Abstract
Background and purpose:
Protein kinase C (PKC) comprises at least twelve isoforms and has an isoform-specific action on cardiac electrical activity. The slow component of delayed rectifier K+ current (I Ks) is one of the major repolarizing currents in the hearts of many species and is also potentiated by PKC activation. Little is known, however, about PKC isoform(s) functionally involved in the potentiation of I Ks in native cardiac myocytes.
Experimental approach:
I Ks was recorded from guinea-pig atrial myocytes, using the whole-cell configuration of patch-clamp method.
Key results:
Bath application of phenylephrine enhanced I Ks concentration-dependently with EC50 of 5.4 μM and the maximal response (97.1±11.9% increase, n=16) was obtained at 30 μM. Prazosin (1 μM) almost totally abolished the potentiation of I Ks by phenylephrine, supporting the involvement of α1-adrenoceptors. The stimulatory action of phenylephrine was significantly, if not entirely, inhibited by the general PKC inhibitor bisindolylmaleimide I but was little affected by Gö-6976, Gö-6983 and rottlerin. Furthermore, this stimulatory effect was significantly reduced by dialyzing atrial myocytes with PKCɛ-selective inhibitory peptide ɛV1-2 but was not significantly affected by conventional PKC isoform-selective inhibitory peptide βC2-4. Phorbol 12-myristate 13-acetate (PMA) at 100 nM substantially increased I Ks by 64.2±1.3% (n=6), which was also significantly attenuated by an internal dialysis with ɛV1-2 but not with βC2-4.
Conclusions and implications:
The present study provides experimental evidence to suggest that, in native guinea-pig cardiac myocytes, activation of PKC contributes to α1-adrenoceptor-mediated potentiation of I Ks and that ɛ is the isoform predominantly involved in this PKC action.
Keywords: IKs, PKC, PKCɛ, phenylephrine, α1-adrenoceptor
Introduction
Protein kinase C (PKC) mediates the regulation of cardiac muscle function by a variety of neurotransmitters, hormones and extracellular signalling molecules. Currently, at least 12 isoforms of PKC have been identified in various tissues and are classified into three groups based on their structure and activation mechanisms (Mellor and Parker, 1998); namely, conventional PKC (α, βI, βII and γ) that have a C2-domain and are activated by Ca2+, diacylglycero1 (DAG) and phosphatidylserine (PS); novel PKC (δ, ɛ, μ, θ and η) that do not have a C2-domain and are activated by DAG and PS; and atypical PKC (ζ and λ) that are regulated by PS. Expression of PKC isoforms in the heart displays species- and/or developmental stage-dependent differences (Pucéat et al., 1994; Rybin and Steinberg, 1994). It has been demonstrated that guinea-pig heart coexpresses conventional (α, βII and γ), novel (ɛ) and atypical (ζ) PKC isoforms (Takeishi et al., 1999). There is accumulating evidence to indicate that PKC isoforms in the heart are differentially regulated by various stimuli under physiological and pathological conditions (Takeishi et al., 1999; Hool, 2000; Ruf et al., 2002) and have different functional roles in the modulation of electrical activity (Johnson and Mochly-Rosen, 1995) and the development of cardiac hypertrophy and heart failure (Bowling et al., 1999; Takeishi et al., 2000). In this regard, it is important to note that PKCɛ has been shown to contribute to cardiac protection associated with ischemic preconditioning (Gray et al., 1997; Qiu et al., 1998; Liu et al., 1999).
The slow component of the delayed rectifier K+ current (IKs) is important for the repolarization of atrial (Sanguinetti and Jurkiewicz, 1991; Wang et al., 1993; Gintant, 1996; Bosch et al., 2003; Ding et al., 2004) and ventricular action potentials (Sanguinetti and Jurkiewicz, 1990; Li et al., 1996; Bosch et al., 1998) in the heart of several mammalian species including humans. IKs also represents a relevant target for the action of the sympathetic neurotransmitters, adrenaline and noradrenaline, and thereby mediates sympathetic control of cardiac excitability and contractility (Sanguinetti et al., 1991). Stimulation of β-adrenoceptors has been shown to markedly enhance IKs via activation of the cyclic AMP-dependent protein kinase (PKA) in some types of myocardial cells, including atrial and ventricular myocytes (Walsh and Kass, 1988; Yazawa and Kameyama, 1990; Matsuura et al., 1996). On the other hand, it has been demonstrated in guinea-pig ventricular myocytes that α1-adrenoceptor stimulation produced a modest (approximately 20–30%) increase in IKs via activation of PKC (Tohse et al., 1992). The elevated sympathetic tone is accompanied by an enhancement of outward K+ current through IKs, which is assumed to counteract the depolarizing effect of the simultaneously potentiated L-type Ca2+ inward current (ICa,L) and thereby prevents the excess prolongation of action potential duration, potentially leading to the occurrence of arrhythmias as well as Ca2+ overload in cardiac muscle. Thus, the stimulatory action of both α1- and β-adrenoceptors on IKs appears to play an important physiological role in protecting the heart from the arrhythmogenic and cardiotoxic effects of excess sympathetic activity.
The present study was undertaken to determine which PKC isoform(s) is functionally involved in mediating the stimulatory action of α1-adrenoceptor activation, as well as PMA, on IKs, with the use of the PKC isoform-selective inhibitory peptides and pharmacological inhibitors. Our results provide experimental evidence supporting a functional role of ɛ isoform of PKC (PKCɛ) in mediating α1-adrenergic potentiation of IKs in native atrial myocytes of guinea-pig hearts.
Materials and methods
Isolation of atrial myocytes
All animal procedures were performed in accordance with the guidelines established by the institution's Animal Care and Use Committee. Single atrial myocytes were enzymatically isolated from adult Hartley guinea-pig hearts using a retrograde Langendorff perfusion method as described previously (Powell et al., 1980; Ding et al., 2004).
Solutions and chemicals
Normal Tyrode solution contained (in mM) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5.5 glucose and 5.0 HEPES (pH adjusted to 7.4 with NaOH). The extracellular bath solution used for measuring IKs was normal Tyrode solution supplemented with 0.4 μM nisoldipine (a generous gift from Bayer AG, Wuppertal-Elberfeld, Germany) and 5 μM E-4031 (Wako, Osaka, Japan). Agents added to the extracellular solution included phenylephrine hydrochloride (Sigma, St Louis, MO, USA), prazosin hydrochloride (Sigma), phorbol 12-myristate 13-acetate (PMA, Sigma), bisindolylmaleimide I (BIS-I, Sigma), Gö-6976 (Calbiochem, San Diego, CA, USA), Gö-6983 (Biomol, Plymouth Meeting, PA, USA), rottlerin (Calbiochem) and thymeleatoxin (Calbiochem). The control pipette solution contained (in mM) 70 potassium aspartate, 50 KCl, 10 KH2PO4, 1 MgSO4, 3 Na2-ATP (Sigma), 0.1 Li2-GTP (Roche Diagnostics GmbH, Mannheim, Germany), 5 EGTA, 0.5 CaCl2 and 5 HEPES (pH adjusted to 7.2 with KOH). The concentration of free Ca2+ and Mg2+ in the pipette solution was calculated to be approximately 1.7 × 10−8 (pCa=7.8) and 3.7 × 10−5 M (pMg=4.4), respectively (Fabiato and Fabiato, 1979; Tsien and Rink, 1980). It should be noted that conventional PKC isoforms can be functionally activated by the presence of intracellular free Ca2+ concentration on the order of 10−8 M in guinea-pig cardiac myocytes (Hool, 2000). In some experiments, peptide translocation inhibitor of PKC isoform βC2-4 or ɛV1-2 (Biomol) was added to the pipette solution and was present throughout the recording period (Ron et al., 1995; Johnson et al., 1996).
Whole-cell patch-clamp technique and data analysis
Whole-cell membrane currents (Hamill et al., 1981) were recorded with an EPC-8 patch-clamp amplifier (HEKA, Lambrecht, Germany), and data were low-pass filtered at 1 kHz, acquired at 5 kHz through an LIH-1600 analog-to-digital converter (HEKA) and stored on hard disc drive, using PATCHMASTER software (HEKA). Patch electrodes had a resistance of 1.8–2.5 MΩ when filled with pipette solutions, and approximately 10 min was allowed to elapse to dialyze the interior of cells with test reagents (βC2-4 or ɛV1-2) in pipette solution. Cells were superfused constantly at 1–2 ml min−1 with extracellular solution at 36±1°C in a 0.5 ml bath chamber.
IKs was activated by depolarizing voltage steps given from a holding potential of −50 mV to various levels, under conditions where the Na+ current was inactivated by setting the holding potential to −50 mV, and ICa,L and the rapid component of delayed rectifier K+ current (IKr) were, respectively, blocked by nisoldipine (0.4 μM) and E-4031 (5 μM) added to the extracellular solution for the measurement of IKs (Ding et al., 2004). The effect of external application of test reagents on IKs was investigated after the initial rundown of IKs within 3−5 min of patch rupture was allowed to reach a steady-state level, and the initial rundown of IKs is not shown in the figures. The period of exposure to various reagents is denoted by the horizontal bar in the figures, and the original current traces recorded at time points indicated by numerals are also illustrated in the inset. The zero-current level is indicated to the left of current traces by the horizontal line.
Voltage-dependent activation of IKs was assessed by fitting the normalized I–V relationship of the tail currents to a Boltzmann equation: IKs,tail=1/(1+exp((V1/2–Vm)/k)), where IKs,tail is the tail current amplitude normalized with reference to the maximum value measured at +50 mV, V1/2 is the voltage at half-maximal activation, Vm is the test potential and k is the slope factor. Concentration–response relationship for the potentiation of IKs by phenylephrine was drawn by least-squares fit of a Hill equation: R=Rmax/(1+(EC50/[agonist])nH), where Rmax represents the maximal degree of potentiation expressed as a percentage, EC50 is the concentration giving half-maximal potentiation and nH is the Hill coefficient.
Statistical analysis
All the averaged data are presented as mean±s.e.m. with the number of experiments given in parentheses. Statistical comparisons were evaluated using either Student's t-test or analysis of variance (ANOVA) with Student–Newman–Keuls (SNK) post hoc analysis, as appropriate. Differences were considered to be statistically significant if a P-value <0.05 was obtained.
Results
Potentiation of IKs by phenylephrine via α1-adrenoceptor in guinea-pig atrial myocytes
We first characterized the stimulatory action of phenylephrine on IKs in guinea-pig atrial myocytes. Figure 1a demonstrates a representative example for the time course of IKs response to 30 μM phenylephrine. IKs was repetitively (once every 20 s) activated by depolarizing voltage steps (2 s in duration) applied from a holding potential of −50 mV to a test potential of +30 mV, and the effect of phenylephrine on IKs was evaluated by measuring the amplitude of the tail current elicited upon return to the holding potential, which reflects the degree of IKs activation at the preceding depolarizing test potential. In a total of 16 myocytes, bath application of 30 μM phenylephrine evoked a marked (97.1±11.9%) increase in the amplitude of IKs in a reversible manner (see also Figures 2d and 4).
Figure 1.
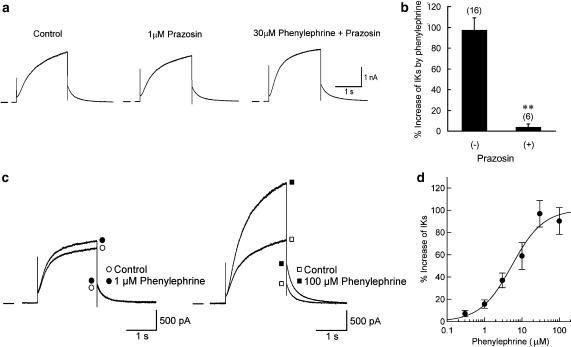
Potentiation of IKs by phenylephrine in guinea-pig atrial myocytes. (a) Time course of changes in the amplitude of IKs tail current during exposure to 30 μM phenylephrine. IKs was repetitively (every 20 s) activated by 2-s depolarizing steps to +30 mV from a holding potential of −50 mV. (b) Superimposed current traces of IKs activated at test potentials of −40 to +50 mV, before and ∼5 min after exposure to 30 μM phenylephrine. a and b were obtained from different myocytes. (c) I–V relationships for IKs tail currents recorded before and during exposure to phenylephrine, obtained from the records in b. (d) I–V relationships for mean values (n=4) of normalized IKs tail currents. Continuous curves through the data points show the least-squares fit of a Boltzmann equation.
Figure 2.
Potentiation of IKs by phenylephrine through α1-adrenoceptor. (a) IKs, recorded in response to 2-s depolarization to +30 mV from a holding potential of −50 mV (the same voltage-clamp protocol as in Figure 1a), under control conditions (left panel), 5 min after exposure to 1 μM prazosin (middle) and 5 min after subsequent addition of 30 μM phenylephrine (right). (b) Percentage increase in IKs tail current, evoked by 30 μM phenylephrine in the absence and presence of 1 μM prazosin. **P<0.01 compared with value in the absence of prazosin. (c) Superimposed current traces of IKs recorded using the same voltage-clamp protocol as in Figure 2a, before and ∼5 min after exposure to phenylephrine at concentration of 1 (left panel) or 100 μM (right). These records were obtained from different myocytes. (d) Concentration–response relationship for the potentiation of IKs by phenylephrine. Percentage increase in IKs tail current was measured for each concentration (0.3–100 μM) of phenylephrine. Each data point represents mean values±s.e.m. of 3–6 myocytes and is fitted with a Hill equation, yielding EC50 of 5.4 μM and nH of 1.03. Only one concentration of phenylephrine was examined in a given myocyte to exclude possible complications of desensitization.
Figure 4.
Summary bar graph showing the percentage increase in the amplitude of IKs tail current by phenylephrine in control and in the presence of pharmacological and peptide inhibitors of PKC isoforms. Effect of each inhibitor was compared with control (*P<0.05 vs control).
Figure 1b shows superimposed current traces of IKs elicited by depolarizing voltage steps given from a holding potential of −50 mV to various test potentials between −40 and +50 mV, before and during exposure to 30 μM phenylephrine. As is evident in Figure 1c, phenylephrine markedly increased the amplitude of IKs tail currents at all test potentials. To elucidate whether phenylephrine affects the voltage dependence of IKs activation, the amplitude of tail current at each test potential was normalized with reference to its maximal value at +50 mV and was then fitted by a Boltzmann equation (Figure 1d). The data points were reasonably well fitted by a Boltzmann equation, yielding V1/2 of 8.6±2.4 mV and k of 12.7±0.7 mV for control, and V1/2 of 5.0±3.2 mV and k of 12.9±0.7 mV for phenylephrine (n=4). There were no significant differences in the values of V1/2 (P=0.133) and k (P=0.850) between control and phenylephrine groups, thus suggesting that phenylephrine has little effect on the voltage dependence of current activation.
In the experiments demonstrated in Figure 2a, the effect of phenylephrine on IKs was examined in the presence of the selective α1-adrenoceptor antagonist prazosin. The atrial myocytes were initially exposed to 1 μM prazosin for ∼5 min, and then to 30 μM phenylephrine in the presence of prazosin. The stimulatory action of phenylephrine on IKs was almost totally abolished by the presence of prazosin (3.7±3.3% increase, n=6 versus 97.1±11.9% increase, n=16; Figure 2b). It should be noted that the amplitude of IKs was only slightly decreased (by 5.3±2.5%, n=6) during exposure to 1 μM prazosin alone (Figure 2a). This observation confirms the view that the action of phenylephrine on IKs is mediated through the α1-adrenoceptor. The potentiation of IKs by phenylephrine was examined at concentrations ranging from 0.3 to 100 μM. Figure 2c illustrates representative examples for the effect of 1 and 100 μM phenylephrine (left and right panels, respectively). Figure 2d depicts the concentration–response relationship for the stimulatory action of phenylephrine on IKs, which could be reasonably well fitted with a Hill equation with EC50 of 5.4 μM and nH of 1.03.
We also evaluated the effect of phenylephrine (30 μM) on the time course of IKs activation during depolarizations in the absence and presence of prazosin. For this purpose, the time to obtain half-maximal activation (T1/2, Stengl et al., 2003; Terrenoire et al., 2005) during 2-s depolarizing voltage steps was compared before and during exposure to phenylephrine. In the absence of prazosin (refer to experiments shown in Figure 1b), T1/2 at test potentials of +10, +30 and +50 mV were, respectively, measured to be 484±56, 342±40 and 305±33 ms (n=5) before phenylephrine and 432±26, 346±34 and 307±44 ms during exposure to phenylephrine. There were no significant differences in the value of T1/2 obtained at each test potential (+10, +30 or +50 mV) before and during exposure to phenylephrine. Similarly, in the presence of 1 μM prazosin (refer to experiments shown in Figure 2a), T1/2 at a test potential of +30 mV (322±10 ms, n=5) was insignificantly affected by further addition of phenylephrine (317±8 ms). These observations are in contrast to an increase in the rate of channel activation during β-adrenergic potentiation of IKs (Han et al., 2001; Stengl et al., 2003; Volders et al., 2003; Terrenoire et al., 2005). Taken together with the results using prazosin (Figure 2b), these data also support a predominant role of α1-adrenoceptors in potentiation of IKs by phenylephrine.
Functional role of novel PKC isoform PKCɛ in potentiation of IKs by phenylephrine
We then evaluated a role for PKC and its isoforms in the potentiation of IKs via α1-adrenoceptors. For this purpose, the stimulatory action of phenylephrine was measured at its maximally effective concentration (30 μM, Figure 2d) in the presence of various pharmacological (bath) and peptide inhibitors (pipette) for PKC isoforms. Figure 3a illustrates a typical example for the action of the general (nonisoform-specific) PKC inhibitor BIS-I (Toullec et al., 1991), which at submicromolar concentrations potently inhibits most PKC isoforms, including conventional (α, βI, βII and γ), novel (δ and ɛ) and atypical (ζ) isoforms (Martiny-Baron et al., 1993). In a total of six myocytes, phenylephrine (30 μM) potentiated the amplitude of IKs by 38.1±12.1% in the presence of BIS-I (100 nM), which is significantly smaller than the degree of IKs potentiation under control conditions (97.1±11.9% increase, n=16; P<0.05; Figure 4). A similar degree of IKs potentiation by phenylephrine (30 μM) was observed in atrial myocytes pretreated for 5–10 min with higher concentration (1 μM) of BIS-I (40.3±6.1% increase, n=5; Figure 4). These results support an involvement of PKC activation in the potentiation of IKs via α1-adrenoceptors in guinea-pig atrial myocytes. Bath application of BIS-I at concentrations of 100 nM and 1 μM alone reduced the amplitude of basal IKs by 10.1±3.2 (n=6) and 11.1±3.0% (n=5), respectively, which appears to be consistent with a recent report showing a modest (12%) reduction of IKs by exposure to BIS-I (1 μM) in guinea-pig ventricular myocytes (Missan et al., 2006).
Figure 3.
Effect of PKC isoform inhibition on the stimulatory action of phenylephrine on IKs. (a, b, c and d) Atrial myocytes were initially exposed to 100 nM BIS-I (a), 100 nM Gö-6976 (b), 100 nM Gö-6983 (c) or 10 μM rottlerin (d) for 5–10 min and then to 30 μM phenylephrine, as indicated by the horizontal bars. (e and f) Atrial myocytes were dialyzed with a pipette solution containing 100 nM βC2-4 (e) or 100 nM ɛV1-2 (f) for approximately 10 min before exposure to 30 μM phenylephrine. IKs was repetitively (every 20 s) activated by depolarizing steps to +30 mV from a holding potential of −50 mV, and amplitude of the tail current, measured upon return to the holding potential was plotted.
Immunoblotting study with the use of isoform-specific antibody has detected the expression of at least five different PKC isoforms, namely α, βII, γ, ɛ and ζ in guinea-pig heart (Takeishi et al., 1999). To elucidate which isoform(s) of PKC is involved in the IKs response to phenylephrine, we used three isoform-selective pharmacological inhibitors of PKC, namely Gö-6976 (Martiny-Baron et al., 1993), Gö-6983 (Gschwendt et al., 1996) and rottlerin (Gschwendt et al., 1994). Figure 3b illustrates a representative example of the IKs response to phenylephrine in the presence of the indolocarbazole Gö-6976, which largely inhibits Ca2+-dependent (α, β and γ) but not Ca2+-independent (δ, ɛ and ζ) isoforms when used at a concentration of 100 nM (Martiny-Baron et al., 1993). The potentiation of IKs was not significantly influenced by the addition of 100 nM Gö-6976 (78.4±9.0% increase, n=6; Figure 4) when compared with control, suggesting that conventional PKC isoforms (α, β and γ) were little, if at all, involved in the response. Similarly, the stimulatory action of phenylephrine was not significantly affected by the bisindolylmaleimide Gö-6983 at 100 nM (Figures 3c and 4; 79.8±11.6% increase, n=6), which at this concentration inhibits conventional (α, β and γ), novel (δ) and atypical (ζ) PKC isoforms (Gschwendt et al., 1996). In addition, IKs response to phenylephrine was practically insensitive to 10 μM rottlerin (Figures 3d and 4; 84.9±8.2% increase, n=6), which potently inhibits PKCδ but not PKCɛ at the concentration tested (Gschwendt et al., 1994). Based on these differences in the sensitivity of phenylephrine action to pharmacological inhibitors, PKCɛ isoform appears to be involved in mediating the stimulatory effect of phenylephrine on IKs in guinea-pig atrial myocytes.
We further tested the effects of the peptide translocation inhibitors of PKC isoforms βC2-4 (Ron et al., 1995) and ɛV1-2 (Johnson et al., 1996) on the stimulatory action of phenylephrine on IKs. It is generally accepted that upon activation, each PKC isoform translocates and binds to the specific anchoring proteins referred to as RACKs (receptors for activated C kinases) within a cell (Mochly-Rosen and Gordon, 1998). The interaction between conventional PKC isoforms and their RACKs is mediated in part by the C2 domain, whereas the binding of the C2-less novel PKC isoforms to their RACKs is via the V1 domain (Mochly-Rosen and Gordon, 1998). The peptide fragment βC2-4, which comprises nine amino acids (SLNPEWNET) derived from the C2 region of PKCβ(218–226), specifically inhibits the translocation of C2-containing conventional PKC isoforms (α, β and γ) but not that of C2-lacking novel PKC isoforms (δ and ɛ; Ron et al., 1995). On the other hand, the peptide ɛV1-2 is composed of eight amino acids (EAVSLKPT) that are derived from the V1 region of PKCɛ and specifically prevents translocation of PKCɛ to its RACKs (Johnson et al., 1996). Previous patch-clamp studies have demonstrated that intracellular application of these peptide inhibitors at a concentration of 100 nM is effective at significantly reducing the action of PKC isoform(s) on ion channels in mammalian cardiac myocytes (Zhang et al., 1997; Hool, 2000; Xiao et al., 2001). After allowing ∼10 min for the pipette solution containing βC2-4 or ɛV1-2 to diffuse into the cell inside, the effect of phenylephrine on IKs was tested. As demonstrated in Figure 3e and f, the stimulatory action of phenylephrine was not appreciably influenced by 100 nM βC2-4 (83.0±16.6% increase, n=6; Figure 4), but was significantly reduced by 100 nM ɛV1-2 (46.4±9.2% increase, n=6; Figure 4). Similarly, IKs response to phenylephrine was significantly reduced by ɛV1-2 (39.2±7.7% increase, n=5) but not by βC2-4 (84.5±12.5% increase, n=5), when applied at higher concentration (2 μM, Figure 4). These experiments with PKC isoform-selective peptide inhibitors again support a significant role for PKCɛ in mediating the potentiation of IKs by phenylephrine.
PKCɛ also mediates the potentiation of IKs by phorbol ester
In the next series of experiments, we explored which isoform(s) of PKC was primarily involved in the IKs response to PMA, by examining the effects of βC2-4 and ɛV1-2 added to a pipette solution. Figure 5a illustrates a representative time course for the potentiation of IKs by bath application of PMA (100 nM) under control conditions. A peak response was typically attained ∼5–10 min after starting the application of PMA and was sustained for at least 5 min even after washout of the compound. In a total of six myocytes, bath application of 100 nM PMA increased IKs by 64.2±1.3% (Figure 5g), when evaluated by the amplitude of tail current at a test potential of +30 mV. This stimulatory action of PMA (100 nM) was almost totally blocked by pretreatment with 100 nM BIS-I (2.7±1.3% increase, n=6; Figure 5b and g), supporting the view that activation of PKC mediates potentiation of IKs by PMA. Figure 5c demonstrates superimposed current traces of IKs recorded during 2-s depolarizing steps to potentials between −40 and +50 mV, before and after ∼10 min exposure to 100 nM PMA. As illustrated in Figure 5d, PMA increased the amplitude of IKs tail currents at all potentials tested. The voltage dependence of IKs activation was little affected by exposure to PMA (control, V1/2=5.0±2.2 mV, k=11.9±1.4 mV; PMA, V1/2=5.9±1.8 mV, k=12.5±1.5 mV, n=4).
Figure 5.
PKCɛ is primarily involved in the stimulatory action of PMA on IKs. (a and b) Time course of changes in the amplitude of IKs tail current during exposure to 100 nM PMA without (a) or with (b) pretreatment with 100 nM BIS-I. IKs was repetitively (every 20 s) activated by 2-s depolarizing steps to +30 mV. (c) Superimposed current traces of IKs activated at test potentials of −40 to +50 mV, before and ∼10 min after exposure to 100 nM PMA. (d) I–V relationships for IKs tail currents, obtained from the experiments shown in (c). (e and f) Time courses in the changes in IKs tail currents recorded from an atrial myocyte dialyzed with a pipette solution containing 100 nM βC2-4 (e) or 100 nM ɛV1-2 (f). (g) Summary bar graph showing the percentage increase in the amplitude of IKs tail currents by PMA, in control and in the presence of BIS-I (100 nM, bath), βC2-4 (100 nM, pipette) or ɛV1-2 (100 nM, pipette). Effect of each inhibitor was compared with control (**P<0.01 vs control).
Figure 5e and f, respectively, shows a typical example of IKs response to 100 nM PMA in an atrial myocyte dialyzed with a pipette solution containing βC2-4 (100 nM) and ɛV1-2 (100 nM). The stimulatory effect of PMA was minimally affected by βC2-4 (56.7±4.7% increase, n=6) but was significantly attenuated by ɛV1-2 (16.5±4.0% increase, n=6), compared with the control value (64.2±1.3% increase, n=6; Figure 5g). These results indicate that PKCɛ is also predominantly involved in the potentiation of IKs by PMA-induced PKC activation in guinea-pig atrial myocytes.
The experimental results obtained using pharmacological and peptide inhibitors for PKC isoforms indicate that activation of conventional PKCs (α, βI, βII and γ) is not primarily involved in the potentiation of IKs by the α1-adrenergic agonist phenylephrine and the phorbol ester PMA in guinea-pig atrial myocytes. To substantiate this view, we examined the response of IKs to thymeleatoxin, a phorbol derivative which predominantly activates conventional PKC isoforms (Ryves et al., 1991). Bath application of 100 nM thymeleatoxin only slightly increased the amplitude of IKs by 14.1±3.4% (n=9; Figure 6a and b), which was usually attained within 5–10 min after starting the application. However, subsequent addition of 100 nM PMA in the continued presence of 100 nM thymeleatoxin caused an additional significant increase in IKs by 60.8±3.6% (n=7) of the baseline amplitude (Figure 6b). This finding again supports a predominant role of novel PKC (PKCɛ) over conventional PKCs in the potentiation of IKs in guinea-pig atrial myocytes.
Figure 6.
Effects of thymeleatoxin and PMA on IKs. (a) Time course of changes in the amplitude of IKs during exposure to PMA in the presence of thymeleatoxin. (b) Summary bar graph showing the percentage increase in the amplitude of IKs tail currents, evoked by thymeleatoxin (100 nM) alone, and PMA (100 nM) in the presence of thymeleatoxin. The values are calculated with reference to the baseline amplitude of IKs tail current. **P<0.01 compared between these two groups.
Discussion
The present study demonstrates that IKs is markedly (approximately twofold) potentiated by phenylephrine (30 μM) through α1-adrenoceptors in guinea-pig atrial myocytes (Figure 2). The stimulatory action of phenylephrine was significantly, if not completely, reduced by the general PKC inhibitor BIS-I (Figures 3a and 4), which strongly suggests that PKC activation plays an important role in mediating the α1-adrenoceptor-induced IKs potentiation. Western-blot analysis using an antibody specific to PKC isoform has suggested that α1-adrenoceptor stimulation with phenylephrine causes translocation and thereby activation of both conventional and novel isoforms of PKC in native cardiac myocytes (Ruf et al., 2002). On the other hand, in the present study we found that the stimulatory action of phenylephrine on IKs was significantly reduced by the PKCɛ-selective inhibitory peptide (ɛV1-2) but was little affected by the peptide inhibitor (βC2-4) for all conventional PKC isoforms (α, β and γ), which favours a preferential role for the novel isoform PKCɛ (Figures 3e, f and 4). This view is also supported by the experiments showing that the stimulatory action of phenylephrine was minimally affected by the indolocarbazole Gö-6976 (Figures 3b and 4), which is believed to have the highest selectivity for conventional PKC isoforms among the pharmacological inhibitors currently available (Martiny-Baron et al., 1993). The present investigation also confirms a substantial enhancement of IKs by phorbol ester PMA (Figures 5 and 6), which directly activates both conventional and novel isoforms of PKC, independent of receptor stimulation. This PMA action on IKs was also significantly reduced by ɛV1-2 but not by βC2-4 (Figure 5), again supporting a functional role of PKCɛ in the regulation of cardiac IKs.
Voltage-clamp study using the Xenopus oocyte expression system has clearly demonstrated that both PKCβII and PKCɛ, but not PKCα, PKCβI, PKCδ and PKCη, are involved in the PMA-induced potentiation of heteromeric KCNQ1/KCNE1 channels (Xiao et al., 2003), molecular constituents of human IKs (Barhanin et al., 1996; Sanguinetti et al., 1996). On the other hand, it was observed in guinea-pig ventricular myocytes that the delayed rectifier K+ current, IK (which seems to largely comprise IKs), is increased by exogenous application of the type III (α) PKC purified from bovine whole brain (Tohse et al., 1990), which resembles the structure of PKCα (Kikkawa et al., 1987). These observations appear to be apparently distinct from the present results, concerning the functional role of PKCα or PKCβII in the regulation of IKs. Individual PKC isoforms (α, βI, βII, δ, ɛ and ζ) in native cardiac myocytes have a differential localization before and after stimulation by norepinephrine or PMA (Disatnik et al., 1994). Whereas PKCα and PKCβII translocate from the cytosolic region to the perinuclear region and/or cell periphery, PKCɛ, upon stimulation, typically translocates to cross-striated regions in ventricular myocytes, which places it near the transverse tubules (t-tubules). Evidence has been presented to indicate that minK (KCNE1) proteins, important function-modifying ancillary β-subunit of the IKs channel, are preferentially localized in the t-tubules in native ventricular myocytes (Furukawa et al., 2001). It is probable that the colocalization of activated PKCɛ with IKs channel proteins underlies the selective potentiation of IKs by PKCɛ in native cardiac myocytes. Further work is required to examine the localization of PKCɛ and IKs channel subunits in atrial myocytes, which lack an extensive t-tubule network.
In the present study, BIS-I at 100 nM almost totally abolishes the potentiation of IKs by PMA (Figure 5b and g) but partially reduces the stimulatory action of phenylephrine on IKs even when applied at 1 μM (Figure 4). We have previously demonstrated that IKs in guinea-pig atrial myocytes is increased by intracellular application of anti-phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) antibody but is decreased by intracellular addition of exogenous PtdIns(4,5)P2 (Ding et al., 2004). These observations suggest that endogenous membrane PtdIns(4,5)P2 exerts an inhibitory action on IKs. We further showed that stimulatory action of extracellular ATP on IKs is due, at least partly, to the reduction of membrane PtdIns(4,5)P2 which is likely to occur following the stimulation of P2Y receptors coupled to a Gq-phospholipase C (PLC) signaling pathway (Ding et al., 2004). It is therefore possible that, in addition to PKC activation, the stimulation of Gq-PLC-coupled α1-adrenoceptors causes other signaling events such as depletion of membrane PtdIns(4,5)P2, to potentiate IKs in atrial myocytes. It is of interest to evaluate the relative contribution of PKC activation and membrane PtdIns(4,5)P2 depletion to the potentiation of IKs observed during the stimulation of various kinds of Gq-PLC-coupled receptors expressed in the heart.
The maximal response of IKs to phenylephrine in atrial myocytes (97.1±11.9% increase) appears to be considerably larger than that in ventricular myocytes of same species (29.9±9.1% increase; Tohse et al., 1992). On the other hand, it has been demonstrated in guinea-pig ventricular myocytes that the amplitude of IKs is potentiated by ∼45–60% by bath application of phorbol esters PMA (also referred to as 12-O-tetradecanoylphorbol 13-acetate, TPA) and phorbol 12,13-dibutyrate (PDBu) (Walsh and Kass, 1988; Tohse et al., 1990; Yazawa and Kameyama, 1990). It is thus likely that IKs can be enhanced by an almost similar degree in atrial and ventricular myocytes of guinea-pigs when PKC is directly activated by phorbol esters. As judged from these observations, it is likely that, following the stimulation of α1-adrenoceptors, PKC may be more effectively activated to potentiate IKs in atrial myocytes. Further studies should be conducted to determine the relative efficiency of α1-adrenoceptor stimulation in potentiating IKs in atrial and ventricular myocytes under the same experimental conditions, including methods to isolate IKs from total IK.
Previous investigators have reported that PKC activation causes a species-specific effect on cardiac IKs; PKC stimulation increases IKs in guinea-pigs but decreases the current amplitude in most of the other mammalian species (Varnum et al., 1993; Robinson et al., 2000). This difference has been accounted for, at least partly, by the sequence variation of minK (KCNE1) protein (Varnum et al., 1993); a PKC phosphorylation site (Ser-102), which is responsible for current reduction, is absent in guinea-pigs but is present in other mammalian species including humans. In recent years, however, Kathöfer et al. (2003) have demonstrated that PKC activation increases membrane current through the human KCNQ1/KCNE1 channel. Furthermore, these authors detected, using site-directed mutagenesis experiments, the PKC phosphorylation sites (Ser-409, Ser-464, Thr-513 and Ser-577) in a pore-forming α-subunit, KCNQ1 protein, which contributes to PKC-dependent increase in KCNQ1/KCNE1 current. Future studies are required to examine whether the PKCɛ-mediated IKs potentiation is present and functions in native cardiac myocytes of various mammalian species including humans.
A number of experimental and/or clinical studies have strongly suggested that, whereas increased expression of PKCβI and/or PKCβII is implicated in the development of cardiomyopathy (Wakasaki et al., 1997) and heart failure (Bowling et al., 1999), the activation and translocation of PKCɛ confers cardioprotection that is associated with ischemic preconditioning (Gray et al., 1997; Qiu et al., 1998; Liu et al., 1999). Furthermore, a recent study has found that intracoronary injection of a selective PKCɛ-activating peptide, ψɛRACK, not only reduces infarct size but also suppresses the occurrence of ventricular tachyarrhythmias caused by ischemia–reperfusion in porcine hearts (Inagaki et al., 2005). In atrial and ventricular myocardium, IKs activation plays a major role in determining the action potential duration that controls the amount of Ca2+ influx through simultaneously activated ICa,L (Pennefather and Cohen, 1990). It seems, therefore reasonable to speculate that activation of PKCɛ and resultant stimulation of IKs can reduce the amount of Ca2+ influx during action potentials and thereby spare energy required for intracellular Ca2+ handling, which can be beneficial to the cardiomyocytes, especially in condition where the oxygen supply to cardiac muscle becomes inadequate (hypoxia or ischemia). It will be interesting to elucidate whether PKCɛ-mediated potentiation of IKs described here in atrial myocytes can be applicable to the IKs regulation in cardiac ventricular myocytes of mammalian species including humans and constitutes, at least in part, the cardioprotective action of PKCɛ during ischemic preconditioning and/or ischemia–reperfusion.
In conclusion, our data indicate that the ɛ isoform of PKC (PKCɛ) is predominantly involved in the stimulatory action of PKC on IKs and contributes to α1-adrenergic potentiation of IKs in native guinea-pig atrial myocytes.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science.
Abbreviations
- BIS-I
bisindolylmaleimide I
- DAG
diacylglycero1
- ICa,L
L-type Ca2+ inward current
- IK
delayed rectifier K+ current
- IKr
rapid component of delayed rectifier K+ current
- IKs
slow component of delayed rectifier K+ current
- PDBu
phorbol 12,13-dibutyrate
- PKA
cyclic AMP-dependent protein kinase
- PKC
protein kinase C
- PLC
phospholipase C
- PMA
phorbol 12-myristate 13-acetate
- PS
phosphatidylserine
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- RACKs
receptors for activated C kinases
- TPA
12-O-tetradecanoylphorbol 13-acetate
Conflict of interest
The authors state no conflict of interest.
References
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Bosch RF, Schneck AC, Csillag S, Eigenberger B, Gerlach U, Brendel J, et al. Effects of the chromanol HMR 1556 on potassium currents in atrial myocytes. Naunyn-Schmiedebergs Arch Pharmacol. 2003;367:281–288. doi: 10.1007/s00210-002-0672-5. [DOI] [PubMed] [Google Scholar]
- Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- Ding WG, Toyoda F, Matsuura H. Regulation of cardiac IKs potassium current by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2004;279:50726–50734. doi: 10.1074/jbc.M409374200. [DOI] [PubMed] [Google Scholar]
- Disatnik M-H, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Furukawa T, Ono Y, Tsuchiya H, Katayama Y, Bang ML, Labeit D, et al. Specific interaction of the potassium channel β-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J Mol Biol. 2001;313:775–784. doi: 10.1006/jmbi.2001.5053. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Two components of delayed rectifier current in canine atrium and ventricle. Does IKs play a role in the reverse rate dependence of class III agents. Circ Res. 1996;78:26–37. doi: 10.1161/01.res.78.1.26. [DOI] [PubMed] [Google Scholar]
- Gray MO, Karliner JS, Mochly-Rosen D. A selective ɛ-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C μ by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Han W, Wang Z, Nattel S. Slow delayed rectifier current and repolarization in canine cardiac Purkinje cells. Am J Physiol. 2001;280:H1075–H1080. doi: 10.1152/ajpheart.2001.280.3.H1075. [DOI] [PubMed] [Google Scholar]
- Hool LC. Hypoxia increases the sensitivity of the L-type Ca2+ current to β-adrenergic receptor stimulation via a C2 region-containing protein kinase C isoform. Circ Res. 2000;87:1164–1171. doi: 10.1161/01.res.87.12.1164. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by ɛ-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an ɛ-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Mochly-Rosen D. Inhibition of the spontaneous rate of contraction of neonatal cardiac myocytes by protein kinase C isozymes. A putative role for the ɛ isozyme. Circ Res. 1995;76:654–663. doi: 10.1161/01.res.76.4.654. [DOI] [PubMed] [Google Scholar]
- Kathöfer S, Röckl K, Zhang W, Thomas D, Katus H, Kiehn J, et al. Human β3-adrenoreceptors couple to KvLQT1/MinK potassium channels in Xenopus oocytes via protein kinase C phosphorylation of the KvLQT1 protein. Naunyn-Schmiedeberg's Arch Pharmacol. 2003;368:119–126. doi: 10.1007/s00210-003-0772-x. [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Ono Y, Ogita K, Fujii T, Asaoka Y, Sekiguchi K, et al. Identification of the structures of multiple subspecies of protein kinase C expressed in rat brain. FEBS Lett. 1987;217:227–231. doi: 10.1016/0014-5793(87)80668-3. [DOI] [PubMed] [Google Scholar]
- Li G-R, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-ɛ is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:1937–1948. doi: 10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Matsuura H, Tsuruhara Y, Sakaguchi M, Ehara T. Enhancement of delayed rectifier K+ current by P2-purinoceptor stimulation in guinea-pig atrial cells. J Physiol (London) 1996;490:647–658. doi: 10.1113/jphysiol.1996.sp021174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missan S, Linsdell P, McDonald TF. Tyrosine kinase and phosphatase regulation of slow delayed-rectifier K+ current in guinea-pig ventricular myocytes. J Physiol (London) 2006;573:469–482. doi: 10.1113/jphysiol.2005.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- Pennefather P, Cohen IS.Molecular mechanisms of cardiac K+-channel regulation Cardiac Electrophysiology from Cell to Bedside 1990WB Saunders: Philadelphia; 17–28.In: Zipes DP, Jalife J (eds) [Google Scholar]
- Powell T, Terrar DA, Twist VW. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol (London) 1980;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- Qiu Y, Ping P, Tang X-L, Manchikalapudi S, Rizvi A, Zhang J, et al. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J Clin Invest. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Liu QY, Rosen MR. Ionic basis for action potential prolongation by phenylephrine in canine epicardial myocytes. J Cardiovasc Electrophysiol. 2000;11:70–76. doi: 10.1111/j.1540-8167.2000.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of β protein kinase C in vivo. J Biol Chem. 1995;270:24180–24187. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- Ruf S, Piper M, Schluter KD. Specific role for the extracellular signal-regulated kinase pathway in angiotensin II- but not phenylephrine-induced cardiac hypertrophy in vitro. Pflügers Arch. 2002;443:483–490. doi: 10.1007/s004240100710. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Evans AT, Olivier AR, Parker PJ, Evans FJ. Activation of the PKC-isotypes α, β1, γ, δ and ɛ by phorbol esters of different biological activities. FEBS Lett. 1991;288:5–9. doi: 10.1016/0014-5793(91)80989-g. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am J Physiol. 1991;260:H393–H399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK, Scott A, Siegl PKS. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circ Res. 1991;68:77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- Stengl M, Volders PGA, Thomsen MB, Spätjens RLHMG, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol (London) 2003;551:777–786. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi Y, Jalili T, Ball NA, Walsh RA. Responses of cardiac protein kinase C isoforms to distinct pathological stimuli are differentially regulated. Circ Res. 1999;85:264–271. doi: 10.1161/01.res.85.3.264. [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. Transgenic overexpression of constitutively active protein kinase C ɛ causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–1223. doi: 10.1161/01.res.86.12.1218. [DOI] [PubMed] [Google Scholar]
- Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res. 2005;96:e25–e34. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- Tohse N, Kameyama M, Sekiguchi K, Shearman MS, Kanno M. Protein kinase C activation enhances the delayed rectifier potassium current in guinea-pig heart cells. J Mol Cell Cardiol. 1990;22:725–734. doi: 10.1016/0022-2828(90)91015-y. [DOI] [PubMed] [Google Scholar]
- Tohse N, Nakaya H, Kanno M. α1-Adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circ Res. 1992;71:1441–1446. doi: 10.1161/01.res.71.6.1441. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tsien RY, Rink TJ. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980;599:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Varnum MD, Busch AE, Bond CT, Maylie J, Adelman JP. The min K channel underlies the cardiac potassium current IKs and mediates species–specific responses to protein kinase C. Proc Natl Acad Sci USA. 1993;90:11528–11532. doi: 10.1073/pnas.90.24.11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders PGA, Stengl M, van Opstal JM, Gerlach U, Spätjens RLHMG, Beekman JDM, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for β-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, et al. Targeted overexpression of protein kinase C β2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci USA. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993;73:276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- Xiao G-Q, Mochly-Rosen D, Boutjdir M. PKC isozyme selective regulation of cloned human cardiac delayed slow rectifier K current. Biochem Biophys Res Commun. 2003;306:1019–1025. doi: 10.1016/s0006-291x(03)01095-7. [DOI] [PubMed] [Google Scholar]
- Xiao G-Q, Qu Y, Sun Z-Q, Mochly-Rosen D, Boutjdir M. Evidence for functional role of ɛPKC isozyme in the regulation of cardiac Na+ channels. Am J Physiol. 2001;281:C1477–C1486. doi: 10.1152/ajpcell.2001.281.5.C1477. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol (London) 1990;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-H, Johnson JA, Chen L, El-Sherif N, Mochly-Rosen D, Boutjdir M. C2 region-derived peptides of β-protein kinase C regulate cardiac Ca2+ channels. Circ Res. 1997;80:720–729. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]