Abstract
Background and purpose:
Recent evidence indicates that carbon monoxide-releasing molecules (CO-RMs) exhibit potential anti-inflammatory properties. In the present study, we have investigated whether tricarbonyl dichloro ruthenium(II) dimer (CORM-2) can control the inflammatory response induced by cytokines in a human colonic epithelial cell line, Caco-2.
Experimental approach:
Caco-2 cells were preincubated with CORM-2 for 30 minutes and then stimulated with interleukin (IL)-1β, tumor necrosis factor-α and interferon-γ for different times. Gene expression was analyzed by real-time PCR. Protein expression was investigated by Western blot and ELISA. Transcription factor activation was determined by the luciferase method.
Key results:
We have shown that CORM-2 significantly decreased the mRNA expression of nitric oxide synthase-2 (NOS-2) and the production of nitrite, in Caco-2 cells stimulated with cytokines. IL-8, IL-6 and metalloproteinase-7 (MMP-7) mRNA and protein were also significantly reduced by CORM-2. Time-course and small interfering RNA studies suggest that inhibition of IL-6 plays a role in the regulation of MMP-7 expression by CORM-2. These effects of CORM-2 can be dependent on the modulation of nuclear factor-κB (NF-κB), activator protein-1, CCAT/enhancer binding protein and the phosphorylated forms of NF-κB inhibitory protein-α, c-Jun N-terminal protein kinase 1/2, p38 and extracellular signal-regulated kinase 1/2.
Conclusions and Implications:
CORM-2 can regulate a number of genes relevant in intestinal inflammation and cancer progression. These findings provide new insights into the anti-inflammatory properties and potential applications of this class of compounds.
Keywords: carbon monoxide-releasing molecules, metalloproteinase-7, cytokines, nuclear factor-κB, Caco-2 cells, inflammation
Introduction
In intestinal epithelial cells, proinflammatory cytokines activate nuclear factor-κB (NF-κB), which regulates the transcription of nitric oxide synthase-2 (NOS-2), interleukin(IL)-8, IL-6, matrix metalloproteinases (MMPs) and other molecules involved in chronic intestinal inflammation and cancer (Roebuck, 1999; Jobin and Sartor, 2000). MMPs can be effector molecules for mucosal injury and play an important role in inflammatory bowel disease (Baugh et al., 1999) and early stages of colorectal carcinogenesis (Nosho et al., 2005). In particular, MMP-7 is produced by epithelial cells in the digestive tract and may mediate a wide range of cellular responses. This enzyme could participate in wound healing (Salmela et al., 2004), leading to migration of epithelial cells after tissue damage. MMP-7 is thought to be a key mediator in intestinal inflammation (Wielockx et al., 2004) and colon cancer (Witty et al., 1994; Newell et al., 2002), as well as a marker of activity related to dysplasia and cancer in patients with ulcerative colitis (Matsuno et al., 2003). Therefore, targeted inhibition of MMP-7 has been proposed as a novel therapeutic option for intestinal inflammation associated with necrotizing enterocolitis (Bister et al., 2005) and several inflammatory disorders (Wielockx et al., 2004).
Heme oxygenase (HO) activity catabolizes heme to biliverdin, carbon monoxide (CO) and iron. The HO-1 isoform is induced by oxidative stress in colonic epithelial cells (Dijkstra et al., 2004) and can be detected in the intestinal epithelium of severely inflamed mucosa of inflammatory bowel disease patients (Paul et al., 2005). Owing to the anti-inflammatory effects observed in animals (Wang et al., 2001; Paul et al., 2005), HO-1 has been proposed as a new therapeutic target for inflammatory bowel disease (Naito et al., 2004). The protective actions of HO-1 in the gut may be mediated by the production of biliverdin, bilirubin (Berberat et al., 2005; Busserolles et al., 2006) or CO (reviewed in Ryter and Otterbein, 2004). Carbon monoxide-releasing molecules (CO-RMs) mimic the biological actions of CO derived from HO activity (Foresti et al., 2005). Recent data have demonstrated the vasoactive (Foresti et al., 2004) and cardioprotective (Clark et al., 2003) effects of this group of compounds. Interestingly, CO released by CO-RMs has been shown to inhibit NO and tumor necrosis factor-α (TNFα) production in mouse macrophage (Sawle et al., 2005) or microglia (Bani-Hani et al., 2006) cell lines. These observations suggest potential anti-inflammatory properties for CO-RMs. The aim of this study was to test the hypothesis that a CO donor, CORM-2 inhibits the inflammatory response elicited by cytokines in a human colonic epithelial cell line, Caco-2. Here we show that this agent inhibits the production of inflammatory mediators through the modulation of NF-κB and other transcription factors. These data demonstrate for the first time the ability of a CO-RM to downregulate MMP-7 expression and support the anti-inflammatory potential of this group of compounds.
Methods
Cell growth and culture
Caco-2 cells from the European Collection of Cell Cultures (ECACC 86010202, Salisbury, UK) were routinely grown in 100 mm plastic dishes at 37°C in a humidified atmosphere of 5% CO2 in air in modified Eagle's medium, supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 1 mM fungizone and 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid. Caco-2 cells were seeded at 105 cells ml−1 and routinely subcultured when about 80% confluent. The culture medium was changed every other day. Cells were used between passages 20 and 40. Cell viability was assessed by the mitochondria1-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) to formazan. After appropriate stimulation times, cells were incubated with MTT (200 μg ml−1) for 180 min. The medium was then removed and the cells solubilized in dimethyl sulfoxide (DMSO) (100 μl) to quantitate formazan at 550 nm (Gross and Levi, 1992). For cell stimulation, Caco-2 cells were plated at 106 cells/well in six-well dishes for 10 days. After medium removal, fresh medium without serum was added. CORM-2 was dissolved in DMSO and then diluted in culture medium (0.1%, v v−1). Control cells were treated with the same vehicle. Cells were preincubated with CORM-2 for 30 min and then stimulated with a mixture of proinflammatory cytokines (cytomix) – interferon-γ (1000 U ml−1), IL-1β (10 ng ml−1) and TNFα (10 ng ml−1) – for different times. As a negative control, cells were treated with RuCl3 (150 μM).
Measurement of NO2− levels
In supernatants from 18 h cytomix-stimulated cells, NO2− was determined fluorometrically in microtiter plates using NaNO2 as a standard (Misko et al., 1993).
Transient transfection and luciferase activity assay
Cells (106/well) were seeded in six-well plates and after 24 h incubation were transiently transfected overnight with either the NF-κB-Luc, activator protein-1 (AP-1)-Luc or CCAT/enhancer binding protein (C/EBP)-Luc plasmid (Stratagene, La Jolla, CA, USA) using Fugene 6 (Roche Applied Science, Indianapolis, IN, USA) according to the supplier's recommendations. Cells were treated with cytomix for 8 h in the presence or absence of CORM-2 at different concentrations. After lysis and centrifugation, 20 μl aliquots of supernatants were used to assay luciferase activity using the kit from Promega Corp. (Madison, WI, USA). Luminescence was measured in a Microbeta counter (Wallac, Turku, Finland) and normalized against protein amount.
Electrophoretic mobility shift assay (EMSA)
Caco-2 cells (106/well) were preincubated with CORM-2 for 30 min and then stimulated with cytomix for 15 or 30 min. Nuclear extracts were prepared as described (López-Collazo et al., 1998). Cells were washed twice with ice-cold phosphate-buffered saline, and then treated with 0.2 ml of buffer A (20 mM Tris–HCl, pH 7.8, 10 mM KCl, 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride and 10 μM leupeptin) for 15 min followed by addition of Nonidet P-40 (0.5%, w v−1). The tubes were vortexed for 15 s, and nuclei were sedimented by centrifugation at 8000 g for 30 s. Aliquots of the supernatant were stored at −80°C (cytoplasmic extract), and the nuclei pellet was resuspended in 50 μl of buffer A supplemented with 0.4 M KCl. After centrifugation at 13 000 g for 15 min, aliquots of the supernatant (nuclear extract) were stored at −80°C. Protein was determined by the DC Bio–Rad protein reagent (Richmond, CA, USA). Double-stranded oligonucleotide containing the consensus NF-κB sequence (Promega Corp., Madison, WI, USA) was end-labeled using T4 polynucleotide kinase (GE Healthcare, Barcelona, Spain) and [γ-32P]ATP (NEN Life Sciences Products Inc. (Boston, MA, USA). Incubations were performed on ice with 6 μg of nuclear extract, 100 000 c.p.m. of labeled probe, 2 μg poly(dI-dC), 5% (v v−1) glycerol, 1 mM ethylenediaminetetraacetic acid, 5 mM MgCl2, 1 mM DTT, 100 mM NaCl and 10 mM Tris–HCl buffer (pH 8.0) for 15 min. Complexes were analyzed by nondenaturating 6% polyacrylamide gel electrophoresis in 0.5 × Tris–borate buffer followed by analysis of the dried gel by the Typhoon 9410 system (GE Healthcare, Barcelona, Spain).
Western blot analysis
For NF-κB inhibitory protein-α (IκBα), cytoplasmic extracts from Caco-2 cells were obtained as indicated in EMSA. Proteins (25 μg) were separated by 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (GE Healthcare, Barcelona, Spain). Membranes were incubated with polyclonal antibodies against IκBα or β-actin. For other proteins, the cell pellets were lysed in 100 μl of buffer (1% Triton X-100, 1% deoxycholic acid, 20 mM NaCl and 25 mM Tris, pH 7.4) and centrifuged at 4°C for 5 min at 10 000 g. Proteins (25 μg) in cell lysates were separated by 12.5% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. Membranes were incubated with specific polyclonal antibodies against phosphorylated Thr180/Tyr185 c-Jun N-terminal protein kinase 1/2, phosphorylated Thr180/Tyr182 p38, phosphorylated Thr183/Tyr185 extracellular signal-regulated kinase (ERK)1/2, the corresponding unphosphorylated proteins, HO-1 or β-actin. Finally, membranes were incubated with peroxidase–conjugated goat anti-rabbit immunoglobulin (Ig)G and the immunoreactive bands were visualized by enhanced chemiluminescence using the AutoChemi image analyzer (UVP Inc., Upland, CA, USA).
Quantification of MMP-7, IL-8 and phosphorylated IκBα
Quantikine CXCL8/IL-8 immunoassay kit (sensitivity 3.5 pg ml−1) was purchased from R&D Biosystems (Abingdon, UK). Pro-MMP-7 protein levels were measured by human Biotrak ELISA kit (sensitivity 97 pg ml−1) from GE Healthcare (Barcelona, Spain). To monitor the amount of phosphorylated IκBα relative to total IκBα protein in Caco-2 cells, we used the ELISA CASE for IκBα-Ser32/Ser36-phospho-IκBα kit from SuperArray Bioscience Corp. (Frederik, MD, USA) according to manufacturer's instructions.
Quantitative real-time PCR
Cells were preincubated with CORM-2 for 30 min and then stimulated with cytomix for different times. Total RNA was extracted using the TRIzol reagent (Life Technologies Inc. Barcelona, Spain) according to the manufacturer's instructions. Reverse transcription was accomplished on 1 μg of total RNA using random primers (TaqMan reverse transcription reagents, Applied Biosystems Spain, Madrid). PCRs were performed using Power SYBR Green PCR Master Mix (Applied Biosystems Spain, Madrid). The primer sequences have been reported elsewhere: IL-8 (Muhlbauer et al., 2004), IL-6 (www.realtimeprimers.org), MMP-7 (Yanagisawa et al., 2005), NOS-2 and β-actin (Thilakawardhana et al., 2005). PCR assays were performed in duplicate on an iCycler Real-Time PCR Detection System (Bio–Rad Laboratories, Richmond, CA, USA) running the cycling conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. Reaction specificity was determined by melt curve analysis which was performed by heating the plate from 55 to 95°C and measuring SYBR Green I dissociation from the amplicons. Cycle threshold (CT) values for each gene were corrected using the mean CT value for β-actin. Relative gene expression for cells stimulated with cytomix either in the presence or absence of drug treatments was calculated with respect to the mean ΔCT of nonstimulated cells.
Data analysis
Results are presented as mean±s.e.m. Statistical analyses were performed using one-way analysis of variance, followed by Dunnett's t-test for multiple comparisons.
Materials
The proinflammatory cytokines were from Peprotech EC Ltd. (London, UK). Anti-active mitogen-activated protein kinase (MAPK), p38 and c-Jun N-terminal protein kinase (JNK)1/2 polyclonal antibodies were purchased from Promega Biotech Iberica (Madrid, Spain), and the antibodies against the corresponding unphosphorylated proteins were from Cell Signaling Technology (Beverly, MA, USA). Polyclonal antibody against IκBα was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and the HO-1 antibody was from Stressgen (Ann Arbor, MI, USA). The peroxidase-conjugated IgGs were purchased from Dako (Copenhagen, Denmark). Predesigned small interfering RNA (siRNA) oligonucleotides were purchased from Ambion Inc. (Austin, TX, USA). Cobalt protoporphyrin IX was purchased from Frontier Scientific Europe Ltd. (Carnforth, UK). CORM-2 and other reagents were from Sigma–Aldrich (St Louis, MO, USA).
Results
CORM-2 inhibits the production of inflammatory mediators in Caco-2 cells stimulated with cytokines
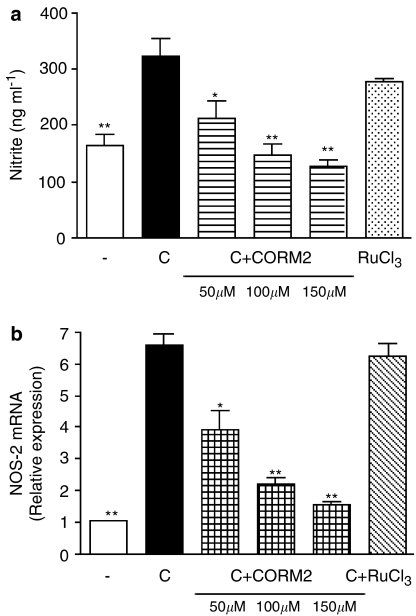
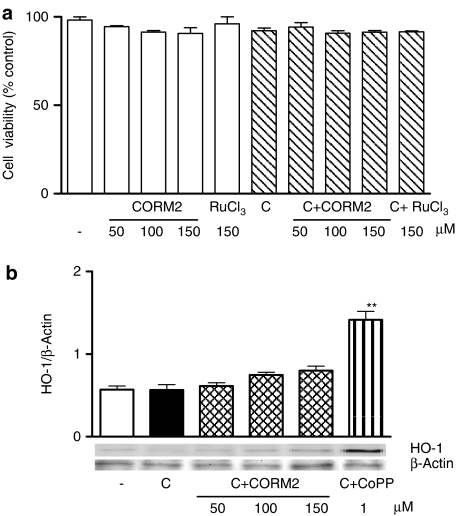
Treatment of Caco-2 cells with CORM-2 significantly reduced NO generation measured as nitrite after 18 h of cytomix stimulation, in a concentration-dependent manner (Figure 1a). In contrast, the negative control without CO groups RuCl3 was ineffective. The inhibition of NO production by CORM-2 appears to correspond to that of NOS-2 mRNA expression (Figure 1b). The concentrations used are in the range of those previously reported in other cell lines (Motterlini et al., 2002) and Caco-2 cells (Busserolles et al., 2006). These effects were not because of cytotoxicity of the compounds, as cell viability was not significantly modified by either CORM-2 (50–150 μM) or RuCl3 (150 μM), as determined by the MTT assay (Figure 2a). As CO and CORM-2 may induce HO-1 (Sawle et al., 2005), which could inhibit NO production (Turcanu et al., 1998), we assessed the effect of CORM-2 on HO-1 protein expression. The known HO-1 inducer, cobalt protoporphyrin IX, was included as positive control. As shown in Figure 2b, CORM-2 treatment did not induce a significant increase in HO-1 expression.
Figure 1.
Effects of CORM-2 on nitrite levels in supernatants (a) and NOS-2 mRNA expression (b) in Caco-2 cells stimulated with cytomix for 18 or 12 h, respectively. Results are the mean±s.e.m. of three separate experiments each consisting of four samples. *P<0.05; **P<0.01 vs control cells stimulated with cytomix (C). (−), nonstimulated control cells.
Figure 2.
Effects of CORM-2 on cell viability (a) and HO-1 protein expression (b). Caco-2 cells were incubated with test compounds for 24 h in the absence or presence of cytomix (C). Viability was expressed as percentage of nonstimulated control cells (−). Results are the mean±s.e.m. of three separate experiments. CoPP, cobalt protoporphyrin IX. The immunoblot is representative of three independent experiments. **P<0.01 vs control cells stimulated with cytomix (C).
Because the CXC-chemokine IL-8 plays an important role in intestinal inflammation (Mazzucchelli et al., 1994), we also tested whether CORM-2 could inhibit IL-8 production. Treatment of Caco-2 cells with cytomix significantly upregulated IL-8 mRNA as well as the release of IL-8 protein. As shown in Figure 3a and b, CORM-2 inhibited IL-8 protein levels in supernatants and mRNA expression in Caco-2 cells.
Figure 3.
Effects of CORM-2 on IL-8 protein levels in supernatants (a) and mRNA expression (b) in Caco-2 cells stimulated with cytomix for 18 or 12 h, respectively. Results are the mean±s.e.m. of three separate experiments, each consisting of four samples. *P<0.05; **P<0.01 vs control cells stimulated with cytomix (C). (−), nonstimulated control cells.
CORM-2 decreases the induction of MMP-7 by cytokines
Consistent with the role of cytokines in inducing MMP-7 in epithelial cells, stimulation of Caco-2 cells with cytomix significantly increased MMP-7 expression. As shown in Figure 4a, CORM-2 inhibited the levels of pro-MMP-7 protein in a concentration-dependent manner, whereas RuCl3 showed no effect. To find out whether CORM-2 reduces MMP-7 gene expression, Caco-2 cells were stimulated with cytomix in the presence or absence of CORM-2 and the relative amounts of mRNA were measured by real-time reverse transcriptase-PCR. Figure 4b shows that treatment with CORM-2 significantly reduced cytomix induction of MMP-7 mRNA.
Figure 4.
Effects of CORM-2 on pro-MMP-7 protein levels in supernatants (a) and mRNA expression (b) in Caco-2 cells stimulated with cytomix for 18 or 12 h, respectively. Results are the mean± s.e.m. of three separate experiments each consisting of three samples. *P<0.05; **P<0.01 vs control cells stimulated with cytomix (C). (−), nonstimulated control cells.
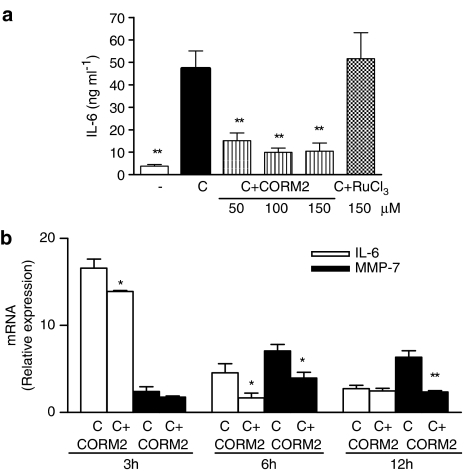
Inhibition of IL-6 may contribute to the regulation of MMP-7 by CORM-2
It is known that the inflammatory mediator IL-6 is produced by the intestinal mucosa and can be induced by IL-1β in Caco-2 cells (Parikh et al., 1997). Cytomix stimulation led to a rapid increase in IL-6 production. As shown in Figure 5a, CORM-2 treatment markedly decreased IL-6 levels in supernatants of cells stimulated with cytomix for 6 h, in a concentration-dependent manner, to approximately the same level as noticed in nonstimulated cells, whereas RuCl3 was inactive. As IL-6 can mediate MMP-7 induction by IL-1β (Maliner-Stratton et al., 2001), we conducted experiments to determine whether the inhibition of IL-6 production plays a role in the effects of CORM-2 on MMP-7. To address the link between IL-6 and MMP-7, we performed a series of real-time PCR experiments looking at changes in relative transcript abundance. IL-6 mRNA was barely detectable in nonstimulated cells, whereas treatment with cytomix rapidly upregulated the expression of this transcript. Figure 5b shows the time course of IL-6 and MMP-7 mRNA induction by cytomix in Caco-2 cells. IL-6 expression stimulated by cytomix peaked by 3 h. This is different from that observed in cytomix-elicited MMP-7 expression, which reaches a plateau after 6 h of cytomix stimulation. Interestingly, CORM-2 treatment reduced the expression of both genes, with the highest effect for IL-6 and MMP-7 at 6 and 12 h after cytomix stimulation, respectively. Therefore, inhibition of IL-6 mRNA expression by CORM-2 preceded that of MMP-7. In addition, the inhibitory effects of CORM-2 on MMP-7 mRNA were abolished if IL-6 was added to the culture medium at the same time as cytomix (Figure 6a). We also observed that pretreatment of Caco-2 cells with a siRNA specific for IL-6 significantly decreased MMP-7 mRNA expression in cytomix-stimulated cells, whereas pretreatment with a nonspecific siRNA had no effect. Furthermore, addition of IL-6 to nonstimulated cells induced MMP-7 mRNA expression which was not inhibited by CORM-2 treatment (Figure 6b).
Figure 5.
Effect of CORM-2 on IL-6 levels in supernatants from cells stimulated with cytomix for 6 h (a), and time course of IL-6 and MMP-7 mRNA expression and effect of CORM-2 (100 μM) (b). Results are the mean±s.e.m. of three separate experiments each consisting of four samples. (−), nonstimulated control cells. *P<0.05; **P<0.01 vs control cells stimulated with cytomix (C).
Figure 6.
Influence of IL-6 modulation on the inhibitory effect of CORM-2 in MMP-7 mRNA expression in Caco-2 cells stimulated with cytomix for 12 h (a). Concentrations used were: CORM-2 (100 μM), IL-6 (1 ng ml−1) and IL-6 or nonspecific siRNA (100 nM). Stimulation of MMP-7 mRNA expression by IL-6 (0.2 or 1 ng ml−1) addition to Caco-2 cells and incubation for 12 h. Effect of CORM-2 (100 μM) (b). Results are the mean±s.e.m. of two separate experiments each consisting of three samples. **P<0.01 vs control cells stimulated with cytomix (C).
CORM-2 inhibits the activation of NF-κB, AP-1 and C/EBP
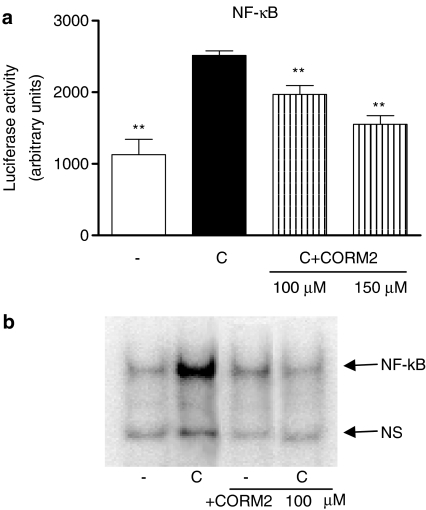
As NF-κB activation is essential for cytokine-mediated inflammation and transcription of NOS-2 and IL-8 in human intestinal epithelial cells (Jobin et al., 1998), and production of IL-6 in Caco-2 cells cells after IL-1β stimulation has been related to the activation of NF-κB, AP-1 and C/EBP (Parikh et al., 1997; Hungness et al., 2000, 2002a), we have tested the hypothesis that CORM-2 mediates its effects through the suppression of the activity of these transcription factors. Cytokine treatment of Caco-2 cells resulted in rapid phosphorylation of IκBα, nuclear translocation of NF-κB, DNA binding and activated transcription. To determine the effect of CORM-2 on cytokine-induced NF-κB-dependent reporter gene expression, we transiently transfected the cells with the NF-κB-regulated luciferase reporter construct, followed by incubation with CORM-2 and cell stimulation with cytomix. Exposure of cells to cytomix for 8 h increased the luciferase activity in the cells transfected with the NF-κB-luciferase reporter construct, whereas treatment with CORM-2 significantly reduced NF-κB activation (Figure 7a). In addition, EMSA experiments revealed that CORM-2 also inhibited NF-κB-DNA binding (Figure 7b). As shown in Figure 8a, IκBα protein expression decreased after cytomix stimulation for 30 min, which was prevented by CORM-2 treatment. In addition, IκBα phosphorylation was significantly inhibited by CORM-2 at 30 min after cytomix stimulation (Figure 8b).
Figure 7.
Effect of CORM-2 on NF-κB promoter activation (a) and NF-κB-DNA binding (b), in Caco-2 cells stimulated with cytomix for 8 h or 30 min, respectively. Results are the mean±s.e.m. of three separate experiments each consisting of three samples. (−), nonstimulated control cells. **P<0.01 vs control cells stimulated with cytomix (C). In (b), the figure is representative of three experiments with consistent results.
Figure 8.
Effect of CORM-2 on IκBα expression (a) and IκBα phosphorylation (b), in cytoplasmic extracts from Caco-2 cells stimulated with cytomix for either 15 or 30 min. Results are the mean±s.e.m. of three separate experiments each consisting of three samples. (−), nonstimulated control cells. **P<0.01 vs control cells stimulated with cytomix (C). In (a), the figure is representative of three experiments with consistent results.
To examine the effects of CORM-2 on the AP-1 and C/EBP transcription activity, Caco-2 cells were transiently transfected with luciferase reporter plasmids containing the corresponding promoters. Treatment with cytomix resulted in an approximately threefold increase in luciferase activity (Figure 9a and b). We found that CORM-2 significantly decreased the activation of these transcription factors by proinflammatory cytokines.
Figure 9.
Effect of CORM-2 on AP-1 (a) and C/EBP (b) promoter activation in Caco-2 cells stimulated with cytomix for 8 h. Results are the mean±s.e.m. of two separate experiments each consisting of three samples. (−), nonstimulated control cells. **P<0.01 vs control cells stimulated with cytomix (C).
Effects of CORM-2 on MAPK enzymes
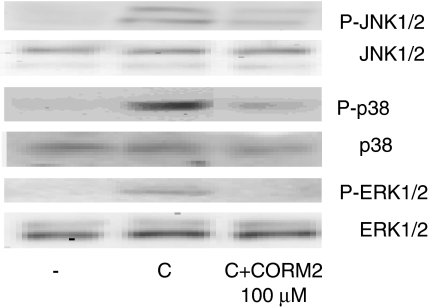
Because activation of MAPK enzymes is an important step of human enterocyte activation by proinflammatory cytokines, we examined the potential involvement of this pathway in the inhibitory effects of CORM-2. We treated cells with cytomix and cell lysates were subjected to Western blot analysis to detect the activated forms of p38, ERK1/2 and JNK1/2, using specific antibodies. As shown in Figure 10, cytomix stimulation rapidly increased the levels of phosphorylated forms of these proteins and in cells treated with CORM-2, the expression of the activated forms of p38, ERK1/2 and JNK1/2 was markedly reduced.
Figure 10.
Effect of CORM-2 on the expression of the phosphorylated forms of JNK1/2, p38 and ERK1/2 in Caco-2 cells stimulated with cytomix for 15 min. (−), nonstimulated control cells. The figure is representative of three experiments with consistent results.
Discussion
In chronic intestinal inflammation, continuous proinflammatory cytokine stimulation leads to NOS-2 upregulation and NO production, a possible risk factor for colorectal cancer (Seidelin and Nielsen, 2005). High levels of NO can damage DNA or inhibit repair (Jaiswal et al., 2000), enhance angiogenesis and favor tumor progression (Ambs et al., 1998; Hellmuth et al., 2004). We have shown that CORM-2 modulated NOS-2 gene expression and NO production, in Caco-2 cells stimulated with proinflammatory cytokines. Studies on the biological activity of CO have shown that the influence of this mediator on NOS-2 expression can be cell and stimulus-dependent. In a model of rat endotoxemia, CO has been shown to prevent the upregulation of NOS-2 in the lung while increasing NOS-2 expression in the liver (Sarady et al., 2004). Our data in Caco-2 cells are in line with previous studies reporting that the anti-inflammatory actions of CO may be related to NOS-2 downregulation in DLD-1 cells and animal models of intestinal inflammation (Dijkstra et al., 2004).
The expression of IL-8 correlates with mucosal inflammation in both ulcerative colitis and Crohn's disease (Daig et al., 1996). Increased production of IL-8 in the presence of bacteria or intestinal inflammation has been related to malignant transformation, cell growth, metastasis and angiogenesis in colon cancer cells (Mizukami et al., 2005). Several studies have revealed the expression of this proinflammatory chemokine in colorectal cancer tissues where it may act as an autocrine or paracrine growth factor. Conversely, the inhibition of IL-8 production can inhibit the proliferation of colon carcinoma cells (Li et al., 2001). We show in this study that IL-8 can be downregulated by the CO donor CORM-2. Given the persistent expression of this chemokine in intestinal inflammatory disorders and colon cancer, these results suggest that the inhibition of IL-8 production by CORM-2 may contribute to the protection of intestinal epithelial cells.
In addition to the participation in inflammatory responses, MMP-7 is a transcriptional target of the src oncogenic pathway in colonic cancer cells, which may contribute to tumorigenicity, cellular invasion and the metastatic potential of cancer cells (Witty et al., 1994; Yamamoto et al., 1995; Rivat et al., 2003). Apart from degradation of extracellular matrix and induction of angiogenic factors, MMP-7 may activate cytokines or growth factors to modify cell growth. Therefore, absence of MMP-7 may inhibit intestinal neoplasia in mice (Wilson et al., 1997) and would reduce the risk of progression from ulcerative colitis-related low-grade dysplasia to cancer (Newell et al., 2002). Our data demonstrate that CORM-2 downregulates MMP-7 expression during the inflammatory response elicited by cytokines in human enterocytes. These studies would support a role for CO in the regulation of MMPs, which is consistent with a recent report on the inhibition of MMP-1 and MMP-2 expression by CORM-2 in the human lung epithelial cell line A549 (Desmard et al., 2005).
Previous work has noted that MMP-7 expression is induced in inflamed human colon epithelia, but the cellular mechanisms responsible for this response are unclear (Newell et al., 2002). In intestinal tumors, MMP-7 expression is regulated by the PEA3 Ets transcription factor (Lynch et al., 2004), which could enhance transcriptional responses to both β-catenin and AP-1 (Crawford et al., 2001). We show that CORM-2 strongly inhibits the production of IL-6, a cytokine involved in the pathogenesis of chronic inflammatory conditions such as inflammatory bowel disease (Gross et al., 1992). The present data suggest that this IL-6 decrease may mediate at least in part the effects of CORM-2 on MMP-7 expression. Production of IL-6 after stimulation of Caco-2 cells by proinflammatory cytokines has been related to activation of NF-κB (Parikh et al., 1997), AP-1 (Hungness et al., 2000) and C/EBP (Hungness et al., 2002a). We found that CORM-2 inhibited the activation of these transcription factors, which could contribute to modulate IL-6 expression. Previous studies have reported that CO inhibited the production of IL-6 in murine macrophages and lung cells (Kim et al., 2005), and a murine model of shock via AP-1 inhibition (Morse et al., 2003).
Activation of NF-κB by cytokines correlates with the degree of mucosal inflammation in inflammatory bowel disease (Rogler et al., 1998) and plays a key role in NOS-2 and IL-8 induction (Nunokawa et al., 1996; Roebuck, 1999). Activation of this transcription factor has been observed in Crohn's disease and ulcerative colitis (reviewed in Jobin and Sartor, 2000), as well as in intestinal tumor development (Greten et al., 2004), human colon cancer cell lines and human colorectal cancer (Lind et al., 2001). Therefore, NF-κB is considered a therapeutic target for intestinal inflammatory diseases and cancer (Viatour et al., 2005).
Our data suggest that inhibition of NF-κB could be responsible for the anti-inflammatory effects of CORM-2 in human enterocytes. Similarly, CO inhalation inhibits NF-κB-binding ability and NOS-2 expression after tracheal transplantation in mice (Minamoto et al., 2005). In contrast, CO shows no effect on this transcription factor in activated endothelial cells (Soares et al., 2004) and the protection against hepatic ischemia/reperfusion injury by CO administration is not associated with inhibition of NF-κB signaling (Kaizu et al., 2005). In cytokine-stimulated Caco-2 cells, inhibition of NF-κB activation by CORM-2 can be dependent on inhibition of p65 nuclear translocation owing to increased stability of the p65-IκBα complex. IκBα undergoes phosphorylation by activation of IκB kinases, which are regulated by many upstream kinases (Karin, 1999; Jobin and Sartor, 2000). We have shown that CORM-2 inhibited-IκBα phosphorylation and subsequent degradation, most likely by interference with one or more of the protein kinases responsible for the phosphorylation of this protein.
Upon activation by stress and inflammatory cytokines, p38 translocates to the nucleus and regulates various transcription factors (Vanden Berghe et al., 1998). It is known that this MAPK may facilitate NF-κB-dependent gene transcription in Caco-2 cells (Garat and Arend, 2003) and may also regulate IL-8 expression independently of NF-κB. Although its precise role in intestinal inflammation is not known, attenuation of p38 may result in anti-inflammatory effects in conditions such as inflammatory bowel disease (Parhar et al., 2003). In addition, the control of JNK1/2 activation by CORM-2 may provide a basis for its inhibitory effects on AP-1 activation. The MAPK signalling pathway is also involved in the activation of the C/EBP family of transcription factors, in Caco-2 cells stimulated with IL-1β (Hungness et al., 2002b). On the other hand, p38 or ERK1/2 can play a role in the stabilization of IL-6 or IL-8 mRNA (Winzen et al., 1999; Choi et al., 2004). Therefore, inhibition of MAPK pathways and transcription factors by CORM-2 may offer several potential mechanisms to control the production of inflammatory mediators in human colonic epithelial cells.
The results reported here indicate that CORM-2 can prevent the increased production of important mediators during an inflammatory situation in colonic cells. Our data suggest that CORM-2 regulates MMP-7 expression induced by cytokines by inhibiting the IL-6 gene. The inhibitory effects of CORM-2 on inflammatory gene transcription may be the result of the regulation of NF-κB, AP-1 and C/EBP transcription factors. Our work also suggests that CORM-2 may exert chemopreventive effects on inflammation-associated colon carcinogenesis. Although additional studies are necessary to define the precise molecular mechanisms involved, the present results provide novel information about the regulation by CORM-2 of MMP-7 expression in colonic cells and the activity of nuclear factors that participate in the transcription of key genes in intestinal inflammation and cancer progression.
Acknowledgments
This work was supported by grants SAF2004-03835 (Ministerio de Educación y Ciencia-FEDER) and ACOMP06/023 (Generalitat Valenciana). J Megías thanks Spanish Ministerio de Educación y Ciencia for a fellowship.
Abbreviations
- AP-1
activator protein-1
- C/EBP
CCAT/enhancer binding protein
- CO-RMs
carbon monoxide-releasing molecules
- CT
cycle threshold
- DTT
dithiothreitol
- EMSA
electrophoretic mobility shift assay
- ERK1/2
extracellular signal-regulated kinase 1/2
- HO-1
heme oxygenase-1
- IL
interleukin
- IκBα
NF-κB, inhibitory protein-α
- JNK1/2
c-Jun N-terminal protein kinase 1/2
- MAPK
mitogen-activated protein kinase
- MMPs
matrix metalloproteinases
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-κB
- NOS-2
nitric oxide synthase-2
- siRNA
small interfering RNA
- TNFα
tumor necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Ambs S, Merriam WG, Ogunfusika MO, Bennett WP, Ishibe N, Hussain SP, et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med. 1998;4:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- Bani-Hani MG, Greenstein D, Mann BE, Green CJ, Motterlini R. Modulation of thrombin-induced neuroinflammation in BV-2 microglia by carbon monoxide-releasing molecule 3. J Pharmacol Exp Ther. 2006;318:1315–1322. doi: 10.1124/jpet.106.104729. [DOI] [PubMed] [Google Scholar]
- Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- Berberat PO, Rahim YI, Yamashita K, Warny MM, Csizmadia E, Robson SC, et al. Heme oxygenase-1-generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis. 2005;11:350–359. doi: 10.1097/01.mib.0000164017.06538.8a. [DOI] [PubMed] [Google Scholar]
- Bister V, Salmela MT, Heikkila P, Anttila A, Rintala R, Isaka K, et al. Matrilysins-1 and -2 (MMP-7 and -26) and metalloelastase (MMP-12), unlike MMP-19, are up-regulated in necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2005;40:60–66. doi: 10.1097/00005176-200501000-00011. [DOI] [PubMed] [Google Scholar]
- Busserolles J, Megias J, Terencio MC, Alcaraz MJ. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. Int J Biochem Cell Biol. 2006;38:1510–1517. doi: 10.1016/j.biocel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Choi EY, Kim EC, Oh HM, Kim S, Lee HJ, Cho EY, et al. Iron chelator triggers inflammatory signals in human intestinal epithelial cells: involvement of p38 and extracellular signal-regulated kinase signaling pathways. J Immunol. 2004;172:7069–7077. doi: 10.4049/jimmunol.172.11.7069. [DOI] [PubMed] [Google Scholar]
- Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Fingleton B, Gustavson MD, Kurpios N, Wagenaar RA, Hassell JA, et al. The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin-LEF-1 to activate matrilysin transcription in intestinal tumors. Mol Cell Biol. 2001;21:1370–1383. doi: 10.1128/MCB.21.4.1370-1383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daig R, Andus T, Aschenbrenner E, Falk W, Scholmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmard M, Amara N, Lanone S, Motterlini R, Boczkowski J. Carbon monoxide reduces the expression and activity of matrix metalloproteinases 1 and 2 in alveolar epithelial cells. Cell Mol Biol (Noisy-le-grand) 2005;51:403–408. [PubMed] [Google Scholar]
- Dijkstra G, Blokzijl H, Bok L, Homan M, van Goor H, Faber KN, et al. Opposite effect of oxidative stress on inducible nitric oxide synthase and haem oxygenase-1 expression in intestinal inflammation: anti-inflammatory effect of carbon monoxide. J Pathol. 2004;204:296–303. doi: 10.1002/path.1656. [DOI] [PubMed] [Google Scholar]
- Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, et al. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Shurey C, Ansari T, Sibbons P, Mann BE, Johnson TR, et al. Reviewing the use of carbon monoxide-releasing molecules (CO-RMs) in biology: implications in endotoxin-mediated vascular dysfunction. Cell Mol Biol (Noisy-le-grand) 2005;51:409–423. [PubMed] [Google Scholar]
- Garat C, Arend WP. Intracellular IL-1Ra type 1 inhibits IL-1-induced IL-6 and IL-8 production in Caco-2 intestinal epithelial cells through inhibition of p38 mitogen-activated protein kinase and NF-kappaB pathways. Cytokine. 2003;23:31–40. doi: 10.1016/s1043-4666(03)00182-0. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Gross SS, Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992;267:25722–25729. [PubMed] [Google Scholar]
- Gross V, Andus T, Caesar I, Roth M, Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992;102:514–519. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- Hellmuth M, Paulukat J, Ninic R, Pfeilschifter J, Muhl H. Nitric oxide differentially regulates pro- and anti-angiogenic markers in DLD-1 colon carcinoma cells. FEBS Lett. 2004;563:98–102. doi: 10.1016/S0014-5793(04)00275-3. [DOI] [PubMed] [Google Scholar]
- Hungness ES, Luo GJ, Pritts TA, Sun X, Robb BW, Hershko D, et al. Transcription factors C/EBP-beta and -delta regulate IL-6 production in IL-1beta-stimulated human enterocytes. J Cell Physiol. 2002a;192:64–70. doi: 10.1002/jcp.10116. [DOI] [PubMed] [Google Scholar]
- Hungness ES, Pritts TA, Luo GJ, Hershko DD, Robb BW, Hasselgren PO. IL-1beta activates C/EBP-beta and delta in human enterocytes through a mitogen-activated protein kinase signaling pathway. Int J Biochem Cell Biol. 2002b;34:382–395. doi: 10.1016/s1357-2725(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Hungness ES, Pritts TA, Luo GJ, Sun X, Penner CG, Hasselgren PO. The transcription factor activator protein-1 is activated and interleukin-6 production is increased in interleukin-1beta-stimulated human enterocytes. Shock. 2000;14:386–391. doi: 10.1097/00024382-200014030-00025. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, et al. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, et al. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229–235. doi: 10.1016/j.surg.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, et al. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- Li A, Varney ML, Singh RK. Expression of interleukin-8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, et al. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- López-Collazo E, Hortelano S, Rojas A, Boscá L. Triggering of peritoneal macrophages with IFN-α/β attenuates the expression of inducible nitric oxide synthase through a decrease in NF-kB activation. J Immunol. 1998;160:2889–2895. [PubMed] [Google Scholar]
- Lynch CC, Crawford HC, Matrisian LM, McDonnell S. Epidermal growth factor upregulates matrix metalloproteinase-7 expression through activation of PEA3 transcription factors. Int J Oncol. 2004;24:1565–1572. [PubMed] [Google Scholar]
- Maliner-Stratton MS, Klein RD, Udayakumar TS, Nagle RB, Bowden GT. Interleukin-1beta-induced promatrilysin expression is mediated by NFkappaB-regulated synthesis of interleukin-6 in the prostate carcinoma cell line, LNCaP. Neoplasia. 2001;3:509–520. doi: 10.1038/sj.neo.7900178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Adachi Y, Yamamoto H, Goto A, Arimura Y, Endo T, et al. The expression of matrix metalloproteinase matrilysin indicates the degree of inflammation in ulcerative colitis. J Gastroenterol. 2003;38:348–354. doi: 10.1007/s005350300062. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, et al. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- Minamoto K, Harada H, Lama VN, Fedarau MA, Pinsky DJ. Reciprocal regulation of airway rejection by the inducible gas-forming enzymes heme oxygenase and nitric oxide synthase. J Exp Med. 2005;202:283–294. doi: 10.1084/jem.20050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- Muhlbauer M, Allard B, Bosserhoff AK, Kiessling S, Herfarth H, Rogler G, et al. Differential effects of deoxycholic acid and taurodeoxycholic acid on NF-kappa B signal transduction and IL-8 gene expression in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1000–G1008. doi: 10.1152/ajpgi.00338.2003. [DOI] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Yoshikawa T. Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:177–184. doi: 10.1111/j.1365-2036.2004.01992.x. [DOI] [PubMed] [Google Scholar]
- Newell KJ, Matrisian LM, Driman DK. Matrilysin (matrix metalloproteinase-7) expression in ulcerative colitis-related tumorigenesis. Mol Carcinog. 2002;34:59–63. doi: 10.1002/mc.10049. [DOI] [PubMed] [Google Scholar]
- Nosho K, Yoshida M, Yamamoto H, Taniguchi H, Adachi Y, Mikami M, et al. Association of Ets-related transcriptional factor E1AF expression with overexpression of matrix metalloproteinases, COX-2 and iNOS in the early stage of colorectal carcinogenesis. Carcinogenesis. 2005;26:892–899. doi: 10.1093/carcin/bgi029. [DOI] [PubMed] [Google Scholar]
- Nunokawa Y, Oikawa S, Tanaka S. Human inducible nitric oxide synthase gene is transcriptionally regulated by nuclear factor-kappaB dependent mechanism. Biochem Biophys Res Commun. 1996;223:347–352. doi: 10.1006/bbrc.1996.0897. [DOI] [PubMed] [Google Scholar]
- Parhar K, Ray A, Steinbrecher U, Nelson C, Salh B. The p38 mitogen-activated protein kinase regulates interleukin-1beta-induced IL-8 expression via an effect on the IL-8 promoter in intestinal epithelial cells. Immunology. 2003;108:502–512. doi: 10.1046/j.1365-2567.2003.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh AA, Salzman AL, Kane CD, Fischer JE, Hasselgren PO. IL-6 production in human intestinal epithelial cells following stimulation with IL-1 beta is associated with activation of the transcription factor NF-kappa B. J Surg Res. 1997;69:139–144. doi: 10.1006/jsre.1997.5061. [DOI] [PubMed] [Google Scholar]
- Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, et al. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol. 2005;140:547–555. doi: 10.1111/j.1365-2249.2005.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C, Le Floch N, Sabbah M, Teyrol I, Redeuilh G, Bruyneel E, et al. Synergistic cooperation between the AP-1 and LEF-1 transcription factors in activation of the matrilysin promoter by the src oncogene: implications in cellular invasion. FASEB J. 2003;17:1721–1723. doi: 10.1096/fj.03-0132fje. [DOI] [PubMed] [Google Scholar]
- Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- Salmela MT, Pender SL, Karjalainen-Lindsberg ML, Puolakkainen P, MacDonald TT, Saarialho-Kere U. Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Scand J Gastroenterol. 2004;39:1095–1104. doi: 10.1080/00365520410003470. [DOI] [PubMed] [Google Scholar]
- Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, et al. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18:854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- Sawle P, Foresti R, Mann BE, Johnson TR, Green CJ, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidelin JB, Nielsen OH. Continuous cytokine exposure of colonic epithelial cells induces DNA damage. Eur J Gastroenterol Hepatol. 2005;17:363–369. doi: 10.1097/00042737-200503000-00017. [DOI] [PubMed] [Google Scholar]
- Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- Thilakawardhana S, Everett DM, Murdock PR, Dingwall C, Owen JS. Quantification of apolipoprotein E receptors in human brain-derived cell lines by real-time polymerase chain reaction. Neurobiol Aging. 2005;26:813–823. doi: 10.1016/j.neurobiolaging.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Turcanu V, Dhouib M, Poindron P. Nitric oxide synthase inhibition by haem oxygenase decreases macrophage nitric-oxide-dependent cytotoxicity: a negative feedback mechanism for the regulation of nitric oxide production. Res Immunol. 1998;149:741–744. doi: 10.1016/s0923-2494(99)80050-9. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, et al. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, et al. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586–G594. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- Wielockx B, Libert C, Wilson C. Matrilysin (matrix metalloproteinase-7): a new promising drug target in cancer and inflammation. Cytokine Growth Factor Rev. 2004;15:111–115. doi: 10.1016/j.cytogfr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, et al. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 1994;54:4805–4812. [PubMed] [Google Scholar]
- Yamamoto H, Itoh F, Hinoda Y, Imai K. Suppression of matrilysin inhibits colon cancer cell invasion in vitro. Int J Cancer. 1995;61:218–222. doi: 10.1002/ijc.2910610213. [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Geironson L, Al Soud WA, Ljungh S. Expression of matrix metalloprotease-2, -7 and -9 on human colon, liver and bile duct cell lines by enteric and gastric Helicobacter species. FEMS Immunol Med Microbiol. 2005;44:197–204. doi: 10.1016/j.femsim.2004.11.009. [DOI] [PubMed] [Google Scholar]