Abstract
Background and purpose:
Calu-3 cells are derived from serous cells of human lung submucosal glands, a prime target for therapy in cystic fibrosis (CF). Calu-3 cells can be cultured to form epithelia capable of transepithelial transport of chloride. A CF Calu-3 cell is not available.
Experimental approach:
A retroviral vector was used to cause persistent down regulation of CFTR using siRNA methodology, in Calu-3 cells. A Calu-3 cell line with CFTR content less than 5% of the original line has been established. Epithelia grown using the modified cells have been used in comparative studies of transporting capability.
Key results:
All aspects of cAMP activated chloride secretion were attenuated in the epithelia with reduced CFTR content. However transporting capability was reduced less than the CFTR content. From studies with the CFTR channel inhibitor, GlyH-101, it was concluded that wild type Calu-3 cells have a reserve of CFTR channels not located in the membrane, but available for replacement, while in the modified Calu-3 cell line there was little or no reserve. Lubiprostone, a putative ClC-2 activator, increased transepithelial chloride secretion in both modified and wild type Calu-3 epithelia. Modified Calu-3 epithelia with the residual CFTR currents blocked with GlyH-101 responded equally well to lubiprostone as those without the blocking agent.
Conclusions and implications:
It appears that lubiprostone is capable of stimulating a non-CFTR dependent transepithelial chloride secretion in Calu-3 monolayers, with obvious implications for CF therapy. Cell lines, however, do not always reflect the behaviour of the native tissue with integrity.
Keywords: Cystic fibrosis transmembrane conductance regulator, human airway submucosal glands, Calu-3 cells, CFTR channel blocker, GlyH-101, lubiprostone, epithelial chloride secretion
Introduction
Calu-3 cells are derived from the serous cells of submucosal glands of human airways. They are able to form epithelial monolayers capable of transepithelial transport (Moon et al., 1997). The serous cells are considered to be of crucial importance in generating fluid that helps maintain mucociliary clearance (Verkman et al., 2003; Salinas et al., 2005). The main process by which serous cells promote fluid secretion is by osmotic drag, following the active secretion of chloride (and bicarbonate) ions into the lumen. The richest source of the cystic fibrosis transmembrane conductance regulator (CFTR), in human airways, is the serous cells of submucosal glands (Engelhardt et al., 1992). In cystic fibrosis (CF), CFTR is absent or present in a form unable to maintain normal function, and fluid production by the glands is reduced. These and other changes in CF result in the accumulation of thick mucus in the airways, bacterial infection and inevitable fibrotic changes, leading eventually to the destruction of lung tissue.
Thus, Calu-3 cells are a focus of interest for agents that can promote secretion without requiring CFTR, with the caveat that Calu-3 is a cell line whose properties may not exactly mimic the functions of serous cells in situ.
For experimental purposes, an ideal test vehicle would be a CF Calu-3 cell line that is capable of forming epithelial monolayers when grown on pervious support membranes. Therefore, the main aim of this study was to produce a Calu-3 cell line with a CF phenotype.
Our approach was to use small inhibitory RNA (siRNA) methods to produce a knockout or knockdown (KD) Calu-3 cell line using a retroviral vector for stable integration and persistent down regulation of CFTR. We show that CFTR content is reduced by at least 95% in our modified Calu-3 cell line. This modified Calu-3 cell line has been used to culture epithelial sheets for comparative functional studies with normal Calu-3 cell monolayers.
Methods
siRNA transfection
Calu-3 cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and were grown on 75 cm2 culture flasks containing Eagle's minimum essential medium (Vitacell, ATCC, VA, USA) with 10% foetal calf serum (Gibco/Invitrogen, Paisley, UK), kanamycin, 100 μM ml−1 (Gibco/Invitrogen) and fungizone, 1.25 mg ml−1 (Gibco/Invitrogen). Cells were incubated at 37°C in humidified air containing 5% CO2.
CFTR-directed siRNA was designed using the pSUPER RNAi system instructions (Oligoengine, Seattle, WA, USA) and subcloned into pSuper.retro.neo+green fluorescent protein (GFP) (Oligoengine) as described previously (Brummelkamp et al., 2002). A pair of unique-to-CFTR oligonucleotides were ligated into the vector to direct the persistent synthesis of siRNA intracellularly, in order to generate a long-term loss-of-function CFTR phenotype in the Calu-3 cell line.
CFTR siRNA-encoding sequence was inserted by using 5′-GATCCCCCAAGGAGGAACGCTCTATCTTCAAGAGAGATAGAGCGTTCCTCCTTGTTTTTGGAAA-3′ (sense) and 5′-AGCTTTTCCAAAAACAAGGAGGAACGCTCTATCTCTCTTGAAGATAGAGCGTTCCTCCTTGGGG-3′ (antisense) oligonucleotides. Calu-3 cells were transfected using Fugene 6 (Roche Applied Science, Burgess Hill, W Sussex, UK) at a ratio of 3:1 (Fugene 6: DNA) according to the manufacturer's instructions in six-well plates. At this ratio, a transfection efficiency of <1% was achieved as determined by the presence of the GFP reporter. At all other ratios, no GFP fluorescence was visible. Within each six-well plate, two wells containing Fugene 6 and water (at a 3:1 ratio) were included to act as controls, and showed no green fluorescence. Cells were left overnight and re-fed in complete medium for 3 days before neomycin selection using a predetermined kill–curve concentration of neomycin (Genticin (G418), Invitrogen, 400 μg ml−1). It is probable that the highly fluorescent cells containing GFP had multiple copies of the expression system, whereas in cells containing a single copy the fluorescence was minimal. The selection medium was then used as standard growth medium for continuous culture. Very few cells survived selection but eventually confluence was reached after circa 6 months. When confluence was reached within the six-well plates cells were passaged into 75 cm2 flasks and maintained thereafter in selection medium. The modified Calu-3 cell line has now been maintained in the modified medium for 18 months.
Western blots of calu-3 cells
SiRNA-transfected Calu-3 and unmodified Calu-3 cells were grown to 90% confluency in T150 flasks. Cells were trypsinized and pelleted, supernatant was removed and cells were washed two times with phospate-buffered saline (PBS). Cells were again pelleted by centrifugation at 3500 g for 5 min at 4°C, supernatant was discarded and cells were resuspended in 200 μl of cell lysis buffer (50 mM Tris HCl pH7.5, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1% Triton X-100 plus broad spectrum protease inhibitor cocktail II (Calbiochem, Beaston, Nottingham, UK)).
Cells were lysed via sonication (Ultrasonic processor XL2020, Heat Systems, Farmingdale, NY, USA) using four times 15 s duration pulses on maximum setting with incubation on ice for 1 min between each pulse. Lysis was completed by incubation on ice for 10 min. Cellular debris was pelleted by centrifugation at 15 000 g for 10 min at 4°C. Cell supernatant was recovered and an equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added. Xenopus oocytes, preinjected 3 days previously with the cDNA for CFTR were used as controls. Ten or 20 pre-injected oocytes were placed into 100 μl of cell lysis buffer as for Calu-3 cells, cell debris was removed by centrifugation at 15 000 g for 3 min at 4°C. Oocytes were lysed by one single 30 s burst on the sonicator and placed on ice for 10 min. Oocyte yolk was separated via centrifugation at 17 250 g for 10 min, supernatant was withdrawn underneath the lipid pellicle and resuspended in SDS-PAGE loading buffer.
Protein concentration was determined using bicinchoninic acid total protein assay (Pierce, Rockford, IL, USA) and 20 μg was loaded into each lane of a 6% SDS-PAGE gel in the presence of running buffer (25 mM Tris Base, 192 mM glycine, 0.1% SDS pH 8.8). Proteins were resolved and transferred to polyvinylidine difluoride membrane using semi dry transfer apparatus (Biorad, Hemel Hempstead, Herts, UK) membranes and filters were pre-incubated in semidry transfer buffer (48 mM Tris Base, 39 mM glycine, 20% methanol, 1.3 mM SDS, pH 9.2) before transfer. Transfer was conducted at 20 mV for 1.5 h.
Membrane was blocked overnight in 5% Marvel, and then washed three times 15 min in PBS containing 0.5% Tween 20. Antihuman C-terminus CFTR antibody (R&D systems Europe Ltd, Abingdon, Oxon, UK) was diluted 1:500 in PBS with 1% Marvel added to the membrane and incubated for 2 h. Wash step was repeated and then membrane was incubated in 1:1500 Goat Anti-Rabbit horseradish peroxidase-conjugated secondary antibody (diluted in PBS with 1% Marvel) for 1 h. Three further washes in PBS 0.5% Tween 20 were carried out and the membrane was then visualized using enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, Bucks, UK) as per the manufacturer's instructions.
Epithelial culture
Cells were collected by trypsinization from 75 cm2 flasks and subcultured either on Snapwell clear polyester membrane inserts for subsequent voltage-clamp experiments (1 cm2, 0.4 mM pore size, VWR International Ltd, Lutterworth, UK) or maintained in 75 cm2 flasks. Cultures were re-fed every 3–4 days; the inserts were used between 5 and 30 days after subculture. All experimental procedures used cells from passages 3–12.
Short circuit current recordings
The Snapwell inserts, bearing the cultured monolayers, were inserted into CHM5 Ussing chambers with associated electrodes (WPI, Herts, UK) and clamped at zero potential using a WPI Dual Voltage clamp. Both sides of the epithelium were bathed in Krebs Henseleit Solution (KHS, 5 ml) that was continually circulated through the half chambers, maintained at 37°C and continuously bubbled with 95%O2/5%CO2. Short circuit currents (SCCs) were continuously recorded using an ADInstruments PowerLab 8SP (NSW 2154, Australia) and displayed on screen. In some experiments, the transepithelial voltage was temporarily set at 1 mV above zero every few minutes. The value of the change in SCC is equivalent to the transepithelial conductance in mS cm2. In experiments where a basolateral to apical chloride gradient was imposed on the epithelia, the NaCl in KHS was replaced with sodium gluconate (see below).
Solutions
KHS had the following composition (mM): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3 and 10 glucose. When NaCl was replaced by an equimolar amount of Na gluconate, the calcium content was doubled.
Materials
Sources of drugs used in the study were as follows: acetazolamide, amiloride, bumetanide, carbachol (CCh) and isobutylmethylxanthine were all from Sigma; forskolin was from Calbiochem; 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazol-2-one (DCEBIO) was from Tocris; (–)-7-[(2R,4aR,5R,7aR0-2-(1,1-difluoropentyl)-2hydroxy-6oxooctahydrocyclopenta[b]pyran-5-yl]heptanoic acid (lubiprostone) (Amitiza) from Takeda Pharmaceuticals America Inc., IL, USA; polypeptide antibiotics from Dr OL Shotwell and N-(2-naphthalenyl)-[(3,5 dibromo-2,4-dihydroxy phenyl)methylene glycine hydrazide] (GlyH-101) from Professor AS Verkman.
Results
Production of a modified Calu-3 cell line
Western blotting showed that CFTR in the modified Calu-3 cell line was less than 5% of that in normal Calu-3 cells. The cell line has now been propagated for over a year in the presence of Geneticin (to confirm that neomycin resistance is present). As the cell line still produces a small amount of CFTR, it is more properly described as a ‘knockdown' rather than a ‘knockout'. Throughout the rest of this paper, we shall refer to the cell line as Calu-3KD when comparing its properties with those of the wild type (Calu3WT). Figure 1 illustrates a Western blot for Calu-3WT and Calu-3KD protein. The bands for oocytes represent a positive control as Xenopus oocytes do not express CFTR unless injected with the cDNA for CFTR several days previously.
Figure 1.
Western blots of CFTR protein from Calu-3WT and Calu-3KD cells together with those for Xenopus oocytes previously injected with the cDNA for CFTR. Densitometer readings indicated that the band for Calu-3KD cells was <5% of that found in Calu-3WT cells.
Activation of adenylate cyclase
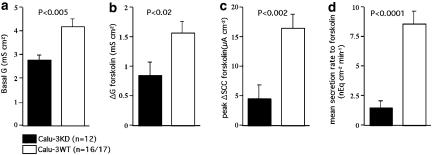
Forskolin was used to stimulate anion secretion in monolayers of both the Calu-3KD and Calu-3WT types. This agent activates adenylate cyclase resulting in an increase of cyclic adenosine monophosphate (cAMP), that in turn activates protein kinase A and hence activation of CFTR. As Calu-3KD grow more slowly than Calu-3WT two types of control monolayer were used; those grown for the same time as the Calu-3KD monolayers and those grown to confluence. No statistical differences were found with the two types of controls. The result of the study is shown in Figure 2. Calu-3KD epithelia were found to have (a) significantly lower basal transepithelial conductance, (b) smaller increases in conductance in response to forskolin, (c) smaller increases in the peak SCC responses to forskolin and (d) smaller increases in secretion rate. The reduction in secretion rate over that found when peak responses were considered was primarily due to the failure of the SCC increase following forskolin to be maintained in Calu-3KD monolayers.
Figure 2.
Comparison of the transporting properties of Calu-3KD and Calu-3WT epithelia. Shown are the effects of forskolin, 1 μM on SCC. Data were collected from 12 Calu-3KD monolayers grown for about 30 days, and were compared with Calu-3WT monolayers of two types, that is, four monolayers also grown for 30 days and 13 monolayers grown to confluence (about 15 days). No significant differences were found between the two groups and the data have been combined. The transepithelial conductances were measured from the change in SCC occurring when the clamp potential was temporarily set at 1 mV above zero. The mean secretion rate was measured during 8 min following the addition of forskolin from the area under curve. Basal conductance values are given, (a) whereas the change in conductance caused by forskolin,1 μM is shown in (b). The peak increase in SCC to forskolin is given in (c) and the secretion rate in (d).
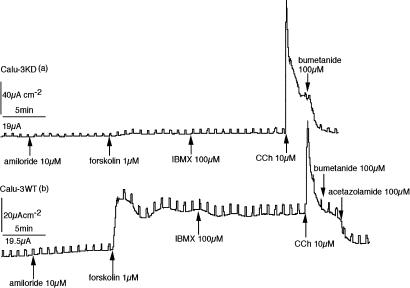
In CF, it is known that the epithelial sodium channels (ENaC) are upregulated in the absence of CFTR in the surface epithelium of the airways (Schwiebert et al., 1999). Although Calu-3WT epithelia show no evidence of the presence of ENaCs, it seemed possible that they might occur in cells where CFTR had been reduced to a low level. In these early experiments amiloride, 10 μM was added to the solution bathing the apical side of all epithelia to block ENaC channels. In no instance was any response to amiloride ever seen and the practice was abandoned. Some of the properties of the epithelia described in this section are illustrated in Figure 3.
Figure 3.
Representative SCC records from a Calu-3KD epithelium (a) on the upper panel and a Calu-3WT epithelium (b) on the lower panel. Neither showed any response to amiloride, whereas the responses to forskolin was minimal in the Calu-3KD tissue. In contrast, the Calu-3KD monolayer showed an enhanced response to CCh (note the differences in scale).
Effects of muscarinic agonists
In intact CF submucosal glands, the responses to muscarinic antagonists are spared, however, it is unclear whether the secretion derives from the serosal cells or from cells in the mucus tubules (Joo et al., 2002). Both types of Calu-3 epithelia retained the property of responding to the muscarinic agonist CCh. Indeed, the responses to CCh were significantly larger in Calu-3KD epithelia (Figure 3a) than in the wild-type version (Figure 3b). The peak heights of responses to CCh, 10 μM were 65.8±6.0 μA cm−2 in control monolayers (n=8), whereas the KD monolayers gave significantly larger responses (111.3±14.7 μA cm−2, n=6, P<0.01). In neither situation was the response to CCh maintained. This is in keeping with the actions of Ca2+ raising agonists. They cause activation of the Ca2+ sensitive K+-channels on the basolateral membranes of epithelia, thus, increasing the driving force on Cl− ions to exit through the apical membrane. In order to determine whether the mechanism of Cl− efflux following CCh was due to residual CFTR channels or an alternative Cl− channel, it was necessary to eliminate residual CFTR channels in Calu-KD epithelia. To do this, we used a novel CFTR channel inhibitor, GlyH-101, that acts by occlusion near to the external pore entrance (Muanprasat et al., 2004). Both types of Calu-3 epithelia failed to respond to CCh, if they were previously incubated with GlyH-101 (50 μM) on the apical side, indicating that chloride exit through the apical faces of the cells in response to CCh required CFTR.
Creating artificial ion channels
Duramycin (Shotwell et al., 1958) is said to form artificial anion channels by insertion into cellular membranes and has been reported to increase chloride secretion in airway epithelial cells (Cloutier et al., 1990). Here, we have examined the effects of duramycin, and the closely related peptide Ro09-0198 (R-009) (Choung et al., 1988) on chloride secretion on Calu-3WT and Calu-3KD epithelia. To enquire whether the peptides introduce a novel chloride conductance when applied to the apical epithelial surface, we have applied bumetanide and acetazolamide to the basolateral face of the epithelia, to prevent transepithelial chloride transport (Cuthbert et al., 2003). The reduction in current provides a measure of chloride entry through the basolateral surface, regardless whether it leaves via polypeptide-induced channels or by conventional means. To increase the likelihood of detecting extra chloride transport, experiments were carried with a basolateral to apical chloride gradient. This was achieved simply by replacing NaCl in the apical solution by sodium gluconate. To validate the system, we used DCEBIO (Singh et al., 2001) to increase current in the presence of a chloride gradient, in the knowledge that the main effect of DCEBIO is to increase the electrical gradient for Cl− efflux. DCEBIO increased the current in Calu3-WT monolayers by 118.2±27.3 μA cm−2 that was reduced by bumetanide/acetazolamide by 104.7±25.9 μA cm−2 (n=3). In contrast, in untreated monolayers, with basal current 19.1±8.4 μA cm−2, bumetanide/acetazolamide reduced the current by only 10.6±7.1 μA cm−2 (n=3). Clearly, the response to bumetanide/acetazolamide is significantly greater after DCEBIO treatment (P<0.03). The same protocol was used for epithelia treated with the polypeptide R-009.
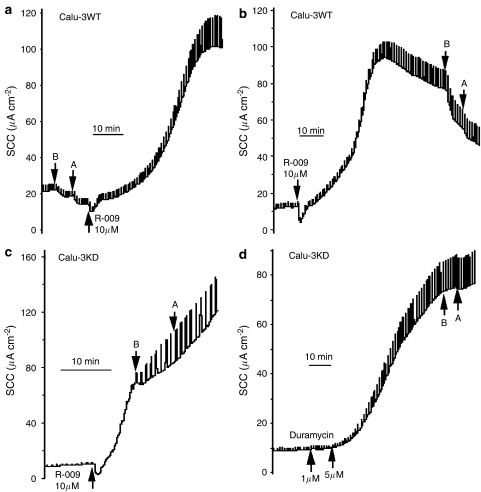
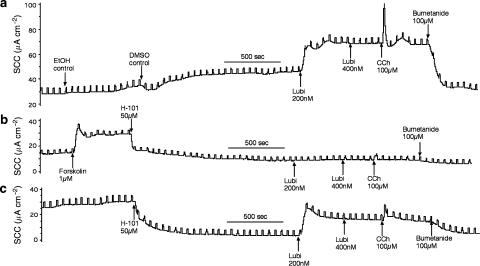
A typical experiment is shown in Figures 4a and b, made with two Calu-3WT epithelia. In Figure 4a, bumetanide/acetazolamide was added at the beginning of the experiment to measure the fraction of the basal current due to Cl− secretion. After apical application of R-009, the current began to increase, accompanied by a striking increase in conductance. Note that the current also increased in the control epithelium, in spite of the continued presence of bumetanide/acetazolamide, suggesting little of the increase in current is due to transepithelial chloride secretion. After the currents had reached a plateau the blockers were added to the test epithelium and the inhibition recorded. The inhibition caused by the blockers was −8.0±1.6 μA cm−2 in the control epithelia before R-009 was added, whereas after R-009, it rose to −23.5±5.0 μA cm−2. Although these two values are significantly different (P<0.03), the increase in chloride secretion caused by R-009 is only 15.5 μA cm−2, representing only 28% of the current increase to R-009 (Figure 5).
Figure 4.
SCC records from Calu-3WT epithelia (a, b) and Calu-3KD epithelia (c, d) exposed to either R-009 or duramycin (applied apically). All experiments were made with a basolateral to apical chloride gradient. (a) and (b) show the differences in the inhibition of SCC by bumetanide/acetazolamide when applied before or after R-009 in Calu-3WT epithelia. (c, d) Show the lack of effect of bumetanide/acetazolamide, applied to Calu-3KD epithelia when added after either R-009 or duramycin. Note that in all instances R-009 and duramycin caused major increases in transepithelial conductance. (B and A) Refer to bumetanide (100 μM applied basolaterally) and acetazolamide (100 μM added both sides).
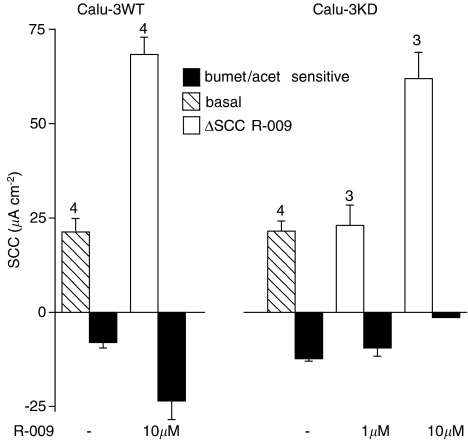
Figure 5.
Summary of the effects of R-009 on Calu-3WT and Calu-3KD epithelia and illustrated in Figure 4. The filled columns show SCC inhibition caused by bumetanide (100 μM added basolaterally) and acetazolamide (100 μM added both sides) under basal conditions and after R-009. Hatched columns show basal SCC, open columns indicate the SCC response to R-009, at either 1 or 10 μM. Note that in Calu-3WT epithelia R-009 significantly increased inhibition by bumetanide/acetazolamide (P<0.03), whereas in Calu-3KD epithelia, the inhibition by bumetanide/acetazolamide never exceeded that found in the basal condition.
Similar paired experiments were made with Calu-3KD epithelia using R-009. These epithelia proved to be more sensitive to R-009. For example, at 10 μM R-009, the current never attained a plateau and addition of blockers had no effect on the further evolution of current (Figure 4c). At lower concentrations, a plateau was achieved, but the current removed by bumetanide/acetazolamide was smaller than found with control conditions before R-009 had been added. Figure 5 summarizes all the data obtained with R-009. It is apparent that only with Calu-3WT monolayers is there any evidence that the polypeptide increased apical chloride permeability and then non-selectively so.
The same pattern emerged when duramycin was used, although we had insufficient material to do a systematic study. Calu-3KD epithelia were sensitive to duramycin, showing a large increase in current and epithelial conductance. After duramycin the epithelia were virtually non-responsive to bumetanide/acetazolamide (Figure 4d). In Calu-3WT epithelia treated with duramycin, the current increase was extremely slow but was accompanied by an increase in the bumetanide/acetazolamide sensitive component.
Clearly, our data provide no support for a selective increase in apical chloride permeability in Calu-3 epithelia with either R-009 or duramycin. Part of the non-bumetanide/acetazolamide, sensitive current after R-009 was sensitive to ouabain (data not shown). If the apical membrane had been non-selectively permeabilized then sodium ions entering the cells would be pumped across the basolateral face by the sodium pump. It is also probable that chloride movement down the concentration gradient, perhaps by the intercellular route, also accounts for some of the current increase following R-009.
Effects of the CFTR channel inhibitor GlyH-101 on responses of Calu-3 monolayers
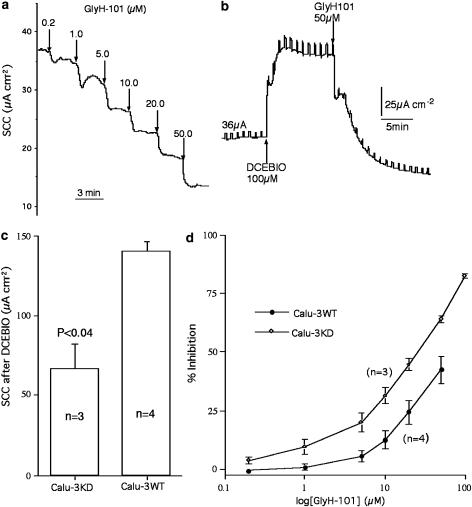
GlyH-101 is a CFTR channel inhibitor that acts from the apical side of epithelial membranes to block chloride secretion (Muanprasat et al., 2004). To examine if the sensitivity to GlyH-101 was different in the two types of epithelia, concentration–response relationships were determined. However, as basal currents are low in non-stimulated Calu-3 monolayers, we needed to increase SCC by an action not involving an action on CFTR. We used DCEBIO (Singh et al., 2001) to do this as it acts to activate KCNN4 channels located in the basolateral membranes of Calu-3 cells. As a result, the cells are hyperpolarized increasing the electrical gradient for chloride exit through any available apical chloride channels. The steady state current caused by DCEBIO was significantly less in Calu-3KD monolayers than in the WT version, as shown in Figure 6c. Also shown is a SCC record of responses to GlyH-101 in a Calu-3KD monolayer (Figure 6a) and concentration–response curves for both types of epithelia (Figure 6d). Because of the limited amount of GlyH-101 available to us, we were unable to pursue responses to higher concentrations of the blocker. In these experiments, a concentration interval of 3 min was chosen, although we were aware that this was something of a compromise, because of the slow decline in current, following high concentrations of GlyH-101 if longer dose intervals were used. This phenomenon was more marked in Calu-3WT epithelia than in the modified version. The data show that Calu-3KD monolayers are more sensitive to the blocker than the wild type. When high concentrations of GlyH-101 was added immediately after DCEBIO, the response showed two phases of inhibition, a rapid phase lasting around 3 min followed by a much slower decline in current to a value close to zero (Figure 6b). Consequently, in other experiments, where we wished to obtain complete blockade of CFTR channels, we have used high concentrations and long exposure times.
Figure 6.
Effects of the CFTR channel blocker, GlyH-101, on Calu-3 epithelia after addition of DCEBIO, 100 μM, basolaterally. (a) Shows SCC responses of a Calu-3KD epithelium to cumulative concentrations of GlyH-101 (added apically) after treatment with DCEBIO. Note that Calu-3KD epithelia gave significantly smaller responses to DCEBIO (shown in (c)). (b) Shows a SCC record from a Calu-3WT epithelium after addition of DCEBIO and GlyH-101, 50 μM. (d) Shows the percentage inhibition of SCC in Calu-3 epithelia of both types to cumulative addition of GlyH-101 after the SCC was increased with DCEBIO.
Actions of lubiprostone
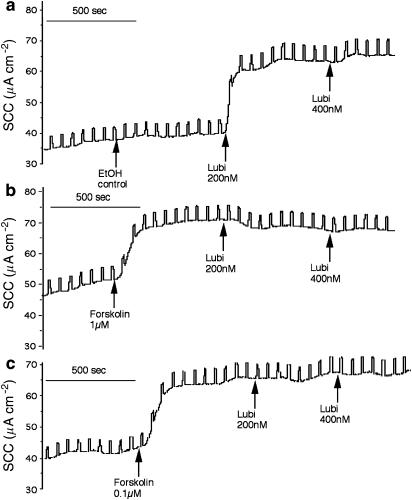
Several authors have suggested that ClC-2 channels might be able to mimic CFTR in epithelia where the latter is absent or non-functional. If this is so then activators of ClC-2 channels may cause chloride secretion in CF epithelia. Lubiprostone is reported to activate ClC-2 channels in cultured T84 epithelia and support transepithelial transport of Cl− (Cuppoletti et al., 2004). We found that lubiprostone activated a bumetanide/acetazolamide sensitive current in Calu-3 WT epithelia, with responses comparable in size to those elicited by forskolin (Figure 7). However, if forskolin was given before lubiprostone, the latter failed to produce any response. Indeed, the current was sometimes inhibited when lubiprostone was given after forskolin (Figure 7b). The converse effect was also found; that is no response was seen to forskolin when given after lubiprostone (Figure 8b). The failure to see a further increase in current when lubiprostone was given after forskolin and vice versa was not because the epithelia were incapable of greater secretion, as addition of the muscarinic agonist, CCh, resulted in a further temporary current increase as seen earlier (Figures 8a and b).
Figure 7.
Records of SCC for three Calu-3WT epithelia from the same batch. Responses to lubiprostone (Lubi) (200 nM applied apically in (a)) were suppressed when forskolin (1 μM (b) or 0.1 μM (c) applied to both sides) were given before lubiprostone. Both forskolin and lubiprostone caused small changes in transepithelial conductance (0.52 ms cm−2 for lubiprostone; 0.34 and 0.56 ms cm−2 for forskolin, 1 and 0.1 μM, respectively). A basolateral to apical chloride gradient was present.
Figure 8.
Records of SCC from two Calu-3WT epithelia from the same batch. Lubiprostone (Lubi) is effective in suppressing the responses to forskolin when former is given first (b). Conductance changes were forskolin 0.8 ms cm−2 (a) and lubiprostone 1.2 ms cm−2 (b). A basolateral to apical chloride gradient was present.
The similar magnitude of the responses to forskolin and lubiprostone suggested they were operating through the same mechanism, most likely to be the CFTR chloride channel. Consequently, experiments were devised to investigate this using GlyH-101 to completely inhibit CFTR channels. Informed by the findings from the earlier section a high concentration (50 μM) of GlyH-101 was used with a long exposure time.
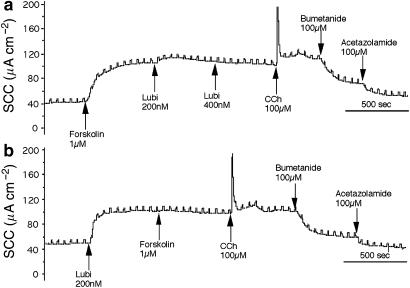
One type of experiment is shown in Figure 9. Here, three Calu-3WT epithelia from the same batch were examined simultaneously. The first (Figure 9a) was used for the addition of solvent controls and showed a normal response to lubiprostone, indicating solvents did not prevent the response to lubiprostone. It is also showed that the epithelium is capable of further secretion after lubiprostone by the addition of CCh and that the secretion is sensitive to bumetanide, indicating Cl− secretion. In Figure 9b, the monolayer was first exposed to forskolin followed by GlyH-101, the latter being allowed to act for 30 min before addition of lubiprostone. As expected, no response to lubiprostone was seen, as forskolin had been given previously. More telling is that there was no response to CCh either, indicating that there were no patent apical chloride conductances through which the hyperpolarization effect could operate, presumably because CFTR was completely blocked by GlyH-101. Further, there was little anion transport remaining as bumetanide had only a minor effect. The results in (b) are to be contrasted with those in (c). In this monolayer, forskolin was omitted from the protocol. Lubiprostone was effective even after exposure to GlyH-101 for 30 min and a small effect of CCh was restored. The experiments illustrated in Figures 7, 8 and 9 were all made in the presence of a basolateral to apical chloride gradient. The reason was to eliminate the possibility that lubiprostone had an acute effect on tight junctions between the epithelial cells, resulting in a current that would be insensitive to loop diuretics, such as bumetanide. All the foregoing experiments in this section were made with Calu-3WT epithelia and the major findings concerning lubiprostone–forskolin interactions and the failure of GlyH-101 to inhibit lubiprostone have been replicated, at least three times, but not necessarily in the format used for illustration.
Figure 9.
SCC records for three Calu-3WT epithelia from the same batch. The upper record (a) was used as a control for the solvents used for forskolin and GlyH-101; these did not affect the responsiveness to lubiprostone (Lubi) or to CCh and bumetanide. The middle record (b) shows an initial response to forskolin following which GlyH-101 (50 μM) was added. After 30 min, there was no response to lubiprostone or CCh and a minimal response to bumetanide. The lower trace (c) repeats the protocol of the middle record, without the addition of forskolin. After 30 min, exposure to GlyH-101 responses to lubiprostone, CCh and bumetanide are apparent. A basolateral to apical chloride gradient was present.
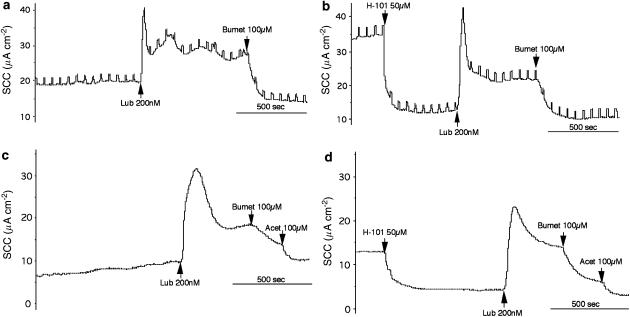
A more crucial test to see if the effects of lubiprostone were independent of CFTR was made using Calu-3KD epithelia, but also in the presence of GlyH-101. Results from two matched pairs of epithelia are given in Figure 10. In the first (a, b) a basolateral to apical chloride gradient was present, whereas in the second pair (c, d) there was no gradient. Clearly, the activity of lubiprostone is retained and the sensitivity to bumetanide infers that the current was due to the transepithelial transport of chloride, and unlikely to be mediated by CFTR. In experiments using matched pairs of epithelia, the charge transfer due to lubiprostone was 34.9±4.0 nEq 5 min−1 in the absence of GlyH-101 and 32.0±6.1 nEq 5 min−1 in its presence (both n=3, NS). Thus, it seems improbable that Calu-3KD cells, in the presence of GlyH-101, would be able to maintain a secretory response not significantly different from controls, in the absence of the inhibitor, by activating any residual CFTR channels.
Figure 10.
SCC records for two pairs of Calu-3KD epithelia. (a and b) are records from two 23-day cultures mounted in the presence of an apical to basolateral chloride gradient, whereas (c) and (d) are two 12-day cultures mounted without a gradient. Two epithelia (b and d) were exposed to GlyH-101 (50 μM) on the apical side. All four epithelia were exposed to lubiprostone (lub) 200 nM as indicated.
Discussion
It is generally believed that replacement of a small amount of CFTR in CF epithelia is sufficient to restore normal function. Human lung xenografts in nu/nu mice showed nearly normal cAMP-stimulated chloride transport, despite only transducing 5% of cells with a recombinant adenovirus, whereas other transporting features were not restored (Goldman et al., 1995). Here, we show that Calu-3 cells, a model human cell system for the serous cells of human airway submucosal glands, in which CFTR had been downregulated by persistent synthesis of siRNA intracellularly, also retained some CFTR-dependent transporting activities. With a maximal CFTR content of 5%, compared with wild-type Calu-3 cells, the modified cells showed 25% of normal cAMP-dependent chloride secretion, measured from peak responses and even less when sustained secretory responses were assessed. However, it is difficult to assess if CF epithelia with only 5% of the cells restored to normal should or would behave equivalently to an epithelium with only 5% of the normal CFTR content in all cells. Nevertheless, the behaviour of both types of epithelium were inferior to native epithelia, suggesting that considerably more CFTR content is required for normal function. Following submission of this paper, we learned that another group (Palmer et al., 2006) had produced a Calu-3 cell line expressing siRNA that showed a 99% reduction in CFTR mRNA and with a 95% reduction in the response to cAMP.
As stated in the introduction, our reasons for generating Calu-3KD cells was to search for agents that would restore chloride secretion to levels as great as those found in Calu-3WT epithelia, but without a requirement for CFTR. Submucosal gland secretion in CF to CCh is partially spared (Joo et al., 2002), but it is unclear from what part of the gland the secretion arises. Our data suggest that it cannot be the serous cells as in the presence of the CFTR channel blocker, GlyH-101, sufficient to completely inhibit all CFTR channels, no response to CCh remained. Thus, the responses in the absence of GlyH-101 must have resulted from the increased driving force due to hyperpolarization forcing Cl− exit through residual CFTR channels. We have no explanation for the significantly increased responses to CCh in Calu-3KD epithelia compared with Calu-3WT epithelia.
The polypeptide antibiotic duramycin was reported to selectively increase chloride permeability in surface airway epithelia (Cloutier et al., 1990). We have examined duramycin and the closely related peptide, R-009, before using both a human colonic cell line (HCA-7) (Roberts et al., 1991) and on lipid bilayers (Sheth et al., 1992). In those earlier studies, we found no evidence of a selective increase in permeability to chloride. In this study, again no evidence for a selective increase in Cl− permeabilty was found, although minor differences in behaviour towards the polypeptides was found in the two types of epithelia.
GlyH-101 is a new CFTR channel blocker that acts from the outside face of the channels (Muanprasat et al., 2004). It has proved useful in a number of instances in this study for its ability to convert either type of Calu-3 epithelium to ‘chemical knock-out models'. However, its blocking actions were not straightforward and pose some intriguing questions for which no straightforward interpretation seems possible. The first problem arises from the leftward shift of the concentration–response curve in Calu-3KD epithelia (Figure 6d). A simple explanation of this finding would be that the affinity of GlyH-101 for the channel is increased in Calu-3KD epithelia over that found in Calu-3WT epithelia. This explanation seems unlikely, as siRNA acts simply by reducing the amount of target protein made by the cells. A second approach is by consideration of the concept of spare receptors, that is can there be, by analogy, ‘spare channels'? Put another way, could there be a channel reserve that can be recruited to the membrane as other channels are blocked, thus, reducing the amount of inhibition expected in the absence of a reserve? It is known that there is continual traffic between CFTR in the membrane and that stored in vesicles just below the surface. It is also known that agents stimulating cAMP-dependent chloride secretion promote an acute insertion-retrieval trafficking process associated with an increase in membrane capacitance (Peters et al., 2001). Thus, Calu-3WT epithelia may have a large channel reserve, but available for replacement. In contrast, Calu-3KD may have little or no store of CFTR, with most of that available located in the apical membrane. This hypothesis is consistent with some of our findings with GlyH-101. The extent of the leftward shift in the concentration–response relationship suggests that Calu-3WT epithelia can maintain an apical channel density three times that of the KD variant, at least at low GlyH-101 concentrations. This ratio is not very different from the ratio of cAMP-dependent chloride secretion in the two types of epithelium (Figure 2). Also, we noted in Calu-3KD monolayers that the responses to forskolin, when present, were not maintained, in contrast to those in Calu-3WT models. That the CFTR content of Calu-3KD epithelia is only 5% of that of wild type (Figure 1) adds force to the arguments made above that Calu-3KD epithelia lack a reserve of CFTR. Thus, when a large concentration of GlyH-101 is added to Calu-3WT epithelia, there is an immediate reduction in current, followed by rapid replacement of channels from internal stores, maintaining the current at a higher level. Afterwards, the current finally wanes, as the supply of fresh channels is depleted (Figure 6b), a pattern not replicated in Calu-3KD monolayers (Figure 10). Further, our hypothesis might provide the explanation for the commonly stated principle that replacement of only a small fraction of CFTR by gene therapy is necessary to restore full function to tissues. But can the responses be maintained? We stress this as a working hypothesis and we are pursuing this by further experiments, not appropriate for inclusion here.
The ClC-2 channel is one of a family of widely distributed human chloride channels (Jentsch et al., 2005). Suggestions have been made that ClC-2 channels might provide an alternative pathway for chloride secretion in CF. ClC-2 channels are found in epithelia but there is no general agreement as to whether they are located apically (Cuppoletti et al., 2004), basolaterally (Pena-Munzenmayer et al., 2005) or are concentrated in the apical aspect of the tight junction complexes (Mohammad-Panah et al., 2001), indeed, their location may vary in different types of epithelia. Lubiprostone has been shown to stimulate chloride secretion in epithelial monolayers of cultured T84 cells, a human colonic cell line, that has been attributed to an action on ClC-2 channels (Cuppoletti et al., 2004). ClC-2 channels are present in Calu-3 cells, but their location is unknown (Cuppoletti et al., 2001).
In this study, it is shown that lubiprostone stimulates a bumetanide sensitive increase in SCC, comparable to that produced by forskolin. The sensitivity to bumetanide strongly supports the view that that transport was transcellular. Further, the similarity of the responses to lubiprostone, whether or not basolateral to apical chloride gradient was present argues against a tight-junction involvement. We were concerned that CFTR was the final effector in the response of Calu-3 epithelia to lubiprostone, especially as the effects of both lubiprostone and forskolin were mutually exclusive. Although we are actively exploring the mechanisms of this interaction, it is enough here to point out that lubiprostone is equally effective in Calu-3WT and Calu3KD epithelia and that Calu-3KD epithelia respond equally to lubiprostone in the presence or absence of the CFTR channel blocker GlyH-101. Thus, it seems likely that lubiprostone activates an alternative chloride conductance in Calu-3 epithelia. If this alternative chloride conductance was located basolaterally it could possibly increase chloride influx through the basolateral aspects of the cells, but presumably would be insensitive to bumetanide. However, another chloride conductance would still be needed on the apical face to allow exit in the presence of GlyH-101. Therefore, we conclude that lubiprostone activates an ‘alternative to CFTR' chloride conductance that must be located, at least partly, in the apical face of Calu-3 epithelia. We cannot conclude definitively that the target for lubiprostone is ClC-2, but it is clearly a likely candidate. Further, we cannot assume that the results with Calu-3 epithelia will be replicated with intact submucosal glands. Nevertheless, our results with lubiprostone raise the possibility that ClC-2 activators could have applicability to therapy for CF.
Finally, CFTR is not only a chloride channel, but an important regulator of other membrane channels (Schwiebert et al., 1999; Wei et al., 1999). We considered that the construction of cells with severely reduced CFTR content might alter its regulatory activity. Specifically, we might expect upregulation of amiloride sensitive sodium channels, ENaC, and calcium sensitive chloride channels, calcium activated chloride channel. No responses to amiloride were apparent in Calu-3KD monolayers and neither did the calcium-dependent responses to CCh survive in the presence of GlyH-101. Thus, neither prediction was fulfilled.
From the data presented here, it would seem that more than one copy of the siRNA construct would need to be incorporated into the Calu-3 genome to produce a knockout Calu-3 cell model. However, the present Calu-3KD cell line in the presence of GlyH-101 appears to be a useful model to investigate non-CFTR dependent drug therapy for CF.
Acknowledgments
We are extremely grateful to Dr Alan S Verkman for a supply of GlyH-101 and to Dr OL Shotwell for samples of polypeptide antibiotics that she supplied years ago for our earlier study. Part of this study was funded by the Cystic Fibrosis Trust.
Abbreviations
- CCh
carbachol
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- DCEBIO
5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazol-2-one
- ENaC
epithelial sodium channel
- GFP
green fluorescent protein
- GlyH-101
N-(2-naphthalenyl)-[(3,5 dibromo-2,4-dihydroxy phenyl)methylene glycine hydrazide]
- KHS
Krebs Henseleit Solution
- lubiprostone
(-)-7-[(2R,4aR,5R,7aR0-2-(1,1-difluoropentyl)-2hydroxy-6oxooctahydrocyclopenta[b]pyran-5-yl]heptanoic acid
- R-009
Ro09-0198
- SCC
short circuit current
- siRNA
small inhibitory RNA
Conflict of interest
The authors state no conflict of interest.
References
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Choung S-Y, Kobayashi J, Inoue J, Takemoto K, Ishisuka H, Inoue K. Haemolytic activity of a cyclic peptide Ro09-0198 isolated from streptoverticillium. Biochim Biophys Acta. 1988;940:171–179. doi: 10.1016/0005-2736(88)90192-7. [DOI] [PubMed] [Google Scholar]
- Cloutier MM, Guernsey L, Mattes P, Koeppen B. Duramycin enhances chloride secretion in airway epithelium. Am J Physiol. 1990;259:C450–C454. doi: 10.1152/ajpcell.1990.259.3.C450. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Malinowska DH, Tewari KP, Li Q-j, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Tewari KP, Sherry AM, Kupert EY, Malinowska DH. ClC-2 channels in human lung epithelia: activation by arachidonic acid, amidation and acid activated omeprazole. Am J Physiol. 2001;281:C46–C54. doi: 10.1152/ajpcell.2001.281.1.C46. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Superan CT, MacVinish LJ. Bicarbonate-dependent chloride secretion in Calu-3 epithelia in response to 7,8-benzoquinoline. J Physiol. 2003;551:79–92. doi: 10.1113/jphysiol.2003.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, et al. Submucosal glands are the predominant sites of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Goldman MJ, Yang Y, Wilson JM. Gene therapy in a xenograft model of cystic fibrosis lung corrects chloride transport more effectively than the sodium defect. Nat Genet. 1995;9:126–131. doi: 10.1038/ng0295-126. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Neagoe I, Scheel O. CLC chloride channels and transporters. Curr Opin Neurobiol. 2005;15:319–325. doi: 10.1016/j.conb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal polypeptide in cystic fibrosis airway glands. J Biol Chem. 2002;277:50710–50715. doi: 10.1074/jbc.M208826200. [DOI] [PubMed] [Google Scholar]
- Mohammad-Panah R, Gymorey K, Rommens J, Choudhury M, Li C, Wang Y, et al. ClC-2 contributes to native chloride secretion by a human intestinal cell line, Caco-2. J Biol Chem. 2001;276:8306–8313. doi: 10.1074/jbc.M006764200. [DOI] [PubMed] [Google Scholar]
- Moon S, Singh M, Krouse ME, Wine JJ. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am J Physiol. 1997;273:L1208–L1219. doi: 10.1152/ajplung.1997.273.6.L1208. [DOI] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJV, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ML, Lee SY, Maniak PJ, Carlson D, Fahrenkrug SC, O'Grady SM. Protease-activated receptor regulation of Cl− secretion in Calu-3 cells requires prostaglandin release and CFTR activation. Am J Physiol. 2006;290:1189–1198. doi: 10.1152/ajpcell.00464.2005. [DOI] [PubMed] [Google Scholar]
- Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, et al. Basolateral localisation of native CLC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–4252. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- Peters KW, Qi J-j, Johnson JP, Watkins SC, Frizzell RA. Role of snare proteins in CFTR and ENaC trafficking. Eur J Physiol. 2001;443:S65–S69. doi: 10.1007/s004240100647. [DOI] [PubMed] [Google Scholar]
- Roberts M, Hladky SB, Pickles RJ, Cuthbert AW. Stimulation of sodium transport by duramycin in cultured human colonic epithelia. J Pharm Exp Ther. 1991;259:1050–1058. [PubMed] [Google Scholar]
- Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, et al. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J. 2005;19:431–433. doi: 10.1096/fj.04-2879fje. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Benos DJ, Egan ME, Stutts J, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79:S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- Sheth TR, Henderson RM, Hladky SB, Cuthbert AW. Ion channel formation by duramycin. Biochim Biophys Acta. 1992;1107:179–185. doi: 10.1016/0005-2736(92)90345-m. [DOI] [PubMed] [Google Scholar]
- Shotwell OL, Stodola FA, Michael WR, Lindenfelsar LA, Dworschack RG, Pridham WR. Antibiotics against plant disease. III Duramycin a new antibiotic from Streptomyces cinnamoneus forma azacoluta. J Am Chem Soc. 1958;80:3912–3915. [Google Scholar]
- Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and obstructive pulmonary disease. J Pharm Exp Ther. 2001;296:600–611. [PubMed] [Google Scholar]
- Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- Wei L, Vankeerberghen A, Cuppens H, Eggermont J, Cassiman JJ, Droogmans G, et al. Interaction between calcium-activated chloride channels and the cystic fibrosis transmembrane conductance regulator. Pflugers Arch. 1999;438:635–641. doi: 10.1007/s004249900108. [DOI] [PubMed] [Google Scholar]