Abstract
Background and Purpose:
Mesalamine is the first-line therapy for colitis, but it lacks potency and is only effective for mild-to-moderate forms of this disease. Hydrogen sulphide has been shown to be a potent, endogenous anti-inflammatory substance, modulating leukocyte-endothelial adhesion and leukocyte migration. The purpose of this study was to determine if an H2S-releasing derivative of mesalamine (ATB-429) would exhibit increased potency and effectiveness in a mouse model of colitis.
Experimental Approach:
Colitis was induced in mice with trinitrobenzene sulphonic acid and the effects of ATB-429 and mesalamine were compared in several treatment regimens. The severity of colitis was determined using several indices, including a disease activity score (comprised of scores for diarrhea, weight loss and fecal blood), colonic myeloperoxidase activity and macroscopic/microscopic scoring of tissue injury.
Key Results:
Irrespective of the treatment regiment, ATB-429 was more effective than mesalamine in reducing the severity of colitis. ATB-429 was particularly effective in reducing granulocyte infiltration into the colonic tissue (by ∼70%), as well as reducing the expression of mRNA for several key proinflammatory cytokines/chemokines (e.g., TNFα, IFNγ). Treatment with ADT-OH, the H2S-releasing moiety of ATB-429, did not affect severity of colitis.
Conclusions and Implications:
ATB-429 exhibits a marked increase in anti-inflammatory activity and potency in a murine model of colitis, as compared to mesalamine. These results are consistent with recently described anti-inflammatory effects of H2S. ATB-429 may represent an attractive alternative to mesalamine for the treatment of inflammatory bowel disease.
Keywords: inflammatory bowel disease, colitis, hydrogen sulphide, mesalamine, inflammation, neutrophil
Introduction
Hydrogen sulfide is increasingly recognized as an important mediator of a number of physiological processes, including vasodilation and neuromodulation (Kimura, 2002; Wang, 2002; Fiorucci et al., 2006). In recent years, several papers have suggested that H2S plays important roles in immune and inflammatory reactions. As is the case with another gaseous mediator, nitric oxide, H2S may exhibit apparently contradictory actions depending on its concentration and the circumstances in which it is generated. For example, recent work suggests that H2S plays a role in protecting gastric mucosal tissue from injury (Fiorucci et al., 2005) and exerts anti-inflammatory actions (Mariggio et al., 1998; Zanardo et al., 2006). On the other hand, the results of some studies point to a contribution of H2S to tissue injury and inflammation (Bhatia et al., 2005; Collin et al., 2005; Zhang et al., 2006).
Based on our findings that H2S is a potent inhibitor of leukocyte adherence to the vascular endothelium (Zanardo et al., 2006), and exerted analgesic activity in a visceral pain model (Distrutti et al., 2006a), we began to investigate the possibility that H2S might be used to enhance the anti-inflammatory properties of certain drugs. Indeed, we recently reported that a derivative of a nonsteroidal anti-inflammatory drug into which we had incorporated an H2S-releasing moiety resulted in an enhancement of anti-inflammatory activity, as well as a profound reduction in gastrointestinal toxicity (Wallace et al., 2007). If H2S does exert beneficial effects in terms of mucosal defence, as well as anti-inflammatory effects, it may have utility in the treatment of inflammatory bowel disease (IBD; Crohn's disease and ulcerative colitis). IBD is a chronic disorder characterized by extensive ulceration and inflammation. The first-line therapy for IBD is mesalamine (5-aminosalicylic acid), the mechanism of action of which is still not well understood (Hanauer, 2006). Mesalamine is effective in inducing remission of mild-to-moderate IBD, but is not very effective in treating more severe IBD. There is a need for more effective and safe alternatives to mesalamine for treatment of IBD (Korzenik and Podolsky, 2006). Other options for therapy, such as glucocorticoids, immunosuppressants and anti-tumor necrosis factor-α antibodies, while often effective, can carry significant risks of adverse effects (sometimes severe) and can be very expensive.
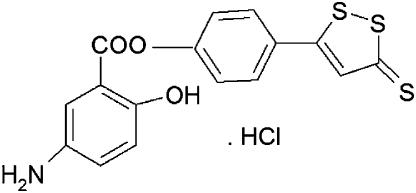
In the present study, we have used the trinitrobenzene sulfonic acid (TNBS) model of colitis in mice to test the hypothesis that an H2S-releasing derivative of mesalamine (5-amino-2-hydroxy-benzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3-yl)-phenyl ester hydrochloride (ATB-429); Figure 1) would exhibit enhanced anti-inflammatory activity relative to mesalamine itself. This comparison was made under several different treatment paradigms and with a range of doses of each drug. The TNBS model is very well characterized, exhibits responsiveness to various therapies similar to those of human IBD and shares many features with IBD in humans, particularly Crohn's colitis (Morris et al., 1989; Wallace et al., 1989; Fiorucci et al., 2002a, 2002b).
Figure 1.
Chemical structure of ATB-429.
Materials and methods
Animals
Six- to eight-week-old female Balb/c mice were obtained from Charles River (Monza, Italy). The mice were fed a standard chow pellet diet, had free access to water and were maintained on a 12-h light/dark cycle. All procedures in this study were approved by the animal care committee at the University of Perugia.
Treatment protocols
Colitis was induced by intrarectal administration of 0.5 mg of TNBS in 0.1 ml of 30% ethanol (Fiorucci et al. 2004), which is modified from that originally described by Morris et al. (1989). The mice were randomized to the various treatment groups (n=5–7 per group). Three treatment protocols were used. In the first, oral treatment with vehicle (1% carboxymethylcellulose), mesalamine (25, 50 or 75 mg kg−1) or the same doses of ATB-429 was initiated 1 h after TNBS administration and continued every 12 h for 7 days. The mice were evaluated, without knowledge of their treatment, on the final day of the study for the presence of diarrhea and fecal occult blood and their body weights were measured. A ‘disease activity score' was calculated based on these data (0–4 scale; Cooper et al., 1993). After killing the mice, a sample of the colon was excised for measurement of myeloperoxidase (MPO) activity, as a marker of granulocyte infiltration.
The second treatment protocol involved oral administration of vehicle, mesalamine (50 mg kg−1) or various doses of ATB-429 (33–130 mg kg−1) in order to determine the magnitude of any increase in potency of the latter. Treatment was initiated 1 day after induction of colitis and was continued every 12 h for 4 days. In this experiment, the disease activity score was determined each day and colonic MPO activity was assessed on the final day. In this study, we also examined the effects of the H2S-releasing moiety of ATB-429, 5-(p-hydroxyphenyl)-1,2-dithione-3-thione (ADT-OH), at a dose equimolar to that of mesalamine. This substance has previously been shown to release H2S spontaneously when incubated with homogenized liver (Distrutti et al., 2006b).
In the third treatment protocol, mice received vehicle, mesalamine (50 mg kg−1) or ATB-429 (50 mg kg−1; 38% of the molar equivalent dose of mesalamine) orally beginning 4 days after TNBS administration and continuing every 12 h for 7 days. The mice were killed with an overdose of halothane 2 h after the final administration of the test drug or vehicle. The severity of colitis was scored, as described above, and, in addition, the extent of mucosal injury was assessed. This involved both macroscopic and microscopic scoring, again without knowledge of the treatment. Colons were examined with a dissecting microscope (magnification, × 5) and graded for macroscopic lesions on a scale from 0 to 10 based on the criteria for inflammation and injury, such as hyperemia, thickening of the bowel and the extent of ulceration (Wallace et al., 1989). For histological scoring, specimens of colon taken 2 cm proximal to the anus were fixed in 10% buffered formalin phosphate, embedded in paraffin, sectioned and stained with hematoxylin and eosin. Coded slides were evaluated under a light microscope and inflammation was graded on a 0–4 scale according to the following criteria: 0, no inflammation; 1, low levels of leukocyte infiltration without ulceration; 2, moderate levels of leukocyte infiltration and epithelial injury; 3, high levels of leukocyte infiltration and vascular density, colon wall thickening; 4, transmural infiltration, loss of goblet cells, high vascular density, colon wall thickening (Fiorucci et al., 2002a, 2002b).
Samples of colonic tissue were also excised for determination of expression of mRNA for a number of cytokines/chemokines: tumor necrosis factor (TNF)-α, interferon gamma (IFN)-γ, interleukin (IL)-1, IL-2, IL-10, IL-12 p40 and regulated on activation normal T cell expressed and secreted (RANTES) (Wallace et al., 1999). Briefly, reverse transcription-polymerase chain reaction was used to detect and quantify mRNA of the particular cytokine/chemokines. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the ‘housekeeping gene' for mRNA expression, as an internal control. For each sample, the ratio of the amplification of the target gene to the amplification of GAPDH (expression of each is measured by performing densitometry on gels) was obtained. Comparisons were then made between the relative amplification (expression) of the target gene in tissues for the treatment groups in comparison to the expression in tissues from healthy controls.
Statistical analysis
All data are presented as the mean±s.e.m. Comparisons among groups of data were made using a one-way analysis of variance followed by the Dunnett's multiple comparison test. An associated probability (P-value) of less than 5% was considered significant.
Materials
TNBS was obtained from Fluka Chimica (Buchs, Switzerland). Kits for measurement of MPO activity were obtained from CytoStore (Calgary, Canada). ATB-429 and ADT-OH (5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione) were synthesized by Antibe Therapeutics Inc. (Toronto, Canada). All other reagents were obtained from Sigma Chemical Co. (St Louis, MO, USA) or Fisher Scientific (Edmonton, Canada).
Results
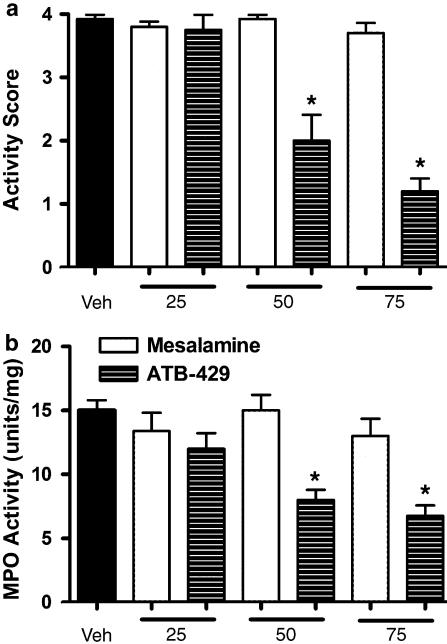
Intracolonic administration of TNBS to mice resulted in the development of extensive mucosal injury and transmural inflammation, as described previously (Morris et al., 1989; Fiorucci et al., 2004). The mice exhibited loss of body weight, diarrhea and blood in the feces, which is reflected by the ‘disease activity score' approaching the maximum of 4 (Figure 2a). Colonic MPO activity in mice with colitis (Figure 2b) was elevated significantly (∼4-fold) above that in samples from healthy controls (3.9±0.6 U mg−1). Treatment with mesalamine (25–75 mg kg−1), beginning 1 h after TNBS administration and continuing every 12 h thereafter for 7 days, did not significantly change the severity of colitis relative to mice treated with the vehicle. Both the disease activity score and colonic MPO activity were unchanged from those in vehicle-treated mice. In contrast, ATB-429 significantly reduced the disease activity score and MPO activity at doses of 50 and 75 mg kg−1.
Figure 2.
Effects of ATB-429 versus mesalamine on disease activity score (a) and colonic MPO activity (b) in TNBS-induced colitis in mice. The mice were treated twice daily for 1 week with the test drugs at doses of 25, 50 or 75 mg kg−1. The data shown were collected on the final day of the study. *P<0.05 versus the vehicle-treated group. Each group consisted of 5–7 mice.
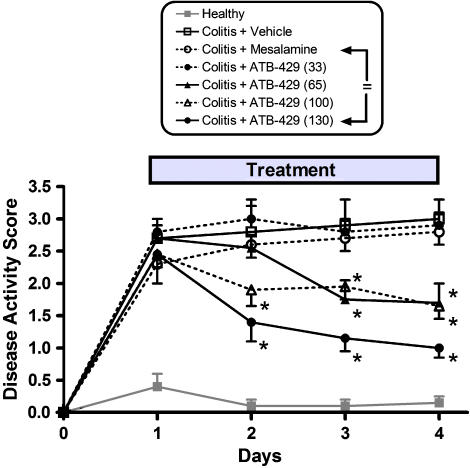
The beneficial effects of ATB-429 could be observed as early as 1 day after initiation of treatment, as shown in Figure 3. Although mesalamine had no significant effect at a dose of 50 mg kg−1, ATB-429 significantly reduced the disease activity score at doses of 130 (equimolar to the mesalamine dose), 100 and 65 mg kg−1. At a dose of 33 mg kg−1, ATB-429 was ineffective. ATB-429 also significantly reduced colonic MPO activity (Figure 4). At doses of 65–130 mg kg−1, MPO activity was reduced to the levels of healthy controls, whereas mesalamine (50 mg kg−1) and the lowest dose of ATB-429 (33 mg kg−1) had no significant effect.
Figure 3.
Effects of ATB-429 versus mesalamine on disease activity score over a 4-day period of twice daily administration of these drugs or vehicle. Colitis was induced on day 0 and drug or vehicle treatment was started on day 1. The disease activity score was assessed each day, without knowledge of the treatment. Mesalamine did not significantly affect the disease activity score, versus vehicle, at any time in the study. ATB-429 at a dose of 33 mg kg−1 also had no significant effect. However, at doses of 66, 100 and 130 mg kg−1, ATB-429 significantly reduced the disease activity score (P<0.05). With each of these doses, a significant effect was observed after the first day of treatment, and thereafter. The 130 mg kg−1 dose of ATB-429 is equimolar to the dose of mesalamine used (50 mg kg−1).
Figure 4.
Effects of ATB-429 versus mesalamine on colonic MPO activity at the end of a 4-day period of twice daily administration of these drugs or vehicle. Colitis was induced on day 0 and drug or vehicle treatment was started on day 1. Samples of distal colon were excised at the end of the experiment for measurement of MPO activity. *P<0.05 versus the vehicle-treated group.
In order to determine if the H2S-releasing moiety of ATB-429 could, by itself, reduce the severity of colitis in mice, we examined the effects of twice daily treatment with ADT-OH versus vehicle, mesalamine and ATB-429 (equimolar doses; n=5–7 per group). Treatment was initiated 1 day after TNBS administration and continued for 3 days. The disease activity score in mice treated with ATB-429 (1.1±0.2) was significantly reduced as compared to vehicle-treated mice (3.0±0.3). Neither mesalamine nor ADT-OH significantly affected the disease activity score (2.9±0.2 and 2.7±0.3, respectively) relative to the vehicle-treated group. The same pattern of results was seen with colonic MPO activity (in U mg−1: vehicle, 10.1±2.3; mesalamine, 8.2±2.4; ADT-OH, 9.7±3.0; ATB-429, 4.8±1. 8, P<0.05 versus vehicle).
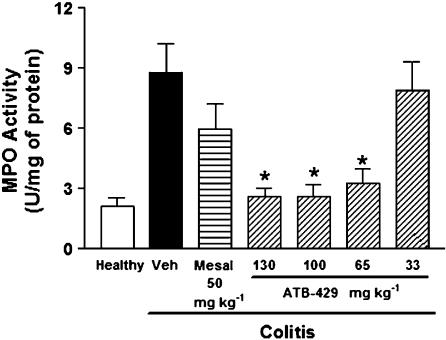
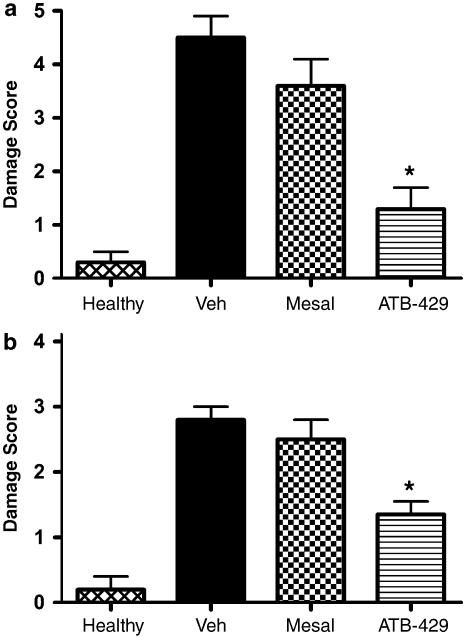
A significant attenuation of the severity of colitis was also observed when treatment with ATB-429 was initiated at a time when colitis was well established (4 days after TNBS administration). Twice daily administration of ATB-429 (65 mg kg−1) resulted in a significant reduction of mucosal damage, as measured by macroscopic (Figure 5a) and histological (Figure 5b) scoring. In contrast, mesalamine (50 mg kg−1) had no significant effect on either measure of mucosal injury. Note that on a molar basis, the dose of ATB-429 represents only 38% of the dose of mesalamine.
Figure 5.
Macroscopic (a) and histological (b) damage scores for mice with colitis (and healthy controls) treated for 1 week with vehicle, mesalamine (50 mg kg−1) or ATB-429 (50 mg kg−1). Twice daily treatment was initiated 4 days after induction of colitis. *P<0.05 versus the vehicle-treated group.
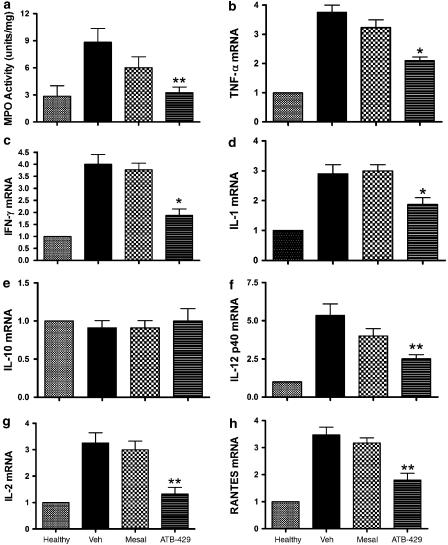
Colonic MPO activity was also significantly reduced by ATB-429, but not by mesalamine (Figure 6a). The expression of a number of cytokines/chemokines was significantly elevated in colonic tissue from mice with colitis as compared to healthy controls (Figure 6b–h). Treatment with mesalamine did not significantly affect the expression of any of the cytokines/chemokines studied. However, ATB-429 treatment resulted in significant reductions of the expression of mRNA for TNFα, IFNγ, IL-1, IL-2, IL-12 p40 and RANTES (Figure 6b–d and f–h). Expression of mRNA for IL-10 was not different in colonic tissue from mice with colitis versus healthy controls, and was not affected by mesalamine or ATB-429 (Figure 6e).
Figure 6.
Effects of treatment for 1 week with ATB-429 versus mesalamine (each at 50 mg kg−1) on colonic MPO activity (a), and cytokine/chemokine mRNA expression (b–h) in mice with colitis. The cytokines/chemokines examined were: TNFα (b), IFNγ (c), IL-1 (d), IL-10 (e), IL-12 p40 (f), IL-2 (g) and RANTES (h). Results are expressed as the fold-increase overexpression in healthy controls, corrected for changes in GAPDH mRNA expression in each sample (n=5–7 per group). Twice daily treatment was initiated 4 days after induction of colitis. *P<0.05 versus the vehicle-treated group.
Discussion
Although widely used as a first-line therapy for IBD, mesalamine is largely ineffective in more severe cases of this disorder. In the present study, doses of mesalamine of up to 75 mg kg−1 were ineffective in reducing the severity of colitis in a mouse model. In sharp contrast, an H2S-releasing derivative of mesalamine, ATB-429, was found to be considerably more effective than the parent drug. ATB-429 was shown to be superior to mesalamine in three different treatment regimens. In addition to reducing mucosal injury and disease activity (body weight loss, fecal blood, diarrhea), ATB-429 markedly reduced colonic granulocyte infiltration (MPO activity) and the expression of mRNA for several important proinflammatory cytokines. Of particular note is the fact that these effects of ATB-429 were observed with a dose that, on a molar basis, was only half of the dose of mesalamine.
ATB-429 consists of a molecule of mesalamine linked via an ester bond to a molecule of ADT-OH. ADT-OH has been shown to liberate H2S when incubated in buffer and even greater generation of H2S was observed when it was incubated in a homogenate of liver (Distrutti et al., 2006b). Interestingly, ATB-429 released significantly more H2S than an equimolar amount of ADT-OH, both in buffer and in the liver homogenate (Distrutti et al., 2006b). H2S released from ATB-429 could contribute to its beneficial effects in colitis in a number of ways. First, H2S is a potent inhibitor of leukocyte adherence to the vascular endothelium (Zanardo et al., 2006), one of the earliest events in an inflammatory reaction. Inhibition of leukocyte adherence by H2S donors was prevented by pretreatment with glibencamide, consistent with the effects being mediated via ATP-sensitive K+ channels (Zanardo et al., 2006). This is consistent with reports that the vascular relaxant effects of H2S are mediated via K+ATP channels (Wang, 2002). The reduction of leukocyte adherence by H2S donors may also be, at least in part, owing to downregulation of expression of adhesion molecules (e.g., P selectin, LFA-1) on the endothelium and/or on leukocytes (Fiorucci et al., 2005). Thus, H2S release from ATB-429 may account for the marked increase in the ability of this compound, as compared to mesalamine, to reduce granulocyte infiltration into the colon. H2S has also been shown to reduce neutrophil-mediated tissue injury, which has been shown previously to be a substantial component of the mucosal damage observed in the TNBS model (Wallace et al., 1992). Thus, H2S can induce neutrophil apoptosis (Mariggio et al., 1998) and can inhibit tissue injury mediated via neutrophil-derived hypochlorous acid (Whiteman et al., 2005).
The intracolonic application of TNBS causes acute and chronic colitis in rodents (Morris et al., 1989; Neurath et al., 1995; Fiorucci et al., 2004). Although there is a prominent neutrophilic infiltration, there is also a marked influx of CCR1+ and CCR5+ macrophages and monocytes, as well as a prominent IL-12- and IFNγ-dependent T-lymphocyte (Th1) activation (Neurath et al., 1995; Fiorucci et al., 2004). In addition to reducing mucosal injury and granulocyte infiltration, ATB-429 also markedly reduced expression of mRNA for several proinflammatory cytokines/chemokines. TNBS colitis, like Crohn's colitis, is generally regarded as being driven by Th1 cytokines, including IL-1, IL-2 TNFα, IFNγ and IL-12 (Neurath et al., 1995; Sartor, 1997; Stallmach et al., 1999). The chemokine RANTES has also been implicated in the pathogenesis of colitis in this model (Ajuebor et al., 2001). Interestingly, expression of the anti-inflammatory cytokine, IL-10, was not affected by ATB-429. The effects of ATB-429 on cytokine/chemokine expression may be mediated by H2S, as it is capable of inhibiting nuclear factor-kappa B (NF-κB) activation (Oh et al., 2006). Transcription factors belonging to the NF-κB family modulate the expression of a range of genes involved in the inflammatory cascade, including TNFα and IFNγ. NF-κB has been identified as a promising target for novel therapies of IBD, particularly Crohn's disease (Neurath et al., 1996).
ADT (5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione; a structural analogue of ADT-OH) has been used clinically as a choleretic and sialogogue for decades, without major adverse reactions being reported (Drukarch et al., 1997). It has also been evaluated as a chemopreventative agent for lung cancer, with positive results (Lam et al., 2002). In the present study, we found that ADT-OH at a dose equimolar to an effective dose of ATB-429 did not significantly alter the severity of colitis in the mouse. Likewise, an equimolar dose of mesalamine was ineffective. As ATB-429 was effective even when given at half the molar dose of mesalamine and ADT-OH, our results suggest that the intact ATB-429 molecule exhibits anti-inflammatory effects beyond any additive effects of its two moieties. One possible explanation for this finding is that ATB-429 is a more effective releaser of H2S than ADT-OH. ATB-429 releases considerably more (>5-fold) H2S than does an equivalent dose of ADT-OH (Distrutti et al., 2006b).
Another attractive feature of ATB-429 with respect to utility in the treatment of IBD is the visceral analgesic effect of this compound that has been recently reported (Distrutti et al., 2006b). Pain is one of the most common and debilitating symptoms in IBD. ATB-429 was more effective than mesalamine in reducing colorectal distention-induced visceral pain in both healthy rats and rats with colitis. The analgesic effects of ATB-429 were blocked by pretreatment with glibenclamide, suggesting that this effect was mediated via K+ATP channels (Distrutti et al., 2006b).
In summary, ATB-429 is a novel, H2S-releasing derivative of mesalamine that exhibits greatly increased anti-inflammatory activity in a murine model of colitis, compared to the parent drug. An analogue of the H2S-releasing moiety of ATB-429 has itself been in clinical use for decades, with a very low incidence of adverse effects (Christen, 1995). ATB-429 therefore appears to represent an attractive alternative for treatment of IBD.
Acknowledgments
This work was supported in part by grants from the Canadian Institutes of Health Research, the Crohn's and Colitis Foundation of Canada and the Alberta Heritage Foundation for Medical Research (AHFMR) Forefront Program. Dr Wallace holds a Canada Research Chair in Inflammation and an AHFMR Senior Scientist award.
Abbreviations
- ATB-429, 5-amino-2-hydroxy-benzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3-yl)-phenyl ester hydrochloride; IBD
inflammatory bowel disease
- MPO
myeloperoxidase
- TNBS
trinitrobenzene sulfonic acid
Conflict of interest
Several of the authors of this paper (SF, GC, VS, GC and JLW) hold shares in Antibe Therapeutics Inc., the company that developed ATB-429.
References
- Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552–558. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen MO. Anethole dithiolethione: biochemical considerations. Meth Enzymol. 1995;252:316–323. doi: 10.1016/0076-6879(95)52034-1. [DOI] [PubMed] [Google Scholar]
- Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006a;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006b;319:447–458. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- Drukarch B, Schepens E, Stoof JC, Langeveld CH. Anethole dithiolethione prevents oxidative damage in glutathione-depleted astrocytes. Eur J Pharmacol. 1997;329:259–262. [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Del Soldato P, Flower RJ, Clark MJ, et al. NCX-1015, a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Proc Natl Acad Sci USA. 2002a;99:15770–15775. doi: 10.1073/pnas.232583599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palazzetti B, Sprague AG, Distrutti E, Morelli A, et al. Importance of innate immunity and collagen binding integrin α1β1 in TNBS-induced colitis. Immunity. 2002b;17:769–780. doi: 10.1016/s1074-7613(02)00476-4. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, et al. A β-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB. New lessons: classic treatments, expanding options in ulcerative colitis. Colorectal Dis. 2006;8 Suppl 1:20–24. doi: 10.1111/j.1463-1318.2006.00988.x. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- Korzenik JR, Podolsky DK. Evolving knowledge and therapy of inflammatory bowel disease. Nat Rev Drug Discov. 2006;5:197–209. doi: 10.1038/nrd1986. [DOI] [PubMed] [Google Scholar]
- Lam S, MacAulay C, le Riche JC, Dyachkova Y, Coldman A, Guilaud M, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Nat Cancer Inst. 2002;94:1001–1009. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- Mariggio MA, Minunno V, Riccardi S, Santacroce R, De Rinaldis P, Fumarulo R. Sulfide enhancement of PMN apoptosis. Immunopharmacol Immunotoxicol. 1998;20:399–408. doi: 10.3109/08923979809034822. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-KB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Rad Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92 Suppl:5S–11S. [PubMed] [Google Scholar]
- Stallmach A, Wittig B, Giese T, Pfister K, Hoffmann JC, Bulfone-Paus S, et al. Protection of trinitrobenzene sulfonic acid-induced colitis by an interleukin 2-IgG2b fusion protein in mice. Gastroenterology. 1999;117:866–876. doi: 10.1016/s0016-5085(99)70345-8. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Buret A, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and enhanced anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Higa A, McKnight GW, MacIntyre DE. Prevention and reversal of experimental colitis by a monoclonal antibody which inhibits leukocyte adherence. Inflammation. 1992;16:343–354. doi: 10.1007/BF00917626. [DOI] [PubMed] [Google Scholar]
- Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vergnolle N, Muscara MN, Asfaha S, Chapman K, McKnight W, et al. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology. 1999;117:557–566. doi: 10.1016/s0016-5085(99)70448-8. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain. Biochem Biophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1193–L1201. doi: 10.1152/ajplung.00489.2005. [DOI] [PubMed] [Google Scholar]