Abstract
Background and purpose:
Methylamine is an endogenous aliphatic amine exhibiting anorexigenic properties in mice. The aim of this work was to show whether methylamine also modifies feeding behaviour in rats and, if so, to identify the mediator(s) responsible for such effects.
Experimental approach:
Microdialysis experiments with the probe inserted in the periventricular hypothalamic nucleus were carried out in 12 h starved, freely moving rats. Collected perfusate samples following methylamine injection (i.c.v.) were analysed for nitric oxide by chemiluminescence and for dopamine and 5-hydroxytryptamine content by HPLC. Kv1.6 potassium channel expression was reduced by antisense strategy and this decrease quantified by semi-quantitative RT-PCR analysis.
Key results:
Methylamine showed biphasic dose-related effects on rat feeding. At doses of 15–30 μg per rat, it was hyperphagic whereas higher doses (60–80 μg) were hypophagic. Methylamine stimulated central nitric oxide (+115% vs. basal) following hyperphagic and dopamine release (60% over basal values) at hypophagic doses, respectively. Treatment with L-NG-nitro-L-arginine-methyl ester (i.c.v. 2 μg 10 μl−1) or with α-methyl-p-tyrosine (i.p. 100 mg kg−1) before methylamine injection, reduced nitric oxide output and hyperphagia, or dopamine release and hypophagia respectively. Moreover, hypophagia and hyperphagia, as well as nitric oxide and dopamine release were significantly reduced by down-regulating brain Kv1.6 potassium channel expression.
Conclusions and implications:
The effects of methylamine on feeding depend on the hypothalamic release of nitric oxide and dopamine as a result of interaction at the Kv1.6 channels. The study of methylamine levels in the CNS may provide new perspectives on the physio-pathogy of alimentary behaviour.
Keywords: voltage-dependent potassium channels; Shaker-like Kv1.6; semicarbazide-sensitive amine oxidase; nitric oxide; dopamine, alimentary behaviour
Introduction
A complex physiological system consisting of central and peripheral signals (brain–gut-adipose tissue axis) controls animal feeding. The homoeostasis of this process is regulated by the hypothalamus through neuronal circuits using specific neurotransmitters. Fluctuation in the levels of mediators in the periventricular hypothalamic (PH) nucleus accounts for hyperphagic or hypophagic behaviour (Stanley et al., 2005). In this respect, increasing levels of nitric oxide (NO) have been recognized as the final and common messenger of several orexigenic neuropeptides (Gaskin et al., 2003; Farr et al., 2005), whereas dopamine and 5-hydroxytryptamine (5-HT) are involved in different physio-pharmacological anorexigenic responses (Parada et al., 1988; Leibowitz et al., 1989; Dawson and Routledge, 1995; Inui, 2000; Frantz et al., 2002). The release of dopamine, 5-HT or other hypophagic mediators has been described following depolarization of central neurons as a result of reduced potassium conductance (Boireau et al., 1991; Dawson and Routledge, 1995; Inui, 2000).
We have recently shown that central or peripheral injection of methylamine, the shortest aliphatic, endogenous amine, reduced feeding in healthy or obese and type 2 diabetic starved mice (Pirisino et al., 2004; Cioni et al., 2006a). In common with other amines of low-molecular weight, methylamine can readily cross the blood–brain barrier (Mitchell and Zhang, 2001).
Tissue levels of methylamine depend on diet, on endogenous and exogenous amine degradation and on the activity of a semicarbazide-sensitive benzylamine oxidase (Bz-SSAO; EC 1.4.3.6) that scavenges methylamine, producing NH3, hydrogen peroxide and formaldehyde (Lyles and McDougall, 1989). Bz-SSAO tissue levels were found to be increased in diabetic tissues and in the brain microvessels of Alzheimer's disease patients (Meszaros et al., 1999; Yu, 2001; Del Mar et al., 2005). Diabetic and Alzheimer's disease patients both suffer from behavioural disorders, including food intake abnormalities (Munoz et al, 2006). As these pathologies are characterized by inflammation of the blood–brain barrier, increased methylamine degradation has been hypothesized to play a role in their pathogenesis (Yu, 2001).
In mice, methylamine-induced hypophagia was found to depend on the brain expression levels of the Shaker-like Kv1.6 subtype potassium channels (Pirisino et al., 2004), suggesting that methylamine could evoke the release of some hypophagic mediator(s) by interacting at these channels. Moreover, both methylamine and NH3 have been reported to have voltage-operated potassium channel blocking activities (Hrnjez et al., 1999; Mundorf et al., 1999). Methylamine hypophagia increased in mice pre-treated with Bz-SSAO inhibitors, suggesting that the enzyme, found in blood vessels and in peripheral tissues, plays an active role in controlling the central levels of methylamine (Banchelli et al., 2001; Pirisino et al., 2001). Despite this suggestive information, the only direct indication of the nature of the mediators involved in the hypophagic activity of methylamine is that the leptin circuit is not involved, as this response to methylamine was conserved in db/db mice, which lack the hypothalamic leptin receptor (Cioni et al., 2006b).
To gain further insight regarding the central activity of methylamine, we investigated its effects on feeding behaviour in rats and the neuromediator(s) involved in the response. Microdialysis experiments were carried out by inserting a probe in the PH of 12 h starved and freely moving rats with different brain levels of Shaker-like Kv1.6 potassium channels as a result of specific antisense treatment. Following intracerebroventricular (i.c.v.) methylamine injection, animal feeding was monitored and perfusates were analysed for their content of some mediators involved in feeding.
Materials and methods
Animals
Male rats (150–200 g) from the Morini breeding farm (San Polo d'Enza, Italy) were used. Five rats were housed per cage. The cages were placed in the experimental room 24 h before the test for adaptation and the rats fasted overnight before the experiment. The animals were kept at 23±1°C with a 12 h light–dark cycle (light on at 0700 h) and were fed a standard laboratory diet with water ad libitum. All experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) for experimental animal care. All efforts were made to minimize animal suffering and to reduce the number of animals used. Unless otherwise indicated, at least 10 animals per group were used in each behavioural protocol.
Evaluation of food consumption
Rats did not have access to food for 12 h, but water was available ad libitum. A weighed amount of food (standard laboratory pellets) was given and the amount consumed (evaluated as the difference between the original amount and the food left in the cage, including spillage) was measured 20, 40, 60, 120, 180 min after i.c.v. administration of saline or methylamine, with an accuracy of 0.1 g. An arbitrary cutoff time of 120 min was used and the total amount of food consumed was expressed in mg per rat 120 min−1. In some experiments, food consumption was evaluated in rats pretreated i.c.v. (2 μmol 10 μl−1) with L-NG-nitro-L-arginine-methyl ester (L-NAME; Sigma-Aldrich, St Louis, MO, USA) 30 min before methylamine (Dobrucki et al., 2001) or with α-methyl-p-tyrosine (Sigma Chemical Company; St Louis, MO, USA) 100 mg kg −1 intraperitoneally (i.p.), 2 h before methylamine injection (Banchelli et al., 2001).
In vivo microdialysis procedures and i.c.v drug administration
Male Wistar rats weighing 180–200 g were anaesthetized with chloral hydrate (400 mg kg−1 i.p.) and placed in a stereotaxic apparatus with the upper incisor bar set to allow the bregma to be at the same height as λ. A CMA/12 microdialysis guide cannula (CMA/Microdialysis, Stockholm, Sweden) was implanted into the PH using the following coordinates from the bregma: AP – 1.40 mm, L=0.15 mm, V=7.0–9.0 mm (Paxinos and Watson, 1982). After surgery, the animals were allowed to recover before the microdialysis experiments. From 5 to 7 days later, the stopper of the guide cannula was removed and the CMA J 12 microdialysis probe (membrane 3 mm, outer diameter 0.5 mm, molecular weight cutoff 20 000 Da) was inserted so that the tip of the membrane arrived at V=7 mm. The rat was placed in a CiVIA/120 system for freely moving animals and the probe was perfused continuously with physiological solution, by means of a CMA/102 microinfusion pump, at a flow rate of 1.5 μl min−1. The solution consisted (in mM) of NaCl (154), KCl (5.63), CaCl2·2H2O (2.18), NaHCO3 (5.95) adjusted to pH 6.8±0.4. Beginning 2–3 h after insertion of the probe, dialysate samples were collected at 20 min intervals.
Samples were collected for 280–300 min after the start of the experiments. At the end of the experiments, the location of the probes in the brain was evaluated by histological examination. The data included in this study refer to rats bearing probes correctly placed within the PH.
An i.c.v. administration was performed in rats, injecting the substances into conscious animals implanted with permanent i.c.v. polyethylene cannulae (5 μl of drug solution+1 μl air +5 μl saline). Implantation of the cannulae in the lateral ventricle (Altaffer et al., 1970) was performed under chloral hydrate anaesthesia (400 mg kg−1 i.p.) at least 5 days before the experiment. To ascertain the exact site of i.c.v. injection, some rats were deeply anaesthetized and injected i.c.v. with 5 or 10 μl of 1:10 diluted India ink and the brains were examined macroscopically after sectioning. The injections were performed randomly into the right or left ventricle. Drug concentrations were prepared in such a way that the necessary dose could be administered in a volume of 10 μl of isotonic (0.9 % NaCl) saline solution to each rat.
Before i.c.v. administration of the compounds used, it was confirmed that the pH values of the compound solutions, ranging from 7.2 to 6.7, did not vary significantly from those of the saline (pH=6.8±0.4).
Analysis of dopamine, 5-HT and NO in dialysate samples
Perfusate was immediately assayed by high-performance liquid chromatography (HPLC) for dopamine and 5-HT by electrochemical detection (Baumann et al., 1994). After stabilization in dialysate, three samples were collected before methylamine hydrochloride (Sigma–Aldrich, St Louis, MO, USA) injection (baseline values). Dopamine, 5-HT and NO concentrations in later samples were expressed as a percentage of the mean baseline values.
Aliquots of the dialysate (5 μl) were injected directly into a microbore HPLC column (Baumann et al., 1994) coupled to an amperometric detector. A glass carbon working electrode was set at a potential of +700 mV relative to an Ag/AgCl reference. A mobile phase consisting of 14.2 g monochloroacetic acid, 6.8 g NaOH, 350 mg sodium octyl-sulphate, 80 mg disodium ethylenediaminetetraacetic acid, 1 ml triethylamine, 6% MeOH, 6% CH3CN per litre of water (final pH 5), was pumped at a rate of 60 ml min−1 with a constant column pressure of 2500–3000 psi. Standard curves of dopamine, constructed before the injection of dialysate samples, were linear over a wide range of concentrations. The lowest limit of assay sensitivity for dopamine was 200 fg in 5 μl of sample.
NO concentrations in dialysate samples were assayed as nitrite (NO2−) with a Nitric Oxide Analyser (Model 280 from Sievers Instruments, Boulder, CO, USA), which allows the measurement of nitrite levels in gaseous and liquid samples (Espey et al., 2000). Each sample (25 μl) was injected into an anaerobic (helium-purged) reaction vessel containing sodium iodide/glacial acetic acid. The production of NO from NO2− was detected by chemiluminescence and was quantified with a photomultiplier tube/computer system. To evaluate the possible oxidation of NO2− to NO3− occurring in our samples, dialysate samples were also incubated for 30 min at 37°C with nitrate reductase (250 mU ml−1; Sigma-Aldrich, St Louis, MO, USA) and reduced nicotinamide adenine dinucleotide phosphate (NADPH) (100 μM; Sigma-Aldrich, St Louis, MO, USA). The reaction mixture was then analysed for NO2− as described above. No significant differences were observed in NO content between treated samples and those not treated with NADPH-nitrate reductase. Standard curves were built by using different concentrations of NaNO2 and the amount of NO2− in each sample was calculated from the standard curve. The detection limit was 5 pmol. Standard curves of NO were linear over a range of concentrations from 5 to 100 pmol.
To block the biosynthesis of NO, rats were pretreated i.c.v. (2 μmol 10 μl−1) with L-NAME (Sigma-Aldrich, St Louis, MO, USA) delivered 30 min before other treatments (Dobrucki et al., 2001).
Antisense oligonucleotide (aODN) treatment
An aODN towards the rat Kv1.6 was designed by targeting the 5′ portion of the rat Kv1.6 mRNA, residues 830–849 of the published cDNA sequence (Yu, 1998) XM575671. The aODN of the following sequence (5′-TTT CTC CGA TCT CAT GTC ac-3′) was capped by a terminal phosphorothioate double substitution.
To evaluate the specific antisense effects of the aODN, a scrambled phosphorodiester phosphorothioate-capped oligonucleotide (sODN) was used as negative control. The sODN, 20-mer (5′-TTC CTT CCA TGT CAT ATC GC-3′) is a collection of about 3 × 1014 different molecular species. Therefore, for the nanomolar–micromolar range concentrations used in the antisense experiments, the sODN was present at the site of action in a subattomolar concentration, which is totally insufficient for any antisense effect.
Both the aODN and the sODN were purified by HPLC; Genosys, The Woodlands, TX, USA.
To favour cell penetration and to minimize the degradation of the oligonucleotides during in vivo experiments, phosphorothioate-cappedphosphorodiester oligonucleotides associated with an artificial cationic lipid (DOTAP =N-(1-(2,3-dioleoyloxy)propyl)-N,N,N-trimethylammonium methyl sulphate; 1.3 μM) proved to be suitable for enhancing cellular uptake. To evaluate the specific effects of the aODN pretreatments on the behavioural response to methylamine administration, each group of rats received a single i.c.v. injection (5 μl; 8 nmol of ODN) on days 1, 4 and 7. sODN did not induce any evident modification in the basal behavioural or neurochemical function of rats, compared to saline- or vector-injected animals (data not shown) and sODN-treated animals were used as controls in all experiments. All the experiments were performed 24 h after the last i.c.v. injection of aODN or sODN, when maximal inhibition of the expression of mKv 1.6 mRNA in the rat brain tissue was detected using RT-PCR analysis.
Semi-quantitative RT-PCR of Kv1.6 and 18 S from rat brain
To quantify mRNA levels of Kv1.6 in rat brain, total RNA was extracted from tissue using the Tri Reagent (Sigma-Aldrich Chemical Company, St Louis, MO, USA). An internal standard-based RT-PCR assay with serial dilution of total RNA was used for the semi-quantitative determination of Kv1.6 mRNA levels, using 18S as reference gene. Total RNA, purified by DNAase treatment, (10 ng) was reverse transcribed and amplified by the SuperScript One-Step RT-PCR System (Invitrogen, Europe). For amplification of the murine Kv1.6, the cDNA sequence was targeted with 5′-CGAGGAGGAAGATGAAGACG-3′ left primer and 5′-ACCAGGAGCTCAAAGGTGAA-3′ right primer; for amplification of the18S, the cDNA sequence was targeted with 5′-AAA CGG CTA CCA CAT CCA AG-3′ left primer and 5′-CCT CCA ATG GAT CCT CGT TA-3′ right primer. The RT-PCR profile for the Kv1.6 gene product was: one cycle of 94°C for 2 min, 30 cycles at 94°C for 30 s, 59°C for 30 s and 72°C for 1 min followed by one cycle at 72°C for 10 min. The RT-PCR profile for the 18S gene product was: one cycle of 94°C for 2 min, 25 cycles of 94°C for 30 min, 57°C for 30 min and 72°C for 1 min followed by one cycle at 72°C for 10 min. Amplification products were run on a 1% agarose gel and the ethidium bromide-stained bands were quantified by densitometric analysis. Within the linear range of amplification, at least three values of Kv1.6 amplification products were normalized to the starting total RNA volumes and referred to the corresponding 18S. The absence of genomic contamination of the isolated RNAs was confirmed by performing PCR reactions on purified RNA samples (DNAase-treated) before their reverse transcription.
Statistical analysis
All experimental results are expressed as the mean±s.e.m. Analysis of variance , followed by Fisher's protected least significant difference procedure for post hoc comparison, were used to verify significance between two means. Data were analysed using the StatView software.
Results
Food intake behaviour in methylamine-treated rats
The 12-h starved, saline-treated rats showed a time-dependent increase in food intake, which reached a plateau 120 min after food readministration (5539±31 mg per rat 120 min−1; n=12). Satiated, saline-treated animals did not consume any appreciable amount of food during the time of the experiment.
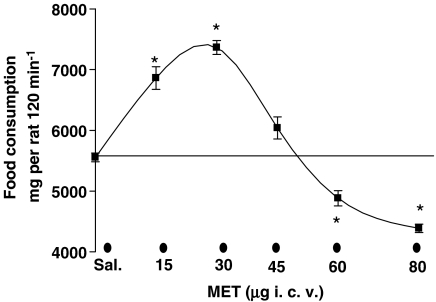
Methylamine, administered i.c.v., biphasically modulated food consumption (Figure 1): doses of 15 and 30 μg were hyperphagic (+ 32% vs saline-treated at 30 μg; *P<0.01 vs saline-treated; n=8) whereas doses of 60 and 80 μg were hypophagic (−20% vs saline-treated at 80 μg; *P<0.05; n=8).
Figure 1.
Methylamine modifies food consumption in starved rats. Starved rats (12 h) were placed in individual cages and injected i.c.v. with methylamine (MET) (15, 30, 60, 80 μg 10 μl−1) or saline, as described in the ‘Materials and methods'. The amount of food consumed was measured over a period of 120 min, with an accuracy of 0.1 g. Results are expressed as mg per rat 120 min−1 and are the mean±s.e.m. of the food consumed by 5–12 animals. *P<0.01 vs saline; n=8.
Microdialysis experiments
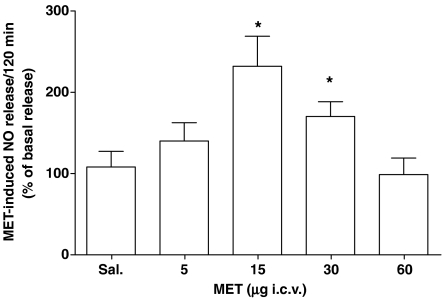
Microdialysates from 12-h fasted, saline-treated rats were found positive for NO content (0.304±0.013 (CL 99% 0.216–0.341) pmol μl−1; n=7). These basal values increased dose–dependently following 5–15 μg methylamine (*P<0.05 vs saline-treated rats; n=4). In this range of doses, 15 μg produced the maximum effect increasing the NO release up to 115% over basal (*P<0.01 vs saline-treated; n=4). However, after 30 μg methylamine, the NO release was lower than that measured following 15 μg and: at higher doses, from 45 to 60 μg, it was indistinguishable from the basal release observed in saline-injected rats (Figure 2). The NO release induced by methylamine was a relatively long-lasting effect, increasing for about 140 min, with a subsequent decline after 160 min following the amine administration (data not shown).
Figure 2.
Methylamine increases NO production in rat hypothalamus. I.c.v. administration of methylamine (MET, 5–60 μg) was performed in conscious rats, as described in the ‘Materials and methods'. Perfusate samples were collected every 20 min for a total of 280 min. In the range of doses injected, 15 μg produced the maximum effect (*P<0.01 vs saline-treated; n=6).
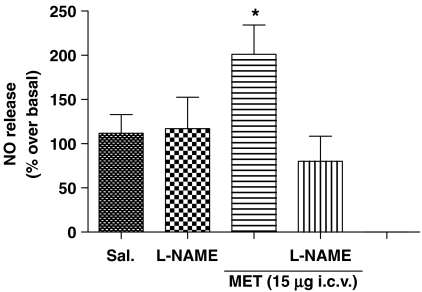
The involvement of NO synthase activity in NO release induced by MET was confirmed by pretreating rats with L-NAME (2 μg 10 μl−1 i.c.v.). Under these conditions, both methylamine (15 μg) -induced NO production (Figure 3) and its hyperphagic effect were significantly reduced (the latter from 6863±186 mg of food per rat to a value not different from saline treated rats, 5261±550 mg of food per rat; P>0.05 vs saline treated, n=9).
Figure 3.
NO production by methylamine is reduced by L-NAME pre-treatment. Twelve-hours-starved mice were pretreated i.c.v. with L-NAME and then injected with methylamine (MET, 15 μg). Perfusates collected after 120 min were analyzed for NO content. Results are expressed as NO release (% over basal) at 120 min. *P<0.05 vs basal; n=4.
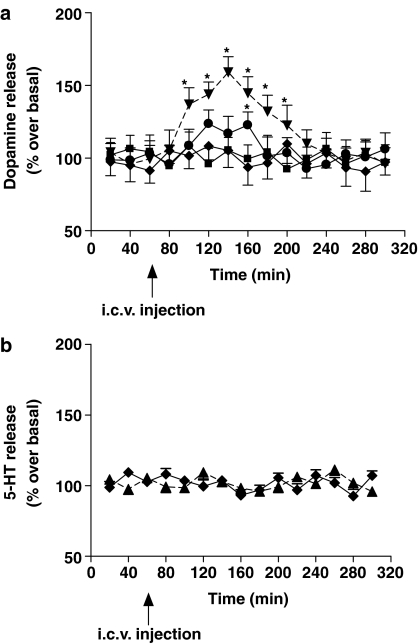
At hypophagic doses (60–80 μg) of methylamine, dopamine was found to be increased in the perfusates from the PH. The maximum extent of dopamine release reached 60% over the basal values, 140 min after methylamine administration (Figure 4a). Moreover, pretreating animals with α-methyl-p-tyrosine (100 mg kg−1 2 h before methylamine injection) reversed methylamine hypophagia from 4066±22 mg rat−1 to 5513±27 mg rat−1 (P>0.05 vs saline treated, n=9).
Figure 4.
Methylamine increases dopamine but not 5-HT release in rat hypothalamus. Time-dependent dopamine (a) and 5-HT (b) release from rat PH was evaluated in rats treated with saline or methylamine (45, 60, 80 μg). Results are expressed as the mean±s.e.m. of four different experiments and as the % over basal release. *P<0.05 vs saline; n=4.
At the highest dose administered (80 μg), methylamine was totally ineffective in changing the local efflux of 5-HT (Figure 4b).
The effect of a pretreatment with aODN towards Kv1.6
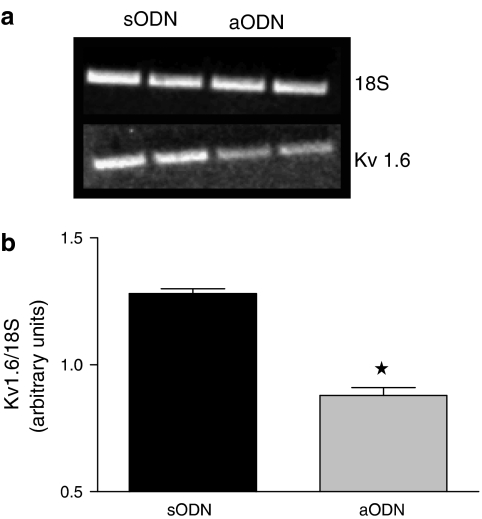
The lowering of Kvl.6 mRNA in whole rat brain homogenates was quantified by RT-PCR analysis (Figure 5a). Densitometric analysis showed that animals treated with aODN had a significant reduction of the ratio between Kvl.6 and 18S of brain mRNA (40% of reduction; *P<0.05 vs sODN-treated rats; n=3; Figure 5b).
Figure 5.
Expression of Kv1.6 channels in rat brain is reduced following aODN administration. Twelve-hours-starved rats were treated with sODN and aODN towards the Kv1.6 potassium channels and total RNA was prepared from the brains. RT-PCR analysis of Kv1.6 and 18S genes were performed in parallel. In (a), a typical picture of RT-PCR products is shown. In (b), densitometry of Kv1.6 gene over that of 18S shows a significant reduction of Kv1.6 expression in aODN vs sODN treated rats. *P<0.05 vs sODN gene expression; results are expressed as the mean±s.e.m. of pixels calculated from three different analyses.
The effect of reducing Kv1.6 brain levels on food consumption, NO and dopamine release
Food consumption after methylamine (30 and 80 μg) i.c.v. injection was evaluated in rats with reduced brain Kv1.6 potassium channel expression.
The hyperphagic and hypophagic effects of 30 and 80 μg methylamine, respectively, were significantly lower in aODN vs sODN-treated rats (*P<0.05 vs n=4; Table 1). In parallel, lower levels of either NO or dopamine following 30 and 80 μg methylamine, respectively, were measured in microdialysis perfusates (Table 1).
Table 1.
Food consumption, nitric oxide and dopamine release, 120 min after methylamine injection, in control and ODN pretreated rats
|
Food consumption (mg rat−1) |
Nitric oxide (% over basal) |
Dopamine (% over basal) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | sODN | aODN | sODN | aODN | sODN | aODN | |||
| Saline 5 μl | 5539±60 | 5571±42 | — | 116±19 | 80±31 | — | 108±9 | 101±9 | — |
| MET 30 μg | 7426°±43 | 7511°±36 | 5628*±32 | 232°±58 | 263°±37 | 163*±33 | — | — | — |
| MET 80 μg | 4134°±32 | 4023°±44 | 5562**±31 | — | — | — | 143°±8 | 157°±9 | 112**±12 |
Abbreviations: aODN, antisense oligonucleotide; sODN, scrambled antisense oligonucleotide
P<0.01 vs saline-treated
P<001 vs MET 30 μg
P<0.01 vs MET 80 μg
Results represent the mean±s.e.m. of at least four rats.
Pretreatment with aODN or sODN (i.c.v.), failed to modify food intake or basal levels of NO or dopamine in perfusates from vehicle-treated mice (data not shown).
Discussion
The main purpose of this work was to investigate the processes involved in the modification by methylamine of feeding behaviour in animals. Microdialysis in the freely moving rat was used to investigate the effects of methylamine on the release of hypothalamic neuromediators that are believed to be involved in alimentary behaviour.
In fasting rats, ‘low' methylamine doses (from 5 to 15 μg) stimulated food consumption and this effect decreased progressively at methylamine doses from 30 to 45 μg. On the other hand, ‘high' doses of methylamine, from 60 to 80 μg, elicited an anorexigenic activity. These results, in the rat, differed from those observed in mice where only a clearcut, dose–related hypophagia is observed (Pirisino et al., 2001; 2004). This suggests that at least two different mediators may be responsible for methylamine-dependent modification of food intake in rats.
In line with this, because of the impossibility of monitoring the presence of any single orexigenic mediator, we decided to analyse our samples for the presence of NO, which might account for the activation of several orexigenic pathways. Moreover, we examined the release of dopamine and 5HT, as they are the mediators, most commonly involved in hypophagic responses (Ghelardini et al., 2003).
Methylamine (15–30 μg) administration resulted in NO formation in the PH, which might explain the observed transitory hyperphagic effect of this amine. It has been reported that various conditions that elevate NO in the hypothalamus can increase food ingestion in rats (Squadrito et al., 1994). Furthermore, NO-synthase inhibitors counteracted the hyperphagic behaviour of genetically obese mice and rats (Squadrito et al., 1993; Morley and Flood, 1994; Inui, 2000). NO-synthase inhibitors were also shown to reduce rat food intake induced by fasting or hyperphagic compounds such as 5-HT1A receptor agonists, 2-deoxy-D-glucose, morphine or chlordiazepoxide (Sugimoto et al., 1999; Yamada et al., 2002). In our experiments, pretreatment (i.c.v.) with L-NAME, at a dose known to nonselectively inhibit brain NOS (Dobrucki et al., 2001), reduced both methylamine-induced NO release and the hyperphagic effect.
This NO-releasing property of methylamine parallels the reported behaviour of NH3, closely related to methylamine from a physico–chemical point of view. The most common hypothesis suggests that NH3 releases NO primarily by increasing glutamate levels which elevates Ca2+, which, in turn, activates NO synthase (Kitano et al., 2004; Monfort et al., 2004). This mechanism might be the consequence of the known ability of NH3 to reduce potassium conductance, a property that it shares with methylamine (Moroni et al, 1998; Hrnjez et al., 1999).
Our results in rats with reduced brain levels of Kv1.6, confirm a key role of the voltage-dependent potassium channels in the methylamine-induced NO release from the PH, further suggesting that the Shaker-Like Kv1.6 channel subtype integrity is essential for the effects of methylamine, as previously described in mice (Pirisino et al., 2004). The bell-shaped dose–response curves of methylamine-induced NO release might be explained by Ca2+-dependent desensitization of brain neurons following sustained reduction of potassium conductance. Similar biphasic responses have been reported after activation of voltage-dependent Ca2+ channels, which depend on the functional status of channel domains, including either the voltage-dependent activated or the voltage or Ca2+-dependent-inactivated gates (Fox, 1981; Di Virgilio et al., 1987). Different stimulatory compounds have been described as inducing Ca2+-related desensitization processes on central neurotransmitter release, including NO (Izumi et al., 1989; Stojilkovic et al., 1989; Crespi and Rossetti, 2004).
The central release of dopamine elicits complex effects in controlling mammalian food intake, depending on the receptor subtype activated (Cooper and Plum, 1987; Cooper et al., 2006). The PH nucleus has been shown to be the area involved in the direct or indirect hypophagic effects elicited by dopaminergic compounds, including amphetamine (Parada et al., 1988; Leibowitz et al., 1989). Serotonin (5-HT), released from neurones projecting from the midbrain dorsal raphe nucleus to PH and to the ventromedial nucleus of the hypothalamus, is also known to control feeding (Parada et al., 1988; Inui, 2000; Frantz et al., 2002). In these areas, amphetamine and, to a greater extent, more selective analogues of 5-HT, are anorexigenic and increase the extracellular levels of 5-HT (Leibowitz et al., 1989; Simansky, 1996). Our results show that methylamine, at the doses of 60–80 μg (i.c.v), elicited a hypophagic effect that is related to increasing extracellular levels of dopamine, but not of 5-HT. The onset of the hypophagic response was prevented in rats pre treated with α-methyl-p-tyrosine. As already observed in mice (Pirisino et al., 2004), both the dopamine release and the hypophagia in rats were lowered by reducing the Kv1.6 potassium channel expression. These results underline the close relationship between methylamine, hypophagia, dopamine efflux and density of brain Kv1.6 subtypes. Increased intracellular Ca2+, consequent on Kv1.6 closure, might be the mechanism responsible for NO and dopamine release that provokes the hyper- and hypophagic effects of MET, respectively. The extent of intraneuronal Ca2+ increase might account for the sequential stimulation of the nitrergic and dopaminergic responses evoked by increasing methylamine doses.
These data also show for the first time that methylamine behaves as an endogenous modulator of central NO production. Further studies on the central levels of methylamine in physiological or pathological conditions should provide insight into the implications of this behaviour.
Conclusions
Methylamine modulates rat feeding behaviour and increases extracellular NO and dopamine in the hypothalamus, probably through interaction with the Kv1.6 potassium channels. Therefore, methylamine should be included in the complex network of mediators that are involved in the hypothalamic control of food intake. Knowledge of the central levels of methylamine might open new perspectives in our understanding of the pathogenesis of eating disturbances.
Acknowledgments
This work was funded by a local grant from the University of Florence (ex 60%).
Abbreviations
- Bz-SSAO
benzylamine oxidase
- L-NAME
L-NG-nitro-L-arginine-methyl ester
- PH
periventricular hypothalamic nucleus
Conflict of interest
The authors state no conflict of interest.
References
- Altaffer FB, Verster FS, Hall S, Long CJ, D'encarnacao P. A simple and inexpensive cannula technique for chemical stimulation of the brain. Physiol Behav. 1970;5:119–121. doi: 10.1016/0031-9384(70)90022-3. [DOI] [PubMed] [Google Scholar]
- Banchelli G, Ghelardini C, Raimondi L, Galeotti N, Pirisino R. Selective inhibition of amine oxidases differently potentiate the hypophagic effect of benzylamine in mice. Eur J Pharmacol. 2001;413:91–99. doi: 10.1016/s0014-2999(01)00739-7. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Char GU, De Costa BR, Rice KC, Rothman RB. GBR12909 attenuates cocaine-induced activation of mesolimbic dopamine neurons in the rat. J Pharmacol Exp Ther. 1994;271:1216–1222. [PubMed] [Google Scholar]
- Boireau A, Richard F, Olivier V, Aubeneau M, Miquet JM, Dubedat P, et al. Differential effects of potassium channel blockers on dopamine release from rat striatal slices. J Pharm Pharmacol. 1991;43:798–801. doi: 10.1111/j.2042-7158.1991.tb03485.x. [DOI] [PubMed] [Google Scholar]
- Cioni L, De Siena G, Ghelardini C, Sernissi O, Alfarano C, Pirisino R, et al. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur J Pharmacol. 2006a;529:179–187. doi: 10.1016/j.ejphar.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Cioni L, De Siena G, Ghelardini C, Sernissi O, Alfarano C, Pirisino R, et al. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: interactive effects with methylamine and alpha-aminoguanidine. Eur J Pharmacol. 2006b;529:179–187. doi: 10.1016/j.ejphar.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev. 1987;67:440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Al Naser HA, Clifton PG. The anorectic effect of the selective dopamine D1-receptor agonist A-77636 determined by meal pattern analysis in free-feeding rats. Eur J Pharmacol. 2006;532:253–257. doi: 10.1016/j.ejphar.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Crespi F, Rossetti ZL. Pulse of nitric oxide release in response to activation of N-methyl-D-aspartate receptors in the rat striatum: rapid desensitization, inhibition by receptor antagonists, and potentiation by glycine. J Pharmacol Exp Ther. 2004;309:462–468. doi: 10.1124/jpet.103.061069. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Routledge C. Differential effects of potassium channel blockers on extracellular concentrations of dopamine and 5-HT in the striatum of conscious rats. Br J Pharmacol. 1995;116:3260–3264. doi: 10.1111/j.1476-5381.1995.tb15133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Mar HM, Esteban M, Szabo P, Boada M, Unzeta M. Human plasma semicarbazide sensitive amine oxidase (SSAO), beta-amyloid protein and aging. Neurosci Lett. 2005;384:183–187. doi: 10.1016/j.neulet.2005.04.074. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Milani D, Leon A, Meldolesi J, Pozzan T. Voltage-dependent activation and inactivation of calcium channels in PC12 cells. Correlation with neurotransmitter release. J Biol Chem. 1987;262:9189–9195. [PubMed] [Google Scholar]
- Dobrucki LW, Cabrera CL, Bohr DF, Malinski T. Central hypotensive action of clonidine requires nitric oxide. Circulation. 2001;104:1884–1886. doi: 10.1161/hc4101.098281. [DOI] [PubMed] [Google Scholar]
- Espey MG, Miranda KM, Pluta RM, Wink DA. Nitrosative capacity of macrophages is dependent on nitric-oxide synthase induction signals. J Biol Chem. 2000;275:11341–11347. doi: 10.1074/jbc.275.15.11341. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Kumar VB, Morley JE. Orexin-A-induced feeding is dependent on nitric oxide. Peptides. 2005;26:759–765. doi: 10.1016/j.peptides.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Fox AP. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci USA. 1981;78:953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, Hansson KJ, Stouffer DG, Parsons LH. 5-HT(6) receptor antagonism potentiates the behavioral and neurochemical effects of amphetamine but not cocaine. Neuropharmacology. 2002;42:170–180. doi: 10.1016/s0028-3908(01)00165-4. [DOI] [PubMed] [Google Scholar]
- Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. 2003;24:913–918. doi: 10.1016/s0196-9781(03)00160-8. [DOI] [PubMed] [Google Scholar]
- Ghelardini C, Quattrone A, Galeotti N, Livi S, Banchelli G, Raimondi L, et al. Antisense knockdown of the Shaker-like Kv1.1 gene abolishes the central stimulatory effects of amphetamines in mice and rats. Neuropsychopharmacology. 2003;28:1096–1105. doi: 10.1038/sj.npp.1300162. [DOI] [PubMed] [Google Scholar]
- Hrnjez BJ, Song JC, Prasad M, Mayol JM, Matthews JB. Ammonia blockade of intestinal epithelial K+ conductance. Am J Physiol. 1999;277:G521–G532. doi: 10.1152/ajpgi.1999.277.3.G521. [DOI] [PubMed] [Google Scholar]
- Inui A. Transgenic approach to the study of body weight regulation. Pharmacol Rev. 2000;52:35–61. [PubMed] [Google Scholar]
- Izumi S, Stojilkovic SS, Catt KJ. Calcium mobilization and influx during the biphasic cytosolic calcium and secretory responses in agonist-stimulated pituitary gonadotrophs. Arch Biochem Biophys. 1989;275:410–428. doi: 10.1016/0003-9861(89)90388-3. [DOI] [PubMed] [Google Scholar]
- Kitano T, Matsumura S, Seki T, Hikida T, Sakimura K, Nagano T, et al. Characterization of N-methyl – aspartate receptor subunits involved in acute ammonia toxicity. Neurochem Int. 2004;44:83–90. doi: 10.1016/s0197-0186(03)00124-4. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Weiss GF, Walsh UA, Viswanath D. Medial hypothalamic serotonin: role in circadian patterns of feeding and macronutrient selection. Brain Res. 1989;503:132–140. doi: 10.1016/0006-8993(89)91713-7. [DOI] [PubMed] [Google Scholar]
- Lyles GA, McDougall SA. The enhanced daily excretion of urinary methylamine in rats treated with semicarbazide or hydralazine may be related to the inhibition of semicarbazide-sensitive amine oxidase activities. J Pharm Pharmacol. 1989;41:97–100. doi: 10.1111/j.2042-7158.1989.tb06401.x. [DOI] [PubMed] [Google Scholar]
- Meszaros Z, Szombathy T, Raimondi L, Karadi I, Romics L, Magyar K. Elevated serum semicarbazide-sensitive amine oxidase activity in non-insulin-dependent diabetes mellitus: correlation with body mass index and serum triglyceride. Metabolism. 1999;48:113–117. doi: 10.1016/s0026-0495(99)90019-7. [DOI] [PubMed] [Google Scholar]
- Mitchell SC, Zhang AQ. Methylamine in human urine. Clin Chim Acta. 2001;312:107–114. doi: 10.1016/s0009-8981(01)00608-8. [DOI] [PubMed] [Google Scholar]
- Monfort P, Munoz MD, Kosenko E, Llansola M, Sanchez-Perez A, Cauli O, et al. Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus: Alterations in hyperammonemia. Neurochem Int. 2004;45:895–901. doi: 10.1016/j.neuint.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Morley JE, Flood JF. Effect of competitive antagonism of NO synthetase on weight and food intake in obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 1994;266:R164–R168. doi: 10.1152/ajpregu.1994.266.1.R164. [DOI] [PubMed] [Google Scholar]
- Moroni A, Bardella L, Thiel G. The impermeant ion methylammonium blocks K+ and NH4+ currents through KAT1 channel differently: evidence for ion interaction in channel permeation. J Membr Biol. 1998;163:25–35. doi: 10.1007/s002329900367. [DOI] [PubMed] [Google Scholar]
- Mundorf ML, Hochstetler SE, Wightman RM. Amine weak bases disrupt vesicular storage and promote exocytosis in chromaffin cells. J Neurochem. 1999;73:2397–2405. doi: 10.1046/j.1471-4159.1999.0732397.x. [DOI] [PubMed] [Google Scholar]
- Munoz AM, Agudelo GM, Lopera FJ. Nutritional condition in patients with Alzheimer-type dementia from the neurosciences' group, Medellin 2004. Biomedica. 2006;26:113–125. [PubMed] [Google Scholar]
- Parada M, Hernandez L, Schwartz D, Hoebel BG. Hypothalamic infusion of amphetamine increases serotonin, dopamine and norepinephrine. Physiol Behav. 1988;44:607–610. doi: 10.1016/0031-9384(88)90325-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: New York; 1982. [Google Scholar]
- Pirisino R, Ghelardini C, Banchelli G, Galeotti N, Raimondi L. Methylamine and benzylamine induced hypophagia in mice: modulation by semicarbazide-sensitive benzylamine oxidase inhibitors and aODN towards Kv1.1 channels. Br J Pharmacol. 2001;134:880–886. doi: 10.1038/sj.bjp.0704316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirisino R, Ghelardini C, Pacini A, Galeotti N, Raimondi L. Methylamine, but not ammonia, is hypophagic in mouse by interaction with brain Kv1.6 channel subtype. Br J Pharmacol. 2004;142:381–389. doi: 10.1038/sj.bjp.0705740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Calapai G, Altavilla D, Cucinotta D, Zingarelli B, Campo GM, et al. Food deprivation increases brain nitric oxide synthase and depresses brain serotonin levels in rats. Neuropharmacology. 1994;33:83–86. doi: 10.1016/0028-3908(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Calapai G, Cucinotta D, Altavilla D, Zingarelli B, Ioculano M, et al. Anorectic activity of NG-nitro-L-arginine, an inhibitor of brain nitric oxide synthase, in obese Zucker rats. Eur J Pharmacol. 1993;230:125–128. doi: 10.1016/0014-2999(93)90422-e. [DOI] [PubMed] [Google Scholar]
- Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131–1158. doi: 10.1152/physrev.00015.2004. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Rojas E, Stutzin A, Izumi S, Catt KJ.Desensitization of pituitary gonadotropin secretion by agonist-induced inactivation of voltage-sensitive calcium channels J Biol Chem 198926410939–10942.[published erratum appears in J Biol Chem 1(90 Jan 5;265(1):594] [PubMed] [Google Scholar]
- Sugimoto Y, Yoshikawa T, Yamada J. Involvement of nitric oxide in the 5-HT1A autoreceptor-mediated hyperphagia in rats. Adv Exp Med Biol. 1999;467:109–111. doi: 10.1007/978-1-4615-4709-9_16. [DOI] [PubMed] [Google Scholar]
- Yamada J, Hirose H, Sugimoto Y. Nitric oxide production in hypothalamus of 2-deoxy – glucose-treated and food deprived mice. Neurosci Let. 2002;327:107–110. doi: 10.1016/s0304-3940(02)00396-8. [DOI] [PubMed] [Google Scholar]
- Yu PH. Deamination of methylamine and angiopathy; toxicity of formaldehyde, oxidative stress and relevance to protein glycoxidation in diabetes. J Neural Transm Suppl. 1998;52:201–216. doi: 10.1007/978-3-7091-6499-0_19. [DOI] [PubMed] [Google Scholar]
- Yu PH. Involvement of cerebrovascular semicarbazide-sensitive amine oxidase in the pathogenesis of Alzheimer's disease and vascular dementia. Med Hypotheses. 2001;57:175–179. doi: 10.1054/mehy.2001.1329. [DOI] [PubMed] [Google Scholar]