Abstract
Background and purpose:
Agonists of the M2 muscarinic acetylcholine receptor (mAChR) increase mRNA for this receptor and mRNA for endothelial and neuronal isoforms of NO synthase (eNOS or nNOS). Here we examine the different signalling pathways involved in such events in rat cardiac atria.
Experimental approach:
In isolated atria, the effects of carbachol on mRNA for M2 receptors, eNOS and nNOS were measured along with changes in phosphoinositide (PI) turnover, translocation of protein kinase C (PKC), NOS activity and atrial contractility.
Key Results:
Carbachol increased mRNA for M2 receptors, activation of PI turnover, translocation of PKC and NOS activity and decreased atrial contractility. Inhibitors of phospholipase C (PLC), calcium/calmodulin (CaM), NOS and PKC prevented the carbachol-dependent increase in mRNA for M2 receptors. These inhibitors also attenuated the carbachol induced increase in nNOS- and eNOS-mRNA levels. Inhibition of nNOS shifted the dose response curve of carbachol on contractility to the right, whereas inhibition of eNOS shifted it to the left.
Conclusions and implications:
From our results, activation of M2 receptors induced nNOS and eNOS expression and activation of NOS up-regulated M2 receptor gene expression. The signalling pathways involved included stimulation of PI turnover via PLC activation, CaM and PKC. nNOS and eNOS mediated opposing effects on the negative inotropic effect in atria, induced by stimulation of M2 receptors. These results may contribute to a better understanding of the effects and side effects of cholinomimetic treatment in patients with cardiac neuromyopathy.
Keywords: M2 mAChR, mRNA, nNOS–eNOS CaM, PKC, PLC, atrial contractility
Introduction
A number of transcription factors that have the capacity to alter the rate of transcription of specific target genes in response to receptor-mediated signaling events at the cell membrane have been identified (Sheng and Greenberg, 1990). Many of these putative transcription factors are rapidly and transiently induced following receptor activation, and, therefore, can be classified as early genes.
A positive regulation of the mRNA for the M1 muscarinic acetylcholine receptor (M1 mAChR) in the early stages after treatment with carbachol has been described in the rat striatum (Chou et al., 1993) and in rat cerebral frontal cortex (Sterin-Borda et al., 2003). Within 1 h of M1 receptor activation, the expression of immediate early genes is induced. These genes play important roles in coupling receptor stimulation to long-term neuronal responses (Albrecht et al., 2000). Three hours after M1 receptor activation, a significantly higher number of genes, some expressing differential patterns, were observed. This other set of genes represents later effector or downstream target genes (von der Kammer et al., 1999). These target genes may be under the control of the immediate early genes. A feedback mechanism whereby upregulated acetylcholinesterase gene expression leads to an acceleration of acetylcholine breakdown at the cholinergic synapse, limiting cholinergic transmission has been demonstrated (von der Kammer et al., 2001).
Most studies conducted to identify early genes targeted by mAChR activation were performed in extracts of the CNS, which contain a high proportion of M1 receptors. This raised the question whether other subtypes of mAChR, such as those expressed in cardiac tissue, also have the capacity to regulate the transcription of specific target genes in response to receptor stimulation.
The negative contractile effect in mammalian heart caused by the activation of mAChRs is mediated mainly by the M2 subtype of receptors (Hosey, 1992; Sterin-Borda et al., 1995). Previously, we have determined the different signaling events involved in the M2 mAChR-dependent inhibition of contractility in rat-isolated atria, which is associated with increased production of nitric oxide (NO). The mechanism appears to occur secondarily to stimulation of phosphoinositide (PI) turnover, via phospholipase C (PLC) activation. This in turn triggers a further cascade of reactions involving calcium/calmodulin (CaM) and protein kinase C (PKC), leading to activation of nitric oxide synthase (NOS) and soluble guanylate cyclase activity (Sterin-Borda et al., 1995).
Recent work has shown that NO is a powerful controller of gene expression, upregulating mRNAs encoding c-fos and microtubule-associated protein-2 and downregulating mRNAs encoding gonadotropin-releasing hormone and CAM-dependent protein kinase II α (Peunova and Enikolopov, 1993; Johnston and Morris, 1995; Belsham et al., 1996; Eigenthaler et al., 1998). It has also been reported that NO is able to alter dramatically the pattern of early gene expression in hippocampal granule cells (Morris, 1995). However, little is known about the reciprocal stimulation of immediate early gene expression between M2 mAChRs and direct enzymic activation of NOS isoforms or an increase in their expression.
Therefore, the aim of these experiments was to determine whether the mAChR agonist carbachol acts as an early positive regulator of M2 receptor, neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS) gene expressions. Moreover, we were interested to see whether there is a reciprocity between the mRNAs for M2 receptors and for isoforms of NOS and the common enzymic pathways involved. Our results showed that activation of M2 receptors induced a complex pattern of gene expression of NOS isoforms that may be involved in the control of cardiac function as well as in the regulation of the mRNA for the M2 receptor.
Methods
Animals
Male Wistar rats (obtained from the Pharmacology Unit, School of Dentistry, University of Buenos Aires, Argentina), and C57B1/6J wild type (WT) and homozygous for the NOS3tm1Unc targeted mutation (eNOS−/−) mice (3–5 months; Jackson Laboratories, Bar Harbor, ME, USA) were used. The animals housed in standard environmental conditions were fed a commercial pellet diet and water ad libitum and kept in automatically controlled lighting (lights on 0800–1900) and uniform temperature (25°C) conditions. Animals were cared for in accordance with the principles and guidelines of the NIH.
mRNA isolation and cDNA synthesis
Total RNA was extracted from rat atria by homogenization using the guanidinium isothiocyanate method (Chomozynski and Saachi, 1987). A 20 μl reaction mixture contained 2 ng of mRNA, 20 units of RNase inhibitor, 1 mM dNTPs and 50 units of Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). First-strand cDNA was synthesized by incubating rat atria in Krebs Ringer bicarbonate (KRB) gassed with 5% CO2 in O2, (pH 7.4) at 37°C for 60 min. In a selected tube, the reverse transcriptase was omitted to control for amplification from contaminating cDNA or genomic DNA.
Quantitative PCR
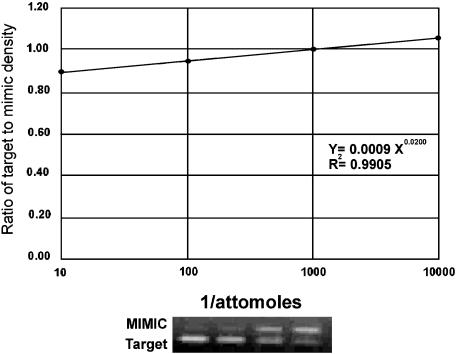
Quantitation of NOS isoforms and M2 mAChR-mRNA levels was performed by a method that involves simultaneous coamplification of both the target cDNA and a reference template (MIMIC) with a single set of primers (Sterin-Borda et al., 2003). MIMIC for nNOS, eNOS, M2 mAChR and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were constructed using a PCR MIMIC construction kit (Clontech Laboratories, Palo Alto, CA, USA). Each PCR MIMIC consists of a heterologous DNA fragment with 5′ and 3′-end sequences recognized by a pair of gene-specific primers. The sizes of PCR MIMIC were distinct from those of the native targets. The sequence of oligonucleotide primer pairs used for construction of MIMIC and amplification of mRNA for the NOS isoforms, M2 mAChR and G3PDH is listed in Table 1. Aliquots were taken from pooled first-strand cDNA from the same group and constituted one sample for PCR. A series of 10-fold dilutions of known concentrations of the MIMIC were added to PCR amplification reactions containing the first-strand cDNA. PCR MIMIC amplification was performed in 100 μl of a solution containing 1.5 mM MgCl2, 0.4 μM primer, dNTPs, 2.5 U Taq DNA polymerase and 0.056 μM Taq Start antibody (Clontech Laboratories). After an initial denaturation at 94°C for 2 min, the cycle conditions were 30 s of denaturation at 94°C, 30 s of extension at 60°C and 45 s for enzymatic primer extension at 72°C for 45 cycles for NOS isoforms and 30 cycles for eNOS. For M2 mAChR, each cycle consisted of 30 s at 94°C, 30 s at 58°C and 45 s at 72°C for 30 cycles. The internal control was the mRNA of the housekeeping gene, G3PDH. PCR amplification was performed with initial denaturation at 94°C for 2 min followed by 30 cycles of amplification. Each cycle consisted of 35 s at 94°C, 35 s at 58°C and 45 s at 72°C. Samples were incubated for an additional 8 min at 72°C before completion. PCR products were subjected to electrophoresis on ethidium bromide-stained gels. Band intensity was quantitated by densitometry using NIH Image software. Levels of mRNA were calculated from the point of equal density of the sample and MIMIC PCR products (Figure 1) (Sterin-Borda et al., 2003). NOS- and M2 receptor mRNA levels were normalized against the levels of G3PDH-mRNA present in each sample, which served as control for variations in RNA purification and cDNA synthesis. The relative mRNA expression of NOS and M2 receptors in each group was compared with those from the respective normal group and reported as a percentage of normal.
Table 1.
Oligonucleotides of primers for PCR
| Gene product | Sense | Antisense | Predicted size (bp) |
|---|---|---|---|
| nNOS | 5′-GCGGA GCAGA GCGGC CTTAT-3′ | 5′-TTTGGT GGGAG GACCG AGGG-3′ | 240 |
| eNOS | 5′-CCGCA CTTCT GTGCC TTTGC TC-3′ | 5′-GCTCG GGTGG ATTTGC TGCTCT-3′ | 360 |
| M2 mAChR | 5′-GATGA AAACAC AGTT TCCAC TTC-3′ | 5′-ATGAT GAAAGC CAACA GGATA GCC-3′ | 280 |
| G3PDH | 5′-ACCAC AGTCCA TGCCAT CAC-3′ | 5′-TCCAC CACCC TGTTG CTGTA-3′ | 452 |
Abbreviations: eNOS, endothelial nitric oxide synthase; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; M2 mAChR, M2 muscarinic acetylcholine receptor; nNOS, neuronal nitric oxide synthase; PCR, polymerase chain reaction.
Neuronal (nNOS) and endothelial (eNOS) nitric oxide synthase (Qi et al., 2001); M2 mAChR (Steel and Buckley, 1993); G3PDH (Bishop-Bailey et al., 1997).
Figure 1.
Analysis of target mRNA levels by competitive PCR. The upper panel shows a quantitative analysis of the competitive PCR experiment. Ordinate, ratio of the target to MIMIC products as determined by band densitometry. Abscissa, inverse amount of MIMIC added to the amplification. When the resultant target and MIMIC densitometric values are equal (y=1), extrapolation can be performed to yield the target amount.The lower panel shows an autoradiogram of neuronal nNOS in digoxigenin-labeled competitive PCR. The same applies for Figures 2, 5, 6 and 7.
Contractility in isolated atria
Rats and mice were killed by decapitation. The left atria were carefully dissected from the ventricles, attached to a glass holder and immersed in a tissue bath containing KRB solution gassed with 5% CO2 in O2 and maintained at pH 7.4 and 30°C. The composition of the KRB solution has been described previously (Pascual et al., 1986). A preload tension of 750 and 500 mg was applied to the rat and mice atria, respectively. Tissues were allowed to equilibrate for 1 h. The initial control values for contractile tension of the isolated atria were recorded by the use of a force transducer coupled to an ink-writing oscillograph. The preparations were paced with a bipolar electrode and an SK4 Grass Stimulator. The stimuli had a duration of 2 ms and the voltage was 10% above threshold. Inotropic effects (expressed as dF/dt) were assessed by recording the maximum rate of isometric force development during electrical stimulation at a fixed frequency of 150 and 250 beats min−1 to the rat and mice atria, respectively. Control values (=100%) refer to the dF/dt before the addition of drugs. The absolute value for dF/dt at the end of the equilibration period (60 min) was 7.8±0.5 and 4.2±0.4 g s−1 for rat and mice atria respectively. Cumulative dose–response curves were obtained according to the method of Van Rossum (1963). A maximal effect was achieved within 5 min after each dose was assayed. Basal values of dF/dt were not affected by the different antagonists or inhibitors at the concentrations used.
Measurement of total labeled PI
Rat-atria slices were incubated for 120 min in 0.5 ml of KRB gassed with 5% CO2 in O2 with 1 mCi [myo-3H]inositol ([3H]MI) (special activiry, 15 Ci mmol−1) from Dupont/New England Nuclear (Boston, MA, USA) and LiCl (10 mM) was added for determination of inositol monophosphate accumulation according to the technique described previously (Sterin-Borda et al., 1995). Carbachol and the blockers were added 60 min before the end of the incubation period. Water-soluble PI were extracted after 120-min incubation. Tissues were washed with KRB and homogenized in 0.3 ml of KRB with 10 mM LiCl and 2 ml chloroform/methanol (1:2, v/v) to stop the reaction. Then, chloroform (0.62 ml) and water (1 ml) were added. Samples were centrifuged at 3000 g for 10 min and the aqueous phase of the supernatant (1–2 ml) was applied to a 0.7-ml column of Bio-Rad AG (Formate form) 1 × 8 anion-exchange resin (100–200 mesh) suspended in 0.1 M formic acid that has been previously washed with 10 mM Tris-formic (pH 7.4). The resin was then washed with 20 volumes of 5 mM myo-inositol followed by 6 volumes of water and PI were eluted with 1 M ammonium formate in 0.1 M formic acid. One-milliliter fractions were recovered and radioactivity was determined by scintillation counting. Peak areas were determined by triangulation and results corresponding to the second peak and were expressed as area mg−1 following previous criteria (Sterin-Borda et al., 1995).
PKC activity assay
Atria were incubated alone or in the presence of each concentration of carbachol, stimulant plus blockers or blockers alone for a total incubation time of 60 min in KRB solution at 30°C and were frozen immediately in liquid nitrogen. PKC activity was purified from subcellular fractions as described previously (Wald et al., 1996); and was assayed on both cytosolic and membrane preparations from atria by measuring the incorporation of 32P from [γ-32P]ATP into histone H1. Incubations were conducted for 30 min at 30°C in a final volume of 85 μl. In final concentrations, the assay mixture contained 25 μM ATP (0.4 μCi), 10 mM magnesium acetate, 5 mM β-mercaptoethanol, 50 μg of histone H1, 20 mM HEPES, [pH 7.4] and unless otherwise indicated, 0.2 mM CaCl2 and 10 μg ml−1 of phosphatidylserine vesicles. The incorporation of 32P-phosphate into histone H1 was linear for at least 30 min. The reaction was stopped by the addition of 2 ml of ice-cold 5% trichloroacetic acid and 10 mM H3PO4. The radioactivity retained on GF/C glass-fiber filters after filtration was determined by counting the filters in 2 ml of scintillation fluid. PKC activity was determined after subtracting the incorporation in the absence of Ca2+ and phospholipids. The data were expressed in picomoles of phosphate incorporated into the substrate per minute and per milligram of protein (pmol min−1 mg−1 protein). Another known PKC substrate peptide, myelin basic protein-4-14 from Life Technologies (Carlsbad, CA, USA), was also used for measuring PKC activity purified from subcellular cardiac fractions, following the instructions of the supplier. PKC specificity was confirmed by means of the PKC pseudosubstrate inhibitor peptide PKC (19–36) provided by Gibco (Wald et al., 1998). The data were expressed in picomoles of phosphate incorporated into the substrate per minute and per milligram of protein (pmol min−1 mg−1 protein).
Determination of NOS activity
NOS activity was measured in atria by production of [U-14C]citrulline from [U-14C]arginine according to the procedure described previously (Sterin-Borda et al., 1995). Briefly, after 20 min preincubation in KRB solution, atria were transferred to 500 μl of prewarmed KRB equilibrated with 5% CO2 in O2 in the presence of [U-14C]arginine (0.5 μCi). Appropriate concentrations of drugs were added, and atria were incubated for 60 min under 5% CO2 in O2 at 37°C. Then tissues were homogenized with an Ultraturrax (IKA-Labortechnik, Staufen, Germany) in 1 ml of medium containing 20 mM HEPES (pH 7.4), 0.5 mM EGTA, 0.5 mM EDTA, 1 mM dithiothreitol, 1 μM leupeptin and 0.2 mM phenylmethanesulfonyl fluoride at 4°C. After centrifugation at 20 000 g for 10 min at 4°C, supernatants were applied to 2 ml columns of Dowex AG 50 WX-8 (sodium form); [14C]-citrulline was eluted with 3 ml of water and quantified by liquid scintillation counting. NOS partially purified using a 2′,5′-ADP-Sepharose column, as described previously (Sterin-Borda et al., 1995). Basal NOS activity in whole atria was inhibited by 95% by 0.5 mM NG-monomethyl-L-arginine (L-NMMA). The results, expressed as picomoles per gram of tissue wet weight (pmol g−1 tissue) obtained for whole atria were expressed as the difference between values in the absence and in the presence of 0.5 mM L-NMMA.
Statistical analysis
Student's t-test for unpaired values was used to determine the levels of significance. When multiple comparisons were necessary, after analysis of variance, the Student–Newman–Keuls test was applied. Differences between means were considered significant if P<0.05.
Drugs
Carbachol, trifluoperazine, L-NMMA, calphostin C, sodium nitroprusside (SNP), isoprotererol and pirenzepine were purchased from Sigma Chemical Company (St Louis, MO, USA); 11-((2-((diethylamino)methyl)-1-piperidinyl)acetyl)-5,11-dihydro-6H-pyrido(2,3-b)(1,4)benzodiazepin-6-one (AF-DX 116), N5-(1-iminoethyl)-L-ornithine dihydrochloride (L-NIO) and N-propyl-L-arginine (NZ) were purchased from Tocris Cookson Inc. (Baldwin, MO, USA); 1-6-17β-3-methoxgestra-1,3,5 (10)-trien-17yl-aminohexyl-1-H-pyrrole-2,5-dione (U-73122) from ICN Pharmaceuticals Inc. (Aurora, OH, USA). Stock solutions were freshly prepared in the corresponding buffers. The drugs were diluted in the bath to achieve the final concentration stated in the text.
Results
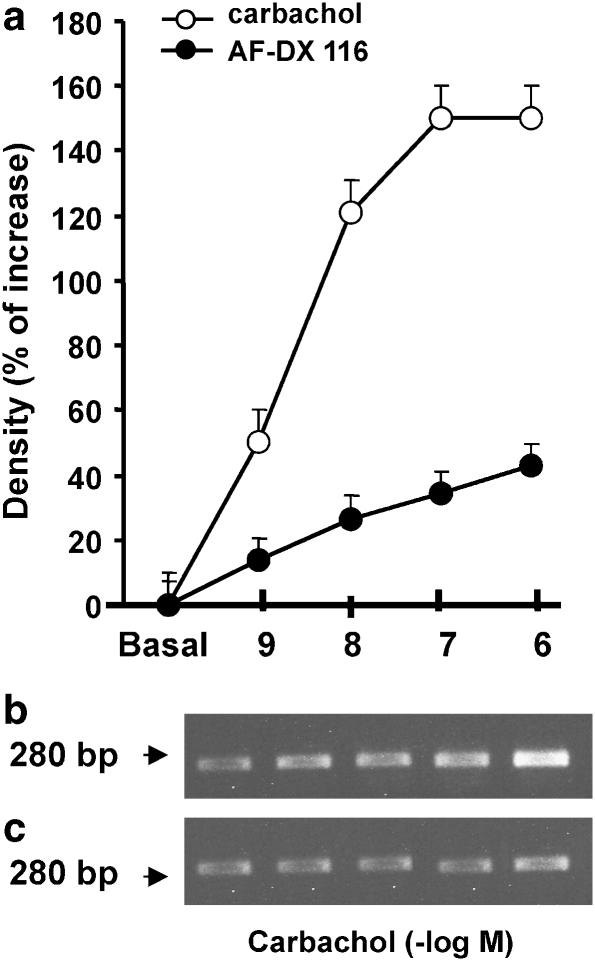
The products of reverse transcription-polymerase chain reaction (RT-PCR) using specific oligonucleotide primers for receptors showed single clear bands of the predicted size (Figure 2). Semi-quantitative RT-PCR analysis demonstrated that stimulation with carbachol for 2 h increased mRNA for M2 receptors in a concentration-dependent manner, with a maximal response at 1 × 10−7 M (Figure 2a and b). Furthermore, a reduction in the carbachol-induced elevation of M2 receptor mRNA levels was observed in the presence of AF-DX 116 (Figure 2a and c). Pirenzepine (1 × 10−7 M) and isoproterenol (from 10−9 to 10−6 M) had no effect (data not shown). This indicates that transcription of the gene for the M2 receptor results specifically from the activation of M2 receptors.
Figure 2.
Effects of carbachol on semi-quantitative RT-PCR analysis of mRNA for M2 receptors from rat atria incubated for 2 h with each concentration of carbachol. (a) Dose–response curve of carbachol in absence or in presence of 1 × 10−7 M AF-DX116. Basal values correspond to mRNA levels after 2-h incubation without carbachol. Values are mean±s.e.m. of six experiments in each group. The RT-PCR products obtained from carbachol dose–response curve in absence (b) and in the presence (c) of AF-DX 116 are shown. P<0.001 between carbachol and carbachol+AF-DX 116 at all concentrations used.
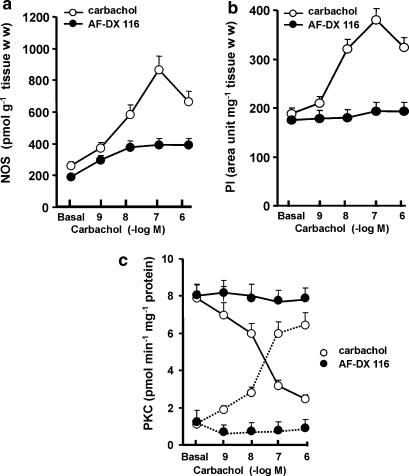
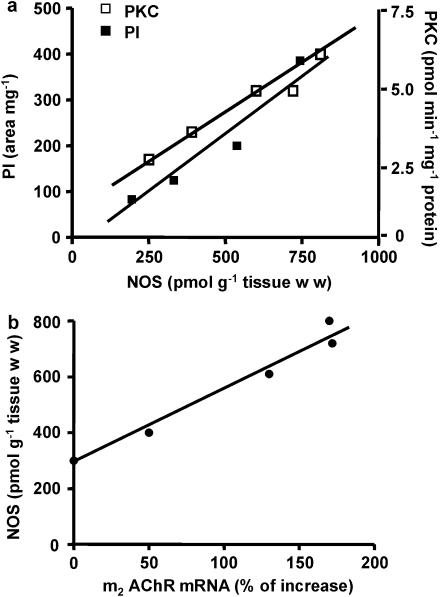
Carbachol stimulation resulted in activation of a number of enzymatic pathways commonly associated with receptor signaling. Figure 3 shows that stimulation of M2 receptors triggered a pronounced increase of NOS activity (a), PI accumulation (b) and membrane PKC translocation (c). The degree of stimulation was directly proportional to agonist concentrations from 1 × 10−9 to 1 × 10−7 M, decreasing thereafter, to values still significantly higher than basal. All these effects of carbachol were inhibited by AF-DX 116 (Figure 3a–c). Figure 4a demonstrates that under identical experimental conditions, there was a significant correlation (α=0.05) between carbachol-stimulated NOS activity and increase of PI accumulation and PKC translocation (Pearson r: PI, 0.9775; PKC, 0.9667). Figure 4b also shows a significant correlation (α=0.05) between the increased mRNA for M2 receptors and NOS stimulation (Pearson r: 0.9850), both induced by carbachol.
Figure 3.
Concentration–response curve of carbachol-induced NOS activity (a) PI production (b) and membrane PKC translocation (c) in rat atria, with or without 1 × 10−7 M AF-DX 116. (c) PKC translocation from cytosol (full line) to membrane (dotted line) is shown. Basal values correspond to data before addition of carbachol. Values are mean±s.e.m. of six experiments in each group.
Figure 4.
(a) Correlation between the stimulatory effects of carbachol (1 × 10−9 to 1 × 10−6 M) on NOS, membrane PKC activities and PI accumulation. NOS was plotted as a function of both PI (r2=0.9556) and PKC (r2=0.9405). Values are mean±s.e.m. of five experiments on each group. (b) Correlation between the stimulatory effect of carbachol (1 × 10−9–1 × 10−6 M) on M2 receptor mRNA and NOS activity. M2 receptor mRNA was plotted as a function of NOS activity (r2=0.9704). Values are mean±s.e.m. of six experiments in each group. For other details see legend of Figure 3.
To determine whether these enzyme activities were dependent on each other, atria were incubated with inhibitors of different enzymic pathways involved in mAChR activation. Table 2 shows that inhibition of PLC by U-73122 (5 × 10−6 M) (Smallridge et al, 1992) attenuated the stimulation of PI accumulation, membrane PKC translocation and NOS activity induced by 1 × 10−7 M carbachol. Trifluoperazine (5 × 10−6 M) and calphostin C (5 × 10−9 M) were used to inhibit CaM (Scharff and Foder, 1984) and PKC (Kobayashi et al., 1989), respectively. Trifluoperazine inhibited carbachol activation of NOS whereas calphostin C prevented the activation of NOS and PKC translocation from cytosol to membrane. As a control, L-NMMA (1 × 10−5 M) inhibited the carbachol-induced increase of NOS activity and L-arginine (1 × 10−4 M) reversed the L-NMMA effect (data not shown).
Table 2.
Effect of carbachol upon enzymatic activities and PI turnover. Influence of enzymatic inhibitors
| Additions | Phosphoinositide (area/mg) | PKC (pmol/min/ mg protein) | NOS (pmol/g tissue wet wt) |
|---|---|---|---|
| Basal | 192±12 | 1.2±0.1 | 240±18 |
| Carbachol | 383±22* | 5.8±0.3* | 848±21* |
| Carbachol+U-73122 | 178±15* | 1.5±0.1** | 250±15** |
| Carbachol+Trifluoperazine | 356±21 | 5.4±0.2 | 485±20** |
| Carbachol+Calphostin | 422±35 | 1.1±0.1** | 323±20** |
| Carbachol+L-NMMA | 356±25 | 5.5±0.2** | 198±15** |
Abbreviations: L-NMMA, NG-monomethyl-L-arginine; NOS, neuronal nitric oxide synthase; PKC, protein kinase C; PI, phosphoinositide; U-73122, 1-6-17β-3-methoxgestra-1,3,5 (10)-trien-17yl-aminohexyl-1-H-pyrrole-2,5-dione.
Values are mean±s.e.m. of five experiments in each group performed in duplicate. Enzyme activities were measured after incubation rat atria after 2 h in the presence of carbachol with or without enzyme inhibitors. Carbachol was used at 1 × 10−7 M and U73122 5 × 10−6 M, TFP 5 × 10−6 M, calphostin 5 × 10−9 M and L-NMMA 5 × 10−5 M.
P<0.001 comparing with basal values (no additions)
P<0.001 comparing with carbachol alone.
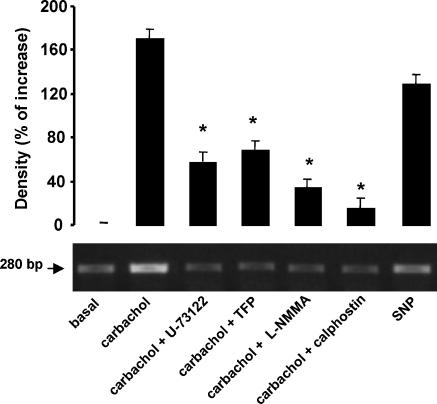
To find out if these enzymic pathways could be involved in the increased M2 receptor gene expression induced by carbachol, atria were incubated with the inhibitory agents at the same concentrations as used above. Figure 5 shows that the inhibition of PLC, CaM, NOS and PKC decreased the effects of carbachol on M2 receptor mRNA levels. Furthermore, SNP increased the levels of this mRNA. These results indicate that activation of M2 receptors increased the mRNA for these receptors as a result of the stimulation of PI turnover, PKC translocation and NOS activation. Thus the endogenous NO signaling system participates in the early gene expression of M2 receptors.
Figure 5.
Carbachol-induced increase in mRNA for M2 receptors in rat atria. Preparations were incubated for 2 h with 1 × 10−7 M carbachol in the absence or in the presence of 5 × 10−6 M U-73122 or 5 × 10−6 M trifluoperazine or 1 × 10−5 M L-NMMA or 5 × 10−9 M calphostin. The effect of 5 × 10−5 M SNP is also shown. Values are mean±s.e.m. of six experiments in each group. *P<0.001 versus carbachol alone. An example of the RT-PCR products obtained from this analysis is shown in the lower panel.
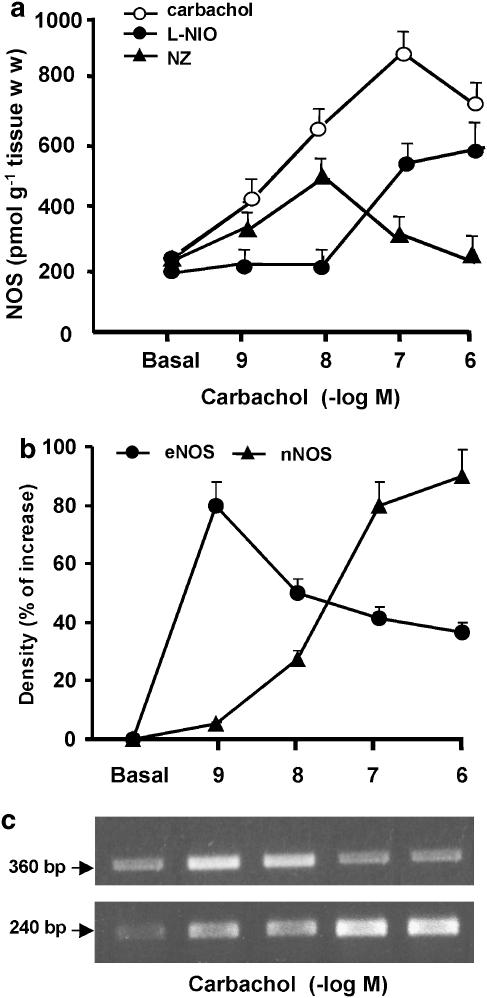
To define the role of different NOS isoforms in the regulation of M2 receptors, isolated rat atria were incubated with selective inhibitors for the different NOS isoforms. As can be see in Figure 6a, eNOS inhibition by L-NIO (5 × 10−6 M) or nNOS inhibition by NZ (5 × 10−6 M) prevented the carbachol-induced stimulation of NOS activity. However, while the inhibition of eNOS was more effective at low agonist concentrations, whereas the inhibition of nNOS was significantly higher at the higher concentrations. The same profile was observed when specific oligonucleotide primers of eNOS and nNOS were used in RT-PCR amplification. Figure 6b shows that carbachol increased nNOS mRNA levels in a concentration-dependent manner. In the case of eNOS, its maximal action was observed at 1 × 10−9 M, decreasing thereafter.
Figure 6.
(a) Concentration–response curve of NOS activity induced by carbachol, in the absence or in the presence of 5 × 10−6 M L-NIO or 5 × 10−6 M NZ. Values are mean±s.e.m. of six experiments in each group. (b) Carbachol effects on semi-quantitative RT-PCR analysis for nNOS and eNOS mRNA expression. Rat atria were incubated for 2 h in the absence (basal) or in the presence of different carbachol concentrations. Values are mean±s.e.m. of seven experiments in each group. (c) RT-PCR products obtained from carbachol dose–response curves are illustrated for eNOS (360 bp) and nNOS (240 bp).
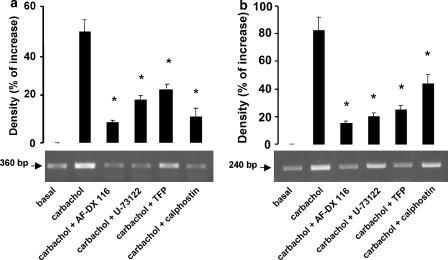
Figure 7 shows that AF-DX 116 (5 × 10−7 M), U-73122 (5 × 10−6 M), trifluoperazine (5 × 10−6 M) and calphostin C (5 × 10−9 M) inhibited the stimulatory action of 1 × 10−7 M carbachol on eNOS- and nNOS-mRNA levels.
Figure 7.
Effects of carbachol on semi-quantitative RT-PCR analysis for eNOS (a), nNOS (b) mRNA levels from rat atria incubated for 2 h with 1 × 10−7 M carbachol in the absence or in the presence of 1 × 10−7 M AF-DX116 or 5 × 10−6 M U-73122 or 5 × 10−6 M trifluoperazine or 5 × 10−9 M calphostin. Basal values correspond to mRNA levels after 2-h incubation without carbachol. Values are mean±s.e.m. of six experiments in each group. The RT-PCR products obtained from carbachol alone or in presence of different blockers are shown in the lower panel. *P<0.001 between carbachol and carbachol+inhibitors.
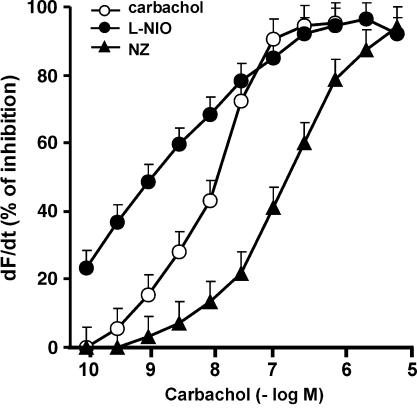
For further studies of the role of NOS isoforms in the effect of carbachol on contractility in atria, we used the selective NOS isoform inhibitors. Figure 8 shows that inhibition of nNOS by NZ (5 × 10−6 M) shifted to the right the dose–response curve of carbachol on atrial contractility (expressed as dF/dt). Contrasting sharply, the dose–response curve of carbachol was shifted to the left by the eNOS inhibitor (L-NIO 5 × 10−6 M).
Figure 8.
Carbachol-induced decrease of contractility (expressed as dF/dt) in rat-isolated atria. Preparations were incubated for 30 min with 5 × 10−6 M NZ, 1−10−6 M L-NIO or vehicle (shown as carbachol) the concentration–response curves of carbachol were plotted out. Values are mean±s.e.m. of six experiments in each group.
All the inhibitory agents at the concentrations used here did not have any effect upon basal values of mRNA for M2 receptors, eNOS or nNOS (Table 3); nor on basal values of dF/dt (Sterin-Borda et al., 2006).
Table 3.
Influence of inhibitory agents upon basal mRNA levels of nNOS, eNOS, iNOS and M2 mAChR
| Inhibitors | M2 (%) | nNOS (%) | eNOS (%) | N |
|---|---|---|---|---|
| None | 100 | 100 | 100 | 12 |
| AF-DX 116 | 98±7 | 93±8 | 93±9 | 5 |
| U-73122 | 110±9 | 91±7 | 108±7 | 5 |
| Trifluoperazine | 95±8 | 93±9 | 109±8 | 5 |
| Calphostin | 118±7 | 101±10 | 102±7 | 5 |
| L-NMMA | 96±8 | 104±9 | 88±6 | 5 |
| L-NIO | 97±8 | 95±6 | 102±9 | 5 |
| NZ | 112±9 | 97±7 | 108±7 | 5 |
Abbreviations: AF-DX 116, 11-((2-((diethylamino)methyl)-1-piperidinyl) acetyl)-5,11-dihydro-6H-pyrido (2,3-b)(1,4)benzodiazepin-6-one; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; L-NMMA, NG-monomethyl-L-arginine; L-NIO, N5-(1-iminoethyl)-L-ornithine dihydrochloride; M2 mAChR, M2 muscarinic acetylcholine receptor; nNOS, neuronal nitric oxide synthase; U-73122, 1-6-17β-3-methoxgestra-1,3,5 (10)-trien-17yl-aminohexyl-1-H-pyrrole-2,5-dione; NZ, N-propyl-L-arginine.
Results are mean±s.e.m. of N number of experiments and are expressed in percentage of variation from controls without drugs (none) taken as 100%.
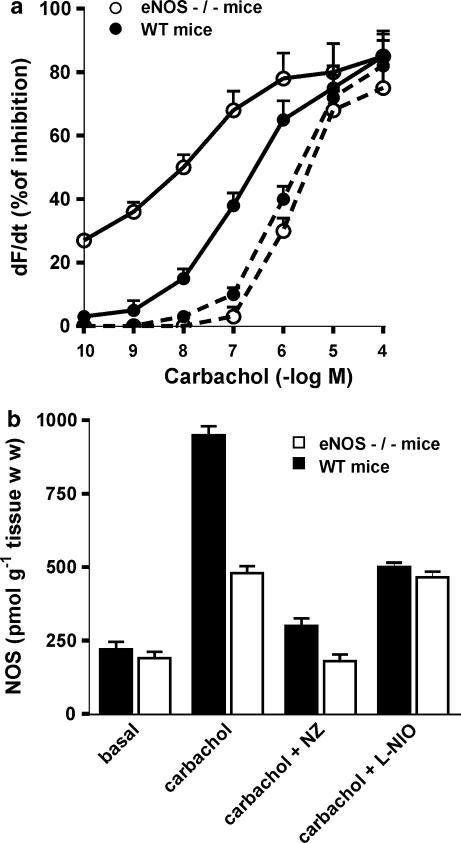
To confirm the role of NOS isoforms in the regulation of atrial contractility by activation of M2 receptors, genetically engineered mice deficient in eNOS (eNOS−/−) were used. Figure 9 shows that there was an almost 10-fold difference in the EC50 values of carbachol in atria from the eNOS−/− mice and in those from the corresponding WT strain. Thus, the EC50 was 1.34 × 10−7 M in WT mice, but in eNOS−/− mice, the EC50 was 1.86 × 10−8 M. The nNOS inhibitor, NZ (5 × 10−6 M) shifted the dose–response curve to the right in atria from both, WT mice (EC50 6.8 × 10−7 M) and eNOS−/− mice (EC50 1.68 × 10−6 M). Figure 9 also shows the effect of carbachol on NOS activity in atria of eNOS−/− and WT mice. Carbachol had a greater effect in WT atria than that in eNOS−/− atria. Further, NZ, but not L-NIO inhibited the stimulatory action of carbachol on NOS activity in atria from eNOS−/− mice, whereas both NZ and L-NIO had an inhibitory effect on NOS activity from WT atria.
Figure 9.
Effects of carbachol on atrial contractility (dF/dt) (a) and NOS activity (b) from WT mice or eNOS−/− mice, in absence (full line) or in presence of 5 × 10−6 M NZ (dotted line). Contractility (dF/dt) is expressed in % of basal values (before addition of the first concentration of the agonists) taken as 100%. Actual basal values were: 4.2±0.4, WT mice (n=5); and 3.9±0.4, eNOS−/− mice (n=5); in the presence of NZ, 4.6±0.5 WT mice (n=5) and 4.2±0.5 eNOS−/− mice (n=5). In (b), the NOS activity triggered by 1 × 10−7 M carbachol alone or in the presence of 5 × 10−6 M NZ and 5 × 10−6 M L-NIO in atria from WT mice or eNOS−/− mice is also shown. Data shown are the means±s.e.m. of five experiments in each group.
Discussion
It has become increasingly clear that agents classically thought to act as neurotransmitters can also alter gene expression. To understand the early events by which agonists at the mAChRs can affect gene expression, we have studied the transcription of several early genes that could be under the control of M2 receptors. A positive feedback mechanism, where carbachol upregulated the mRNA for M2 receptors, nNOS and eNOS was observed. Our present data indicated that the endogenous NO signaling system was involved in this positive feedback between activated M2 receptors and the increase in mRNA for these M2 receptors. The results also pointed to a role for PKC and Ca2+ mobilization in rapid activation of NOS and in the expression of early genes.
A positive regulation of mRNA for M1 receptors soon after treatment with carbachol has been described in rat cerebral frontal cortex (Sterin-Borda et al., 2003). The present work shows that M1 receptor subtype seems not to be involved in the induction of the mRNA by carbachol for M2 receptors, as pirenzepine was not able to modify this effect. This is in agreement with the fact that mRNA for the M1 receptors represents only 0.8% whereas the mRNA for the M2 receptor represents 94% of total mAChR-mRNA in rat atria (Krejci and Tucek, 2002). Moreover, all the carbachol-mediated biological effects on adult rat atria are coupled to M2 and not to M1 receptors (Sterin-Borda et al., 1995; Borda et al., 1997).
The induction of immediate early genes could play an important role in coupling receptor stimulation to long-term responses (Albrecht et al., 2000). At the same time, long-term treatment of cardiac cell cultures with carbachol decreased the levels of mRNA for M2 receptors (Mckinnon et al., 1997) as a result of the activation of a different set of genes that represents later effector or downstream target genes. These target genes may be under the control of the immediate-early genes (von der Kammer et al., 1999).
It has been suggested that events triggered within the first minute of agonist binding to mAChRs are sufficient for induction of gene expression (Trejo and Brown, 1991). However, different pathways for the regulation of mAChR responsiveness by short- and long-term agonist exposure have been described. The mobilization of intracellular Ca2+, and activation of PKC were crucial in inducing immediate early gene expression, while inhibition of cAMP was associated with long-term responses (Trejo and Brown, 1991). It has been reported that NO also is able to alter dramatically the pattern of early gene expression (Morris, 1995). However, little is known about the reciprocal stimulation of immediate early gene expression between M2 receptors and direct enzymatic activation of NOS isoforms or an increase in their expression.
In this paper, we propose a novel insight into the mechanism for the regulation of the positive feedback, where carbachol upregulates the mRNA for M2 receptors, nNOS and eNOS. In rat-isolated atria, we observed that the activation of M2 mAChR was able to promote nNOS and eNOS gene expression, and vice versa, that the gene for M2 receptors was under the control of the endogenous NO signaling system. The mechanism by which carbachol increased mRNA for M2 receptors in atria seemed to involve stimulation of PI hydrolysis by activation of PLC, which in turn, induced CaM, PKC and NOS activation. Two lines of evidence support this statement. On the one hand, we showed that agents known to interfere with these enzyme activities inhibited the stimulatory effect of carbachol on M2 receptor mRNA levels. On the other, we found that, under identical experimental conditions, carbachol stimulated NOS activity, increased PI accumulation and PKC translocation, showing a significant correlation between activation of these enzymes by carbachol. Moreover, a correlation between the increase in mRNA for M2 receptors and NOS activity was demonstrated. All these results indicate that activation of atrial M2 receptors caused the increase in mRNA for M2 receptors as a result of increased NOS activity. The fact that an NO donor (SNP) mimicked the carbachol effect provided more evidence that NO was the key player in the autoregulation of expression of the M2 receptors.
All this raises the question whether other agonists, capable of raising intracellular Ca2+ concentration and NO, could similarly increase M2 receptor expression. In this paper, we demonstrate that β-adrenergic stimulation did not modify M2 receptor gene expression. Moreover, the stimulation of β-adrenoceptors selectively activated nNOS without affecting eNOS (Sterin-Borda et al., 2006). Thus, agonists at other receptors capable of raising intracellular Ca2+ and enhancing NOS gene expression and activation did not lead to increased mRNA for M2 receptors.
The effect of the isoforms of NOS, induced at different agonist concentrations, appeared to be a pivotal factor in the regulation of gene expression. Our findings showed a positive correlation between agonist concentration and the mRNA and activity of nNOS. However, eNOS decreased with increasing concentrations of carbachol. Thus, a negative cross-talk between eNOS and nNOS was observed. A similar negative feedback regulation of eNOS activity and endothelial cell function by NO has been described (Buga et al., 1993; Schwatz et al., 1997).
The subcellular localization of NOS isoforms and the effector molecules is an important regulatory mechanism on their influence upon several biological parameters, including cardiac contraction (Fang et al., 2000, Massion et al., 2003). Stimulation of eNOS activity has been identified as a key inducer of plasmalemma caveolae budding and the internalization of the mAChR–eNOS complex, which terminates mAChR–eNOS coupling in cardiac myocytes (Dessy et al., 2000). The M2 mAChR is translocated in calveolae after agonist stimulation (Feron et al., 1997). In cardiac myocytes transfected with acylation-deficient eNOS, which does not translocate to caveolae (Feron et al., 1998), carbachol was unable to inhibit the spontaneous beating rate of the mycocytes showing that caveolar location of eNOS was essential for the NO-dependent effect of the M2 receptor agonist. Thus, the interaction between eNOS and caveolin may not only restrain basal NO production, but may also concentrate the enzyme in discrete locations, both promoting activation by agonist and the subsequent termination of signaling (Feron and Kelly, 2001).
On the basis of subcellular location of both NOS isoforms, our hypothesis is that the divergent effects on contractility in response to M2 receptor activation observed in this paper could be because of the altered myocyte Ca2+ cycling (Petroff et al., 2001). Our striking current finding is that intracrine NO production, depending on which NOS isoform is its source, has opposite effects on the contractile response to M2 agonists. Thus, inhibition of nNOS activity shifted the dose–response curve for carbachol to the right, whereas inhibition of eNOS activity enhanced the effect of low concentrations of carbachol. These results were corroborated by those from eNOS−/− mice, where an enhanced contractile response to carbachol was demonstrated. Moreover, inhibition of nNOS resulted in an inverse inotropic effect. In the heart, NO inhibits the L-type of Ca2+ channels (Mery et al., 1993), but stimulates sarcoplasmic reticulum Ca2+ release (Petroff et al., 2001), leading to variable effects on myocardial contractility. Our results suggest that nNOS may act as a negative cardiac regulator, whereas eNOS acts as a positive cardiac regulator. Accordingly, in rat atria, nNOS acts in a countervailing mechanism limiting the positive chronotropic and inotropic effect of β-adrenoceptor agonists (Sterin-Borda et al., 2006), because of the inhibition of Ca2+ influx (Vandecasteele et al., 1999; Sears et al., 2003). Although this study supports the view that eNOS attenuates mAChR-mediated control of the heart, this is challenged by opposite results (Han et al., 1996; Feron et al., 1998). These conflicting results might be the consequence of various factors, such as differences in mammalian species and the anatomical region within the myocardium itself (Massion et al., 2003). Thus, both eNOS and nNOS enzymes are constitutively expressed in rat atria, but in rat ventricle, the nNOS does not appear to be expressed constitutively (Sterin-Borda et al., 2006). The lack of nNOS in ventricle probably accounts for the discrepancies between the contributions of eNOS to mAChR signaling pathways in atria or ventricle.
Strikingly, calphostin C, known to inhibit PKC activity, was able to inhibit both eNOS and nNOS signaling pathways, triggered by mAChR activation. This suggests that PKC is an important activator of constitutive NOS in atria. The requirement of CaM and PKC activation observed for the mAChR agonist-induced stimulation of the NO-mediated pathway in rat atrium, revealed in this paper, is consistent with reports showing CaM kinase and PKC-mediated phosphorylation and regulation of NOS (Nakane et al., 1991; Bredt et al., 1992).
Myocardial production of NO is one element in a wide range of autonomic controls of normal cardiac contraction. It is also one of the pathogenic mediators of the loss of such controls in a failing heart (Massion et al., 2003). The discovery of the selective modulation of cardiac contractility by NOS isoforms has stimulated considerable interest in the possibility of reversing cardiac dysfunction with isoform-selective inhibitors of NOS.
Acknowledgments
We thank Mrs Elvita Vannucchi and Mrs Fabiana Solari for their excellent technical assistance. We also thank W Rasband for NIH Image. This research was funded by UBACYT (University of Buenos Aires, Argentina) and CONICET (Argentine National Research Council, Buenos Aires, Argentina).
Abbreviations
- AF-DX 116
11-((2-((diethylamino)methyl)-1-piperidinyl) acetyl)-5,11-dihydro-6H-pyrido (2,3-b)(1,4)benzodiazepin-6-one
- CaM
calcium/calmodulin
- eNOS
endothelial nitric oxide synthase
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- KRB
Krebs Ringer bicarbonate
- L-NIO
N5-(1-iminoethyl)-L-ornithine dihydrochloride
- L-NMMA
NG-monomethyl-L-arginine
- M2 mAChR
M2 muscarinic acetylcholine receptor
- [3H]MI
[myo-3H]inositol
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NZ
N-propyl-L-arginine
- PCR
polymerase chain reaction
- PI
phosphoinositide
- PKC
protein kinase C
- PLC
phospholipase C
- SNP
sodium nitroprusside
- U-73122
1-6-17β-3-methoxgestra-1,3,5 (10)-trien-17yl-aminohexyl-1-H-pyrrole-2,5-dione
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Albrecht C, Von Der Kammer H, Mayhaus M, Klaudiny J, Schweizer M, Nitsch RM. Muscarinic acetylcholine receptors induce the expression of the immediate early growth gene CYR61. J Biol Chem. 2000;275:28929–28936. doi: 10.1074/jbc.M003053200. [DOI] [PubMed] [Google Scholar]
- Belsham DD, Wetsel WC, Mellon PL. NMDA and nitric oxide act through the cGMP signal transduction pathway to repress hypothalamic gonadotropin-releasing hormone gene expression. EMBO J. 1996;15:538–547. [PMC free article] [PubMed] [Google Scholar]
- Bishop-Bailey D, Larkin SW, Warner TD, Chen G, Mitchell JA. Characterization of the induction of nitric oxide synthase and cyclo-oxygenase in rat aorta in organ culture. Br J Pharmacol. 1997;121:125–133. doi: 10.1038/sj.bjp.0701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda ES, Perez Leiros C, Camusso JJ, Bacman S, Sterin-Borda L. Differential cholinoceptor subtype-dependent activation of signal transduction pathways in neonatal versus adult rat atria. Biochem Pharmacol. 1997;53:959–967. doi: 10.1016/s0006-2952(96)00866-0. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. J Biol Chem. 1992;267:10976–10981. [PubMed] [Google Scholar]
- Buga GM, Griscavage JM, Rogers NE, Ignarro LJ. Negative feedback regulation of endothelial cell function by nitric oxide. Circ Res. 1993;73:808–812. doi: 10.1161/01.res.73.5.808. [DOI] [PubMed] [Google Scholar]
- Chomozynski P, Saachi N. Single step method of RNA isolation by acid guanidium thiocianate-phenol-chloroform extraction. Ann Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou H, Ogawa N, Asanuma M, Hirata H, Kondo Y, Mori A. Rapid response of striatal muscarinic M1-receptor mRNA to muscarinic cholinergic agents in rat brain. Mol Brain Res. 1993;19:211–214. doi: 10.1016/0169-328x(93)90028-n. [DOI] [PubMed] [Google Scholar]
- Dessy C, Kelly RA, Balligand JL, Feron O. Dynamin mediates caveolar sequestration of muscarinic cholinergic receptors and alteration in NO signaling. EMBO J. 2000;19:4272–4280. doi: 10.1093/emboj/19.16.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenthaler M, Lohmann SM, Walter U, Pilz RB. Signal transduction by cGMP-dependent protein kinases and their emerging roles in the regulation of cell adhesion and gene expression. Rev Physiol Biochem Pharmacol. 1998;135:174–209. doi: 10.1007/BFb0033673. [DOI] [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras 1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res. 2001;88:129–131. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- Feron O, Smith TW, Michel T, Kelly RA. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J Biol Chem. 1997;272:17744–17748. doi: 10.1074/jbc.272.28.17744. [DOI] [PubMed] [Google Scholar]
- Han X, Kobzik L, Balligand JL, Kelly RA, Smith TW. Nitric oxide synthase (NOS3)-mediated cholinergic modulation of Ca2+ current in adult rabbit atrioventricular nodal cells. Circ Res. 1996;78:998–1008. doi: 10.1161/01.res.78.6.998. [DOI] [PubMed] [Google Scholar]
- Hosey MM. Diversity of structure, signaling and regulation within the family of muscarinic cholinergic receptors. FASEB J. 1992;6:845–852. [PubMed] [Google Scholar]
- Johnston HM, Morris BJ. NMDA and nitric oxide regulate the expression of calcium/calmodulin-dependent kinase II in the hippocampal dentate gyrus. Mol Brain Res. 1995;31:141–150. doi: 10.1016/0169-328x(95)00046-u. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Krejci A, Tucek S. Quantification of mAChRs for M1 to M5 subtypes of muscarinic receptor in rat heart and brain cortex. Mol Pharmacol. 2002;61:1267–1272. doi: 10.1124/mol.61.6.1267. [DOI] [PubMed] [Google Scholar]
- Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- Mckinnon LA, Rosoff M, Hamilton SE, Schlador ML, Thomas SL, Nathanson NM. Regulation of muscarinic receptor expression and function in cultured cells and in knock-out mice. Life Sci. 1997;60:1101–1104. doi: 10.1016/s0024-3205(97)00053-2. [DOI] [PubMed] [Google Scholar]
- Mery PF, Pavoine C, Belhassen L, Pecker F, Fischmeister R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J Biol Chem. 1993;268:26286–26295. [PubMed] [Google Scholar]
- Morris BJ. Stimulation of immediate early gene expression in striatal neurons by nitric oxide. J Biol Chem. 1995;270:24740–24744. [PubMed] [Google Scholar]
- Nakane N, Mitchell J, Forstermann V, Murad F. Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem Biophys Res Commun. 1991;150:1396–1402. doi: 10.1016/s0006-291x(05)81351-8. [DOI] [PubMed] [Google Scholar]
- Pascual J, Borda E, Cossio P, Arana R, Sterin-Borda L. Modification of sarcolemmal enzymes of chagasic IgG and its effect on cardiac contractility. Biochem Pharmacol. 1986;35:3839–3845. doi: 10.1016/0006-2952(86)90673-8. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, et al. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Peunova N, Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993;364:450–453. doi: 10.1038/364450a0. [DOI] [PubMed] [Google Scholar]
- Qi WN, Yan ZQ, Whang PG, Zhou Q, Chen LE, Seaber AV, et al. Gene and protein expressions of nitric oxide synthases in ischemia-reperfused peripheral nerve of the rat. Am J Physiol Cell Physiol. 2001;281:C489–C856. doi: 10.1152/ajpcell.2001.281.3.C849. [DOI] [PubMed] [Google Scholar]
- Scharff O, Foder B. Effect of trifluoroperazine, compound 48/80, TMB-8 and verapamil on the rate of calmodulin binding to erythrocyte calcium ATPase. Biochem Biophys Acta. 1984;772:29–36. doi: 10.1016/0005-2736(84)90514-5. [DOI] [PubMed] [Google Scholar]
- Schwatz D, Menconca M, Schwartz I, Xia Y, Satriao J, Wilson CB, et al. Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J Clin Invest. 1997;100:439–448. doi: 10.1172/JCI119551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, et al. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:52–59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Smallridge RG, Kiang JG, Gist ID, Feim HG, Galloway RJ. U-73122, an aminosteroid phospholipase C antagonist, noncompetitively inhibits thyrotropin-releasing hormone effects in GH3 rat pituitary cells. Endocrinology. 1992;131:1883–1888. doi: 10.1210/endo.131.4.1396332. [DOI] [PubMed] [Google Scholar]
- Steel MC, Buckley NJ. Differential regulation of muscarinic receptor mRNA levels in neuroblastoma cells by chronic agonist exposure: a comparative polymerase chain reaction study. Mol Pharmacol. 1993;43:694–701. [PubMed] [Google Scholar]
- Sterin-Borda L, Bernabeo G, Ganzinelli S, Joensen L, Borda E. Role of nitric oxide/cyclic GMP and cyclic AMP in beta3 adrenoceptor-chronotropic response. J Mol Cell Cardiol. 2006;40:580–588. doi: 10.1016/j.yjmcc.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Sterin-Borda L, Ganzinelli S, Berra A, Borda E. Novel insight into the mechanisms involved in the regulation of the m1 muscarinic receptor, iNOS and nNOS mRNA levels. Neuropharmacology. 2003;45:260–269. doi: 10.1016/s0028-3908(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Sterin-Borda L, Vila-Echague A, Perez-Leiros C, Genaro A, Borda E. Endogenous nitric oxide signalling system and the cardiac muscarinic acetylcholine receptor-inotropic response. Br J Pharmacol. 1995;115:1525–1531. doi: 10.1111/j.1476-5381.1995.tb16646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J, Brown JH. c-fos and c-jun are induced by muscarinic receptor activation of protein kinase C but are differentially regulated by intracellular calcium. J Biol Chem. 1991;266:7876–7882. [PubMed] [Google Scholar]
- Van Rossum JM. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Vandecasteele G, Eschenhagen T, Scholz H, Stein B, Verde I, Fischmeister R. Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med. 1999;5:331–334. doi: 10.1038/6553. [DOI] [PubMed] [Google Scholar]
- Von Der Kammer H, Albrecht C, Mayhaus M, Hoffmann B, Stanke G, Nitsch RM. Identification of genes regulated by muscarinic acetylcholine receptors: application of an improved and statistically comprehensive mRNA differential display technique. Nuclei Acid Res. 1999;27:2211–2218. doi: 10.1093/nar/27.10.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Kammer H, Demiralay C, Andersen B, Albrecht C, Mayhaus M, Nitsch RM. Regulation of gene expression by muscarinic acetylcholine receptors. Biochem Soc Symp. 2001;67:131–140. doi: 10.1042/bss0670131. [DOI] [PubMed] [Google Scholar]
- Wald MR, Borda ES, Sterin-Borda L. Mitogenic effect of erythropoietin on neonatal rat cardiomyocytes: signal transduction pathways. J Cell Physiol. 1996;167:461–468. doi: 10.1002/(SICI)1097-4652(199606)167:3<461::AID-JCP10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wald MR, Borda ES, Sterin-Borda L. Participation of nitric oxide and cyclic GMP in the supersensitivity of acute diabetic rat myocardium by cholinergic stimuli. Biochem Pharmacol. 1998;55:1991–1999. doi: 10.1016/s0006-2952(98)00069-0. [DOI] [PubMed] [Google Scholar]