AICAR or metformin was also capable of inducing the apoptotic program via c-Jun N-terminal kinase in MIN6 and primary rat β-cells (Kefas et al., 2003a, 2003b, 2004). Conflicting reports were published employing INS-1 cells, indicating a protective effect of metformin during gluco-lipotoxic culture conditions (El-Assaad et al., 2003) and possibly via a poorly characterized anti-oxidant mechanism in human islets (Marchetti et al., 2004). Indeed, AMPK has been given a protective role against palmitate-induced cell death of glial cells and hyperglycemia-stimulated endothelial cell death (Blazquez et al., 2001; Ido et al., 2002). Although the underlying cause of the discrepancies between the reports of metformin's effects on apoptosis in β-cells is unknown, it may be related to a dual role for AMPK in growth regulation (Shaw et al., 2004; Luo et al., 2005). Thus, depending on environmental conditions, the duration that stimuli are present and the cell type, AMPK may function to preserve cell viability over the short-term, but chronic AMPK activation would lead to programmed cell death. Under standard culture conditions, we have been unable to reproduce any direct protective effects of metformin, yet consistently observe significant β-cell death at time points of 24 h and longer, including at lower concentrations than those used here. It may be a relevant preliminary observation by Evans et al. (2005) that metformin treated type II diabetic patients appear to have a lower risk for cancer development, and that metformin prevented carcinogen-induced malignant lesions in the pancreata of high fat fed hamsters (Schneider et al., 2001).
The initiation of research into AMPK activity in β-cells further raises questions as to the peripheral effects of metformin. It has been widely accepted that improved insulin sensitivity in muscle, liver and adipose tissue is via a direct action of metformin on these tissues, likely to be via AMPK. Studies using AICAR to activate AMPK in rodent islets and insulinoma cells have suggested an inhibitory role for the kinase on insulin release at stimulatory glucose concentrations and either no effect or augmentation of basal release under reduced glycemia (Salt et al., 1998; da Silva Xavier et al., 2003; Leclerc et al., 2004). Use of adenoviral overexpression of constitutively active AMPK in MIN6 cells or mammalian islets also suppressed glucose-induced insulin secretion (Salt et al., 1998; Leclerc et al., 2004; Richards et al., 2005), confirming that the effects of pharmacological activation were the result of specific AMPK activation.
Metformin would be predicted to inhibit insulin secretion by reducing ATP:ADP and thus promoting KATP channel open state, but it may also be expected that indirect pharmacological activation of AMPK using metformin would similarly suppress insulin release from β-cells. However, the effect of metformin on insulin secretion has been controversial. An early study using isolated rat islets found an inhibitory effect of metformin on insulin release (Schatz et al., 1972b), whereas later studies on hamster insulinoma (HIT-T15 cells) found no effect (Moore and Cooper, 1991), and subsequent in vitro release experiments using isolated human islets reported a potentiation of glucose-stimulated insulin release (Lupi et al., 1997). A more recent re-evaluation of metformin's influence on islet hormone release supported an inhibitory effect of the biguanide, correlating well with AMPK activation, in both MIN6 cells and human islets (Leclerc et al., 2004). Because of the complex pharmacodynamics of metformin in vitro (electrogenic mitochondrial accumulation), the disparate results may simply be owing to differences in experimental protocols. Despite the commonly held opinion that the improvement in glucose tolerance is due to peripheral sensitising actions of biguanides – and not because of a direct effect on the islet – several published human trials on this drug class have demonstrated improved glucose tolerance yet reduced insulin profiles (Grodsky et al., 1963; Abramson and Arky, 1967; Schatz et al., 1972a; Defronzo and Goodman 1995). Two main approaches to anti-diabetic therapy have been to augment insulin secretion from β-cells (e.g. sulphonylureas) or to increase peripheral insulin sensitivity (e.g. biguanides, glitazones). Surprisingly in pre-clinical studies, it has been shown that agents which act directly upon β-cells to suppress insulin secretion, such as diazoxide and its analogues, counterintuitively improve glycaemia, the working hypothesis being that without persistently high serum insulin levels, the peripheral tissues can begin to re-sensitise to the hormone as well as allow the β-cell time to regranulate during a period of relative quiescence (Carr et al., 2003; Dabrowski et al., 2003; Yoshikawa et al., 2004). It will be important to establish if metformin may be acting partially via this direct β-cell mechanism to mediate its beneficial effects in treated humans and rodent models in vivo.
In conclusion, the current study has aimed to clarify the direct effects of metformin on the β-cell. Results clearly demonstrate a mitochondrial effect of this drug on β-cells, leading to AMPK activation, which is consistent with studies of other tissues. The dual nature of AMPK, however, casts some uncertainty on independent duplication of results depending on precise environmental factors and experimental design. In vitro data must be regarded with caution, as neither β-cell protection nor destruction has been consistently observed in the human therapeutic use of this compound, and results observed in vitro appear to depend largely on the experimental conditions. The dual action of AMPK may also underlie the inconsistent data published on direct effects of metformin on insulin secretion. The results presented in the current manuscript reconcile the data showing cell death in vitro but not in vivo, as explained by the availability and utilization of metabolic fuels able to bypass the mitochondrial complex 1.
External data objects
Acknowledgments
Simon Hinke is the recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research. This work was supported by Grants from the Scientific Research Fund Flanders (FWO-G.0357.03 and 101/8 to GM, who is aspirant FWO), by the Inter-University Poles of Attraction Program (IUAP P5/17) from the Belgian Science Policy, and by the Brussels Free University, VUB (OZR-898&1161). Geert Stangé and Erik Quartier are acknowledged for excellent technical assistance.
Abbreviations
- α-KIC
α-ketoisocaproic acid
- AICAR
5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside
- AMPK
AMP-activated protein kinase
- AMPKK
AMP-activated protein kinase kinase (LKB1)
- APS
ammonium persulphate
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethylsulphoxide
- DTNB
5,5′-dithiobis-(2-nitrobenzoic acid)
- DTT
dithiothreitol
- EC50
half-maximal effective concentration
- EDTA
ethylenediamine tetraacetic acid
- EGTA
ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′ tetra acetic acid
- FCS
foetal calf serum
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- KRBH
Kreb's ringer bicarbonate HEPES buffer
- LDH
lactate dehydrogenase
- MOPS
3-(N-morpholino) propanesulfonic acid
- MTT, 3-(4,5-dimethylthiazolyl-2)-2
5-diphenyltetrazolium bromide
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulphate
- T2DM
type 2 diabetes mellitus
- TBST
Tris-buffered saline with 0.1% Tween-20
- TCA
trichloroacetic acid
- TEMED
1,2-bis(dimethylamino)ethane
- TI
toxicity index
Conflict of interest
The author state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Abbud W, Habinowski S, Zhang JZ, Kendrew J, Elkairi FS, Kemp BE, et al. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch Biochem Biophys. 2000;380:347–352. doi: 10.1006/abbi.2000.1935. [DOI] [PubMed] [Google Scholar]
- Abramson E, Arky RA. Treatment of the obese diabetic. A comparative study of placebo, sulfonylurea and phenformin. Metabolism. 1967;16:204–212. doi: 10.1016/0026-0495(67)90169-2. [DOI] [PubMed] [Google Scholar]
- Ainscow EK, Zhao C, Rutter GA. Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. doi: 10.2337/diabetes.49.7.1149. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Jetton TL, Ying G, Magnuson MA, Piston DW. Quantitative subcellular imaging of glucose metabolism within intact pancreatic islets. J Biol Chem. 1996;271:3647–3651. doi: 10.1074/jbc.271.7.3647. [DOI] [PubMed] [Google Scholar]
- Bergmeyer H, Bernt E.Lactate dehydrogenase: UV-assay with pyruvate and NADH Methods of Enzymatic Analysis 1974Verlag Chemie Weinheim, Academic Press, Inc.: New York; 574–579.In: Bergmeyer H (ed) [Google Scholar]
- Blazquez C, Geelen MJ, Velasco G, Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–153. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions. Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- Carr RD, Brand CL, Bodvarsdottir TB, Hansen JB, Sturis J. NN414, a SUR1/Kir6.2-selective potassium channel opener, reduces blood glucose and improves glucose tolerance in the VDF Zucker rat. Diabetes. 2003;52:2513–2518. doi: 10.2337/diabetes.52.10.2513. [DOI] [PubMed] [Google Scholar]
- Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004;23:4037–4040. doi: 10.1038/sj.onc.1207502. [DOI] [PubMed] [Google Scholar]
- Cidad P, Almeida A, Bolanos JP. Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through 5′-AMP-activated protein kinase. Biochem J. 2004;384:629–636. doi: 10.1042/BJ20040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva Xavier G, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, et al. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci USA. 2000;97:4023–4028. doi: 10.1073/pnas.97.8.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowski M, Larsen T, Ashcroft FM, Bondo Hansen J, Wahl P. Potent and selective activation of the pancreatic beta-cell type K(ATP) channel by two novel diazoxide analogues. Diabetologia. 2003;46:1375–1382. doi: 10.1007/s00125-003-1198-1. [DOI] [PubMed] [Google Scholar]
- Davidoff F. Effects of guanidine derivatives on mitochondrial function 3. The mechanism of phenethylbiguanide accumulation and its relationship to in vitro respiratory inhibition. J Biol Chem. 1971;246:4017–4027. [PubMed] [Google Scholar]
- Defronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsi J. NADH:Ubiquinone Oxidoreductase Inhibition Following Metformin Exposure in MIN6 Cells [M Sc. Dissertation]. Department of Metabolism and Endocrinology. Vrije Universiteit Brussel, Brussels, Belgium; 2004. p. 44. [Google Scholar]
- Grodsky GM, Karam JH, Pavlatos FC, Forsham PH. Reduction by phenformin of excessive insulin levels after glucose loading in obese and diabetic subjects. Metabolism. 1963;12:278–286. [PubMed] [Google Scholar]
- Gutmann I, Wahlefeld W.L-(+)-Lactate determination with lactate dehydrogenase and NAD Methods of Enzymatic Analysis 1974Verlag Chemie Weinheim, Academic Press, Inc.: New York; 1456–1468.In: Bergmeyer H (ed) [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- Hinke SA, Hellemans K, Schuit FC. Plasticity of the beta cell insulin secretory competence: preparing the pancreatic beta cell for the next meal. J Physiol. 2004;558:369–380. doi: 10.1113/jphysiol.2004.064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorens A, Van De Casteele M, Kloppel G, Pipeleers D. Glucose promotes survival of rat pancreatic beta cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest. 1996;98:1568–1574. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- Janjic D, Wollheim CB. Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia. 1992;35:482–485. doi: 10.1007/BF02342448. [DOI] [PubMed] [Google Scholar]
- Jewess PJ, Devonshire AL. Kinetic microplate-based assays for inhibitors of mitochondrial NADH:ubiquinone oxidoreductase (complex I) and succinate:cytochrome c oxidoreductase. Anal Biochem. 1999;272:56–63. doi: 10.1006/abio.1999.4168. [DOI] [PubMed] [Google Scholar]
- Jimenez AI, Fernandez P, Dominguez O, Dopazo A, Sanchez-Cespedes M. Growth and molecular profile of lung cancer cells expressing ectopic LKB1: down-regulation of the phosphatidylinositol 3′-phosphate kinase/PTEN pathway. Cancer Res. 2003;63:1382–1388. [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, et al. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004;68:409–416. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, et al. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol. 2003a;30:151–161. doi: 10.1677/jme.0.0300151. [DOI] [PubMed] [Google Scholar]
- Kefas BA, Heimberg H, Vaulont S, Meisse D, Hue L, Pipeleers D, et al. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia. 2003b;46:250–254. doi: 10.1007/s00125-002-1030-3. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- Klepser TB, Kelly MW. Metformin hydrochloride: an antihyperglycemic agent. Am J Health Syst Pharm. 1997;54:893–903. doi: 10.1093/ajhp/54.8.893. [DOI] [PubMed] [Google Scholar]
- Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Woltersdorf WW, Da Silva Xavier G, Rowe RL, Cross SE, Korbutt GS, et al. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E1023–E1031. doi: 10.1152/ajpendo.00532.2003. [DOI] [PubMed] [Google Scholar]
- Luo Z, Saha a K, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Lupi R, Marchetti P, Giannarelli R, Coppelli A, Tellini C, Del Guerra S, et al. Effects of glibenclamide and metformin (alone or in combination) on insulin release from isolated human pancreatic islets. Acta Diabetol. 1997;34:46–48. doi: 10.1007/s005920050065. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- Martens GA, Cai Y, Hinke S, Stange G, Van De Casteele M, Pipeleers D. Glucose suppresses superoxide generation in metabolically responsive pancreatic beta cells. J Biol Chem. 2005;280:20389–20396. doi: 10.1074/jbc.M411869200. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Srere PA. Kinetic studies of citrate synthase from rat kidney and rat brain. J Biol Chem. 1973;248:8022–8030. [PubMed] [Google Scholar]
- Meisse D, Van De Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, et al. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- Mena JA, Ramirez OT, Palomares LA.Titration of non-occluded baculovirus using a cell viability assay Biotechniques 200334260–262.264 [DOI] [PubMed] [Google Scholar]
- Moore CX, Cooper GJ. Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun. 1991;179:1–9. doi: 10.1016/0006-291x(91)91325-7. [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Part 3:607–614. [PMC free article] [PubMed] [Google Scholar]
- Rafaeloff-Phail R, Ding L, Conner L, Yeh WK, Mcclure D, Guo H, et al. Biochemical regulation of mammalian AMP-activated protein kinase activity by NAD and NADH. J Biol Chem. 2004;279:52934–52939. doi: 10.1074/jbc.M409574200. [DOI] [PubMed] [Google Scholar]
- Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic ß-cell function in vivo. J Endocrinol. 2005;187:225–235. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Da Silva Xavier G, Leclerc I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003;375:1–16. doi: 10.1042/BJ20030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335 Part 3:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz H, Doci S, Hofer R. The effect of dimethylbiguanide on glucose tolerance, serum insulin and growth hormone in obese patients. Diabetologia. 1972a;8:1–7. doi: 10.1007/BF01219979. [DOI] [PubMed] [Google Scholar]
- Schatz H, Katsilambros N, Nierle C, Pfeiffer EE. The effect of biguanides on secretion and biosynthesis of insulin in isolated pancreatic islets of rats. Diabetologia. 1972b;8:402–407. doi: 10.1007/BF01212167. [DOI] [PubMed] [Google Scholar]
- Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- Segu VB, Li G, Metz SA. Use of a soluble tetrazolium compound to assay metabolic activation of intact beta cells. Metabolism. 1998;47:824–830. doi: 10.1016/s0026-0495(98)90120-2. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, Depinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Vaidya AB. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother. 1999;43:1334–1339. doi: 10.1128/aac.43.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangé G, Van De Casteele M, Heimberg H. Purification of rat pancreatic B-cells by fluorescence-activated cell sorting. Methods Mol Med. 2003;83:15–22. doi: 10.1385/1-59259-377-1:015. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Williams RH. Respiratory inhibition and hypoglycemia by biguanides and decamethylenediguanidine. Biochim Biophys Acta. 1958;30:329–340. doi: 10.1016/0006-3002(58)90058-1. [DOI] [PubMed] [Google Scholar]
- Tiainen M, Vaahtomeri K, Ylikorkala A, Makela TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1) Hum Mol Genet. 2002;11:1497–1504. doi: 10.1093/hmg/11.13.1497. [DOI] [PubMed] [Google Scholar]
- Van De Casteele M, Kefas BA, Cai Y, Heimberg H, Scott DK, Henquin JC, et al. Prolonged culture in low glucose induces apoptosis of rat pancreatic beta-cells through induction of c-myc. Biochem Biophys Res Commun. 2003;312:937–944. doi: 10.1016/j.bbrc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Ma Z, Bjorklund A, Grill V. Short-term intermittent exposure to diazoxide improves functional performance of beta-cells in a high-glucose environment. Am J Physiol Endocrinol Metab. 2004;287:E1202–E1208. doi: 10.1152/ajpendo.00255.2004. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.