Abstract
Background and purpose:
Aortic complications account for the major mortality in Marfan syndrome (MFS), a connective tissue disorder caused by mutations in FBN1 encoding fibrillin-1. We hypothesized that MFS impaired endothelial function and nitric oxide (NO) production in the aorta.
Experimental approach:
Mice (at 3, 6, 9 and 12 months of age) heterozygous for the Fbn1 allele encoding a cysteine substitution (Fbn1 C1039G/+, Marfan mice, n=75), the most common class of mutation in MFS, were compared with age-matched control littermates (n=75). Thoracic and abdominal aortas from the two groups were studied.
Key results:
Isometric force measurements revealed that relaxation to ACh (but not to sodium nitroprusside) was diminished in the phenylephrine-precontracted Marfan thoracic aorta at 6 months of age (pEC50=6.12±0.22; maximal response, Emax=52.7±6.8%; control: pEC50=7.34±0.19; Emax=84.8±2.2%). At one year, both inhibition of NO production with Nω-nitro-L-arginine methyl ester, or denudation of endothelium increased the phenylephrine-stimulated contraction in the control thoracic aorta by 35%, but had no effect in the Marfan aorta, indicating a loss of basal NO production in the Marfan vessel. From 6 months, a reduced phosphorylation of endothelial NOS (eNOS)Ser1177 and AktThr308 detected by Western blotting was observed in the Marfan thoracic aorta, which was accompanied by decreased levels of cGMP. Expressions of Akt and eNOS in the abdominal aorta were not different between the two groups.
Conclusions and Implications:
MFS impairs endothelial function and signaling of NO production in the thoracic aorta, suggesting the importance of NO in the age-related progression of thoracic aortic manifestations.
Keywords: Marfan syndrome; endothelium-dependent relaxation; nitric oxide; thoracic aorta; acetylcholine; Akt, age-related disease progression
Introduction
Marfan syndrome (MFS), with incidence of 2–3 per 10 000 individuals, is an autosomal-dominant disorder of the connective tissue caused by mutations in FBN1-encoding fibrillin (Fbn)-1 (Dietz et al., 1991; Pyeritz, 2000). Fbn-1 is the major component of microfibrils, which act as an organizing scaffold in the formation of elastic fibers in the extracellular matrix of large arteries. Abnormality in elastic fibers alters load bearing by the aorta and predisposes to microdissection, and degeneration and fibrosis of the media in the aorta (Dietz et al., 1991; Pereira et al., 1999; Pyeritz, 2000), and aortic aneurysm and rupture account for of the major cause of mortality in MFS (Pyeritz, 2000).
Patients with MFS display increases in aortic stiffness index and pulse wave velocity (Sandor et al., 2003; Bradley et al., 2005; Vitarelli et al., 2006). Endothelial dysfunction, which is also suggested by elevated plasma levels of von Willebrand factor antigen and thrombomodulin in patients with MFS (Wilson et al., 1999), may be involved in the above effects as removal of the endothelium increases large artery stiffness (Boutouyrie et al., 1997; Wilkinson et al., 2002). Indeed, alteration of Fbn-1 in MFS impairs the integrity of elastic fibers within the endothelial layer (Davis, 1994). In addition, endothelial permeability is reported to be impaired in MFS (Sheremet'eva et al., 2004).
The inhibitory effect of endothelium-derived nitric oxide (NO) on smooth muscle contractility is important in the regulation of vascular tone. The basal arterial production of NO is constitutively provided by a calcium-dependent endothelial NO synthase (eNOS), whose activity is positively controlled by the serine/threonine protein kinase Akt, through phosphorylation on Ser1177 (Dimmeler et al., 1999; Wyatt et al., 2004). Activity of eNOS can be stimulated by pharmacological agonists such as acetylcholine (ACh), and by flow-induced shear forces on the endothelial cell wall (Cocks et al., 1985; Pohl et al., 1986). It has been shown that the maximum forearm blood flow in response to ACh (Nakamura et al., 2000), and the flow-mediated vasorelaxation in the brachial artery in MFS patients were lower than those of healthy controls (Wilson et al., 1999). However, the regulation of endothelial function and NO signaling in the development of aortic complications in this disorder remains unexplored.
In the present study, the animal model used was heterozygous for a cysteine substitution (C1039G) in a calcium-binding epidermal growth factor – like domain in Fbn1 (Fbn1C1039G/+), the most common class of mutation observed in MFS (Judge et al., 2004; Ng et al., 2004; Habashi et al., 2006). We show that MFS is associated with impaired endothelial NO secretion and propose that the endothelial dysfunction plays a role in the progression of aortic complications in MFS.
Methods
Experimental animals and tissue preparation
Heterozygous (Fbn1C1039G/+) mice were mated with C57BL/6 mice to produce equal numbers of Fbn1C1039G/+ ‘Marfan' subjects (n=75) and wild-type ‘control' (n=75). Marfan mice were previously backcrossed to C57BL/6 for nine or more generations (Judge et al., 2004; Ng et al., 2004; Habashi et al., 2006) and genotyped as described (Judge et al., 2004). Both strains were housed in the institutional animal facility (University of British Columbia, Child and Family Research Institute) under standard animal room conditions (12 h light–12 h dark, at 25°C, 2–5 in a cage, Purina Chow diet), and all animal procedures were approved by the Institutional Animal Ethics Board. Mice at ages of 3 (n=40), 6 (n=40), 9 (n=40) and 12 (n=30) months were anesthetized with a mixture of ketamine hydrochloride (80 mg kg−1) and xylazine hydrochloride (12 mg kg−1) given intraperitoneally.
Endothelial function and NO signaling were assessed at functional and molecular levels. The thoracic aorta (TA) (from the aortic root, arch and along the descending TA above the diaphragm) and the abdominal aorta (AA) (between the diaphragm and the common iliac arteries) were isolated and cleaned of connective tissues and blood, with special care taken to preserve the endothelium (Okon et al., 2003). In isometric force measurements, proper mounting and force assessment required a parallel vessel segment (2 mm in length), so only the straight part of the TA (i.e., the descending TA), instead of the curved segments (i.e., ascending TA or arch) was used. For the AA, the segment below the renal artery was used. The remaining TA and AA segments were flash-frozen for Western immunoblotting and quantification of cyclic guanosine monophosphate (cGMP).
Measurement of isometric force
TA/AA segments (2 mm) were mounted isometrically in a small vessel myograph (A/S Danish Myotechnology, Aarhus N, Denmark) for measuring generated force (Okon et al., 2003). Aortic segments were stretched to the optimal tension (obtained from preliminary experiments, the maximal force generation given in response to phenylephrine and 80 mM KCl buffer; for TA=6.0 mN; for AA=3.5 mN, and which were the same for both control and Marfan mouse aorta) for 20 min. The vessels were thereafter challenged twice with 80 mM KCl, then contracted with 3 μM phenylephrine before making cumulative applications of ACh or sodium nitroprusside (SNP) (0.01 nM–10 μM). The percent of relaxation compared to the initial phenylephrine-induced contraction was recorded at different concentrations of ACh and concentration–response curves were constructed. The negative logarithm (pD2) of the concentration of ACh giving half-maximum response (EC50) was assessed by linear interpolation on the semilogarithm concentration–response curve (pD2=−log(EC50)). To study the effect of Nω-nitro-L-arginine methyl ester (L-NAME, 200 μM), vessels were incubated with this compound for 30 min before continuing with the protocol. In some experiments, the endothelium was mechanically removed by passing a 40-μm-diameter stainless wire through the vessel lumen.
Western immunoblotting
Because of the limited sample from each mouse, TA and AA were pooled in groups with the same strain and age. Procedures of Western blotting have been previously described (Okon et al., 2005). Briefly, flash-frozen aortic segments were ground with liquid nitrogen in a stainless-steel motor and pestle. Tissue powder was mixed in nine volumes of ice-cold lysis buffer and samples were homogenized by a glass homogenizer (Okon et al., 2005). Supernatant was collected by centrifugation at 14 000 g for 20 min at 4°C. Protein samples (10 μg) were run on an 8% (for eNOS) or 11% (for Akt) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and the separated proteins were transferred to polyvinyldifluoride membranes. Nonspecific-binding sites were blocked. The membranes were then incubated with the appropriate antibodies as follows: monoclonal antibody to eNOS (1 μg ml−1), rabbit polyclonal antibody to phospho-eNOSSer1177 (1:1000), rabbit polyclonal antibody to Akt (1:1000) or rabbit polyclonal antibody to phospho-AktThr308 or phospho-AktSer473 (1:1000). Afterwards, membranes were incubated with anti-rabbit or anti-mouse immunoglobulin (IgG) peroxidase-conjugated secondary antibodies (1:2500). Bound antibodies were detected by the ECL Western blotting detection kit (Amersham Life Sciences, Arlington Heights, IL, USA). To ensure equal protein loading, membranes were stripped and reprobed with anti-β-actin antibody (1:3000).
Aortic cGMP levels
cGMP content was measured as an indicator of eNOS activity (Rapoport et al., 1983; Wyatt et al., 2004). The aortic segments pooled from each animal at each age group were ground to fine powder and homogenized as described in the manufacturer's instruction. cGMP content in the reconstituted aortic extract was measured using the Correlate-EIA cGMP Enzyme Immunoassay Kit (Assay Designs, Inc, Ann Arbor, MI, USA).
Calcium signals in the endothelial cells of aorta
Segments (2 mm in length) of TA and AA maintained in physiological saline solution with buffered with HEPES were carefully inverted and the endothelial cells were loaded with fluo-4 AM (5 μM) plus pluronic acid (5 μM) for 15 min at room temperature (Wang et al., 1995; Ohata et al., 1997). The aortic segment was then mounted in a wire myograph chamber filled with the above solution. Data were acquired using an Olympus BX50WI microscope fitted with an Ultraview Nipkow confocal (Perkin–Elmer, Waltham, MA, USA). Images were captured with a × 60 water-dipping lens (numerical aperture 0.90) at 300 nm intervals.
Statistics
Data were reported as mean±s.e.m. from at least four independent experiments. Statistical analysis and construction of concentration–response curves were performed using GraphPad Prism software. Differences between control and Marfan groups were assessed by two-tailed Student's t-test. Differences between concentration–response curves were analyzed by two-way analysis of variance (ANOVA). Statistical significance was defined as P-values <0.05.
Drugs
Ketamine hydrochloride and xylazine hydrochloride (Research Biochemicals International, Natick, MA, USA); phenylephrine, ACh, KCl, SNP, L-NAME, pluronic acid, HEPES, chemicals for preparing Krebs solution, anti-rabbit and anti-mouse IgG peroxidase-conjugated secondary antibodies (Sigma, Oakville, Canada); polyvinyldifluoride membranes (BioRad, Hercules, USA); anti-eNOS antibody (BD BioSciences, Mississauga, Canada), anti-phospho-eNOSSer1177, Akt, phospho-AktThr308 and phospho-AktSer473 antibodies (Cell Signaling, Beverly, Massachusetts).
Results
Animal model
Marfan mice developed kyphosis at about 2 months of age. About 92% of them showed dilatation along the TA of variable severity from 6 months of age. About 5% died suddenly between 5 and 8 months old, in association with hemopericardium. About 9% of Marfan mice developed an aneurysm along the AA.
Endothelium-dependent relaxation
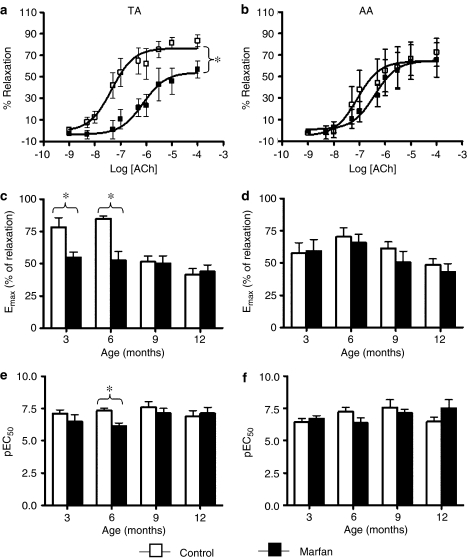
In phenylephrine (3 μM)-precontracted aortas from control and Marfan mice, addition of ACh-reduced force in a concentration-dependent manner (Figure 1a and b). The maximal responses of ACh-induced relaxation (Emax) in Marfan TA at 3 and 6 months were only 70.3 and 62.1%, respectively, of that in the controls (Figure 1c). Emax values tended to decline with age in the control TA, but this association of endothelial function with the age was weakened in the Marfan aorta (Figure 1c). Values of pEC50 for ACh that represented the sensitivity of endothelium to this agonist indicated that at 6 months of age the Marfan TA was less sensitive to ACh than the controls (Figure 1e). This difference disappeared between 9 and 12 months owing to an age-dependent deterioration in the controls. The Emax and pEC50 of ACh in AA were similar in control and Marfan mice, and did not alter during aging (Figure 1d and f).
Figure 1.
Concentration–response curves of ACh-induced relaxation in phenylephrine (3 μM)-precontracted (a) TA and (b) AA from control (open bars) and Marfan (closed bars) mice at 6 months of age. In the TA, the ACh-induced maximal relaxation response (Emax) is significantly higher than that in the control (n=8–14, *P<0.05). The concentration-response curve of Marfan TA was right-shifted compared with the control, indicating a decrease in the sensitivity to ACh-induced relaxation. Similar observations were not made in the AA. Values of Emax and pEC50 at different age groups are presented in the bar graphs (c–f).
Removal of endothelium completely abolished the ACh-stimulated relaxation (data not shown). ACh stimulates not only endothelial NO production but also the release of a number of other vasodilators such as prostacyclin, and endothelium-derived hyperpolarizing factor. We assessed the ACh response in the presence of L-NAME (200 μM) and found that the effect of ACh was inhibited by pretreatment of L-NAME by more than 90%, indicating that the major portion of the response to ACh was related to NO production.
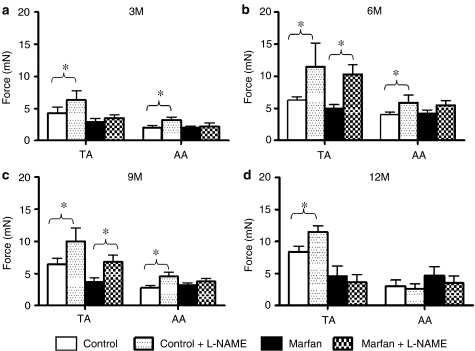
To examine the role of endogenous NO in vasoconstriction in control and Marfan aortas, the Emax values of phenylephrine (3 μM) were compared before and after the pretreatment of L-NAME (200 μM). L-NAME potentiated the phenylephrine Emax in the TA from control mice at 3-, 6-, 9- and 12-month old by 47, 83, 55 and 38%, respectively (Figure 2). This not only demonstrated a significant level of NO production in the control TA, but also indicated a delayed gradual loss of basal endothelial-dependent vasorelaxation during aging. In the Marfan TA, L-NAME had no significant effect on the PE-induced contraction at 3-months, suggesting that, in young mice, the disease progression associated with pronounced reduction of endothelial NO production. Basal NO secretion was detected at 6 months as L-NAME potentiated the phenylephrine Emax by 207%. However, this compensatory mechanism started to decline at 9 months as L-NAME increased phenylephrine Emax by only 83%, and at 12 months it had no effect on phenylephrine-induced contraction (Figure 2). In the AA, L-NAME enhanced the phenylephrine Emax by 59, 47 and 62% in the control at 3, 6 and 9 months of age, respectively, but had no effect in the Marfan AA.
Figure 2.
Effect of L-NAME (200 μM) on phenylephrine-induced contraction in aortas from Marfan and control mice. Bar graphs showing Emax (mN) in response to phenylephrine stimulation (3 μM) plus and minus L-NAME at the age of (a) 3, (b) 6, (c) 9 and (d) 12 months (n=8–16, *P<0.05).
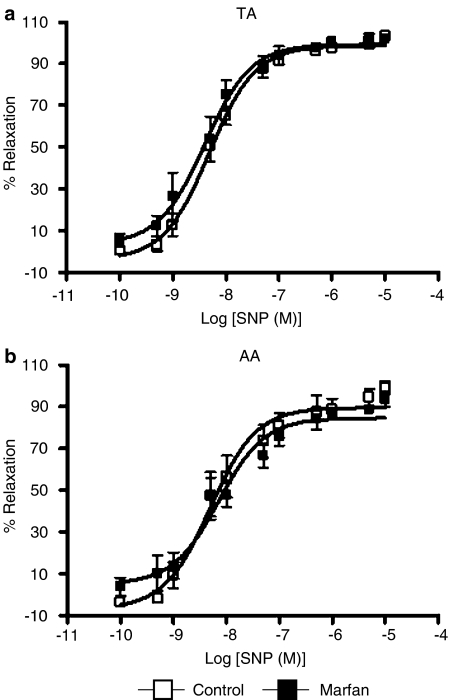
Endothelium-independent vasodilatation induced by the addition of SNP, a NO donor which bypasses endogenous NO production by endothelial cells, completely dilated phenylephrine-precontracted control and Marfan aortas, without a significant difference in Emax and pEC50 (Figure 3). This indicates that the sensitivity of smooth muscle to NO was not altered in MFS.
Figure 3.
Concentration–response curves of SNP-induced relaxation in phenylephrine precontracted (a) TA and (b) AA from control and Marfan mice at 6 months of age. The endothelium-independent relaxation stimulated by SNP was not different between two animal groups, suggesting that the sensitivity of smooth muscle to NO was not altered in MFS.
Aortic levels of cGMP
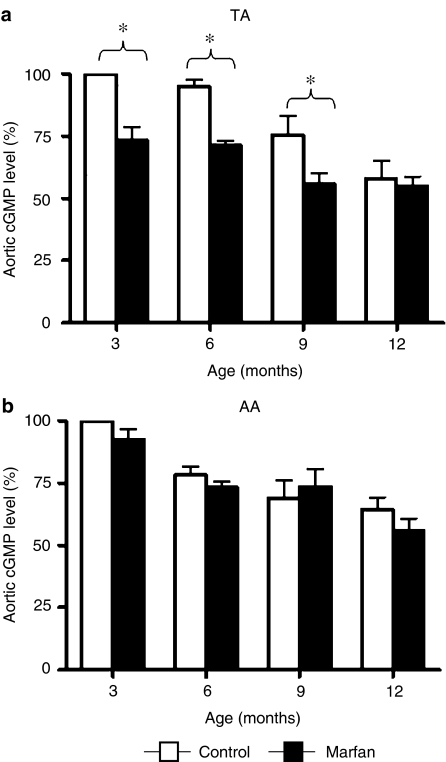
NO activates guanylyl cyclase and elevates the cellular levels of cGMP, which mediates most of the biological actions of NO in vascular smooth muscle cells (Rapoport et al., 1983; Wyatt et al., 2004). We found that the levels of cGMP in the TA and AA homogenates from the two animal groups were gradually decreased with age (Figure 4). However, a profound difference between Marfan and control TA was observed at 3, 6 and 9 months, whereas the difference disappeared at 1 year owing to an age-dependent loss in the controls, which lagged behind that of Marfan mice (Figure 4a). A similar phenomenon was not found in the AA (Figure 4b).
Figure 4.
Bar graphs presenting the levels of cGMP in the (a) TA and (b) AA homogenate from control (open bars) and Marfan (closed bars) mice. Values shown, expressed as %, are normalized to the control level at 3 months of age (n=4, *P<0.05, vs age-matched control groups).
Calcium signals in the TA endothelial cells
As eNOS is a calcium-dependent enzyme, we investigated if the intracellular calcium level is modified by MFS. Regardless of the age, ACh (1.0 μM) induced a transient increase in the intracellular calcium concentration ([Ca2+]i) in endothelial cells of TA and AA from both strains without a significant difference.
Signaling of NO production – eNOS and Akt
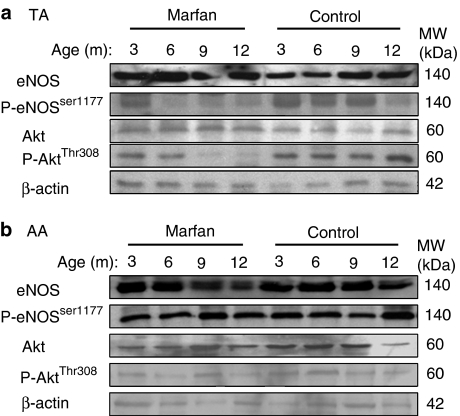
The activity of eNOS is upregulated via phosphorylation at Ser1177 by Akt phosphorylated at Thr308 or Ser473 (Dimmeler et al., 1999). We found that the phosphorylation of eNOSSer1177 in the Marfan TA was greatly downregulated from the age of 6 months compared to the control (Figure 5b). At 1 year a reduced phosphorylated eNOSSer1177 level was observed in the control group. Phosphorylated AktThr308 remained at a constant level in the control during aging, whereas it diminished in the Marfan group starting at 9 months. Level of phosphorylated AktSer473 was not different between Marfan and control and was not changed during aging (data not shown). The basal levels of eNOS and Akt were not different between the two groups.
Figure 5.
Mechanisms of impaired NO production in TA: Western immunoblotting showing the protein expression of eNOS, phospho-eNOSSer1177, Akt, phospho-AktThr308 and β-actin in the (a) TA and (b) AA from control and Marfan mice at different ages. Expression of β-actin served as loading control. Because of the limited aortic samples from each mouse, TA and AA were pooled from each group of animals at different ages.
Discussion
Using a genetically defined and validated mouse model of MFS, we were able to show for the first time that endothelium-dependent relaxation in the TA is impaired in MFS, and that the endothelial dysfunction is likely owing to the downregulation of Akt/eNOS-induced production of NO. To study the age-related disease progression, we used appropriate control littermates to distinguish between observations owing to the pathogenesis of MFS from those owing to the physiological process of aging. We demonstrated that the endothelial function and NO signaling of TA and AA during disease development are greatly different, which might be associated with the higher prevalence of complications in the TA. We suggest that NO is crucial in the pathogenesis of TA manifestations in MFS.
MFS demonstrates highly variable clinical manifestations affecting the cardiovascular (progressive aortic dilatation associated with aortic valve incompetence, mitral valve prolapse and incompetence), ocular (lens dislocation and myopia) and skeletal (arachnodactyly, pectus deformities and scoliosis) systems (Pyeritz, 2000). Patients with MFS have an increased propensity for cardiovascular complications in the form of aortic root dilatation, aneurysm and rupture, leading to morbidity and sudden death (Dietz et al., 1991; Pyeritz, 2000).
Abnormal microfibrils formation and degeneration of the elastic laminae in the aortic media render the vessel wall less able to sustain the pulsatile blood pressure in the aorta, as the already ‘fragile' elastic fibres are susceptible to further fragmentation, predisposing it to aortic root dilatation and dissection over time. In the clinical assessment of patients with MFS, aortic distensibility, stiffness index and pulse-wave velocity are predictive for dilatation and dissection (Sandor et al., 2003; Bradley et al., 2005; Vitarelli et al., 2006). The endothelium constitutively releases a number of vasoactive mediators including NO to regulate smooth muscle contractility and thus vascular smooth muscle tone and mechanical properties (Ramsey et al., 1995; Boutouyrie et al., 1997; Wilkinson et al., 2002). We observed that, in the Marfan TA, endothelium-dependent relaxation stimulated by ACh was compromised and this was not owing to the loss of sensitivity of smooth muscle cells to NO, as the addition of SNP still caused complete relaxation. Blockade of NO production by L-NAME had no effect in Marfan TA in some age groups (3 and 12 months), indicating the disease progression at early stage of life (3 months), which was followed by the restoration of endogenous NO secretion (6 months). However, such compensatory mechanism started to decline during aging (9 months) and the basal NO-mediated relaxation was absent at old age (12 months). The reduction of NO production in TA correlated with the decreased levels of cGMP, a downstream second messenger of NO signaling. In MFS patients the forearm resistance vessel blood flow in response to ACh was lower (Nakamura et al., 2000), which was suggested to be associated with an elevation of the pulse wave pressure in the ascending aorta (Yin et al., 1989; Ceravolo et al., 2003). Conflicting data were reported in a recent study as the ACh/bradykinin-mediated dilatations of the brachial arteries were similar in MFS patients and healthy controls (Wilson et al., 1999). However, reduced flow-mediated vasodilatation was evident in MFS patients and this was suggested to be related to the impaired mechano–transduction caused by abnormal Fbn-1, which disturbs the functional relationship between blood flow and endothelial function (Wilson et al., 1999). In addition, it has been shown that MFS and aortic aneurysm are associated with elevated plasma levels of homocysteine, which attenuates endothelial function and limits NO bioavailability (Giusti et al., 2003; Jiang et al., 2005). The impaired vasorelaxation owing to decreased NO production may limit vessel responsiveness and large-artery distensibility. Such increased vascular stiffness adds greater afterload stress on the heart (Yin et al., 1989; Jeremy et al., 1994). Structural stiffness also could alter endothelial function and thereby further enhance stiffening (Peng et al., 2003).
The underlying mechanism of endothelial dysfunction and impairment of NO synthesis during the progression of MFS has never been studied. However, endothelium is likely to be affected in MFS, as Fbn-1-rich microfibrils are abundant in the connective tissue matrix, intermediately subjacent to arterial endothelial cells (Kielty et al., 1996). It has been reported that microfibrils composed of Fbn-1 are important in the integration of the endothelial cell layer during the development of vascular architecture (Davis, 1994). In patients with MFS, endothelial dysfunction was suggested by the elevated plasma levels of von Willebrand factor antigen and thrombomodulin (Wilson et al., 1999). Stimulation of endothelial cells by receptor-dependent agonists or by shear stress elevates [Ca2+]i, which leads to increased eNOS activity and NO release (Wyatt et al., 2004). However, in the present study we excluded the possibility of aberrant calcium handling in the regulation of eNOS function in aortic endothelial cells as we did not detect any changes in [Ca2+]i responses to ACh measured by fluorescence confocal microscopy. Instead, we detected downregulation of eNOSSer1177, accompanied by a reduced AktThr308 phosphorylation in Marfan TA. The compromised NO production could be attributed to over activation of transforming growth factor-β signaling which is known to negatively regulate activity of eNOS in endothelial cells (Neptune et al., 2003; Ng et al., 2004; Schwartz et al., 2005; Habashi et al., 2006). We suggest that NO donors, such as glyceryl trinitrate, which reduces brachial arterial stiffness (Bank et al., 1999) and pulse wave reflection (Yaginuma et al., 1986) in humans, might be a therapeutic option for the treatment of MFS. Mild exercise, which increases NO production possibly via upregulation of vascular eNOS expression and eNOSSer1177 phosphorylation might also be beneficial for patients with MFS (Kojda and Hambrecht, 2005).
The differences between control and Marfan TA in response to ACh-induced relaxation disappeared with age owing to the fact that endothelial function and Akt/eNOS/NO/cGMP signaling are progressively impaired during aging (Ueda and Moritoki, 1991; Tao et al., 2004; Smith et al., 2006). Advancing age is associated with derangement of endothelial cells leading to a decrease in NO production (Gerhard et al., 1996).
Interestingly, the effect of MFS is not homogenous along the aorta, as we observed profound differences between Marfan and control groups with respect to the endothelial-dependent relaxation and the eNOS/Akt signaling pathway mainly in the TA but not in the AA. Endothelial dysfunction and downregulated NO signaling particularly observed in the TA would contribute to the increased vessel stiffness and reduced distensibility (Yin et al., 1989; Jeremy et al., 1994; Boutouyrie et al., 1997; Wilkinson et al., 2002), and this might explain the high incidence of thoracic aortic dilatation (92%) in the Marfan mice. In humans, most of the clinical manifestations and mortality of MFS are related to different severity and types of thoracic aortic aneurysm and dissection (Dietz et al., 1991; Pyeritz, 2000). Aneurysmatic dilatations are usually confined to sections I and II of the TA, whereas aneurysms in sections IV and V are much more rare (Wolfgarten et al., 2001). TA and AA are largely different in their inherent structural properties and composition, as well as in the hemodynamics that they are exposed to (Wolinsky and Glagov, 1969; Halloran et al., 1995; Orsi et al., 2004). Possible reasons for the higher susceptibility of TA to disease manifestations in MFS include the following: (1) The relatively high proportion of elastin content and the highly organized structure of elastic lamella in TA (Wolinsky and Glagov, 1969; Halloran et al., 1995; Orsi et al., 2004). (2) The particularly high and pulsatile blood pressure predisposes the ascending part of the aorta to aneurysm. In patients with MFS, aortic aneurysm and dissection generally begin in the ‘root' of the ascending aorta, then ‘tear' along the whole aorta over time. (3) NO has been suggested to be the major vasodilatory mediator in TA (Castillo et al., 1997). Therefore, a reduction of its production is likely to increase the susceptibility to aortic complications. (4) The mechanisms of aneurysm production are suggested to be different between the ascending and AA. Atheroma is the dominant lesion in the AA and genetic abnormalities predisposing to ‘mediacystic necrosis' are more frequently observed in ascending aortic aneurysms. Some ascending aortic aneurysms are associated with bicuspid aortic valve defects, some of which are familial. Familial factors however are less evident in abdominal aortic aneurysms (Jondeau et al., 2003).
In conclusion, we have demonstrated that MFS compromised endothelial-dependent relaxation in the TA and that the related pathogenesis was age-dependent. Endothelial dysfunction was attributed to the downregulation of eNOS/Akt signaling-induced NO production. During the progression of MFS, endothelial function and the NO signaling pathways in the TA were greatly different from those in the AA, consistent with the high prevalence of TA manifestations of the disorder in humans. Our discovery of decreased NO production in TA of Marfan mice suggests a novel therapeutic strategy, which might slow or reverse the extensive vascular remodeling and aortic complications in MFS.
Acknowledgments
This work was funded by the Canadian Marfan Association. AC is the recipient of the Heart and Stroke Foundation of Canada/AstraZeneca Canada Research Fellowship Award and the Michael Smith Foundation for Health Research/St's Paul Hospital Foundation Trainee Award. KAY is supported by Child and Family Research Institute Summer Studentship. A Fellowship for SC is generously provided by CNPq. HCD is supported by NIH grants (AR41135 and AR049698) and the National Marfan Foundation. We greatly thank Dr James E Potts for his assistance in obtaining the Marfan mouse model.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- Fbn
fibrillin
- L-NAME
Nω-nitro-L-arginine methyl ester
- MFS
Marfan syndrome
- SNP
sodium nitroprusside
Conflict of interest
The authors state no conflict of interest.
References
- Bank AJ, Kaiser DR, Rajala S, Cheng A. In vivo human brachial artery elastic mechanics: effects of smooth muscle relaxation. Circulation. 1999;100:41–47. doi: 10.1161/01.cir.100.1.41. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Bezie Y, Lacolley P, Challande P, Chamiot-Clerc P, Benetos A, et al. In vivo/in vitro comparison of rat abdominal aorta wall viscosity. Influence of endothelial function. Arterioscler Thromb Vasc Biol. 1997;17:1346–1355. doi: 10.1161/01.atv.17.7.1346. [DOI] [PubMed] [Google Scholar]
- Bradley TJ, Potts JE, Potts MT, DeSouza AM, Sandor GG. Echocardiographic Doppler assessment of the biophysical properties of the aorta in pediatric patients with the Marfan syndrome. Am J Cardiol. 2005;96:1317–1321. doi: 10.1016/j.amjcard.2005.06.080. [DOI] [PubMed] [Google Scholar]
- Castillo C, Reyes G, Escalante B, Lopez P, Castillo EF. Endothelium-dependent vasodilatation in rat aorta is mainly mediated by nitric oxide. Proc West Pharmacol Soc. 1997;40:39–40. [PubMed] [Google Scholar]
- Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, et al. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol. 2003;41:1753–1758. doi: 10.1016/s0735-1097(03)00295-x. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Angus JA, Campbell JH, Campbell GR. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985;123:310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Davis EC. Immunolocalization of microfibril and microfibril-associated proteins in the subendothelial matrix of the developing mouse aorta. J Cell Sci. 1994;107:727–736. doi: 10.1242/jcs.107.3.727. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Giusti B, Porciani MC, Brunelli T, Evangelisti L, Fedi S, Gensini GF, et al. Phenotypic variability of cardiovascular manifestations in Marfan syndrome. Possible role of hyperhomocysteinemia and C677T MTHFR gene polymorphism. Eur Heart J. 2003;24:2038–2045. doi: 10.1016/j.ehj.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BG, Davis VA, McManus BM, Lynch TG, Baxter BT. Localization of aortic disease is associated with intrinsic differences in aortic structure. J Surg Res. 1995;59:17–22. doi: 10.1006/jsre.1995.1126. [DOI] [PubMed] [Google Scholar]
- Jeremy RW, Huang H, Hwa J, McCarron H, Hughes CF, Richards JG. Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am J Cardiol. 1994;74:369–373. doi: 10.1016/0002-9149(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr, Randhawa JK, et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondeau G, Muti C, Boileau C. Aortic aneurysms excluding Marfan's syndrome. Arch Mal Coeur Vaiss. 2003;96:1074–1080. [PubMed] [Google Scholar]
- Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Wilson DG, Stuart G, Musumeci F, Jones CJ, Davies S, et al. Fibrillin expression and deposition by vascular endothelial cells: implications for the Marfan syndrome. Circulation. 1996;94:I-350. [Google Scholar]
- Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy. Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Itoh S, Makita S, Ohira A, Arakawa N, Hiramori K. Peripheral resistance vessel dysfunction in Marfan syndrome. Am Heart J. 2000;139:661–666. doi: 10.1016/s0002-8703(00)90045-0. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata H, Ujike Y, Momose K. Confocal imaging analysis of ATP-induced Ca2+ response in individual endothelial cells of the artery in situ. Am J Physiol. 1997;272:C1980–C1987. doi: 10.1152/ajpcell.1997.272.6.C1980. [DOI] [PubMed] [Google Scholar]
- Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- Okon EB, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40:520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- Orsi AM, Stefanini MA, Crocci AJ, Simoes K, Ribeiro AA. Some segmental features on the structure of the aortic wall of the dog. Anat Histol Embryol. 2004;33:131–134. doi: 10.1111/j.1439-0264.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–381. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Pyeritz RE. The Marfan syndrome. Annu Rev Med. 2000;51:481–510. doi: 10.1146/annurev.med.51.1.481. [DOI] [PubMed] [Google Scholar]
- Ramsey MW, Goodfellow J, Jones CJ, Luddington LA, Lewis MJ, Henderson AH. Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation. 1995;92:3212–3219. doi: 10.1161/01.cir.92.11.3212. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306:174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Sandor GG, Hishitani T, Petty RE, Potts MT, Desouza A, Desouza E, et al. A novel Doppler echocardiographic method of measuring the biophysical properties of the aorta in pediatric patients. J Am Soc Echocardiogr. 2003;16:745–750. doi: 10.1016/S0894-7317(03)00407-3. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremet'eva GF, Ivanova AG, Belov Iu V, Gens AP, Kocharian EZ. A comparative study of the aortic wall in patients with Marfan's syndrome and Erdheim's disease. Angiol Sosud Khir. 2004;10:22–29. [PubMed] [Google Scholar]
- Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 2006;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- Tao J, Jin YF, Yang Z, Wang LC, Gao XR, Lui L, et al. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 2004;17:654–659. doi: 10.1016/j.amjhyper.2004.03.678. [DOI] [PubMed] [Google Scholar]
- Ueda H, Moritoki H. Possible association of decrease of ATP-induced vascular relaxation with reduction of cyclic GMP during aging. Arch Int Pharmacodyn Ther. 1991;310:35–45. [PubMed] [Google Scholar]
- Vitarelli A, Conde Y, Cimino E, D'Angeli I, D'Orazio S, Stellato S, et al. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol. 2006;97:571–577. doi: 10.1016/j.amjcard.2005.09.089. [DOI] [PubMed] [Google Scholar]
- Wang X, Lau F, Li L, Yoshikawa A, van Breemen C. Acetylcholine-sensitive intracellular Ca2+ store in fresh endothelial cells and evidence for ryanodine receptors. Circ Res. 1995;77:37–42. doi: 10.1161/01.res.77.1.37. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- Wilson DG, Bellamy MF, Ramsey MW, Goodfellow J, Brownlee M, Davies S, et al. Endothelial function in Marfan syndrome: selective impairment of flow-mediated vasodilation. Circulation. 1999;99:909–915. doi: 10.1161/01.cir.99.7.909. [DOI] [PubMed] [Google Scholar]
- Wolfgarten B, Kruger I, Gawenda M. Rare manifestation of abdominal aortic aneurysm and popliteal aneurysm in a patient with Marfan's syndrome: a case report. Vasc Surg. 2001;35:81–84. doi: 10.1177/153857440103500118. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circ Res. 1969;25:677–686. doi: 10.1161/01.res.25.6.677. [DOI] [PubMed] [Google Scholar]
- Wyatt AW, Steinert JR, Mann GE. Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem Soc Symp. 2004;71:143–156. doi: 10.1042/bss0710143. [DOI] [PubMed] [Google Scholar]
- Yaginuma T, Avolio A, O'Rourke M, Nichols W, Morgan JJ, Roy P, et al. Effect of glyceryl trinitrate on peripheral arteries alters left ventricular hydraulic load in man. Cardiovasc Res. 1986;20:153–160. doi: 10.1093/cvr/20.2.153. [DOI] [PubMed] [Google Scholar]
- Yin FC, Brin KP, Ting CT, Pyeritz RE. Arterial hemodynamic indexes in Marfan's syndrome. Circulation. 1989;79:854–862. doi: 10.1161/01.cir.79.4.854. [DOI] [PubMed] [Google Scholar]