Abstract
Background and purpose:
Up-regulation of proteinase-activated receptor-2 (PAR2) is a factor in a number of disease states and we have therefore examined the signalling pathways involved in the expression of the receptor.
Experimental approach:
We investigated the effects of tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), trypsin and the PAR2 activating peptide, 2-furoyl(2f)-LIGKV-OH on both mRNA and functional expression of PAR2 in human umbilical vein endothelial cells (HUVECs). The effect of specific chemical inhibitors and dominant negative adenovirus constructs of the mitogen-activated protein kinase (MAPK) cascade and the nuclear factor kappa B (NF-κB) signalling pathway was assessed. Methods included semi-quantitative and quantitative RT-PCR, [3H]inositol phosphate (IP) accumulation and Ca2+-dependent fluorescence.
Key results:
The above agonists induced both mRNA and functional expression of PAR2; PAR4 mRNA, but not that for PAR1 or PAR-3, also increased following TNFα treatment. Inhibition of p38 MAP kinase reduced PAR2 and PAR4 expression, whilst inhibition of MEK1/ERK/JNK was without effect. A similar dependency upon p38 MAP kinase was observed for the expression of PAR4. TNFα -induced enhancement of PAR2 stimulated [3H]-inositol phosphate accumulation (IP) and Ca2+ signalling was abolished following SB203580 pre-treatment. Infection with adenovirus encoding dominant-negative IKKβ (Ad.IKKβ+/−) and to a lesser extent dominant-negative IKKα (Ad.IKKα+/−), substantially reduced both control and IL-1β- induced expression of both PAR2 and PAR4 mRNA and enhancement of PAR2-stimulated IP accumulation and Ca2+ mobilisation.
Conclusions and implications:
These data reveal for the first time the signalling events involved in the upregulation of both PAR2 and PAR4 during pro-inflammatory challenge.
Keywords: Adenovirus, 2-furoyl(2f)-LIGKV-OH, Human umbilical vein endothelial cells, inhibitory kappa B kinase, nuclear factor kappa B, p38 MAP kinase, proteinase-activated receptor
Introduction
The protease-activated receptors (PARs) are a family of G-protein coupled receptors (GPCRs) activated by a unique proteolytic mechanism, whereby the N-terminus is cleaved by a serine protease and activation of the receptor is achieved intramolecularly (Vu et al., 1991; Coughlin, 2002). Of the four members currently identified (PARs 1–4) (Macfarlane et al., 2001; Hollenberg and Compton, 2002), PAR2 has been the subject of considerable recent attention (Nystedt et al., 1995). It is believed to play important roles in the regulation of skin development and pigmentation (Derian et al., 1997; Seiberg et al., 2000), intestinal function (Kawabata et al., 1999, 2000a, 2000b) and angiogenesis, and is implicated in a number of inflammatory conditions including inflammatory pain (Steinhoff et al., 2000), arthritis (Ferrell et al., 2003) and type IV dermatitis (Kawagoe et al., 2002).
A consistent and striking feature of PAR2 within disease models within the vasculature and elsewhere is the marked increase in receptor expression. Inflammatory agents such as lipopolysaccharide (LPS) and TNFα have been shown previously to increase mRNA for the receptor in HUVECs (Nystedt et al., 1996), whereas in rats LPS pretreatment in vivo enhances PAR2-mediated endothelium-dependent vasodilation in vitro (Cicala et al., 1999). PAR2 expression is also upregulated in malignant tissue (D'Andrea et al., 2001), following radiation injury (Wang et al., 2003) or exposure to ultraviolet light (Scott et al., 2001) in asthma (Knight et al., 2001), following viral lung infection (Lan et al., 2004) or as a result of smoking (Miotto et al., 2002). These data suggest a mechanism whereby, during inflammation, enhanced release of PAR2 activator, coupled with increased receptor expression increases PAR2-mediated pro-inflammatory responses. To date, no information is available regarding the mechanism(s), which regulate the induction of the receptor and this may be an important site for inhibition of the pro-inflammatory actions of PAR2. A similar paucity of information exists regarding the other novel PARs, in particular PAR4, which is also activated by PAR2 effectors such as trypsin (Xu et al., 1998) and is also upregulated following inflammatory challenge (Hamilton et al., 2001).
In this study, we addressed the role of a number of candidate signalling events which may regulate cytokine-induced upregulation of PAR2 expression and to a lesser extent PAR4. This includes the major mitogen-activated protein (MAP) kinases (ERK, p38 and JNK), known to be intracellular effectors of cytokine receptor stimulation and the NFκB signalling pathway, including the potentially divergent roles of the upstream kinases inhibitory kappa B kinases (IKK)-α and -β. Our results reveal a crucial role for p38 MAP kinase in the expression of PAR2 and PAR4, and the NFκB pathway principally through the activation of IKKβ.
Materials and methods
Cell culture
HUVECs were grown in endothelial basal media, supplemented with endothelial growth media (EGM-2) containing single aliquots (2% foetal bovine serum, 0.2 ml hydrocortisone, 2 ml hFGF-B, 0.5 ml VEGF, 0.5 ml R3-insulin like growth factor-1, 0.5 ml ascorbic acid, 0.5 ml hEGF, 0.5 ml GA 1000, 0.5 ml heparin) purchased from Cambrex. All experiments were performed between passages 3 and 10. Clone G cells (NCTC-2544 expressing PAR2) were maintained in M199 media supplemented with 10% FCS, L-glutamine (27 mg ml−1), penicillin (250 units ml−1), streptomycin (25 μg ml−1) and geneticin (400 μg ml−1). Cells were incubated at 37°C in humidified air with 5% CO2.
Generation of recombinant dominant-negative adenoviral constructs
Recombinant replication-deficient adenoviral vectors encoding dominant-negative (DN) p38β (Ad.p38β+/−), IKKβ(K44A) (Ad.IKKβ+/−) (Oitzinger et al., 2001) and wild-type IκBα (Ad.IκBα) were a generous gift from Rainer de Martin (University of Vienna, Vienna, Austria). An adenoviral vector encoding DN IKKα (K44A) (Ad.IKKα+/−) was created in-house using the Adeno-X system from Clontech (CA, USA). The IKKα+/− plasmid (24) was originally a gift from Dr D Goeddel (Tularik Inc., CA, USA). Large-scale production of high-titre recombinant adenovirus was performed as described by Nicklin and Baker (1999).
Infection of HUVECs
HUVECs, when approximately 70% confluent, were incubated with Ad.p38β+/−. Ad.IKKα+/−, Ad.IKKβ+/− or Ad.IκBα+/− at a multiplicity of infection (m.o.i.) of 300 for 16 h in endothelial growth media after which the medium was replaced. Cells were stimulated with agonist 40 h after infection and changes in PAR2 gene expression were determined by semi-quantitative and quantitative reverse transcription-polymerase chain reaction (RT-PCR).
RNA extraction and RT-PCR
Total RNA was extracted from cells using the QIAGEN RNeasy minikit (Qiagen, Crawley, West Sussex, UK) as per manufacturers instruction. About 4–5 μg of total RNA was reverse transcribed into cDNA in a total volume of 20 μl using the Superscript first-strand synthesis system for RT-PCR (Invitrogen, San Diego, CA, USA) using 1 μl of oligo (dT) primers (0.5 μg μl−1). About 1 μl of resulting cDNA was subjected to 35 cycles of PCR for amplification of PAR2 and cytoplasmic β-actin. PCR was performed in a 50 μl reaction volume containing 1 μM of each primer, 0.2 mM dNTP and 2.5 U Klentaq DNA polymerase (Clontech Laboratories Ltd., Mountain View, CA, USA). The PCR conditions for both primers sets were 94°C for 2 min followed by 35 cycles of 94°C for 45 s, 60°C for 1 min, 68°C for 2 min and a final extension of 68°C for 10 min. The nucleotide sequence of the primers used were 5′-AAC CAA GCT TTC TCG GTG CGT CCA GT-3′ (Forward) 5′-GCT CTA GAC TGC AAT TCC CAT CTG AGG-3′ (Reverse) for PAR2 and 5′-CGT GGG GCG CCC CAG GCA CCA-3′ (Forward), 5′-CCC CCT GAA CCC CAA GGC CAA-3′ (Reverse) for cytoplasmic β-actin, both primer sets were purchased from Invitrogen Ltd. (Renfrew, UK). DNA samples were run on a 0.8% agarose gel with a 1 kB DNA hyperladder to determine the size of PCR products. The DNA bands were visualized on a transilluminator and a digital image captured.
TaqMan RT-PCR (quantitative RT-PCR)
Reverse transcription was performed using a GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA, USA) as recommended by the manufacturer's instructions. TaqMan RT-PCR was also performed according to the manufacturer's instructions (Applied Biosystems). Oligonucleotide primers and probes for human PARs were designed using the Primer Express program and were synthesized by Applied Biosystems. Primers and probes used for TaqMan RT-PCR were: the following for PAR1 5′-TTG CTT CGG ACC CAC AAA C-3′ (Forward) 5′-CCT CTG TGG TGG AAG TGT GAG A-3′ (Reverse) 5′-TCC TCC TGA TTG CGC ATT ACT CAT TCC T-3′ (Probe). For PAR2: 5′-GGC AAC ATG TAC TGT TCC ATT CTC-3′ (Forward) 5′-GGT TCA CGA TGA CCC AAT ACC T-3′ (Reverse) 5′-TCA TGA CCT GCC TCA GTG TGC A-3′ (Probe). For PAR3: 5′-GGC CAC CAC AGT CAT CTT CTA TG-3′ (Forward) 5′-GCG GTT GAT GCT GAT GCA-3′ (Reverse) 5′-CAA CAT GTA CTG CTC CAT TCT GCT C-3′ (Probe). For PAR4 5′-CTG CGT GGA TCC CTT CAT CT-3′ (Forward) 5′-CCT GCC CGC ACC TTG TC-3′ (Reverse) 5′ TAC TAC GTG TCG GCC GAG TTC AG-3′ (Probe). Sequence-specific amplification was detected with an increased fluorescent signal of 6-carboxy fluorescein (6-FAM) during the amplification cycles. Amplification of human rRNA (ribosomal RNA) was used in the same reaction of all samples as an internal control. Gene-specific mRNA was subsequently normalized to rRNA. Primers and probe for rRNA were purchased from Applied Biosystems.
Indirect Immunofluorescent microscopy
HUVECs were grown to 50% confluency on No. 0 glass coverslips (Merck Biosciences, Nottingham, England) before the experiments. Cells were washed × 3 with ice-cold phosphate-buffered saline (PBS) and fixed with ice-cold methanol for 20 min at room temperature. Methanol was washed off, and the coverslips were incubated with PBS containing 1% (w/v) bovine serum albumin for 30 min at room temperature. Primary PAR-2 antibody B5 (Seatter et al., 2004), 1:400, was added for 60 min and then following three washes in PBS incubated with secondary antibody for 60 min in the dark at room temperature. The coverslips were washed and mounted in Moviol onto slides and analysed using a Nikon Eclipse E600 fluorescent microscope (Sloss et al., 2005).
Measurement of inositol phosphate accumulation
The cellular accumulation of total [3H]inositol phosphates 1–4 ([3H]IP) was assessed in cells prelabelled with 1–3 μCi ml−1 of [3H]2-myo-inositol for 24 h and preincubated with 10 mM LiCl for 30 min before stimulation. Cells were washed in PBS, and inositol phosphates were extracted by partition in 2:1 methanol/chloroform. After further addition of deionized water and chloroform, the inositol phosphate-containing aqueous phase was loaded onto prewashed Dowex formate (AG 1-X8 resin, 200–400 mesh formate) anion-exchange resin, and [3H]IP were eluted using anion-exchange chromatography as outlined previously (Plevin et al., 1994).
Calcium signalling assay
HUVECs were grown to confluency on 13 mm circular glass cover slips. On the day of the experiment, growth mediim was replaced with 1 ml of bath solution (NaCl 150 mM, KCl 5.4 mM, MgCl2 1.2 mM, CaCl 1.8 mM, glucose 10 mM, HEPES 10 mM, pH 7.4 (NaOH)), containing 5 μM Fura-2(AM) and incubated for 60 min at 37°C in humidified air with 5% CO2. Fluorescence measurements were performed on a Perkin Elmer LS50B spectrophotometer with an excitation wavelength of 360 and 380 nm and emission recorded at 510 nm; results are expressed as a ratio of 360/380 nm.
Western blotting
Proteins were fractionated by SDS-polyacrylamide gel electrophoresis and electrophoretically transferred onto nitrocellulose membrane (Protran, Whatman, GmbH, Germany). Membranes were probed with antibodies raised against phospho-ERK, phospho-38 MAP kinase, IκBα, total p38 and total ERK, and protein expression determined by chemiluminescence.
Data analysis
Results are expressed as means±s.e.m. from the number of experiments shown. Statistical analysis was by analysis of variance followed by Dunnett's test for multiple comparisons. P values <0.05 were taken to show significant differences between means.
Reagents
All materials used were of the highest commercial grade available and were purchased from Sigma (Dorset, UK) unless otherwise stated. The p38 MAP kinase inhibitor, SB203580, JNK inhibitor, SP600125 and MEK inhibitors, PD98059 and U0126, were obtained from Calbiochem (San Diego, CA, USA). Antibodies raised against phosphorylated forms of ERK and p38 MAP kinase were purchased from Biosource (CA, USA), anti-IκBα, anti-ERK1/2 and p38 MAP kinase antibodies were purchased from Santa Cruz Biotechnology (CA, USA), interleukin-1β (IL-1β) and tumour necrosis factor-α (TNFα) were purchased from Insight Biotechnology (Middlesex, UK). The PAR2 activating peptide 2f-LIGKV-OH was a kind gift of the Kowa Company Ltd. (Tokyo, Japan).
Results
Cytokine-mediated upregulation of PAR2 and PAR4 mRNA and functional responses in HUVECs
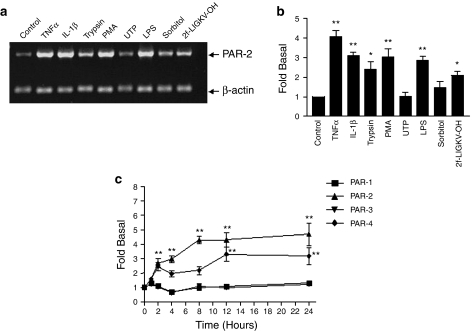
In initial experiments, a number of endothelial cell agonists were assessed for their ability to increase PAR2 mRNA expression using semi-quantitative PCR (Figure 1). TNFα (10ng ml−1) stimulated a significant increase in PAR2 expression which peaked between 8 and 12 h (data not shown) and stayed elevated for 24 h, the time point is represented in panels a and b. IL-1β and PMA, but not uridine triphosphate (UTP), also stimulated a strong increase in the expression of PAR2, approximately three-fold of basal values. In addition, activation of PAR2 itself either using trypsin or the selective PAR2-activating peptide (AP), 2f-LIGKV-OH (Kawabata et al., 2004), also significantly enhanced the expression of PAR2 mRNA. Quantitative PCR confirmed that TNFα increased PAR2 expression over a 24 h time course (Figure 1c). No change in PAR1 and 3 was detected; however, PAR4 mRNA was also enhanced by TNFα pre-treatment, albeit with somewhat slower kinetics than PAR2. Nevertheless, levels of both PAR2 and PAR4 mRNA were similar by 24 h after stimulation.
Figure 1.
Effect of endothelial cell agonists on expression of PAR2 mRNA in HUVECs. HUVECs were stimulated with TNFα (10 ng ml−1), IL-1β (10 ng ml−1), trypsin (30 nM), PMA (100 nM), UTP (50 μM), LPS (200 ng ml−1), sorbitol (0.5 M) and 2f-LIGKV-OH (100 μM) for 24 h (a and b) or for the times indicated (c). In panel a, cells were assessed for PAR2 mRNA expression by semi-quantitative PCR as outlined in Materials and methods. In panel b, gels were semi-quantified by densitometry. Cytoplasmic β-actin was used to standardize each sample. In panel c, HUVECs were stimulated with TNFα (10 ng ml−1) for 24 h, and PAR1, PAR2, PAR3 and PAR4 gene expression were determined by real-time quantitative RT-PCR. The relative expression levels of PARs mRNAs were determined using an endogenous reference (rRNA) and appropriate standard curves. Each value represents the mean±s.e.m. from at least three experiments. Statistical analysis was performed by Dunnett's test for multiple comparisons. **P<0.01 vs control; *P<0.05 vs control.
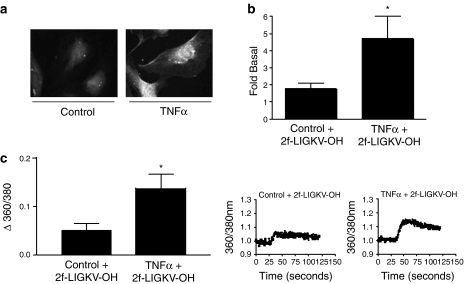
The cytokine-mediated increase in PAR2 expression was further examined using immunofluorescence (Figure 2a). In non-stimulated cells there was considerable staining with a substantial amount within the cytosol, presumably in the Golgi. However, following TNFα treatment there was a marked increased in staining at the plasma membrane confirming increased PAR2 expression. However, Western blotting of PAR2 in either whole-cell extracts or purified plasma membranes generated multiple non-specific bands with no observed increase in any relevant bands following cytokine pre-treatment (data not shown). Thus, to confirm that TNFα and also other agents increased functional PAR2 receptor expression at the membrane, stimulation of [3H]IP accumulation and Ca2+ mobilization was assessed (Figure 2b and c). In control HUVECs stimulation with 2f-LIGKV-OH for 30 min increased [3H]IP accumulation, but 24 h before treatment with TNFα caused the 2f-LIGKV-OH-stimulated [3H]IP accumulation to increase further above the relevant control (Figure 2b). IL-1β and LPS had a similar effect to TNFα; however, following PMA pre-treatment, no significant change in 2f-LIGKV-OH-stimulated [3H]IP accumulation was observed over that for the untreated control (1.33±0.64-fold of basal). Furthermore, despite increasing PAR-2 mRNA, pretreatment of the cells with trypsin did not significantly augment subsequent peptide stimulation of [3H]IP accumulation (2.40±0.40 versus 1.77±0.6).
Figure 2.
The effect of cytokine pretreatment on PAR2-mediated stimulation of [3H]IP accumulation and Ca2+ mobilization. HUVECS were pre-treated with TNFα (10 ng ml−1, panels a–c) for 24 h before stimulation with 2f-LIGKV-OH (b and c). In panel a, cells were stained for PAR2 expression using B5 antibody. In panel b, cells were also prelabelled with 2 μCi ml−1 [3H]2-myo-inositol overnight before experiments; in panel c cells were preincubated with 5 μM Fura-2(AM). [3H]inositol phosphate accumulation was measured over a 30-min period, whereas [Ca2+]i over 120 s as outlined in the Materials and methods. Each value represents the mean±s.e.m. from at least three experiments. Statistical analysis for panel b was performed by Dunnett's test for multiple comparisons, analysis of panel c was performed by a paired t-test. *P<0.05 vs 2f-LIGKV-OH.
When intracellular Ca2+ mobilization was assessed using the Ca2+ fluorescent dye Fura-2(AM), a similar pattern was obtained (Figure 2c), cytokine pretreatment, as exemplified by TNFα, was found to enhance significantly Ca2+ signalling in responses to 2f-LIGKV-OH. In preliminary control experiments, we used UTP as a PAR2 agonist and found that TNFα failed to enhance the Ca2+ signal in response to this agonist (data not shown), suggesting that the effect of TNFα in these experiments was associated with effects upon PAR2 upregulation. Attempts to assess PAR4 upregulation by assessing immunofluorescence, Western blotting, IP accumulation and Ca2+ mobilization were not successful; either immunofluorescence or Western blotting revealed non-specific binding, and PAR4 stimulation gave no increase in IP accumulation or Ca2+ mobilization (data not shown).
The role of p38 MAP kinase in the induction of PAR2 and PAR4 in HUVECs
The activation of candidate MAP kinases and NFκB signalling by agonists that regulate PAR2 expression is shown in Figure 3. TNFα and IL-1β were found to be strong activators of p38 MAP kinase (Figure 3a), which on some occasions gave a double-band indicative of activation of two isoforms of the enzyme. JNK was also strongly activated (data not shown) but ERK phosphorylation was increased differently, with TNFα being largely ineffective (Figure 3b). Both cytokines gave a strong activation of IκBα loss, indicative of NFκB activation (Figure 3c). In contrast, trypsin or the PAR2-AP (data not shown) while giving a moderate activation of p38 MAP kinase (Figure 3a), induced no loss of IκBα expression (Figure 3c).
Figure 3.
Activation of ERK, p38 MAP kinase and IκBα degradation by TNFα, IL-1β and trypsin in HUVECs. Cells were stimulated with TNFα (10 ng ml−1), IL-1β (10 ng ml−1) and trypsin (30 nM) for the times and at the concentrations outlined. Cell lysates were assessed by Western blotting for p38 MAP kinase (a) and ERK activation (b) using specific anti-phospho antibodies, IκBα levels using an anti-IκBα antibody (c), or total ERK and p38 MAP kinase (d) as outlined in the Materials and methods. Blots were semi-quantified by densitometry. Each blot is representative of at least three others. Statistical analysis was performed by Dunnett's test for multiple comparisons. **P<0.01 vs control; *P<0.05 vs control.
The effect of kinase inhibition upon PAR2 expression was then assessed by semi-quantitative PCR (Figure 4). Three recognized inhibitors of MAP kinase activation were used over concentration ranges used previously in our laboratory (Cameron et al., 2003). Pretreatment of cells with SB203580 markedly reduced PAR2 mRNA levels, suggesting a role for p38 MAP kinase in the regulation of PAR2 expression (Figure 4a and e). In contrast, inhibition of ERK or JNK by pretreatment with PD098059 and U1026: both MEK-1 inhibitors and the JNK inhibitor, SP100625, did not significantly decrease PAR2 mRNA expression (Figure 4a, b and e). Infection of cells with Adv. DN-p38 MAP kinase β (Figure 4d and e) also significantly reduced the TNFα-mediated enhanced PAR2 expression, confirming a role for p38 MAP kinase. The 2f-LIGKV-OH-mediated increase in PAR2 expression was also significantly inhibited by SB203580 (Figure 4c and e).
Figure 4.
Effect of MAP kinase inhibition upon PAR2 expression in HUVECs. Cells were treated with DMSO (0.2%), SB203580 (10 μM) and SP10025 (10 μM). PD098059 (50 μM) or U0126 (50 μM) for 30 min before stimulation with TNFα (10 ng ml−1) (a and b) or 2f-LIGKV-OH (100 μM) (c) for 24 h. In d, cells were infected with 300 m.o.i. of vector or Ad.p38β+/− for 40 h. Samples were assessed for PAR2 expression by semi-quantitative RT-PCR as outlined in Material and methods. In panel e, gels were semi-quantified by densitometry using cytoplasmic β-actin to standardize each sample. Clone G is a positive control sampled from a PAR2-transfected cell lines. Each value represents the mean±s.e.m. from at least three experiments. Statistical analysis was performed by Dunnett's test for multiple comparisons. **P<0.01 vs TNFα; *P<0.05 vs TNFα.
The effect of p38 MAP kinase inhibition upon the expression of other PARs was examined further in Figure 5 using quantitative PCR. Confirming the results in Figure 4, PAR2 expression was strongly inhibited by SB203580 over a range of concentrations. However, we found that PAR4 expression was also completely abolished, showing a greater sensitivity than PAR2, to inhibition of p38 MAP kinase. In contrast, mRNA expression for PAR1 and PAR3 was not significantly altered by addition of SB203580, suggesting a lack of p38 MAP kinase involvement in the regulation of expression of these receptors (data not shown).
Figure 5.
Effect of p38 MAP kinase inhibition upon expression of PARs1–4 in HUVECs assessed by quantitative PCR. HUVECs were pretreated with SB203580 at concentrations of 1, 10 and 20 μM 30 min before 24 h TNFα treatment. The relative expression levels of PAR2 and PAR4 mRNAs were determined using endogenous reference (rRNA) and appropriate standard curves. Data are mean±s.e.m. from three separate experiments and are expressed as relative expression level compared with the control. Statistical analysis was performed by Dunnett's test for multiple comparisons. ***P<0.001, **P<0.01 vs TNFα stimulation.
The role of p38 MAP kinase in the regulation of functional PAR2 receptor expression was assessed by measuring PAR2-AP-stimulated [3H]inositol phosphate accumulation (Figure 6a) and intracellular Ca2+ mobilization (Figure 4b) following treatment with SB203580. TNFα potentiated the increases in [3H]IP accumulation and [Ca2+]i stimulated by PAR2-AP. However, in cells incubated with SB203580 before pretreatment with TNFα, this potentiation was lost. A similar result was also observed for IL-1β (panel A) at the level of IP accumulation and in preliminary results at the level of Ca2+ mobilization (data not shown).
Figure 6.
Effect of SB203580 upon TNFα-induced potentiation of PAR2-mediated [3H]IP accumulation and Ca2+ mobilization. HUVECS were treated with SB203580 (10 μM) for 30 min before treatment with TNFα for 24 h. In a, cells were stimulated with 50 μM 2f-LIGKV-OH for a further 30 min in the presence of 10 mM LiCl, and [3H]IP accumulation was assayed as outlined in Materials and methods. In b, cells were preincubated with 5 μM Fura-2(AM) and [Ca2+]i measured in response to 50 μM 2f-LIGKV-OH over 120 s as outlined in Materials and methods. Individual traces were analysed and quantified. Each value represents the mean±s.e.m. from at least three experiments. Statistical analysis was performed by Dunnett's test for multiple comparisons. **P<0.01, *P<0.05 vs TNFα plus 2f-LIGKVOH.
The role of NFkB signalling in PAR2 and PAR4 induction in HUVECs
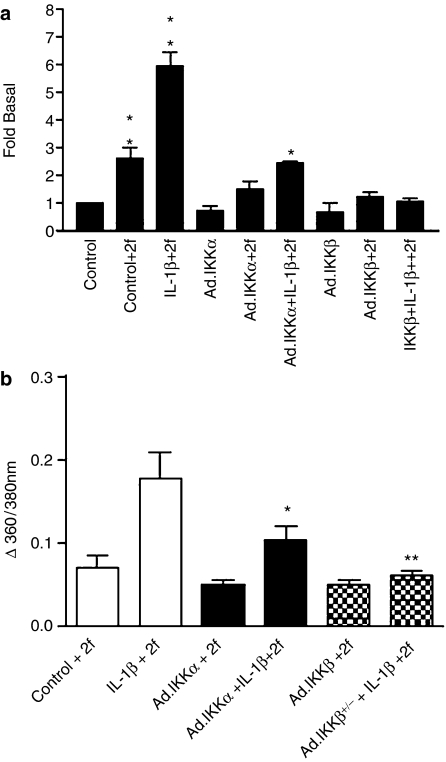
As TNFα and other endothelial cell agonists were also able to activate NFκB signalling as assessed by IκBα degradation, the role of NFκB signalling in the regulation of PAR2 expression was also examined (Figure 7). Cells were infected with vector control, which had no significant effect upon PAR2 expression (Figure 7a), or Ad.IKKα-/+, Ad.IKKβ+/− and Ad.IκBα (Figure 7b), and subsequently stimulated with IL-1β rather than TNFα, as TNFα is known to initiate cellular apoptosis under conditions of NFκB inhibition (Senftleben et al., 2001b). Under conditions shown to give greater than 90% cellular infection (Liu et al., 2001), infection with Ad.IKKβ+/− was found to inhibit substantially IL-1β-stimulated PAR2 expression (Figure 7d). Infection with Ad.IKKα+/− and Ad.IκBα also reduced PAR2 expression, but to a lesser extent than that observed for Ad.IKKβ+/−. Basal expression was also reduced following infection with any of the three constructs; however, fold stimulation remained significantly reduced for DN-IKKβ+/− (Figure 7d). Pharmacological inhibition of IKKβ using the IKKβ inhibitor SC514 (Gomez et al., 2005) also resulted in a substantial inhibition of both control and IL-1β-induced PAR2 expression (Figure 7c and d), again supporting a role for IKKβ.
Figure 7.
Effect of NFκB inhibition upon PAR2 expression in HUVECs. HUVECs were infected with vector (a), Ad.IKKα+/−, Ad.IKKβ+/− or Ad.IκBα (b) at an m.o.i. of 300 for 40 h before stimulation with 10 ng ml−1 IL-1β for a further 24 h. In c, cells were incubated with 300 μM SC514, 1 h prior to IL-1β addition. In panels a–c, cells were assessed for PAR2 expression by semi-quantitative PCR as outlined in Materials and methods. Data are mean±s.e.m. from three separate experiments and are expressed as fold of unstimulated control (d). Statistical analysis was performed by Dunnett's test for multiple comparisons. **P<0.01 vs IL1β; *P<0.05 vs IL-1β.
A similar profile was also obtained for PAR4 when assessed in conjunction with PAR2 using real-time PCR (Figure 8). Figure 8a shows an almost identical profile in PAR2 expression following adenovirus expression as that shown previously using semi-quantitative PCR; Ad.IKK β+/− was the most effective inhibitor of PAR2 expression. In panel B, IL-1β-stimulated PAR4 upregulation was found to be significantly reduced following infection with Ad.IKKβ+/− and Ad.IκBα. All three constructs also decreased basal expression of PAR4, suggesting that both basal- and agonist-stimulated expression are affected by inhibition of the NFκB pathway.
Figure 8.
Effect of NFκB inhibition PAR2 and PAR4 expression assessed by quantitative PCR. HUVECs were infected with Ad.IKKα+/−, Ad.IKKβ+/− or Ad.IκBα at an m.o.i. of 300 for 40 h before stimulation with 10 ng ml−1 IL-1β for a further 24 h. Expression levels of mRNA for PAR2 (a) and PAR4 (b) were determined by real-time quantitative RT-PCR. The relative expression levels of the PAR mRNAs were determined using endogenous reference (rRNA) and appropriate standard curves. Data are mean±s.e.m. from three separate experiments and are expressed as fold of unstimulated control. Statistical analysis was performed by Dunnett's test for multiple comparisons. *P<0.05 vs IL-1β.
The effects of NFκB inhibition on functional PAR2 receptor expression as assessed by either [3H]inositol phosphate accumulation or intracellular Ca2+ activation are shown in Figure 9. In control cells, stimulation by 2f-LIGKV-OH of [3H]inositol phosphate accumulation was significantly enhanced by IL-1β pre-treatment (Figure 9a). Infection with either Ad.IKKα+/− or Ad.IKKβ+/− substantially reduced the potentiated response; Ad.IKKβ+/− was more effective and reduced the 2f-LIGKV-OH response following IL-1β treatment to basal values, although a significant AP-stimulated response was still obtained following treatment of the Ad.IKKα+/−-infected cells with IL-1β and AP. Similarly, infection with Ad.IKKβ+/− essentially abolished all AP-induced Ca2+ responses in pretreated cells but with Ad.IKK+/− infection, a residual AP response remained.
Figure 9.
Effect of NFκB inhibition on functional expression of PAR2 in HUVECs. HUVECs were infected with Ad.IKKα+/−, Ad.IKKβ+/− or Ad.IκBα at an m.o.i. of 300 for 40 h and pre-treated with IL-1β (10 ng ml−1) for a further 8 h before stimulation with 2f-LIGKV-OH (50 μM). Cells were assessed for either [3H]inositol phosphate accumulation (a) or [Ca2+]i fluorescence (b) as outlined in Materials and methods. Each value is the mean±s.e.m. of triplicate determinations from an experiment representative of three others. Statistical analysis was performed by Dunnett's test for multiple comparisons. **P<0.01, *P<0.05 vs IL-1β plus 2f.
Discussion
In this study we have, for the first time, elucidated the intracellular mechanisms by which cytokines and GPCRs, including PAR2 itself, regulate the expression of PAR2 in endothelial cells. Both semi- and quantitative PCR revealed selective upregulation of PAR2 and PAR4 in HUVECs occurring with similar kinetics, PAR2 expression being maximal by 8 h, whereas PAR4 expression did not reach maximal levels until 12 h. Cytokine-mediated PAR2 mRNA induction was accompanied by a marked increase in functional activation of the receptor, an effect previously observed in vivo following LPS treatment (Cicala et al., 1999) and indicative of an increase in receptor expression. In contrast, we found that despite increases in PAR4 mRNA, similar increases in functional response for this receptor could not be reproduced. Increases in [3H]IP or [Ca2+]i in response to PAR4 peptide were not observed, not even when pretreated with TNFα. The functional expression of PAR4 on endothelial cells is controversial, as vessel-specific responses have been noted. However, recent reports have shown PAR4-mediated responses in HUVECs (Syeda et al., 2006) and, in one report, NO release in the absence of increases in Ca2+ (Momota et al., 2006). Thus, despite early reports indicating PAR4 coupling to Ca2+ (Kahn et al., 1999), this may not be the case for all cell types.
Of the candidate MAP kinases that were thought likely to regulate PAR2 expression, our studies suggested that p38 MAP kinase was the principal kinase involved. Empirically, agents which strongly activate p38 MAP kinase also stimulated PAR2 expression and this was also shown in another cell type tested, EAhy926, shown previously to be responsive to TNFα (Paul et al., 2000) (results not shown). Thus, as expected, TNFα, IL-1 and LPS were strong inducers of PAR2 receptor expression (Figure 1). However, this analysis also applied to other agents including PMA and trypsin itself, acting via PAR2, both generating a strong p38 MAP kinase signal. PAR2 coupling to p38 MAP kinase has previously been shown by ourselves (Kanke et al., 2001) and others; however, in some cell types activation of this pathway is minimal (Sabri et al., 2000).
Further experiments using the p38 MAP kinase inhibitor SB203580 or the DN mutant kinase, p38β+/−, identified a central role of p38 MAP kinase in the regulation of the functional expression of PAR2 as both mRNA expression and coupling to [3H]IP and [Ca2+]i were strongly inhibited. In contrast, neither ERK nor JNK was implicated. Pretreatment with PD98059, a MEK inhibitor, did reduce PAR2 expression to a minor extent. However, this action was not reproduced by another inhibitor U0126, suggesting a non-specific action of PD98059 in our system. PAR2-mediated induction of PAR2 was also regulated specifically by p38 MAP kinase, demonstrating for the first time a role for this kinase in homologous receptor upregulation. Cytokine-mediated increases in PAR4 expression were also abolished by SB203580, suggesting that activation of p38 MAP kinase is a common mechanism for the upregulation of GPCRs in inflammatory disease. A previous study has shown upregulation of bradykinin B1 receptors to involve p38 MAP kinase and to a lesser extent ERK (Larrivee et al., 1998), data broadly consistent with our hypothesis.
The role of NFκB in the regulation of PAR2 and PAR4 expression was also examined. In particular, we focussed upon the major IKKs, IKKα and IKKβ, which have been demonstrated to have regulatory roles in different aspects of NFκB-dependent gene transcription and cellular function (Hu et al., 1999, 2001; Li et al., 1999a). In our hands we have found pharmacological inhibitors of the NFκB pathway to display questionable specificity (MacKenzie et al., 2003); while the recently synthesized selective IKKβ inhibitors (Kishore et al., 2003) have not been used in a large number of cellular studies. At present there are no selective IKKα inhibitors available, so we initially utilized DN adenoviral versions of IKKα and IKKβ, used previously in our laboratory (MacKenzie et al., 2003). Our results clearly indicate a predominant role for IKKβ in the regulation of the functional expression of PAR2, as not only was mRNA expression abolished, but coupling to both [3H]IP accumulation and [Ca2+]i was also significantly diminished. The effect of Ad.IKKβ+/− is likely to be through the inhibition of the IκBα/NFκB axis, as infection with Ad.IκBα was also effective in reducing PAR2 expression. Our results were consistent with previous studies, which implied an essential role for IKKβ in cytokine-mediated NFκB DNA-binding (Li et al., 1999b; Schwabe et al., 2001). Pharmacological inhibition of IKKβ using the novel inhibitor SC514, previously characterized in our laboratory in human cells (Gomez et al., 2005), did confirm the specific role of this kinase in the induction process. While recent studies have implicated NFκB in the upregulation of B1 receptors (Passos et al., 2004), NK1 receptors (Simeonidis et al., 2003; Weinstock et al., 2003), adenosine A1 receptors (Nie et al., 1998) and other GPCRs by pharmacological inhibition, ours is the first study to use both adenoviral and pharmacological approaches to successfully identify a specific role for IKKβ in the expression of both PAR2 and PAR4.
It was difficult, however, to determine if any other modulatory role for IKKα was involved in regulating PAR2 expression for example through a nuclear action. Histone modification (Anest et al., 2003), phosphorylation of p65 NFκB (Madrid et al., 2000) or processing of larger NFκB precursors (Senftleben et al., 2001a) are possibilities, although there is no confirmation of these functions in cells other than those from IKKα-deficient mice, so far. Nevertheless, as IL-1β and other major cytokines activate IKKα and IKKβ, the most parsimonious interpretation is that IKKα plays a permissive role in PAR2 induction, at least at a basal level. So far we have been unable to identify any genes in HUVECs, which are selectively regulated by IKKα, suggesting that the two kinase subunits both impinge upon the NFκB pathway, albeit at different sites.
From the results in this study, we would conclude that a combination of pathways is likely to be required, including a strong activation of p38 MAP kinase and at least a partial activation of NFκB or vice versa. In models of disease in which PAR2 expression is increased, it is likely that these events occur. This includes arthritis (Ferrell et al., 2003), cancer (D'Andrea et al., 2001), lung disease (Knight et al., 2001; Miotto et al., 2002; Lan et al., 2004) and neuronal trauma (Striggow et al., 2001). In fibroblasts, for example, both platelet-derived growth factor and extracellular matrix stimulate PAR2 expression (Gruber et al., 2004) and both factors are strong activators of p38 MAP kinase (Matsumoto et al., 1999). We have for the first time elucidated the major mechanistic pathways involved in the regulation of PAR2 and PAR4 expression following inflammatory challenge, and given the importance of these receptors in inflammation, and the absence of selective, potent antagonists, inhibition of PAR2 or PAR4 expression by inhibition of regulatory kinase pathways may represent a novel therapeutic strategy.
Grants
Elwyn Ritchie was in receipt of a British Heart Foundation studentship sponsored by R.P. and R.M.D. We gratefully acknowledge the kind gift of reagents from Kowa Company Ltd., Tokyo, Japan.
Abbreviations
- Ad.IκBα
wild-type IκBα
- Ad.IKKα+/−
IKKα dominant-negative adenoviral construct
- Ad.IKKβ+/−
IKKβ dominant-negative adenoviral construct
- Ad.p38β+/−
p38β dominant-negative adenoviral construct
- ERK
extra-cellular regulated kinase
- FCS
foetal calf serum
- GPCR
G-protein coupled receptor
- IKK
inhibitory kappa B kinase
- JNK
c-jun-N-terminal kinase
- HUVECs
human umbilical vein endothelial cells
- IL-1β, interleukin-1β; IP
inositol phosphate
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- MEK1
MAP/ERK kinase-1
- PAGE
polyacrylamide gel electrophoresis
- PAR2-AP
2-furoyl-LIGKV-OH
- PAR
proteinase-activated receptor
- PBS
phosphate-buffered saline
- PMA
phorbol myristate acetate
- RT-PCR
reverse transcription-polymerase chain reaction
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Cameron P, Smith SJ, Giembycz MA, Rotondo D, Plevin R. Verotoxin activates mitogen-activated protein kinase in human peripheral blood monocytes: role in apoptosis and proinflammatory cytokine release. Br J Pharmacol. 2003;140:1320–1330. doi: 10.1038/sj.bjp.0705560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, et al. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in the cardiovascular system. Cold Spring Harb Symp Quant Biol. 2002;67:197–208. doi: 10.1101/sqb.2002.67.197. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001;158:2031–2041. doi: 10.1016/S0002-9440(10)64675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian CK, Eckardt AJ, Andrade-Gordon P. Differential regulation of human keratinocyte growth and differentiation by a novel family of protease-activated receptors. Cell Growth Differ. 1997;8:743–749. [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AB, MacKenzie C, Paul A, Plevin R. Selective inhibition of inhibitory kappa B kinase-beta abrogates induction of nitric oxide synthase in lipopolysaccharide-stimulated rat aortic smooth muscle cells. Br J Pharmacol. 2005;146:217–225. doi: 10.1038/sj.bjp.0706308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Santiago-Schwarz F, Martin CA, Zhang J, Kew RR. Protease-activated receptor-2 (PAR-2) expression in human fibroblasts is regulated by growth factors and extracellular matrix. J Invest Dermatol. 2004;123:832–839. doi: 10.1111/j.0022-202X.2004.23445.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JR, Frauman AG, Cocks TM. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res. 2001;89:92–98. doi: 10.1161/hh1301.092661. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Hu YL, Baud V, Delhase M, Zhang PL, Deerinck T, Ellisman M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKK alpha subunit of I kappa B kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Kahn ML, NakanishiMatsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanke T, Macfarlane SR, Seatter MJ, Davenport E, Paul A, McKenzie RC, et al. Proteinase-activated receptor-2-mediated activation of stress-activated protein kinases and inhibitory kappa B kinases in NCTC 2544 keratinocytes. J Biol Chem. 2001;276:31657–31666. doi: 10.1074/jbc.M100377200. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kanke T, Yonezawa D, Ishiki T, Saka M, Kabeya M, et al. Potent and metabolically stable agonists for protease-activated receptor-2: evaluation of activity in multiple assay systems in vitro and in vivo. J Pharmacol Exp Ther. 2004;309:1098–1107. doi: 10.1124/jpet.103.061010. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kuroda R, Nishikawa H, Kawai K. Modulation by protease-activated receptors of the rat duodenal motility in vitro: possible mechanisms underlying the evoked contraction and relaxation. Br J Pharmacol. 1999;128:865–872. doi: 10.1038/sj.bjp.0702755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata A, Morimoto N, Nishikawa H, Kuroda R, Oda Y, Kakehi K. Activation of protease-activated receptor-2 (PAR-2) triggers mucin secretion in the rat sublingual gland. Biochem Biophys Res Commun. 2000a;270:298–302. doi: 10.1006/bbrc.2000.2404. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Nishikawa H, Kuroda R, Kawai K, Hollenberg MD. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br J Pharmacol. 2000b;129:1808–1814. doi: 10.1038/sj.bjp.0703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe J, Takizawa T, Matsumoto J, Tamiya M, Meek SE, Smith AJ, et al. Effect of protease-activated receptor-2 deficiency on allergic dermatitis in the mouse ear. Jpn J Pharmacol. 2002;88:77–84. doi: 10.1254/jjp.88.77. [DOI] [PubMed] [Google Scholar]
- Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- Lan RS, Stewart GA, Goldie RG, Henry PJ. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L388–L398. doi: 10.1152/ajplung.00286.2003. [DOI] [PubMed] [Google Scholar]
- Larrivee JF, Bachvarov DR, Houle F, Landry J, Huot J, Marceau F. Role of the mitogen-activated protein kinases in the expression of the kinin B1 receptors induced by tissue injury. J Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- Li QT, VanAntwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the I kappa B kinase 2 gene. Science. 1999a;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu WM, Hu YL, Delhase M, Deerinck T, Ellisman M, et al. The IKK beta subunit of I kappa B kinase (IKK) is essential for nuclear factor kappa B activation and prevention of apoptosis. J Exp Med. 1999b;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Paul A, MacKenzie CJ, Bryant C, Graham A, Plevin R. Nuclear factor kappa B is involved in lipopolysaccharide-stimulated induction of interferon regulatory factor-1 and GAS/GAF DNA-binding in human umbilical vein endothelial cells. Br J Pharmacol. 2001;134:1629–1638. doi: 10.1038/sj.bjp.0704404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MacKenzie CJ, Paul A, Wilson S, de Martin R, Baker AH, Plevin R. Enhancement of lipopolysaccharide-stimulated JNK activity in rat aortic smooth muscle cells by pharmacological and adenovirus-mediated inhibition of inhibitory kappa B kinase signalling. Br J Pharmacol. 2003;139:1041–1049. doi: 10.1038/sj.bjp.0705330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJG, Baldwin AS, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappa B. Molec Cellular Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Yokote K, Tamura K, Takemoto M, Ueno H, Saito Y, et al. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J Biol Chem. 1999;274:13954–13960. doi: 10.1074/jbc.274.20.13954. [DOI] [PubMed] [Google Scholar]
- Miotto D, Hollenberg MD, Bunnett NW, Papi A, Braccioni F, Boschetto P, et al. Expression of protease activated receptor-2 (PAR-2) in central airways of smokers and non-smokers. Thorax. 2002;57:146–151. doi: 10.1136/thorax.57.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momota F, Hirano K, Hirano M, Nishimura J, Kanaide H. Involvement of Gi/o in the PAR-4-induced NO production in endothelial cells. Biochem Biophys Res Commun. 2006;342:365–371. doi: 10.1016/j.bbrc.2006.01.165. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Baker AH.Simple methods for preparing recombinant adenoviruses for high efficiency transduction of vascular cells Vascular Disease: Molecular Biology and Gene Transfer Protocols (Methods in Molecular Medicine) 1999Humana Press: New York, NY; In: Baker AH (ed) [DOI] [PubMed] [Google Scholar]
- Nie Z, Mei Y, Ford M, Rybak L, Marcuzzi A, Ren H, et al. Oxidative stress increases A1 adenosine receptor expression by activating nuclear factor kappa B. Mol Pharmacol. 1998;53:663–669. doi: 10.1124/mol.53.4.663. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells – comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- Oitzinger W, Hofer-Warbinek R, Schmid JA, Koshelnick Y, Binder BR, de Martin R. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97:1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- Passos GF, Fernandes ES, Campos MM, Araujo JG, Pesquero JL, Souza GE, et al. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J Immunol. 2004;172:1839–1847. doi: 10.4049/jimmunol.172.3.1839. [DOI] [PubMed] [Google Scholar]
- Paul A, Torrie LJ, McLaren GJ, Kennedy C, Gould GW, Plevin R. P2Y receptor-mediated inhibition of tumor necrosis factor alpha -stimulated stress-activated protein kinase activity in EAhy926 endothelial cells. J Biol Chem. 2000;275:13243–13249. doi: 10.1074/jbc.275.18.13243. [DOI] [PubMed] [Google Scholar]
- Plevin R, Kellock NA, Wakelam MJ, Wadsworth R. Regulation by hypoxia of endothelin-1-stimulated phospholipase Dactivity in sheep pulmonary artery cultured smooth muscle cells. Br J Pharmacol. 1994;112:311–315. doi: 10.1111/j.1476-5381.1994.tb13070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri A, Muske G, Zhang HL, Pak E, Darrow A, Andrade-Gordon P, et al. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ Res. 2000;86:1054–1061. doi: 10.1161/01.res.86.10.1054. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bennett BL, Manning AM, Brenner DA. Differential role of I kappa B kinase 1 and 2 in primary rat hepatocytes. Hepatology. 2001;33:81–90. doi: 10.1053/jhep.2001.20799. [DOI] [PubMed] [Google Scholar]
- Scott G, Deng A, Rodriguez-Burford C, Seiberg M, Han R, Babiarz L, et al. Protease-activated receptor 2, a receptor involved in melanosome transfer, is upregulated in human skin by ultraviolet irradiation. J Invest Dermatol. 2001;117:1412–1420. doi: 10.1046/j.0022-202x.2001.01575.x. [DOI] [PubMed] [Google Scholar]
- Seatter MJ, Drummond R, Kanke T, Macfarlane SR, Hollenberg MD, Plevin R. The role of the C-terminal tail in protease-activated receptor-2-mediated Ca2+ signalling, proline-rich tyrosine kinase-2 activation, and mitogen-activated protein kinase activity. Cell Signal. 2004;16:21–29. doi: 10.1016/s0898-6568(03)00095-0. [DOI] [PubMed] [Google Scholar]
- Seiberg M, Paine C, Sharlow E, AndradeGordon P, Costanzo M, Eisinger M, et al. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp Cell Res. 2000;254:25–32. doi: 10.1006/excr.1999.4692. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001a;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Li ZW, Baud V, Karin M. IKKbeta is essential for protecting T cells from TNFalpha-induced apoptosis. Immunity. 2001b;14:217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, et al. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci USA. 2003;100:2957–2962. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloss CM, Cadalbert L, Finn SG, Fuller SJ, Plevin R. Disruption of two putative nuclear localization sequences is required for cytosolic localization of mitogen-activated protein kinase phosphatase-2. Cell Signal. 2005;17:709–716. doi: 10.1016/j.cellsig.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, et al. Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-kappaB pathways. J Biol Chem. 2006;281:11792–11804. doi: 10.1074/jbc.M509292200. [DOI] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Cloning of the platelet thrombin receptor reveals a novel mechanism of receptor activation. Clin Res. 1991;39:A251. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Hollenberg MD, Wijesuriya SJ, Ou X, Hauer-Jensen M. Up-regulation and activation of proteinase-activated receptor 2 in early and delayed radiation injury in the rat intestine: influence of biological activators of proteinase-activated receptor 2. Radiat Res. 2003;160:524–535. doi: 10.1667/rr3080. [DOI] [PubMed] [Google Scholar]
- Weinstock JV, Blum A, Metwali A, Elliott D, Arsenescu R. IL-18 and IL-12 signal through the NF-kappa B pathway to induce NK-1R expression on T cells. J Immunol. 2003;170:5003–5007. doi: 10.4049/jimmunol.170.10.5003. [DOI] [PubMed] [Google Scholar]
- Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, et al. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]