Abstract
Background and purpose:
Central 5-HT-containing pathways are known to be important in cardiovascular regulation and a crucial area for this regulation is the nucleus tractus solitarius (NTS), which contains many of the known 5-HT receptor subtypes. In this study the role of 5-HT1B and 5-HT1D receptors, targets for the antimigraine drugs known collectively as triptans, was examined in the NTS.
Experiment approach:
Extracellular recordings were made, in anaesthetized rats, from 109 NTS neurones that were excited by electrical stimulation of the vagus and drugs were applied ionophoretically to these neurones.
Key results:
The 5-HT1B/1D receptor agonist sumatriptan applied to 64 neurones produced a 64% reduction in the firing rate of 54 of these neurones. Ketanserin, a 5-HT1D/2A receptor antagonist, alone had little effect, but co-applied with sumatriptan significantly attenuated this inhibition, whilst co-application of the 5-HT1B receptor antagonist GR55562 resulted in potentiation of this inhibition. Sumatriptan also caused a 25% reduction in vagal afferent evoked activity as well as that caused by stimulation of cardiopulmonary afferents. In another 41 neurones the 5-HT1B receptor agonist CP-93 129 produced a doubling of the background firing rate in 31 of these neurones and a significant increase in both vagal afferent evoked activity and that evoked by cardiopulmonary afferent activation.
Conclusions and implications:
Activation of 5-HT1B and 5-HT1D receptors have opposing actions on NTS neurones of excitation and inhibition, respectively. As both receptors are negatively coupled to adenylate cyclase this would indicate that they have different anatomical locations within NTS.
Keywords: nucleus tractus solitarius, 5-hydroxytryptamine, serotonin, 5-HT1B receptors, 5-HT1D receptors, sumatriptan, CP-93 129, ketanserin, GR55562, vagal C-fibre afferents
Introduction
Central 5-HT1A, 5-HT3 and 5-HT7 receptors have been demonstrated to play a major role in the reflex regulation of the cardiovascular system (see Ramage, 2001; Jordan, 2005; Kellett et al., 2005a). Furthermore, depletion of central 5-HT causes an increase in resting blood pressure and attenuates the baroreceptor gain (Kellett et al., 2005b). One of the major brain sites involved in the regulation of the cardiovascular system is the nucleus tractus solitarius (NTS) (see Jordan and Spyer, 1986). This nucleus has been shown to contain many of the 14 identified 5-HT receptor subtypes: 5-HT1A and 5-HT1B (Manaker and Verderame, 1990; Thor et al., 1992; Castro et al., 1997; Longmore et al., 1997), 5-HT1D (Longmore et al., 1997), 5-HT1F (Castro et al., 1997), 5-HT2A and 5-HT2C (Pompeiano et al., 1994), 5-HT3 (Leslie et al., 1994; Miquel et al., 2002), 5-HT5A (Oliver et al., 2000) and 5-HT7 receptors (Gustafson et al., 1996). At present, there is evidence suggesting that 5-HT3 (Jeggo et al., 2005) and 5-HT7 (Kellett et al., 2004), but probably not 5-HT1A (Ramage and Mifflin, 1998), receptors in the NTS participate in the reflex pathways controlling cardiovascular reflexes. Furthermore, 5-HT2A/B and 5-HT2C receptors have also been found to affect NTS neurone activity and have opposing actions, excitation and inhibition, respectively (Sévoz-Couche et al., 2000). However, central blockade of 5-HT2 receptors does not seem to interfere with cardiovascular (Bogle et al., 1990) or airway (Bootle et al., 1996, 1998) reflexes mediated by the NTS. In addition, activation of central 5-HT2 receptors by DOI failed to affect airway reflexes (Bootle et al., 1998). Interestingly, the archetypical 5-HT1B/1D receptor agonist and antimigraine drug sumatriptan, acting centrally, has been shown to inhibit these cardiovascular (Dando et al., 1998) and airway (Bootle et al., 1998) reflexes. Thus, the present study was carried out to investigate the effects of 5-HT1B and 5-HT1D receptor agonists on neuronal activity in the NTS. Preliminary accounts of some of this work have been published (Wang et al., 1998; Jeggo et al., 2000).
Methods
Experiments were performed on 27 male Sprague–Dawley rats (280–350 g body weight) anaesthetized with pentobarbitone sodium (initially 60 mg kg−1, intraperitoneal (i.p.), supplemented when necessary with 10 mg kg−1, intravenous (i.v.)). These experiments were carried out under the Animals (Scientific Procedures) Act 1986. At the end of each experiment, all animals were humanely killed by an overdose of pentobarbitone sodium (i.v.). A tracheotomy was performed low in the neck, and a femoral artery and vein cannulated for measurement of arterial blood pressure and administration of supplemental anaesthetic and drugs. A silicone cannula filled with phenylbiguanide (PBG; 200 μg ml−1) was advanced through the right jugular vein until it lay within the right atrium. Tracheal and arterial pressures were measured with pressure transducers (model P23XL, Statham, Hato Rey, PR, USA). To record electrocardiograph (ECG), two leads attached to opposite limbs were connected to an amplifier (NL 104, Neurolog, Digitimer Ltd, Welwyn Garden City, Hertfordshire, UK; × 5k) and filter unit (NL125, Neurolog, 0.5–5.0 kHz). Animals were ventilated with oxygen-enriched room air using positive pressure ventilator (Harvard Rodent ventilator, Harvard Apparatus, Ltd, Edenbridge, Kent, UK, model 683). Arterial blood samples were taken regularly and blood gases and pH monitored with a pH/Blood gas analyser (model 238, Ciba Corning Diagnostics Ltd, Halstead, UK). Blood gases were maintained in the following ranges: PO2 155±5 mmHg, PCO2 37±1 mmHg, pH 7.35±0.01, by slow i.v. infusions of sodium bicarbonate (1.0 M) and/or adjustments of the respiratory pump. Rectal temperature was monitored and maintained at 37°C with a Harvard homeothermic blanket system (Harvard Apparatus, South Natick, MA, USA).
The rats were fixed in a stereotaxic frame and the right cervical vagus nerve was dissected free from the sympathetic trunk low in the neck using a dorsolateral approach. The nerve was then placed on bipolar silver wire electrodes for electrical stimulation (50–500 μA, 1 ms, 0.3–1 Hz) by an isolated stimulator (Digitimer DS2A) that was triggered by a programmer (Digitimer 4030). The exposed length of nerve was covered in paraffin wax and fixed in place with dental impression material (Super-Dent light body dental polyvinylsiloxane). To expose the dorsal surface of the caudal brainstem, the nuchal muscles were removed from the back of the neck, the occipital bone removed and the dura overlying the brainstem cut and reflected laterally. In some cases, during single-cell recordings, the animals were immobilized by administration of a neuromuscular blocker, either decamethonium bromide (3 mg kg−1, i.v.; initial dose followed by 3 mg kg−1 h−1, i.v.) or a single dose of α-bungarotoxin (140 μg kg−1, i.v.). The depth of anaesthesia was assessed during neuromuscular blockade by monitoring the stability of the arterial blood pressure and heart rate and the cardiovascular responses to pinching the paw.
Protocol
Extracellular recordings were made from neurones in the medial regions of the NTS (<1 mm lateral to midline) within 2 mm caudal to obex using five or seven barrelled microelectrodes made from borosilicate glass (Clarke Electromedical, Reading, UK) or compound electrodes constructed by gluing a single-barrelled glass recording electrode (tip diameter 1 μm) to a multibarrelled glass electrode (tip diameter 3–7 μm, Wang et al., 1998). The recording barrel contained NaCl (0.5–4 M), whereas the other barrels contained pontamine sky blue dye, the excitant amino acid DL-homocysteic acid (DLH) and a combination of ligands for 5-HT1B/1D-receptors. Neuronal recordings were amplified × 1000– × 5000 (Axoclamp 2A, Axon Instruments, CA, USA) and filtered (0.5–5.0 kHz; NL 125, Neurolog). NTS neurones were identified by their orthodromic response to electrical stimulation of the cervical vagus nerve at 2 × threshold for evoking activity (Wang et al., 1995; Jeggo et al., 2005). NTS neurones receiving non-myelinated vagal input mediate a diverse range of functions. In the present study, one functional subpopulation of these neurones was identified by their response to cardiopulmonary afferent stimulation induced by right atrial administration of PBG (12–24 μg kg−1, 60–120 μl kg−1; Kay and Armstrong, 1990). Ligands were applied to the vicinity of the recorded neurones by ionophoresis (Neurophore, Medical Systems, Digitimer Ltd). Between drug ejection periods a retaining current of 10–15 nA was applied to each drug barrel. When neuronal firing rate was steady, the effects of agonist and/or antagonist ligands given alone or in combination were tested. In all experiments, any possible current artefacts were minimized by using the automatic current balancing device available on the Neurophore system. In some experiments, recording sites were marked by ionophoretic ejection of pontamine sky blue dye. At the end of the experiment, the brainstem was removed and fixed in 10% formal saline. Frozen sections (50 μm) were cut and the location of the recording sites visualized and mapped onto standard sections of a rat brainstem (Paxinos and Watson, 1986).
Analysis of data
Arterial blood pressure, tracheal pressure, ECG and neuronal activity were recorded on videotape via a digital interface (Instrutech, VR100A, Digitimer Ltd) and/or on a PC hard disk accessed via an A-D interface (Cambridge Electronic Design (CED 1401plus, Science Park, Milton Road, Cambridge, UK). Off-line analysis of recorded data was made using Spike2 software (CED). Single unit activity was discriminated using a Spike Processor (D130, Digitimer Ltd) and displayed as a rate histogram. To investigate the effects of ligands on ongoing NTS neuronal activity, baseline and ligand-evoked neuronal firing rates (averaged over a 10–20 s period) were measured and compared. Peri-stimulus time histograms (PSTHs, 20 stimuli) were constructed to investigate the effect of ligands on the vagal-evoked response of NTS neurones. The total numbers of evoked spikes before and during ionophoretic application of the ligands were compared. The response of NTS neurones to cardiopulmonary afferent stimulation by PBG injected into the right atrium was analysed by counting the total number of spikes evoked from the beginning of the excitatory burst until activity returned to pre-injection level. Ligands were classed as evoking excitation or inhibition if activity increased or decreased, respectively, by more than 20% (Wang et al., 1995; Jeggo et al., 2005). All data are presented as mean±s.e.m. except where indicated. Comparisons between means were made with Student's paired t-test.
Drugs and solutions
The following drugs were freshly dissolved in 0.9% saline, except where stated, and their pH adjusted by addition of drops of either 0.1 M HCl or 0.1 M NaOH and administered by ionophoresis: DLH (100 mM, pH 8.5) from Sigma-Aldrich, Poole, Dorset; sumatriptan (20 mM, pH 4) was a gift from Glaxo SmithKline, Harlow; ketanserin (10 mM, pH 4), GR55562 (3-[3-(dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide; 10 mM, pH 4) and CP-93 129 (1,4-dihydro-3-[1,2,3,6-tetrahydrp-4-pyridinyl]-5H-pyrrol[3,2-b] pyridine-5-one dihydrochloride; 10 mM, pH 4) from Tocris Cookson Ltd. CP-93 129 was dissolved in 50 μl 1% ascorbic acid, then made up to 1 ml with 0.9% saline. Pontamine sky blue dye (20 mg ml−1; from BDH, Poole, Dorset) was dissolved in 0.5 M sodium acetate. The following drugs were also used; PBG and α-bungarotoxin, both from Sigma-Aldrich, Poole, Dorset.
Results
Identification of NTS neurones
NTS neurones (n=102) were identified by their orthodromic excitatory responses to electrical stimulation of the cervical vagus nerve (50–500 μA, 1 ms, 0.3–1 Hz). The latency of this input ranged from 24 to 45 ms (mean 31.7±0.43 ms), indicating that the conduction velocity was within the C-fibre range (0.9–1.7 m s−1, mean 1.3±0.02 m s−1). Eight of these neurones also received short latency inputs (4–9 ms, mean 5.8±0.57 m s−1), indicating that they also had a myelinated input (conduction velocities of 5.6–10 m s−1, mean 7.9±0.56 m s–1), whereas a further seven neurones received short latency input alone (4–7 ms, mean 5.9±0.46 ms; conduction velocity range 5.7–10 m s−1, mean 7.1±0.62 ms). In addition, 39 (38%) of those neurones receiving longer latency input had a very small variability in onset latency indicating that they are likely to include (but not exclusively) those receiving monosynaptic inputs, whereas the remainder of neurones (62%) with a greater variability of onset latency are more likely to be activated polysynaptically. Furthermore, 52 (51%) of all neurones recorded exhibited spontaneous activity (2.1±0.2 spikes s−1), a similar proportion to that seen in a previous study (Sévoz-Couche et al., 2000). In experiments, where the effects of 5-HT1B/1D receptor ligands were investigated, activity was in some cases induced by ionophoretic administration of low currents (2–15 nA) of the excitant amino acid DLH.
Effects of 5-HT1B/1D receptor ligands on baseline neuronal activity
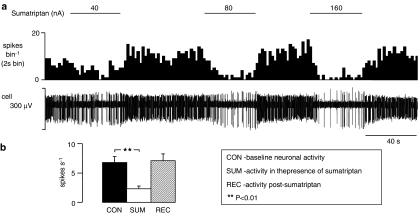
The 5-HT1B/1D receptor agonist sumatriptan (20–160 nA) significantly reduced baseline activity from a mean of 6.8±1.0 to 2.3±0.5 spikes s−1 in 84% (54/64) of neurones tested (Figure 1). Following removal of sumatriptan, activity recovered to 7.1±1.2 spikes s−1. In eight further neurones sumatriptan had no significant effect (5.3±0.8 vs 5.1±0.8 spikes s−1), whereas in only two of the 64 neurones it had an excitatory action (1.7–4.3 spikes s−1).
Figure 1.
Current-dependent inhibition of the activity of a NTS neurone, in anaesthetized rats, evoked by ionophoretic administration of sumatriptan. (a) The top trace shows a rate histogram (spikes bin−1) and the bottom trace raw data of extracellular recording of neuronal activity (cell). The bars show when sumatriptan was being applied at the currents stated. (b) Histograms of the combined data of NTS neuronal activity (n=54) showing means with vertical lines representing s.e.m. From left to right: baseline activity, activity evoked by ionophoretic administration of sumatriptan (20–160 nA), and recovery of activity. Comparison of neuronal activity alone and in the presence of sumatriptan was carried out using Student's paired t-test.
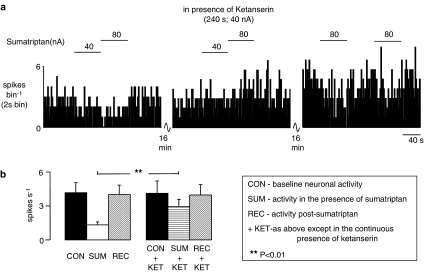
When administered alone, the 5-HT1D/2A receptor antagonist ketanserin (10–40 nA) was without effect on 43% (6/14) of the neurones tested (4.8±1.3–4.7±1.5 spikes s−1) and produced a non-significant decrease in activity in 50% (7/14) of neurones (4.8±1.8–2.5±0.8 spikes s−1). In only one neuron increased activity was seen (0.3–1.5 spikes s−1). However, during application of ketanserin, the inhibitory action of sumatriptan (4.1±0.8–1.2±0.3 spikes s−1) was significantly attenuated (3.9±1.0–2.8±0.6 spikes s−1) in 78% (11/14) of neurones tested (Figure 2b). Interestingly, in three of these 11 neurones the inhibition in baseline activity evoked by sumatriptan (2.2±0.4–1.1±0.2 spikes s−1) was reversed in the presence of ketanserin to a non-significant increase in activity (2.3±0.03–3.0±0.3 spikes s−1, Figure 2a).
Figure 2.
The effect of ionophoretic administration of ketanserin on the inhibitory effect evoked by ionophoretic administration of sumatriptan in anaesthetized rats. (a) The top trace shows a rate histogram (spikes bin−1). The bars show when sumatriptan was being applied at the currents stated in the absence and presence of ketanserin. (b) Histograms of the combined data of NTS neuronal activity showing means with vertical lines representing s.e.m. From left to right: baseline activity (n=11), activity evoked by ionophoretic administration of sumatriptan (20–160 nA; n=11), recovery (n=11), baseline activity in the presence of ketanserin (10–40 nA; n=11), activity evoked by sumatriptan in the presence of ketanserin (10–40 nA; n=11) and recovery (n=5). Comparison of inhibition caused by sumatriptan alone and in the presence of ketanserin was carried out using Student's paired t-test.
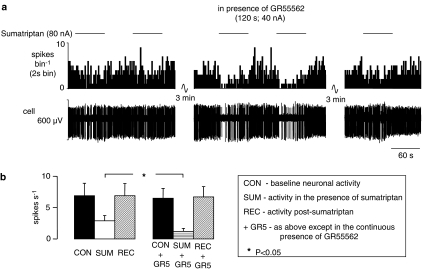
Furthermore, in 55% (6/11) of neurones tested, the inhibitory effects of sumatriptan (6.9±2.0–2.9±0.8 spikes s−1) were significantly increased in the presence of the 5-HT1B receptor antagonist GR55562 (6.5±1.6–1.2±0.5 spikes s−1; Figure 3) without any change in baseline activity. In the remaining neurones (45%; 5/11), the sumatriptan-evoked inhibition was unaffected by GR55562. GR55562 itself reduced baseline activity in one group of NTS neurones. In 63% (20/32) of neurones tested, activity decreased from 4.7±1.0 to 2.6±0.5 spikes s−1, whereas in the remaining neurones (37%; 12/32) activity was unaffected (4.8±1.2 vs 4.3±0.9 spikes s−1). In neurones that GR55562 had reduced baseline activity, activity was increased back to baseline level by applying DLH before repeating sumatriptan-evoked inhibition.
Figure 3.
The effect of ionophoretic administration of GR55562 on the inhibitory effect evoked by ionophoretic administration of sumatriptan in anaesthetized rats. (a) The top trace shows a rate histogram (spikes bin−1) and the bottom depicts the raw extracellular recording of neuronal activity (cell). The bars show when sumatriptan was being applied at the currents stated in the absence and presence of GR55562. (b) Histograms of the combined data of NTS neuronal activity showing means with vertical lines representing s.e.mean. From left to right: baseline activity (n=6), activity evoked by ionophoretic administration of sumatriptan (20–160 nA; n=6), recovery (n=6), baseline activity in the presence of GR55562 (10–80; n=6), activity evoked by sumatriptan in the presence of GR55562 (10–80 nA; n=6) and recovery (n=4). Comparison of inhibition caused by sumatriptan alone and in the presence of GR55562 was carried out using Student's paired t-test.
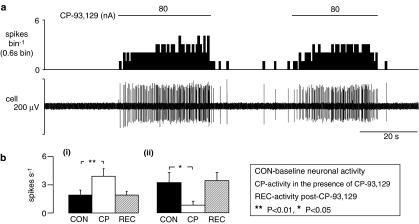
The 5-HT1B receptor agonist CP-93 129 had a predominantly excitatory effect on NTS neurones. In 76% (31/41) of neurones tested, CP-93 129 (40–160 nA) evoked a significant increase in baseline firing rate from 1.9±0.5 to 3.8±0.8 spikes s−1 (Figure 4). In 28 of these, recordings were maintained until recovery occurred (1.9±0.4 spikes s−1). In a further 12% (5/41) of neurones it reduced activity (3.2±1.1–0.8±0.4 spikes s−1; Figure 4b) and in the other 12% (5/41) it did not (4.4±1.3–4.6±1.3 spikes s−1). Although the differences in the mean baseline activity of these groups could indicate different populations of neurones, in actual fact neurones with background activity similar to and indeed higher than the activity of neurones in the inhibitory responding group can be found in the excitatory responding group.
Figure 4.
The effects evoked by ionophoretic administration of CP-93 129 on the activity of NTS neurones in anaesthetized rats. (a) The top trace shows a rate histogram (spikes bin−1) and the bottom depicts the raw extracellular recording of neuronal activity (cell). The bars show when CP-93 129 was being applied at the currents stated. (b) Histograms of the combined data of NTS neuronal activity (n = 41) showing means with vertical lines representing s.e.m. From left to right: baseline activity, activity evoked by ionophoretic administration of CP-93 129 (40–240 nA), and recovery of activity. (i) The combined data from 28 neurones that were excited whereas (ii) represents the combined data from five neurones that showed inhibition. Comparisons were carried out using Student's paired t-test.
Ionophoretic administration of saline at pH 4 (the vehicle for sumatriptan, ketanserin and GR55562) had no significant effect in 92% (11/12) of neurones tested (8.2±2.8–8.2±2.8 spikes s−1), with only one neurone showing an increase in activity of >20%. Similarly, 1% ascorbic acid at pH 4 (the vehicle for CP-93 129) had no significant effect on the baseline firing rate of the majority of neurones tested (5/6; 2.1±0.8–2.2±0.8 spikes s−1) with the activity of the only affected neurone being reduced.
Effects of 5-HT1B/1D receptor ligands on vagal afferent evoked responses
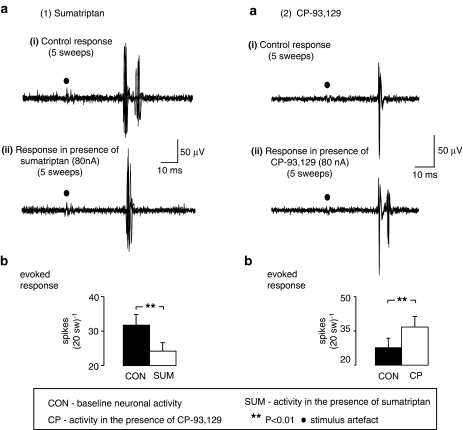
Ionophoretic application of sumatriptan (20–160 nA) decreased vagal afferent evoked activity (31.7±3.1–24.2±2.5 spikes 20 sweeps−1) in 87% (20/23) of neurones tested (Figure 5). In addition, it decreased cardiopulmonary afferent input (Figure 6) in two of the three neurones tested, the input of the remaining neurone being unaffected by sumatriptan. In contrast, ionophoretic application of CP-93 129 increased vagal afferent evoked activity (27.6±4.3–36.7±4.6 spikes 20sweeps−1) in 88% (22/25) of neurones tested (Figure 5) and increased the cardiopulmonary afferent (Figure 6) evoked input (4.7±1.3–16.0±4.0 spikes) in 60% (3/5) of neurones, with the input of the remaining two neurones unaffected.
Figure 5.
Effect of ionophoretic administration of (1) sumatriptan and (2) CP-93 129 on vagal afferent-evoked (electrically) activation of NTS neurones in anaesthetized rats. Both panels in (a) show two traces of five superimposed traces of vagal afferent electrically (100 μA, 1 ms) evoked activity in a NTS neurone in the (i) presence and (ii) absence of ionophorectically applied sumatriptan or CP-93 129 at the currents indicated. Both panels in (b) show histograms of the mean data of vagal afferent-evoked activity with vertical lines representing s.e.mean. From left to right: control evoked activity which is mean response per 20 sweeps and evoked activity during the ionophoretic administration of either sumatriptan (30–60 nA; n=20) or CP-93 129 (40–160 nA; n=22). Comparisons were carried out using Student's paired t-test.
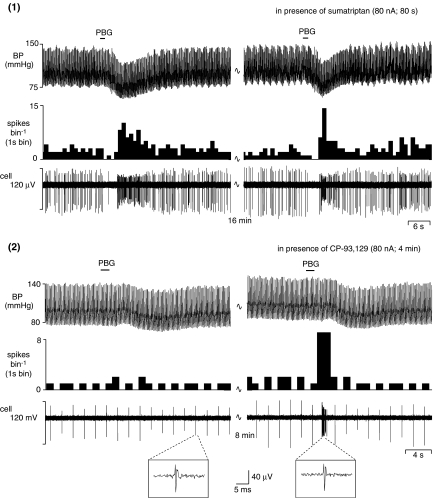
Figure 6.
Traces showing the effects of ionophoretically applied (1) sumatriptan and (2) CP-93 129 to the vicinity of a NTS neurone (in anaesthetized rats), that had been excited by cardiopulmonary afferent activation (induced by injecting PBG (18 μg kg−1; 30 μl) into the right atrium) on blood pressure (BP), continuous rate histogram of neuronal activity (spikes bin−1) and a raw recording of neuronal activity. In (2) it should be noted that the background activity is vagal evoked activity (0.5 Hz) and demonstrates the stability of the recording of the neurone over time. Cell identity between panels is confirmed by the expanded sections.
Discussion
The results for the present experiments demonstrate that in anaesthetized rats the activity of NTS neurones receiving input from vagal afferents is reduced by ionophoretic application of the 5-HT1B/1D receptor ligand sumatriptan, which has a pKD at the r5-HT1B receptor of 6.74 and at 5-HT1D receptors of 7.95 (Bruinvels et al., 1993). Conversely, ionophoretic administration of the selective 5-HT1B receptor agonist CP-93 129 (Macor et al., 1990), which has a pKD at 5-HT1D receptors of 8.2 compared with 6.2 at the r5-HT1B (Bruinvels et al., 1993) increased the activity of these neurones. Further, in the presence of ketanserin, which is a highly selective 5-HT1D receptor antagonist (pKi of 7) compared with pKi of <5 at the h5-HT1B receptor (Kaumann et al., 1994), the inhibitory effects of sumatriptan were found to be reduced and in some cases reversed. It should be noted that ketanserin is also a 5-HT2A (Bonhaus et al., 1995), an α1-adrenoceptor and a H1 receptor antagonist (Leysen et al., 1985). These data support the view that sumatriptan causes inhibition of neuronal activity by activating 5-HT1D receptors and that this inhibitory effect is counteracted by the activation of excitatory 5-HT1B receptors by sumatriptan. This interpretation is further supported by the observation that in the presence of GR55562, a h5-HT1B receptor antagonist (pKi of 8.8, whereas at 5-HT1D receptors it has a pKi of 6.2; Longmore et al., 1997), the sumatriptan-evoked inhibition was found to be potentiated in just over 50% of the neurones tested. It should be noted that some of these effects of sumatriptan could also be mediated by activation of 5-HT1F receptors (Adham et al., 1993). However, at present, there are no available selective antagonists for this receptor subtype and thus this possibility cannot be investigated.
The ability of sumatriptan to inhibit baseline and the vagal activation of NTS neurones is consistent with observations that it attenuates cardiovascular (Dando et al., 1998) and airway (Bootle et al., 1998) reflexes that are mediated by vagal afferents. This can occur both centrally and at the level of sympathetic ganglia (Jones et al., 1995). Furthermore, the excitation of NTS neurones evoked by stimulation of Aδ afferents from the superior sagittal sinus has also been found to be inhibited by the ionophoretic application of 5-HT1B/1D receptor agonists (Hoskin et al., 2004), as had previously been shown for trigeminal neurones (Storer and Goadsby, 1997). The inhibition of trigeminal neurones is also mediated by 5-HT1D receptors (Travagli and Williams, 1996; Jennings et al., 2004). However, the physiological role of these receptors is not clear. Although ketanserin had a strong tendency to reduce ongoing activity this probably reflects an action at other receptor subtypes (see above) rather than 5-HT1D receptors, especially as the present data suggest that blockade of tonically activated 5-HT1D receptors induces excitation. Thus, overall the present antagonist data suggest that in the ‘resting' state, at least in anaesthetized animals, these 5-HT1D receptors in the NTS are not tonically activated. Thus, the question arises as to their physiological function. However, the selective 5-HT1B/1D receptor antagonist GR127935 (Skingle et al., 1996), given i.c., has been found to have a tendency to potentiate the cardiovascular responses evoked by stimulation of the upper airways (Dando et al., 1998). This at least suggests that 5-HT1D receptors may have some physiological role and the variable results observed with GR127935 would be consistent with its ability to block both receptor subtypes. This also indicates that 5-HT1B receptors may receive a tonic input. However, further experiments need to be carried out to determine if these 5-HT receptor subtypes have a physiological role at the level of the NTS.
The present data, indicating that 5-HT1B and 5-HT1D receptors have opposing actions on NTS neurones, are somewhat surprising as both receptors inhibit cyclic AMP formation. This would suggest that within the NTS these receptors are not located at the same site. In this respect, 5-HT1D receptors have been located on the central terminals of trigeminal primary afferents fibres of rat (Potrebic et al., 2003) and human (Smith et al., 2002) and on afferents in the human solitary tract and nucleus (Longmore et al., 1997). Thus, if 5-HT1D receptors are located on afferent terminals in the NTS, they would act to inhibit the release of glutamate, as indicated by the ability of non-NMDA glutamate antagonists to attenuate monosynaptic afferent excitation of NTS neurones (Zhang and Mifflin, 1988), from such terminals. For 5-HT1B receptors, the overall evidence indicates that they can act as auto- and heteroreceptors causing excitation or inhibition (see Sari, 2004). As these receptors are negatively coupled to adenylate cyclase, it is likely that at the level of the NTS they inhibit a tonic inhibitory, presumably GABAergic, input onto these neurones similar to 5-HT1B receptors in the substantia nigra (Stanford and Lacey, 1996). Thus, they facilitate vagal input, as indicated in the present experiments.
In conclusion, the results from the present study demonstrate that yet another set of 5-HT receptors can influence activity of NTS neurones. However, the role that these receptors play in the control of cardiovascular and airway reflexes by central 5-HT-containing neurones remains to be determined. The present data also indicate that the administration of triptans to treat migraine could affect cardiovascular reflexes. Therefore, some of the unexpected cardiovascular effects observed with these drugs could be due to an action within the NTS.
Acknowledgments
RDJ was supported by a British Heart Foundation PhD Studentship and YW was supported by the Wellcome Trust (grant 050894/z). We are also grateful for the technical assistance of Mr S Wilkinson.
Abbreviations
- 5-HT
5-hydroxytryptamine
- CON
baseline activity
- CP
CP–93129
- DLH
DL-homocysteic acid
- GR5
GR55562
- KET
ketanserin
- NTS
nucleus tractus solitarius
- PBG
Phenylbiguanide
- PSTHs
Peri-stimulus time histograms
- REC
recovery
- SUM
sumatriptan
Conflict of interest
The authors state no conflict of interest.
References
- Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle RG, Pires JGP, Ramage AG. Evidence that central 5-HT1A receptors play a role in the von Bezold–Jarisch reflex in the rat. Br J Pharmacol. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. Involvement of central 5-HT1A receptors in the reflex activation of pulmonary vagal motoneurones by inhaled capsaicin in anaesthetized cats. Br J Pharmacol. 1996;117:724–728. doi: 10.1111/j.1476-5381.1996.tb15250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. The role of central 5-HT receptors in the bronchoconstriction evoked by inhaled capsaicin in anaesthetised guinea-pigs. Neuropharmacol. 1998;37:243–250. doi: 10.1016/s0028-3908(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localization of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- Castro ME, Pascual J, Roman T, del Arco C, del Olmo E, Pazos A. Differential distribution of [3H]sumatriptan binding sites (5-HT1B, 5-HT1D and 5-HT1F receptors) in human brain: focus on brainstem and spinal cord. Neuropharmacol. 1997;36:535–542. doi: 10.1016/s0028-3908(97)00061-0. [DOI] [PubMed] [Google Scholar]
- Dando SB, Skinner MR, Jordan D, Ramage AG. Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br J Pharmacol. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br J Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin KL, Lambert GA, Donaldson C, Zagami AS. The 5-hydroxytryptamine1B/1D/1F receptor agonists eletriptan and naratriptan inhibit trigeminovascular input to the nucleus tractus solitarius in the cat. Brain Res. 2004;998:91–99. doi: 10.1016/j.brainres.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Jeggo RD, Kellett DO, Wang Y, Ramage AG, Jordan D. The role of central 5-HT3 receptors in cardiopulmonary reflex inputs to neurones in the nucleus tractus solitarius of anaesthetised rats. J Physiol. 2005;566:939–953. doi: 10.1113/jphysiol.2005.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. The effects of 5-HT1B/1D/1F receptor ligands on the activity of nucleus tractus solitarius (NTS) neurones in anaesthetized rats: an in vivo ionophorectic study. Br J Pharmacol. 2000;129:63P. [Google Scholar]
- Jennings EA, Ryan RM, Christie MJ. Effects of sumatriptan on rat medullary dorsal horn neurons. Pain. 2004;111:30–37. doi: 10.1016/j.pain.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Jones JFX, Martin GR, Ramage AG. Evidence that 5-HT1D receptors mediate inhibition of sympathetic ganglionic transmission in anaesthetized cats. Br J Pharmacol. 1995;116:1715–1717. doi: 10.1111/j.1476-5381.1995.tb16651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: Central serotonergic (5-HT) mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Jordan D, Spyer KM. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Frenken M, Posival H, Brown AM. Variable participation of 5-HT1-like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary arteries. 5-HT1-like receptors resemble cloned 5-HT10 β receptors. Circulation. 1994;90:1141–1153. doi: 10.1161/01.cir.90.3.1141. [DOI] [PubMed] [Google Scholar]
- Kay IS, Armstrong DJ. Phenylbiguanide not phenyldiguanide is used to evoke the pulmonary chemoreflex in anaesthetized rabbits. Exp Physiol. 1990;75:383–389. doi: 10.1113/expphysiol.1990.sp003413. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Excitation of rat nucleus tractus solitarius (NTS) neurones by vagal afferents involves central 5-HT7 and AMPA receptors. J Physiol. 2004;560P:C10. [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Central 5-HT7 receptors are critical for the reflex activation of cardiac vagal drive in anaesthetised rats. J Physiol. 2005a;563:319–331. doi: 10.1113/jphysiol.2004.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett DO, Stanford SC, Machado BH, Jordan D, Ramage AG. Effect of 5-HT depletion on cardiovascular vagal reflex sensitivity in awake and anesthetized rats. Brain Res. 2005b;1054:61–72. doi: 10.1016/j.brainres.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27:600–611. [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJM, Newberry NR.Localization of 5-HT3 receptors 5-Hydroxytryptamine-3 Receptor Antagonists 1994CRC Press: Ann Arbor, MI; 79–96.In: King FD, Jones BJ, Sanger GJ (eds) [Google Scholar]
- Longmore J, Shaw D, Smith D, Hopkins R, McAllister G, Pickard JD, et al. Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- Macor JE, Burkhart CA, Heym JH, Ives JL, Lebel LA, Newman ME, et al. 3-(1,2,5,6-Tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl)indole. J Med Chem. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, et al. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJ. Localization of 5-HT5A receptor-like immunoreactivity in the rat brain. Brain Res. 2000;867:131–142. doi: 10.1016/s0006-8993(00)02273-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 1986Academic Press Inc: San Diego; 2nd ed [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosc. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Mifflin SW. Vagal-evoked excitation of a sub-population of neurones in the nucleus of the solitary tract (NTS) involves 5-HT3 receptors in the anaesthetized rat. J Physiol. 1998;509:129P. [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sévoz-Couche C, Spyer KM, Jordan D. In vivo modulation of vagal-identified dorsal medullary neurones by activation of different 5-Hydroxytryptamine2 receptors in rats. Br J Pharmacol. 2000;131:1445–1453. doi: 10.1038/sj.bjp.0703722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skingle M, Beattie DT, Scopes DI, Starkey SJ, Connor HE, Feniuk W, et al. GR127935: a potent and selective 5-HT1D receptor antagonist. Behav Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- Smith D, Hill RG, Edvinsson L, Longmore J. An immunocytochemical investigation of human trigeminal nucleus caudalis: CGRP, substance P and 5-HT1D-receptor immunoreactivities are expressed by trigeminal sensory fibres. Cephalalgia. 2002;22:424–431. doi: 10.1046/j.1468-2982.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer RJ, Goadsby PJ. Microiontophoretic application of serotonin 5-HT1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997;120:2171–2177. doi: 10.1093/brain/120.12.2171. [DOI] [PubMed] [Google Scholar]
- Thor KB, Blitz-Siebert A, Helke CJ. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse. 1992;10:217–227. doi: 10.1002/syn.890100305. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Williams JT. Endogenous monoamines inhibit glutamate transmission in the spinal trigeminal nucleus of the guinea-pig. J Physiol. 1996;491:177–185. doi: 10.1113/jphysiol.1996.sp021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jones JFX, Ramage AG, Jordan D. Effects of 5-HT and 5-HT1A receptor agonists and antagonists on dorsal vagal preganglionic neurones in anaesthetized rats: An ionophoretic study. Br J Pharmacol. 1995;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sévoz-Couche C, Jordan D, Ramage AG. Activation of 5-HT1B and 5-HT1D receptors modulates the excitatory vagal afferent input to nucleus tractus solitarius (NTS) neurones in anaesthetized rats. J Physiol. 1998;513:78P. [Google Scholar]
- Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol. 1988;511:733–745. doi: 10.1111/j.1469-7793.1998.733bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]