Abstract
Background and purpose:
F15063 is a high affinity D2/D3 antagonist, D4 partial agonist, and high efficacy 5-HT1A agonist, with little affinity (40-fold lower than for D2 receptors) at other central targets. Here, the profile of F15063 was evaluated in models of positive symptoms of schizophrenia and motor side-effects.
Experimental approach:
Rodent behavioural tests were based on reversal of hyperactivity induced by psychostimulants and on measures of induction of catalepsy and ‘serotonin syndrome'.
Key results:
F15063 potently (ED50s: 0.23 to 1.10 mg kg−1 i.p.) reversed methylphenidate-induced stereotyped behaviors, blocked d-amphetamine and ketamine hyperlocomotion, attenuated apomorphine-induced prepulse inhibition (PPI) deficits, and was active in the conditioned avoidance test. In mice, it reversed apomorphine-induced climbing (ED50 = 0.30 mg kg−1 i.p.). F15063, owing to its 5-HT1A agonism, did not produce (ED50 > 40 mg kg−1 i.p.) catalepsy in rats and mice, a behavior predictive of occurrence of extra-pyramidal syndrome (EPS) in man. This absence of cataleptogenic activity was maintained upon sub-chronic treatment of rats for 5 days at 40 mg kg−1 p.o. Furthermore, F15063 did not induce the ‘serotonin syndrome' in rats (flat body posture and forepaw treading: ED50 >32 mg kg−1 i.p.).
Conclusions and implications:
F15063 conformed to the profile of an atypical antipsychotic, with potent actions in models of hyperdopaminergic activity but without inducing catalepsy. These data suggest that F15063 may display potent antipsychotic actions with low EPS liability. This profile is complemented by a favourable profile in rodent models of negative symptoms and cognitive deficits of schizophrenia (companion paper).
Keywords: 5-HT1A agonist, atypical antipsychotic, dopamine, D2 antagonist, D4 partial agonist, schizophrenia
Introduction
Schizophrenia is a disease with non-pathognomic symptoms, characterized by a multitude of facets, most notably psychosis, negative symptoms and cognitive deficits. Currently available antipsychotics alleviate the positive symptoms of psychosis and, to a more limited extent, also improve the negative signs (Tandon et al., 1993; Breier et al., 1994; Buchanan et al., 1998; Rosenheck et al., 1999). This limited spectrum of efficacy is, in turn, thought to hamper proper societal functioning of patients and their re-insertion into society (Freedman, 2003; Green et al., 2004). Beneficial effects on psychosis are considered to result from blockade of dopamine D2 receptors (Kapur and Remington, 2001). Unfortunately, this blockade also gives rise to troublesome motor side effects, clustered under the name of extrapyramidal syndrome (EPS). Adding to antagonism at dopamine D2 receptors an additional property that would minimize EPS and afford therapeutic activity against negative symptoms and cognitive dysfunction would thus constitute a definite advantage.
There is ample evidence that agonist activity at the 5-HT1A receptor offers the possibility of controlling EPS associated with blockade of dopamine D2 receptors. 8-OH-DPAT, the prototypical 5-HT1A receptor agonist, blocked catalepsy produced by haloperidol (Broekkamp et al., 1988; Invernizzi et al., 1988) and reduced dyskinesia in monkeys chronically treated with dopamine D2 receptor antagonists (Liebman et al., 1989; Christoffersen and Meltzer, 1998). In the clinic, buspirone and tandospirone, two partial agonists at the 5-HT1A receptor, have been shown to decrease parkinsonian signs or tardive dyskinesia in schizophrenic patients treated with antipsychotics (Goff et al., 1991; Moss et al., 1993; Yoshida et al., 1998).
Combining a 5-HT1A receptor agonist with dopamine D2 antagonist activity is anticipated to bring additional benefits: 5-HT1A receptor activation increases dopamine release in rat prefrontal cortex (Rollema et al., 1997, 2000; Millan et al., 1998; Sprouse et al., 1999; Ichikawa and Meltzer, 2000; Diaz-Mataix et al., 2005), indicative of beneficial activity against negative symptoms and cognitive disturbances of schizophrenia (Kapur and Remington, 1996). Also, it has been reported that buspirone and tandospirone substantially ameliorate cognitive performance in schizophrenic patients treated with haloperidol (Sumiyoshi et al., 2000, 2001). Lastly, 5-HT1A receptor agonists exert antidepressant- and anxiolytic-like properties (Blier and Ward, 2003; Celada et al., 2004), of particular interest to schizophrenic patients, an appreciable proportion of whom suffer from co-morbid anxiety and/or depression (Buchanan et al., 2002). Thus, combined 5-HT1A agonist and D2 antagonist properties would be expected to have a wider spectrum of activity than currently used antipsychotics and, in particular, exhibit greater efficacy against negative/cognitive symptoms with reduced EPS liability (Millan, 2000; Bantick et al., 2001; Ichikawa et al., 2001).
These considerations have led to the hypothesis that a dual dopamine D2 antagonist/5-HT1A agonist could represent a new therapeutic approach for the treatment of schizophrenia. Indeed, several new third generation compounds in development conform to this profile: bifeprunox (Feenstra et al., 2001; Wolf, 2003), SSR181507 (Claustre et al., 2003; Depoortère et al., 2003; Boulay et al., 2004; Terranova et al., 2005), SLV313 (Feenstra et al., 2002; McCreary et al., 2002) and sarizotan (now developed as an anti-dyskinetic: Bibbiani et al., 2001; Rabiner et al., 2002; Bartoszyk et al., 2004).
In a companion paper (Newman-Tancredi et al., 2007, and in abstract form: Newman-Tancredi et al., 2006), we have reported on the binding and neurochemical profiles of F15063 (N-[(2,2-dimethyl-2,3-dihydro-benzofuran-7-yloxy)ethyl]-3-(cyclopent-1-enyl)-benzylamine; mono-tartrate), synthesized by the Medicinal Chemistry department of Pierre Fabre Recherche (Vacher et al., 2002). This compound presents a marked affinity for human dopamine D2L (pKi=9.44), D2S (pKi=9.25), D3 (pKi=8.95) and D4 (pKi=8.81) receptors, and a somewhat lesser affinity for 5-HT1A receptors (pKi=8.37). It presents low affinity at a multitude of other receptors, including 5-HT2A and 5-HT2C, α1 and α2 adrenoceptors, muscarine M1 and histamine H1 receptors. In functional in vitro tests, it behaves as an antagonist at dopamine D2 receptors (contrary to other preferential D2/5-HT1A antipsychotics such as bifeprunox and SSR181507 that act as partial agonists at these receptors: Bruins Slot et al., 2006; Cosi et al., 2006), and as a partial agonist at D4 and agonist at 5-HT1A receptors.
The present set of experiments assessed the behavioural profile of F15063 in tests considered to be predictive of antipsychotic activity in mice (apomorphine-induced climbing/sniffing behaviours) and in rats (methylphenidate (MPD)-induced stereotypies, hyperlocomotion produced by d-amphetamine or ketamine, active avoidance and deficit of apomorphine-induced prepulse inhibition (PPI) of the startle reflex). Finally, F15063 was evaluated in models predictive of unwanted side effects, such as the catalepsy test in mice and rats (predictive of occurrence of EPS in humans) and the induction of the serotoninergic syndrome in rats. Comparative data on a series of reference antipsychotics have been presented elsewhere (Bardin et al., 2006a, 2006b, in press). The behavioural profile of F15063 in tests indicative of activity against negative symptoms and cognitive deficits of schizophrenia, reflecting agonist activity at 5-HT1A and dopamine D4 receptors, is the subject of a separate paper (Depoortère et al., 2007), but has been reported in an abstract form (Depoortère et al., 2006).
Materials and methods
Animals
Male Sprague–Dawley rats and NMRI mice (180–200 and 20–24 g at the start of the experiments) were supplied by Iffa-Credo (Les Oncins, France). Animals were kept in temperature- and humidity-controlled rooms (21±1°C, relative humidity: 55±5%) on a 12:12 h light:dark cycle (lights on at 0700 hours). Food (standard A04 rodent chow; Animal Food and Engineering, Epinay sur Orge, France) and filtered water (0.22 μm pore diameter) were available ad libitum. Animals were handled and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, ML, USA) and the European Directive 86/609. In addition, the protocols were carried out in compliance with French regulations and the local ethical committee guidelines for animal research.
Apomorphine-induced climbing and sniffing behaviours in mice
A detailed description is given by Bardin et al. (2006a). Briefly, mice were injected with F15063 or saline (intraperitoneal (i.p.) or per os (p.o.)), followed 45 min later by subcutaneous (s.c.) injection of apomorphine (2.5 mg kg−1) or vehicle. They were then placed into cylindrical wire-mesh cages and observed (55–65 min after the first injection) for 10 s every minute for the presence or absence of climbing (i.e., all four paws on the cage, above the floor). Mice were injected and tested on a single occasion. Sniffing was scored when the animal showed uninterrupted sniffing for at least 3 s during these 10 s sampling periods. Thus, the score for climbing or sniffing could vary from 0 to 10 for the entire observation period.
Data (climbing and sniffing scores) were analysed with a one-way analysis of variance (ANOVA) with the treatment dose as the between-subjects factor, followed by post hoc comparisons with the control group using Bonferroni's tests.
MPD-induced behaviours in rats
A detailed description of these methods is given by Koek and Colpaert (1993). Briefly, rats were treated twice, first with F15063 or vehicle (i.p.), followed 30 min later by MPD (40 mg kg−1, i.p.) or saline. Observations were made during a 10 min period starting 30 min after the second injection. Every minute, rats were observed sequentially during a 10 s period for the presence or absence of:
locomotion (all four limbs moving);
rearing (standing on hindlimbs, body fully extended);
sniffing;
gnawing (the cage or body);
licking (the cage).
A behaviour was considered present if the animal showed uninterrupted signs of it for at least 3 s.
This cycle of observation was repeated 10 times during this 10 min period; thus, the incidence of a particular behaviour could vary from 0 to 10 for the entire observation period. Data are expressed as the percentage of animals showing a reduction of gnawing and normalization of behaviour (see Koek and Colpaert, 1993 for in-depth definition of terms and discussion of methodology). Effective dose for 50% response (ED50) values and their associated confidence limits were calculated with the Litchfield and Wilcoxon probit analysis procedure (Tallarida and Murray, 1987).
Conditioned avoidance response in rats
Rats were trained to avoid delivery of an electric foot-shock (0.8 mA, 33 Hz) using a two-compartment shuttle box apparatus (MED Associates, East Fairfield, VT, USA). During training sessions, the rat was required to avoid the shocks delivered through the grid floor of the compartment in which it was present by moving to the other ‘safe' compartment, in response to the illumination of a cue light (conditioned stimulus). Training sessions consisted of 30 trials with an interval of 40 s between the start of each trial. A trial began with the illumination of the cue light for a maximum period of 10 s. A conditioned avoidance response (CAR) was recorded if the rat moved to the ‘safe' compartment during the illumination of the cue light, but before shock delivery. If the animal did not move to the other compartment during this period, an electric shock was delivered to the floor of the compartment in which the animal was present, for a maximal duration of 10 s. In that case, an avoidance failure was recorded: this parameter is not reported, as it is the difference between the number of CARs and the number of escape failure responses (EFRs; see below). If the animal remained in the electrified compartment after the end of this 10 s period, an EFR was recorded. On a test day, animals were injected with F15063 or vehicle (i.p.) 60 min before the start of the session.
Data (number of CARs and of EFRs) were analysed with a one-way ANOVA with the treatment dose as the between-subjects factor, followed by post hoc Bonferroni's tests with the vehicle-injected group as the control.
In order to investigate the modulatory influence of the 5-HT1A receptor agonist component of F15063 on conditioned avoidance, the 5-HT1A receptor antagonist WAY100,635 (0.63 mg kg−1) or saline were administered s.c., 15 min before F15063, that is, 75 min before the start of the experiment. Data were analysed with a two-way ANOVA, with the pretreatment (WAY100,635 or vehicle) and the dose of F15063 as the between-subjects factor, followed by a Newman–Keuls post hoc test.
d-Amphetamine or ketamine-induced locomotor activity in rats
Rats were treated twice, first with F15063 or vehicle (i.p.), followed 45 min later by saline (for recording of spontaneous locomotor activity), d-amphetamine (0.63 mg kg−1, s.c.) or ketamine (40 mg kg−1, s.c.). After the second injection, the home cage was placed in an automated animal activity monitor (Multi-Varimex, Columbus Instruments, Columbus, OH, USA) and infrared light beams interruptions were counted for 1 h (between 16 and 75 min).
Data (number of beam breaks) were analysed with a one-way ANOVA with the treatment dose as the between-subjects factor, followed by post hoc Bonferroni's tests for comparison with the vehicle/saline vehicle/d-amphetamine or vehicle/ketamine groups.
Deficit of PPI of the startle reflex induced by apomorphine in rats
A full description is given by Auclair et al. (2006a). Rats were pre-tested in startle chambers (SR LAB, San Diego Instruments, San Diego, CA, USA) 1 h 45 min before the pharmacological challenge (test) session. This pre-test session was used to accustom rats to the procedure. Three different trial types were presented against a continuous 70 dB background noise: no pulse (NP), 118 dB pulse (pulse alone; PA) and 78 dB prepulse (pp) followed by a 118 dB pulse (prepulse-pulse; ppP). The PA duration was 40 ms, the pp duration 20 ms, and the interval between the end of the pp and the onset of the PA is 80 ms. Sessions started with a 5-min adaptation period after which the animals were exposed to 10 PA (included to induce habituation to startle, such that habituation during the following PPI assessment would be minimized: these trials were not used for data analysis). These 10 PA trials were followed by 10 PA, 10 ppP and three NP trials presented in a pseudo-random order. The interval between trials was variable but with a median of 15 s.
At the end of the pre-test session, animals were treated i.p. with F15063 or its vehicle, and 45 min later with apomorphine (0.63 mg kg−1) or saline s.c. They were then 15 min later subjected to a test session, in all respect similar to the pre-test session (vide supra).
For each test session, the median of the amplitude of the startle responses for the last 10 PA trials and for the 10 ppP was calculated. The percentage PPI was calculated as follows:
Data (percentage PPI) were analysed with a one-way ANOVA with the treatment dose as the between-subjects factor, followed by a Dunnett's post hoc test for comparison with the vehicle/apomorphine group.
Induction of catalepsy in rats
A detailed description is given by Kleven et al. (2005). Rats were injected i.p. or p.o. with F15063 or vehicle 60 min before measuring catalepsy, first in the crossed-legs position (CLP) and immediately thereafter in the bar test. In the CLP test, each hindlimb was placed over its ipsilateral forelimb by the experimenter, and the time during which the animal remained in this position was determined up to a maximum of 30 s. In the bar test, forelimbs were placed on a horizontal, cylindrical metal bar, and the time during which both forelimbs remained on the bar was determined up to a maximum of 30 s. Both tests were repeated 3 and 6 min later; between each trial, the animal was returned to its home cage.
Involvement of the 5-HT1A receptor agonist component of F15063 in catalepsy was assessed by pretreatment with WAY100,635 (0.63 mg kg−1, or saline as a control), administered s.c., 15 min before F15063, that is, 75 min before recording catalepsy. Data (mean time spent in catalepsy for the three trials) were analysed with a two-way ANOVA, with the pretretatment (WAY100,635 or vehicle) and the dose of F15063 as the between-subjects factor, followed by a Newman–Keuls post hoc test.
To assess if catalepsy develops following repeated administration, F15063 (40 mg kg−1) or saline were administered p.o. daily for 4 consecutive days. On the 5th day, 60 min after an acute challenge with the same dose of F15063, rats were tested for catalepsy, first in the CLP test, and immediately thereafter in the bar test. A separate group of rats received a pretreatment with either saline or WAY100,635 (0.63 mg kg−1, s.c.) 15 min before administration of the acute challenge with F15063 on day 5. Data (mean time spent in catalepsy for the three trials) were analysed with a two-way ANOVA, with the chronic treatment (saline or F15063) and the acute pretreatment (WAY100,635 or vehicle) as the between-subjects factor, followed by a Newman–Keuls post hoc test.
Induction of catalepsy in mice
A detailed description is given in Bardin et al. (2006a). Mice were injected with F15063 or saline (i.p.) 60 min before measurement of catalepsy. Forelimbs were placed on a cylindrical metal bar and the time during which both forelimbs remained on the bar was recorded up to a maximum of 30 s. The test was repeated three times (inter-trial interval: 1 min). In order to investigate the effects of 5-HT1A receptors on catalepsy, WAY100,635 (2.5 mg kg−1) or saline was administered s.c., 15 min before F15063, that is, 75 min before recording catalepsy. Data (mean time spent in catalepsy for the three trials) were analysed with a two-way ANOVA, with the pretreatment (WAY100,635 or vehicle) and the dose of F15063 as the between-subjects factor, followed by a Newman–Keuls post hoc test.
Induction of the serotonin syndrome in rats
Rats were treated i.p. or p.o. with F15063 or its vehicle. Behavioural observations were made at two time points, from 10 to 20 min or from 55 to 65 min post-injection for i.p. and p.o. administration, respectively. Four animals were observed individually during each 10 min period; the four rats were observed in turn, every 15 s with a period of 10 s of observation per animal. During each of these observation periods, the presence (1), or absence (0) of forepaw treading (FPT) and lower lip retraction (LLR) was recorded. The studied behaviour was considered present if the animal showed uninterrupted signs for at least 3 s. This cycle was repeated 10 times during a 10 min period; thus, the incidence of a particular behaviour could vary from 0 to 10 for any observation period. Flat body posture (FBP) was scored present (1) if it occurred during the entire observation period, otherwise, the score was 0. Data are presented as the percentage of rats presenting FPT, LLR and FBP.
Effects on plasma prolactin levels in rats
Plasma prolactin levels were assessed as described in detail by Cosi et al. (2006). Briefly, rats were treated p.o. with F15063 or its vehicle 60 min before killing by decapitation. Prolactin levels were assessed with a commercially available radioimmunoassay kit (Rat Prolactin [125I] Biotrak Assay System with Magnetic Separation, RPA553; Amersham Biosciences, Little Chalfont, UK).
Statistical analyses
Results were analysed by ANOVA (one- or two-way) with appropriate post hoc tests (Dunnett's, Bonferroni or Newman–Keuls). The details of the statistical treatment of each data set are given at the end of the description of the methods used to generate the data.
Drugs
F15063 ((N-[(2,2-dimethyl-2,3-dihydro-benzofuran-7-yloxy)ethyl]-3-(cyclopent-1-enyl)-benzylamine; mono-tartrate) and methylphenidate HCl were synthesized by Bernard Vacher/Stephane Cuisat and Jean Louis Maurel, respectively (Medicinal Chemistry, Centre de Recherche Pierre Fabre, Castres, France). Ketamine HCl and apomorphine were purchased from Sigma RBI (St Quentin Fallavier, France) and d-amphetamine HCl from Calaire Chimie (Calais, France). d-Amphetamine, ketamine, methylphenidate and apomorphine were dissolved in distilled water and administered s.c. or i.p. (methylphenidate) at a volume of 10 ml kg−1. F15063 was administered at a volume of 10 ml kg−1 in distilled water+Tween 80 (0.1% v/v) for both routes of administration. Doses refer to the weight of the free base.
Results
Antagonism of apomorphine-induced climbing and sniffing in mice
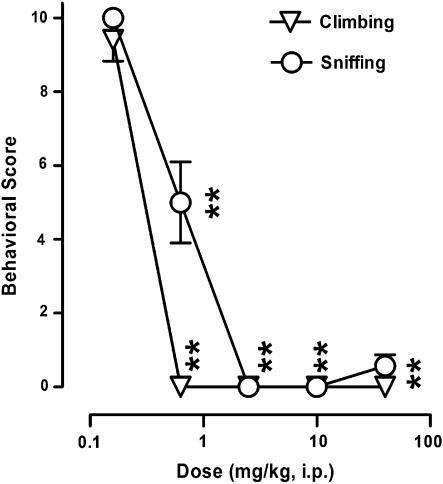
In a model considered to detect of antipsychotic activity, using a direct dopaminergic receptor agonist, F15063 dose-dependently and significantly reduced both climbing scores in mice injected with apomorphine (Figure 1), with ED50 values of 0.30 and 0.37 mg kg−1 i.p. for climbing and sniffing, respectively.
Figure 1.
Antagonism by F15063 of climbing and sniffing behaviours induced by apomorphine in mice. Each symbol represents the mean behavioural score (±s.e.m.) obtained during observation periods (10 s every min, from 55 to 65 min after F15063 administration). Apomorphine (2.5 mg kg−1 s.c.) was injected 45 min after F15063. **P<0.01, compared with the vehicle/apomorphine group (scores were 9.45±0.2 and 9.94±0.04 for climbing and sniffing, respectively, n=84), Bonferroni's post hoc tests following significant one-way ANOVA (climbing (F(5,123)=139.6, P<0.0001)) and sniffing (F(5,123)=600.6, P<0.0001). N=7 mice per group.
Normalization of methylphenidate-induced behaviours in rats
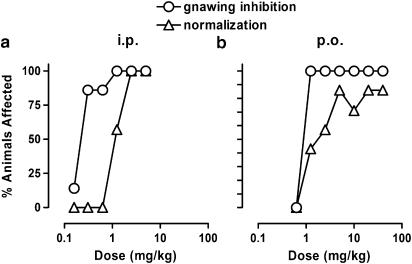
Following i.p. administration, F15063 dose-dependently (Figure 2a) increased the percentage of animals free from gnawing (ED50: 0.24 mg kg−1) and presenting a complete normalization of behaviours induced by the dopamine releaser methylphenidate (ED50: 1.10 mg kg−1), a model of antipsychotic activity using an indirect dopaminergic receptor agonist.
Figure 2.
Antagonism by F15063 of stereotypies induced by methylphenidate in rats after i.p. (a) or p.o. (b) administration. Each symbol represents the percentage of rats showing a gnawing score less than 9, or a complete normalization of stereotyped behaviour induced by 40 mg kg−1 i.p. methylphenidate, administered 30 min after F15063 or vehicle. For both panels, N=7 rats per group.
F15063 was also found to be active via the p.o. route (Figure 2b), with ED50 values of 0.93 and 2.5 mg kg−1 for gnawing inhibition and normalization of behaviour, respectively, yielding p.o./i.p. ratios of ∼4 and ∼2 for gnawing and normalization of behaviour, respectively.
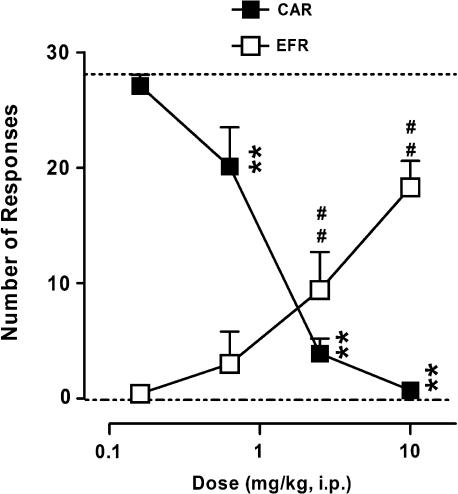
Antagonism of CAR in rats
In a classical model of antipsychotic-like activity, F15063 reduced the number of CARs in a dose-dependent and significant manner, with a minimal effective dose (MED)=0.63 mg kg−1 i.p. (Figure 3). Although the compound also increased the number of EFRs, it did so less potently (MED=2.5 mg kg−1). This was further confirmed by an almost threefold difference in the ED50 values for CARs (0.33 mg kg−1) and EFRs (0.96 mg kg−1).
Figure 3.
Reduction by F15063 of two-way active avoidance behaviour in rats. Each symbol represents the mean (±s.e.m.) number of CARs (out of a maximum of 30) or EFRs. F15063 or vehicle was administered 60 min before the start of the session. **P<0.01, compared with the vehicle-treated group (mean represented by the upper dotted line) for CAR; ##P<0.01, compared with the vehicle-treated group (mean represented by the lower dotted line) for EFR, Bonferroni's post hoc tests following significant one-way ANOVA (CAR (F(4,52)=90.8, P<0.0001); EFR (F(4,52)=25.2, P<0.0001)). N=7 rats per group.
In a separate experiment, pretreatment with the 5-HT1A receptor antagonist WAY100,635 (0.63 mg kg−1) did not influence the potency of F15063 to either decrease the number of CARs or to augment the number of EFRs (Table 1). This was confirmed by the statistical analysis, with a significant F15063 dose factor but nonsignificant pretreatment and interaction factors.
Table 1.
The potency of F15063 in the conditioned avoidance response model is not influenced by pretreatment with the 5-HT1A receptor antagonist WAY100,635 (0.63 mg kg−1 s.c.)
| Pretreatment | F15063 (mg kg−1 i.p.) | CAR | EFR |
|---|---|---|---|
| Vehicle | 0.63 | 14.6±3.1 | 5.3±3.3 |
| 1.25 | 3.3±2.6 | 14.1±3.5 | |
| 2.50 | 1.3±1.1 | 16.6±3.5 | |
| WAY100,635 | 0.63 | 15.7±4.2 | 1.6±1.3 |
| 1.25 | 5.0±2.1 | 7.7±2.5 | |
| 2.50 | 0.13±0.2 | 19.3±2.7 |
Abbreviations: ANOVA, analysis of variance; CAR, conditioned avoidance response; EFR, escape failure response; s.c., subcutaneous.
Results shown are the means±s.e.m.
Significant ANOVA, dose of F15063 versus number of CARs, F(2,36)=12.5, P<0.001, and versus EFRs, F(2,36)=16.6, P<0.001.
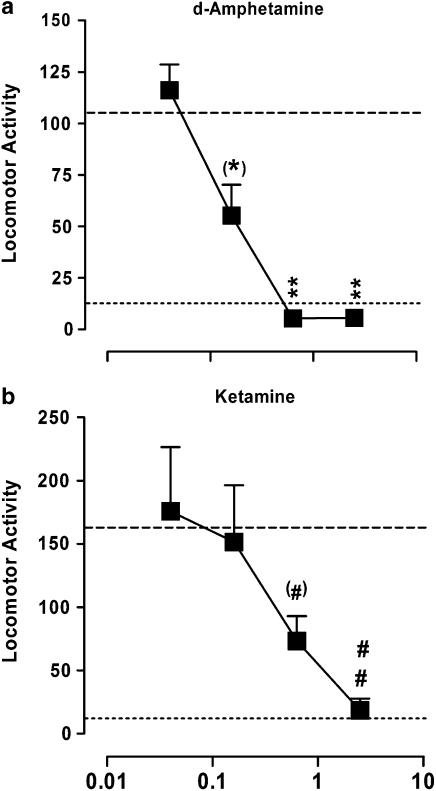
Reversal of locomotor hyperactivity induced by d-amphetamine or ketamine in rats
F15063 was active in two models of rat hyperactivity induced by psychotomimetic agents. First, animals injected with d-amphetamine (0.63 mg kg−1 s.c.) showed a large increase in the number of infrared beam breaks (105.4±6.0 versus 13.5±1.3 for vehicle/vehicle-treated rats, dotted lines in Figure 4a). F15063 dose-dependently and significantly antagonized this d-amphetamine-induced hypermotility, with the level of motility at 0.63 and 2.5 mg kg−1 being close to that obtained in vehicle/vehicle-injected control rats. The ED50 value for reversal of d-amphetamine-induced hyperactivity was 0.23 mg kg−1 i.p., that is, 4–5 times less than the ED50 for inhibition of spontaneous locomotor activity (1.08 (0.34–3.46) mg kg−1 i.p., with: 94.1±4.5, 97.9±15.8, 82.3±10.1, 45.3±9.2 and 15.6±2.9, for vehicle, 0.04, 0.16, 0.63 and 2.5 mg kg−1 F15063, respectively), suggesting that antagonism of d-amphetamine effects was distinct from nonspecific motor and/or sedating effects. F15063 was also active via the p.o. route, reducing d-amphetamine-induced hyperlocomotion with an ED50 of 1.66 mg kg−1, thus giving a p.o./i.p. ratio of about 7.
Figure 4.
Reversal by F15063 of hyperlocomotor activity induced by d-amphetamine (a) or ketamine (b) in rats. Each symbol represents the mean (±s.e.m.) number of infrared beam interruptions recorded 16–75 min following an s.c. injection of d-amphetamine (0.63 mg kg−1) or ketamine (40 mg kg−1). Bottom and top dotted lines represent the average locomotor score for vehicle- and psychotomimetic-injected rats, respectively. (*)P=0.05, **P<0.01, compared with the vehicle/d-amphetamine group, (#)P=0.06, ##P<0.01, compared with the vehicle/ketamine group, Bonferroni's post hoc tests following significant one-way ANOVA (versus amphetamine (F(4,133)=10.6, P<0.0001); versus ketamine (F(4,137)=4.3, P<0.001)). N=7 rats per group.
Rats treated with ketamine (40 mg kg−1, s.c.) also showed a marked hyperlocomotor activity (162.3±10.0 versus 13.5±1.3 for control rats, Figure 4b), which was similarly dose-dependently and significantly attenuated by F15063, with an ED50 of 0.96 mg kg−1 i.p.
Diminution of deficit of PPI of the startle reflex induced by apomorphine in rats
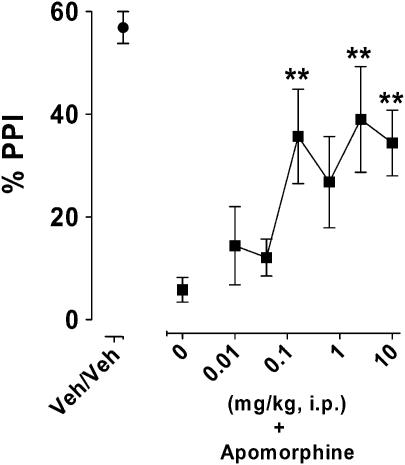
In a rodent model of sensory-motor gating deficit, control animals presented a PPI of about 60% (veh/veh, Figure 5), which was reduced to a 10th of this control value by treatment with apomorphine (leftmost square, Figure 5). F15063 attenuated this deficit, with the first significant dose being 0.16 mg kg−1.
Figure 5.
Diminution by F15063 of a deficit of the PPI of the startle reflex induced by apomorphine in rats. Each symbol represents the mean (±s.e.m.) percentage of PPI, recorded 60 min after i.p. injection of F15063 or vehicle. Apomorphine (0.63 mg kg−1 s.c.) was administered 15 min pre-test. **P<0.01, compared with the vehicle/apomorphine vehicle control group, Dunnett's post hoc tests following significant one-way ANOVA (F(6,231)=5.9, P<0.001). N=13 rats per group.
Lack of cataleptogenic potential of F15063 is due to its 5-HT1A receptor agonist activity: interaction with the 5-HT1A receptor antagonist WAY100,635
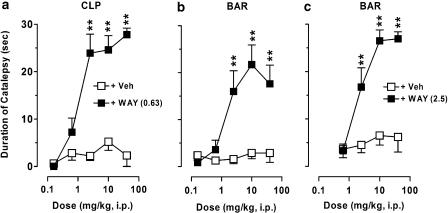
In a measure predictive of induction of EPS in rodents, F15063, up to a dose of 40 mg kg−1 i.p., did not produce notable catalepsy in rats, whether tested in the CLP or the bar test (open squares, Figure 6a and b). However, when rats were pretreated with the 5-HT1A receptor antagonist WAY100,635 (0.63 mg kg−1 s.c.), a marked cataleptogenic activity was observed: at 2.5, 10 and 40 mg kg−1 i.p. catalepsy scores were above 24 s. Under these conditions, ED50 for induction of catalepsy was 1.20 (0.55–2.80) mg kg−1, very similar to that observed for normalization of MPD-induced behaviours (1.10 mg kg−1, see above). Statistical analysis showed that there were significant effects of treatment (dose of F15063), pretreatment (WAY100,635) and interaction for both catalepsy tests. Likewise, in both tests, there was minimal catalepsy following p.o. administration of F15063 (from 0.8±0.6 to 8.2±3.1 s).
Figure 6.
Lack of cataleptogenic activity of F15063 in rats and mice is due to activation of 5-HT1A receptors: Interaction with the 5-HT1A receptor antagonist WAY100,635. Each symbol represents the mean (±s.e.m.) time spent in a cataleptic position in the CLP (a) and in the bar test in rats (b) and mice (c), measured 60 min post F15063 administration. WAY100,635 (WAY) was injected s.c. (0.63 mg kg−1) 15 min before F15063. **P<0.01, compared with the vehicle (Veh)/F15063 group at the corresponding dose of F15063, Bonferroni's post hoc tests following significant two-way ANOVA (significant treatment, pretreatment and interaction factors: all F's>5.9, all P's<0.0001). N=7 rats or mice per group.
A similar pattern was observed in the bar test in mice, with F15063 being devoid of cataleptogenic activity of its own (Figure 6c). Again, following pretreatment with 2.5 mg kg−1 s.c. of WAY100,635, there was a gradual dose-dependent augmentation of the time spent in a cataleptic position (ED50: 2.00 (0.55–2.80) mg kg−1).
Lack of cataleptogenic potential of F15063 is preserved following semichronic treatment: interaction with the 5-HT1A receptor antagonist WAY100,635
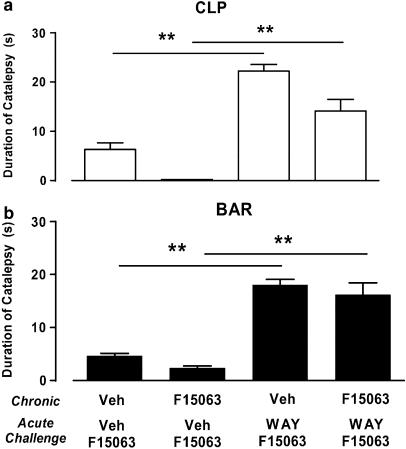
Following treatment with 40 mg kg−1 p.o. of F15063 once a day for 4 days, the same dose of F15063 given acutely on day 5 did not produce more catalepsy than in control groups of rats chronically treated with vehicle. Statistical analysis showed that there was a just significant chronic treatment effect (two-way ANOVA: F(1,19)=4.5, P=0.05) for the CLP test only. Indeed, rats chronically treated with F15063 showed a level of catalepsy lower than that of rats treated with vehicle (first versus second bar, starting from the left, Figure 7a), although this difference was not significant.
Figure 7.
Lack of cataleptogenic activity of F15063 in rats is preserved following repeated treatment. Interaction with the 5-HT1A receptor antagonist WAY100,635. Each symbol represents the mean (±s.e.m.) time spent in a cataleptic position in the CLP (a) and in the bar test (b) in rats. F15063 (40 mg kg−1) or vehicle were administered p.o. daily for 4 consecutive days and, on the 5th day all rats received an acute challenge with the same dose of F15063 60 min before testing. Vehicle or WAY100,635 (0.63 mg kg−1 s.c.) were administered 15 min before the acute challenge with F15063. **P<0.01, Newman–Keuls post hoc test following significant two-way ANOVA (CLP (F(1,19)=19.1, P<0.001); bar test (F(1,19)=21.6, P<0.001)). N=7 rats per group.
However, as was observed in the acute experiment above, pretreatment with 0.63 mg kg−1 s.c. of the 5-HT1A receptor antagonist WAY100,635 resulted in an increased amount of time spent in a cataleptic position (first versus third bar, and second versus fourth bar, starting from the left, Figure 7a and b). Statistical analysis showed that, for both tests, there were significant effects of the pretreatment.
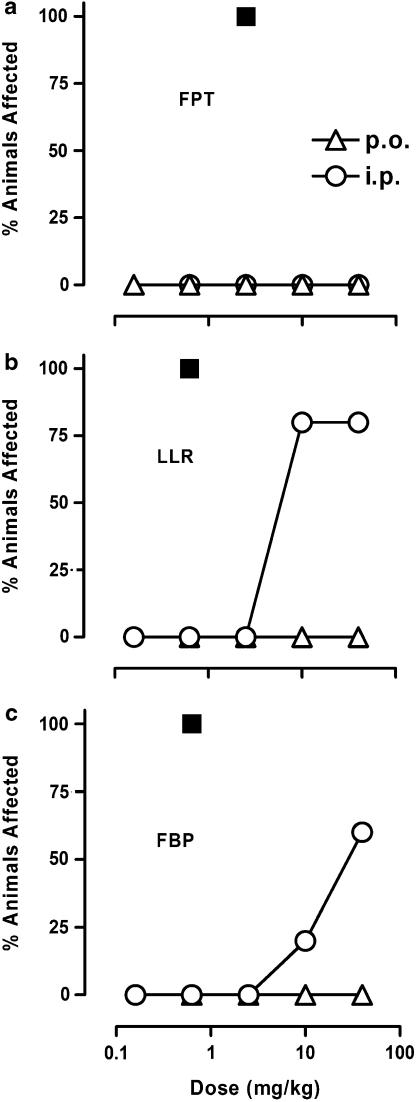
F15063, at ‘therapeutically relevant' doses does not induce the serotonin syndrome in rats
F15063, up to 40 mg kg−1 i.p., produced no FPT (Figure 8a). At the highest doses tested (10 and 40 mg kg−1 i.p., i.e., doses at least 10–40 times higher than those active in models predictive of antipsychotic-like efficacy), F15063 only produced LLR and FBP (Figure 8b and c). Given p.o. (triangles in Figure 8), none of the doses tested induced any sign of the serotonergic syndrome. The prototypical 5-HT1A receptor agonist 8-OH-DPAT was more potent than F15063 in producing LLR and FBP (maximal effect at 0.63 mg kg−1 p.o.), and contrary to the latter, produced FPT in all animals tested at 2.5 mg kg−1 p.o. (filled squares, Figure 8).
Figure 8.
F15063 does not produce the serotonin syndrome in rats at ‘therapeutically relevant' doses. Each symbol represents the percentage of rats showing FPT (a), LLR (b) and FBP (c). Behavioural observations were made from 10 to 20 min and from 55 to 65 min post i.p. or p.o. administration of F15063. N=5 rats per group. (▪) Data for 8-OH-DPAT (p.o. administration), from Koek et al. (1998).
Effects on plasma prolactin levels in rats
F15063, administered p.o., produced a significant (Kruskal–Wallis: H=37.84, P<0.001) increase in prolactin plasma levels, with a MED of 0.63 mg kg−1 (4.6±1.0, 17.0±4.3, 34.4±10.6, 56.2±10.0, 68.9±14.7, 61.0±6.9 and 91.4±9.2 ng/ml, for vehicle, 0.04, 0.16, 0.63, 2.5, 10 and 40 mg kg−1 F15063, respectively).
Table 2 summarizes the pharmacological activity of F15063 in tests predictive of activity against the positive symptomatology of schizophrenia. It can be observed that ED50 values following i.p. administration are around or below 1 mg kg−1, indicating that the compound is a potent in vivo dopamine D2 receptor blocker. It also shows that the compound is active orally at an ED50 3–5 times higher than the corresponding ED50 via the i.p. route. By contrast, the ED50 for activity of F15063 in test predictive of occurrence of side effects (EPS and serotonin syndrome, Table 3) are well above those reported in Table 2, suggesting that at therapeutically meaningful doses, F15063 should be free of these side effects.
Table 2.
Summary of pharmacological activity of F15063 in models predictive of activity against positive symptoms of schizophrenia
| Species | Model | Route | Response | Parameter | Dose (mg kg−1) |
|---|---|---|---|---|---|
| Rat | Methylphenidate-induced behaviours | i.p. | Blocks gnawing | ED50 | 0.24 (0.14–0.41) |
| Normalizes behaviour | ED50 | 1.10 (0.70–1.80) | |||
| p.o. | Blocks gnawing | ED50 | 0.93 (0.66–1.30) | ||
| Normalizes behaviour | ED50 | 2.50 (1.00–6.20) | |||
| Amphetamine hyperlocomotion | i.p. | Blocks hyperlocomotion | ED50 | 0.23 (0.15–0.31) | |
| p.o. | Blocks hyperlocomotion | ED50 | 1.66 (0.56–4.93) | ||
| Ketamine hyperlocomotion | i.p. | Blocks hyperlocomotion | ED50 | 0.96 (0.28–3.30) | |
| Conditioned avoidance | i.p. | Blocks behaviour | ED50 | 0.33 (0.19–0.55) | |
| Apomorphine-induced PPI deficit | i.p. | Attenuates deficit | MED | 0.16 | |
| Mouse | Apomorphine stereotypies | i.p. | Blocks climbing | ED50 | 0.30 (0.14–0.63) |
| Blocks sniffing | ED50 | 0.37 (0.18–0.75) |
Abbreviations: ED50, dose (mg kg−1) producing a significant difference from control in 50% of the animals tested; i.p., intraperitoneal; MED, minimal effective dose; p.o., per os.
Confidence intervals are given in parentheses.
Table 3.
Summary of pharmacological activity of F15063 in models predictive of side effects relevant to antipsychotic treatment
| Symptoms | Species | Model | Route | Response | Parameter | Dose (mg kg−1) |
|---|---|---|---|---|---|---|
| EPS | Rat | Catalepsy | i.p. | Absence of catalepsy | ED50 | >40 |
| p.o. | Absence of catalepsy | ED50 | >40 | |||
| Catalepsy after 4 day treatment | p.o. | Absence of catalepsy | 40/day | |||
| Mouse | Catalepsy | i.p. | Absence of catalepsy | ED50 | >40 | |
| Serotonin syndrome | Rat | Characteristic behaviours | p.o. | All signs | ED50 | >40 |
| i.p. | Lower lip retraction | ED50 | 7 (2–30) | |||
| Flat body posture | ED50 | 32 (10–102) | ||||
| Forepaw treading | ED50 | >40 |
Abbreviations: ED50, dose producing a significant difference from control in 50% of the animals tested; i.p., intraperitoneal; p.o., per os.
Confidence intervals are given in parentheses.
Discussion
The main findings can be summarized as follows: (1) F15063 induced behavioural effects consistent with dopamine D2 receptor blockade and agonist activity at the 5-HT1A receptor; (2) a well-balanced combination of dopamine D2 receptor antagonism and 5-HT1A receptor agonism confers a favourable behavioural profile on F15063, characterized by efficacy in models predictive of antipsychotic activity (dopamine D2 receptor antagonism), together with a lack of cataleptogenic activity (5-HT1A receptor activation) or serotonin syndrome induction (partial agonism at 5-HT1A receptor combined with dopamine D2 receptor blockade).
F15063 is active in models predictive of activity against positive symptomatology of schizophrenia
F15063 had clear antagonist activity in rat behavioural models used to detect antipsychotic potential: it dose-dependently diminished d-amphetamine-induced hyperlocomotor activity, fully normalized at higher doses stereotyped behaviours produced by methylphenidate, and reduced CAR. In a parallel study that compared a dozen reference or putative antipsychotics (Bardin et al., in press), it was found that there was a positive correlation between the potency of compounds in these three tests and their affinity at dopamine D2 receptors. F15063 was active in these tests at fairly low doses (ED50 ranged from 0.23 to 1.10 mg kg−1 i.p.), similar to those of other potent reference or putative antipsychotics such as risperidone, bifeprunox and SLV313. This shows that F15063 has potent in vivo dopamine D2 receptor blocking activity. Likewise, F15063 blocked ketamine-induced hyperactivity; however, we found that there was no correlation between the ability of antipsychotics to antagonize ketamine-induced hyperlocomotion and their affinity at either dopamine D2 or 5-HT1A rat receptors, suggesting that interaction with other receptors is necessary (Bardin et al., in press). Some reports suggest that the 5-HT2A antagonist activity of some antipsychotics is responsible for the blockade of N-methyl-D-aspartate antagonist-induced hypermotility (Gleason and Shannon, 1997; Martin et al., 1997; Millan et al., 1999). However, F15063 has no appreciable affinity for rat 5-HT2A receptors (pKi ∼6.6, Newman-Tancredi et al., 2007), and antagonizes DOI-induced head-twitches in mice only at high doses (MED: 10 mg kg−1 p.o., unpublished data). The neurochemical characteristics that underlie the activity of F15063 against ketamine-induced hyperlocomotor activity remain to be explored.
Similarly, F15063 potently (ED50 ∼0.30 mg kg−1 i.p.) antagonized apomorphine-induced climbing and sniffing in mice, again with a potency comparable to that of haloperidol, risperidone, and greater than that of olanzapine, clozapine and ziprasidone (Bardin et al., 2006a). These in vivo results are in line with central dopamine D2 receptor occupancy data in mice (Assie et al., 2006), in which F15063 displaced [3H]nemonapride binding, with an ED50 of 0.55 mg kg−1 i.p. at 1 h post-injection. In a further model of antipsychotic-like activity, F15063, like haloperidol, risperidone, clozapine and olanzapine (Auclair et al., 2006a), attenuated apomorphine-induced PPI disruption, a model of gating deficits observed in schizophrenic patients (Swerdlow et al., 1986). To summarize, all these findings suggest that F15063, because of its potent in vivo dopamine D2 receptor blocking profile, should control positive symptoms of schizophrenia.
Activity of F15063 in this model of apomorphine-induced PPI disruption is most likely to be related to the dopamine D2 receptor antagonist properties of F15063 and contrasts with the effects of aripiprazole and ziprasidone, which were minimal in this model (Auclair et al., 2006a). Selective dopamine D2/5-HT1A compounds exhibiting marked 5-HT1A receptor agonist properties, such as sarizotan, SSR181507 and SLV313, failed to reverse apomorphine-induced PPI disruption, because of their marked 5-HT1A agonist actions. In effect, SSR181507 and SLV313 were able to reverse apomorphine-induced deficit in the presence of a selective 5-HT1A receptor antagonist (Auclair et al., 2006a), suggesting that the precise balance of D2 antagonism versus 5-HT1A agonism is a key element in the pharmacological activity of new generation antipsychotics targeting both of these receptors.
Further, sarizotan, SSR181507, bifeprunox, and to a lesser extent SLV313, were found to have robust PPI-disrupting effects of their own, because of their marked agonist activity at 5-HT1A receptors (Auclair et al., 2006b). Such a disruption is potentially problematic, considering that schizophrenic patients already suffer from diminished basal PPI levels (Braff et al., 1978; for a review, Swerdlow et al., 2000), and it would thus be desirable for an antipsychotic not to further affect basal PPI. Interestingly although, F15063, over a wide dose-range (0.01–10 mg kg−1 i.p.), did not modify basal PPI level (Depoortère et al., 2007). This is thought to be related to the preferential affinity of F15063 for dopamine D2 over 5-HT1A receptors, as well as to an antagonistic activity at the former. This again emphasizes the importance of the balance between dopamine D2 blocking properties and 5-HT1A receptor agonist activity.
However, several reports have highlighted either the neutral (Depoortère et al., 2003; present results) or the positive influence (Wadenberg and Ahlenius, 1991; Prinssen et al., 1996) of activating 5-HT1A receptors in a model predictive of antipsychotic activity (conditioned avoidance). In mice, the coadministration of the 5-HT1A receptor antagonist SL88.0338 with SSR181507 did not modify the activity of the latter (Depoortère et al., 2003). On the other hand, some authors (Wadenberg and Ahlenius, 1991; Prinssen et al., 1996) have reported that the 5-HT1A receptor agonist 8-OH-DPAT potentiated the action of dopamine D2 receptor blockers such as haloperidol or raclopride. Thus, these interaction studies with the conditioned avoidance model highlight the notion that a 5-HT1A receptor agonist activity does not seem to interfere with the desired therapeutic effects of dopamine D2 receptor blockade.
F15063 is not cataleptogenic, indicating a low propensity for EPS in the clinic
Up to a dose (40 mg kg−1) that was between 35 and 200 times higher than the ED50 in tests predictive of activity against positive symptoms, F15063 did not induce catalepsy in either rats or mice. Among other reference antipsychotics tested under the same experimental conditions, such a wide margin was only found with SSR181507, SLV313, bifeprunox and aripiprazole, the former three compounds belonging to the new generation of dopamine D2/5-HT1A antipsychotics (Bardin et al., 2006a). Other atypical antipsychotics, with more moderate or no agonist activity at 5-HT1A receptors had a lower margin, especially when taking into account the CLP test of catalepsy. Indeed, the cataleptogenic properties of antipsychotics in the CLP test, but less so in the bar test, was inversely correlated (r=0.75, P<0.01) with their affinities at rat 5-HT1A receptors (Bardin et al., in press).
At 40 mg kg−1, occupation of dopamine D2 receptors in the striatum by F15063 is probably maximal (Assie et al., 2006), and in the case of selective dopamine D2 antagonists this level of occupancy would translate into catalepsy, which is thought to occur when striatal occupancy is greater than 80% (Wadenberg et al., 2000). The involvement of 5-HT1A agonist activity in this lack of catalepsy is demonstrated by the observation that co-treatment of F15063 with the 5-HT1A receptor antagonist WAY100,635 produced catalepsy, in both species and in both tests in rat, consistent with previous interaction studies with dopamine D2/5-HT1A antipsychotics and WAY100,635 (Kleven et al., 2005; Bardin et al., 2006a). Expression of agonist activity at 5-HT1A receptors in an in vivo model is in line with neurochemical data showing that F15063 reduced 5-HT release in rat hippocampus (Newman-Tancredi et al., 2006) and reversed phencyclidine (PCP)-induced social interaction deficits between a dyad of adult rats, because of its 5-HT1A agonist component (Depoortère et al., 2007). It is also in agreement with reports that activation of the 5-HT1A receptor with full or partial agonists opposes catalepsy produced by various dopamine D2 receptor blockers (Broekkamp et al., 1988; Invernizzi et al., 1988). Interestingly, in the presence of WAY100,635, acute treatment with F15063 induced catalepsy with an i.p. ED50 (1.20 mg kg−1) very close to that observed for normalization of MPD-induced behaviours (1.10 mg kg−1). This indicates that the absence of cataleptogenic activity at ‘therapeutically relevant' doses is attributable to its agonist activity at 5-HT1A receptors. The experiment in which blockade of 5-HT1A receptors with WAY100,635 on day 5 of the sub-chronic regimen produced catalepsy, shows that absence of catalepsy after several days of treatment with F15063 does not result from a diminished antagonist activity at dopamine D2 receptors, but from a persistent activation of 5-HT1A receptors that prevents the deleterious effects of potent blockade of the former.
Catalepsy is an animal model considered to be predictive of occurrence of extra-pyramidal signs (EPS), and the absence of catalepsy with F15063 suggests that this compound should be free from such motor side effects in patients. Indeed, clinical data have shown that augmentation of antipsychotic therapy with the 5-HT1A receptor partial agonists buspirone (Goff et al., 1991; Moss et al., 1993) and tandospirone (Yoshida et al., 1998) decreased EPS and/or akathisia and/or tardive dyskinesia scores.
F15063 does not produce the serotonin syndrome at ‘therapeutically relevant' doses
Acute activation of 5-HT1A receptors has been generally associated with occurrence of the serotonin syndrome (FPT and FBP) as well as other signs such as LLR in rats, seen following treatment with full or partial agonists at 5-HT1A receptors, such as 8-OH-DPAT and buspirone (Goodwin et al., 1986; Koek et al., 1998). It should be emphasized, though, that tachyphylaxis rapidly develops to the serotonin syndrome (see DeVry, 1995 for a review, Prinssen et al., 2000). F15063, given acutely at doses active in models predictive of therapeutic efficacy, did not give rise to this syndrome and only produced some behavioural signs at much higher (10–40 times) doses. The absence of FBP at low doses is likely to result from its partial agonist activity at 5-HT1A receptor, combined with its dopamine D2 receptor blocking properties. Indeed, it has been shown that other 5-HT1A receptor partial agonists such as SSR181507 produce minimal serotonin syndrome (Depoortère et al., 2003), and that dopamine D2 receptor antagonists such as spiperone block this 8-OH-DPAT-induced behaviour (Berendsen et al., 1990). Although not very common, the serotonin syndrome has been described in the clinic (restlessness, myoclonus, hypertension, shivering, etc.: see review by Gillman, 1999), and can be observed with a wide variety of drugs affecting the central 5-HT system. However, the present data suggest that F15063 has low or negligible liability to produce the serotonin syndrome.
Other effects of F15063
F15063, at doses active in the various antipsychotic tests (0.01–2.5 mg kg−1 i.p.), was free from deleterious effects in the elevated plus maze (data not shown), indicating a lack of anxiogenic potential. In a rotarod task in rats, F15063 started to significantly decrease the drop-off time at 2.5 mg kg−1 p.o. only, indicating that, at therapeutically active doses, it should not produce significant sedation and/or myorelaxation (data not shown). The compound augmented plasma prolactin levels, a classical effect resulting from blockade of dopamine D2 receptors located on the pituitary gland (prolactin release is tonically inhibited by dopamine acting on its D2 receptors: Ben-Jonathan, 1985). This effect is observed with all potent antipsychotics (Cosi et al., 2006). Finally, F15063 is devoid of affinity (Newman-Tancredi et al., 2007) at a variety of receptors thought to mediate adverse effects (Richelson, 1999) such as 5-HT2c receptors (possibly responsible for body weight gain associated with some newer antipsychotics: Reynolds et al., 2005), α1 adrenoceptors and histaminergic H1 receptors (implicated in sedation), α2 adrenoceptors (responsible for autonomic effects) and at muscarinic M1 receptors (whose blockade exerts deleterious effects on memory). This suggests that F15063 exhibits low liability for giving rise to troublesome side effects that are thought to contribute to poor compliance.
Additional properties of F15063
Present data indicate a favourable profile for antipsychotic-like versus cataleptogenic potential, but this favourable balance of dopamine D2/5-HT1A properties also extends to other activities of F15063. In fact, F15063 was, among several reference antipsychotics including clozapine, the only one found to be active in each of three different models of cognitive impairment: PCP-induced working and reference memory deficits in the hole-board spatial memory task, scopolamine-induced social memory deficits in the adult/juvenile social recognition model and PCP-induced memory reacquisition deficit in a ‘reversal learning' operant conditioning paradigm (active doses: 0.04–0.63 mg kg−1 i.p.: Bardin et al., 2006b; Depoortère et al., 2006, 2007). Similarly, F15063 alleviated (MED: 0.04 mg kg−1 i.p.), through 5-HT1A receptors activation, PCP-induced deficit of social interaction between adult rats. This result, in conjunction with the increase in dopamine in prefrontal cortex of rats (Newman-Tancredi et al., 2006), underlines the potential of F15063 to combat negative symptoms of schizophrenia. Of further interest was the observation that in each of these behavioural tests, F15063 exhibited activity at doses overlapping with those efficacious in tests predictive for activity against positive symptoms (this paper).
Conclusions
F15063 was highly active and potent in preclinical models of antipsychotic activity, and showed an innovative receptor affinity profile, characterized by selective dopamine D2/D3 receptor antagonism, and partial agonism at dopamine D4 and 5-HT1A receptors. F15063 was also active in diverse models of negative and cognitive symptoms of schizophrenia (Depoortère et al., 2006, 2007), at dose-ranges overlapping with those in tests predictive of activity against positive signs. In addition, F15063 did not induce catalepsy, a model predictive of EPS liability, and did not induce signs of the serotonin syndrome at ‘antipsychotic' doses. These data provide compelling evidence that F15063 possesses a profile that distinguishes it from currently commercialized antipsychotic drugs, with a well-balanced profile of affinity/activity at D2 and 5-HT1A receptors, consistent with potent antipsychotic-like properties and low side effect liability.
Acknowledgments
We thank M Barreto, C Barret-Grevoz, J Besnard, A Galinier and N Malfetes and for their expert technical assistance.
Abbreviations
- CAR
conditioned avoidance response
- CLP
crossed-legs position
- ED50
effective dose for 50% response
- EFR
escape failure response
- EPS
extrapyramidal syndrome
- FPT
forepaw treading
- FBP
flat body posture
- i.p.
intraperitoneal
- LLR
lower lip retraction
- MPD
methylphenidate
- NP
no pulse
- PA
pulse alone
- PCP
phencyclidine
- p.o.
per os
- pp
prepulse
- PPI
prepulse inhibition
- ppP
prepulse-pulse
- s.c.
subcutaneous
Conflicts of Interest
The present study was funded by Pierre Fabre Médicament. All authors are or were employees of the Centre de Recherche Pierre Fabre at the time when the experiments were conducted.
References
- Assie MB, Bardin L, Auclair A, Consul-Denjean N, Sautel F, Kleven MS, et al. F15063, a novel antipsychotic with dopamine D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: III) Duration of brain D2-like receptor occupancy, antipsychotic-like activity and plasma concentration in rodents Neuropsychopharmacology 200616Suppl 45432P3D007 [Google Scholar]
- Auclair A, Kleven MS, Besnard J, Depoortère R, Newman-Tancredi A. Actions of novel antipsychotic agents on apomorphine-induced PPI disruption: influence of combined serotonin 5-HT1A receptor activation and dopamine D2 receptor blockade. Neuropsychopharmacology. 2006a;31:1900–1909. doi: 10.1038/sj.npp.1301015. [DOI] [PubMed] [Google Scholar]
- Auclair A, Newman-Tancredi A, Depoortère R. Comparative analysis of typical, atypical, and novel antipsychotics with preferential D2/D3 and 5-HT1A affinity in rodent models of cognitive flexibility and sensory gating: (II) The reversal learning task and PPI of the startle reflex. Int J Neuropsychopharmacol. 2006b;9 Suppl 1:P01.167. [Google Scholar]
- Bantick RA, Deakin JF, Grasby PM. The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics. J Psychopharmacol. 2001;15:37–46. doi: 10.1177/026988110101500108. [DOI] [PubMed] [Google Scholar]
- Bardin L, Auclair A, Kleven MS, Prinssen EPM, Koek W, Newman-Tancredi A, et al. Pharmacological profiles in rats of novel antipsychotics with combined dopamine D2/serotonin 5-HT1a activity: comparison with typical and atypical conventional antipsychotics Behav Pharmacol(in press) [DOI] [PubMed]
- Bardin L, Kleven MS, Barret-Grevoz C, Depoortère R, Newman-Tancredi A. Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology. 2006a;31:1869–1879. doi: 10.1038/sj.npp.1300940. [DOI] [PubMed] [Google Scholar]
- Bardin L, Newman-Tancredi A, Depoortère R. Comparative analysis of typical, atypical, and novel antipsychotics with preferential D2/D3 and 5-HT1A affinity in rodent models of cognition and memory deficits: (I) The hole-board and the social recognition tests. Int J Neuropsychopharmacol. 2006b;9 Supp 1:P01.166. [Google Scholar]
- Bartoszyk GD, Van Amsterdam C, Greiner HE, Rautenberg W, Russ H, Seyfried CA. Sarizotan, a serotonin 5-HT1A receptor agonist and dopamine receptor ligand. 1. Neurochemical profile. J Neural Transm. 2004;111:113–126. doi: 10.1007/s00702-003-0094-7. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985;6:564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL, Van Delft AM. Antagonism of 8-OH-DPAT-induced behaviour in rats. Eur J Pharmacol. 1990;187:97–103. doi: 10.1016/0014-2999(90)90344-6. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology. 2001;57:1829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression. Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortère R, Louis C, Perrault G, Griebel G, Soubrié P. SSR181507, a putative atypical antipsychotic with dopamine D2 antagonist and 5-HT1A agonist activities: improvement of social interaction deficits induced by phencyclidine in rats. Neuropharmacology. 2004;46:1121–1129. doi: 10.1016/j.neuropharm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Broekkamp CLE, Oosterloo SK, Berendsen HHG, van Delft AML. Effects of metergoline, fenfluramine, and 8-OH-DPAT on catalepsy induced by haloperidol or morphine. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;338:191–195. doi: 10.1007/BF00174869. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, De Vries L, Newman-Tancredi A, Cussac D. Differential profile of antipsychotics at serotonin 5-HT1A and dopamine D2S receptors coupled to extracellular signal-regulated kinase. Eur J Pharmacol. 2006;534:63–70. doi: 10.1016/j.ejphar.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Kreyenbuhl J, Zito JM, Lehman A. Relationship of the use of adjunctive pharmacological agents to symptoms and level of function in schizophrenia. Am J Psychiatry. 2002;159:1035–1043. doi: 10.1176/appi.ajp.159.6.1035. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig V, Armagos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. Rev Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Christoffersen CL, Meltzer LT. Reversal of haloperidol-induced extrapyramidal side effects in cebus monkeys by 8-hydroxy-2-(di-n-propylamino)tetralin and its enantiomers. Neuropsychopharmacology. 1998;18:399–402. doi: 10.1016/S0893-133X(97)00176-0. [DOI] [PubMed] [Google Scholar]
- Claustre Y, De Peretti D, Brun P, Gueudet C, Allouard N, Alonso R, et al. SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist. I: neurochemical and electrophysiological profile. Neuropsychopharmacology. 2003;28:2064–2076. doi: 10.1038/sj.npp.1300262. [DOI] [PubMed] [Google Scholar]
- Cosi C, Carilla-Durand E, Assié MB, Ormière AM, Maraval M, Leduc N, et al. Partial agonism of the antipsychotics SSR181507, aripiprazole and bifeprunox at D2 receptors: G-protein activation and prolactin release. Eur J Pharmacol. 2006;535:135–144. doi: 10.1016/j.ejphar.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Auclair AL, Bardin L, Bruins Slot L, Kleven M, Newman-Tancredi A.F15063 a compound with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (III) Activity in models of cognition and negative symptoms Br J Pharmacol 2007 10.1038/sj.bjp.0707160E-pub ahead of print: 20 March 2007 [DOI] [PMC free article] [PubMed]
- Depoortère R, Bardin L, Auclair AL, Bruins-Slot L, Kleven M, Newman-Tancredi A. F15063, an innovative antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (II) Behavioural profile in models of positive, negative symptoms and cognitive deficits of schizophrenia. Int J Neuropsychopharmacol. 2006;9 Supp 1:P01.165. [Google Scholar]
- Depoortère R, Boulay D, Perrault G, Bergis O, Decobert M, Françon D, et al. SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist. II: Behavioral profile in tests predictive of antipsychotic, anxiolytic and antidepressant activities. Neuropsychopharmacology. 2003;28:1889–1902. doi: 10.1038/sj.npp.1300261. [DOI] [PubMed] [Google Scholar]
- DeVry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Diaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci. 2005;25:10831–10843. doi: 10.1523/JNEUROSCI.2999-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra RW, de Moes J, Hofma JJ, Kling H, Kuipers W, Long SK, et al. New 1-aryl-4-(biarylmethylene)piperazines as potential atypical antipsychotics sharing dopamine D2-receptor and serotonin 5-HT1A-receptor affinities. Bioorg Med Chem Lett. 2001;11:2345–2349. doi: 10.1016/s0960-894x(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Feenstra RW, Long SK, Kuipers W, van der Heyden JA, Tulp MT, Kruse CG. New approaches for psychosis treatment: design, synthesis and SAR of ligands binding to dopamine D2 and serotonin 5-HT1A receptors. Drugs of the future. XVIIth International Symposium on Medicinal Chemistry. 2002;27 Supp A:237. [Google Scholar]
- Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol. 1999;13:100–109. doi: 10.1177/026988119901300111. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology. 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- Goff DC, Midha KK, Brotman AW, McCormick S, Waites M, Amico ET. An open trial of buspirone added to neuroleptics in schizophrenic patients. J Clin Psychopharmacol. 1991;11:193–197. [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Wood AJ, Green AR. The enhancement by lithium of the 5-HT1A mediated serotonin syndrome produced by 8-OH-DPAT in the rat: evidence for a post-synaptic mechanism. Psychopharmacology. 1986;90:488–493. doi: 10.1007/BF00174066. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY. The effect of serotonin1A receptor agonism on antipsychotic drug-induced dopamine release in rat striatum and nucleus accumbens. Brain Res. 2000;858:252–263. doi: 10.1016/s0006-8993(99)02346-x. [DOI] [PubMed] [Google Scholar]
- Invernizzi RW, Cervo L, Samanin R. 8-Hydroxy-2-(di-n-propylamino) tetralin, a selective serotonin1A receptor agonist, blocks haloperidol-induced catalepsy by an action on raphe nuclei medianus and dorsalis. Neuropharmacology. 1988;27:515–518. doi: 10.1016/0028-3908(88)90134-7. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D2 receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Barret-Grevoz C, Bruins Slot LB, Newman-Tancredi A. Novel antipsychotic agents with 5-HT(1A) agonist properties: role of 5-HT(1A) receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology. 2005;49:135–143. doi: 10.1016/j.neuropharm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Koek W, Colpaert FC. Inhibition of methylphenidate-induced behaviors in rats: differences among neuroleptics. J Pharmacol Exp Ther. 1993;267:181–191. [PubMed] [Google Scholar]
- Koek W, Patoiseau JF, Assie MB, Cosi C, Kleven MS, Dupont-Passelaigue E, et al. F 11440, a potent, selective, high efficacy 5-HT1A receptor agonist with marked anxiolytic and antidepressant potential. J Pharmacol Exp Ther. 1998;287:266–283. [PubMed] [Google Scholar]
- Liebman JM, Gerhardt SC, Gerber R. Effects of 5-HT1A agonists and 5-HT2 antagonists on haloperidol-induced dyskinesias in squirrel monkeys: no evidence for reciprocal 5-HT-dopamine interaction. Psychopharmacology. 1989;97:456–461. doi: 10.1007/BF00439547. [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, Carlsson A, Carlsson ML. The apparent antipsychotic action of the 5-HT2a receptor antagonist M100907 in a mouse model of schizophrenia is counteracted by ritanserin. J Neural Transm. 1997;104:561–564. doi: 10.1007/BF01277672. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Glennon J, Tuinstra T, Herremans AHJ, Van der Heyden JAM, Feenstra R, et al. SLV313: a novel antipsychotic with additional antidepressant and anxiolytic-like actions. Eur Neuropsychopharmacol. 2002;12:S274. [Google Scholar]
- Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)1A receptors. J Pharmacol Exp Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet J, et al. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Veiga S, Cistarelli L, Melon C, Gobert A. WAY 100,635 enhances both the ‘antidepressant' actions of duloxetine and its influence on dialysate levels of serotonin in frontal cortex. Eur J Pharmacol. 1998;341:165–167. doi: 10.1016/s0014-2999(97)01445-3. [DOI] [PubMed] [Google Scholar]
- Moss LE, Neppe VM, Drevets WC. Buspirone in the treatment of tardive dyskinesia. J Clin Psychopharmacol. 1993;13:204–209. [PubMed] [Google Scholar]
- Newman-Tancredi A, Assié M-B, Martel J-C, Cosi C, Bruins Slot L, Palmier C, et al. F15063, a potential antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (I) in vitro receptor affinity and efficacy profile Br J Pharmacol 2007 10.1038/sj.bjp.0707158E-pub ahead of print: 20 March 2007 [DOI] [PMC free article] [PubMed]
- Newman-Tancredi A, Assié M-B, Martel J-C, Cosi C, Heusler P, Bruins Slot L, et al. F15063, an innovative antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (I) in vitro, neurochemical and neuroendocrine profiles. Int J Neuropsychopharmacol. 2007;9 Supp 1:P01.164. [Google Scholar]
- Prinssen EP, Kleven MS, Koek W. Effects of dopamine antagonists in a two-way active avoidance procedure in rats: interactions with 8-OH-DPAT, ritanserin, and prazosin. Psychopharmacology. 1996;128:191–197. doi: 10.1007/s002130050124. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, Koek W, Colpaert FC, Kleven MS. Repeated treatment with 8-OH-DPAT induces tolerance to its ability to produce the 5-HT1A behavioural syndrome, but not to its ability to attenuate haloperidol-induced catalepsy. Behav Pharmacol. 2000;11:299–305. doi: 10.1097/00008877-200006000-00013. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Gunn RN, Wilkins MR, Sedman E, Grasby PM. Evaluation of EMD 128 130 occupancy of the 5-HT1A and the D2 receptor: a human PET study with [11C]WAY-100635 and [11C]raclopride. J Psychopharmacol. 2002;16:195–199. doi: 10.1177/026988110201600301. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Templeman LA, Zhang ZJ. The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1021–1028. doi: 10.1016/j.pnpbp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry. 1999;60 Suppl 10:514. [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT1A receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry. 2000;48:229–237. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Zorn SH. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur J Pharmacol. 1997;338:3–5. doi: 10.1016/s0014-2999(97)81951-6. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Dunn L, Peszke M, Cramer J, Xu W, Thomas J, et al. Impact of clozapine on negative symptoms and on the deficit syndrome in refractory schizophrenia. Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. Am J Psychiatry. 1999;156:88–93. doi: 10.1176/ajp.156.1.88. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Reynolds LS, Braselton JP, Rollema H, Zorn SH. Comparison of the novel antipsychotic ziprasidone with clozapine and olanzapine: inhibition of dorsal raphe cell firing and the role of 5-HT1A receptor activation. Neuropsychopharmacology. 1999;21:622–631. doi: 10.1016/S0893-133X(99)00057-3. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, et al. Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry. 2001;158:1722–1725. doi: 10.1176/appi.ajp.158.10.1722. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Uehara T, Kurachi M, et al. Effect of adjunctive treatment with serotonin-1A agonist tandospirone on memory functions in schizophrenia. J Clin Psychopharmacol. 2000;20:386–388. doi: 10.1097/00004714-200006000-00019. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA, Koob GF. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biol Psychiatry. 1986;21:23–33. doi: 10.1016/0006-3223(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RG. Manual of Pharmacological Calculations with Computer Programs. Springer Verlag: New York; 1987. [Google Scholar]
- Tandon R, Ribeiro SC, DeQuardo JR, Goldman RS, Goodson J, Greden JF. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34:495–497. doi: 10.1016/0006-3223(93)90242-6. [DOI] [PubMed] [Google Scholar]
- Terranova J-P, Chabot C, Barnouin M-C, Perrault G, Depoortère R, Griebel G, et al. SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist, alleviates disturbances of novelty discrimination in rats, a model of selective attention deficit. Psychopharmacology. 2005;181:134–144. doi: 10.1007/s00213-005-2268-5. [DOI] [PubMed] [Google Scholar]
- Vacher B, Cuisiat S, Koek W, Colpaert F.3-(Cyclopenten-1-yl)-benzyl-or 3-(Cyclopenten-1-yl)-heteroarylmethyl-amine derivatives and use thereof as medicines for treating schizophrenia 2002. Patent # WO 2004/035561 A1. Priority 16 October 2002
- Wadenberg ML, Ahlenius S. Antipsychotic-like profile of combined treatment with raclopride and 8-OH-DPAT in the rat: enhancement of antipsychotic-like effects without catalepsy. J Neural Transm (Gen Sect) 1991;83:43–53. doi: 10.1007/BF01244451. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Kapur S, Soliman A, Jones C, Vaccarino F. Dopamine D2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology. 2000;150:422–429. doi: 10.1007/s002130000466. [DOI] [PubMed] [Google Scholar]
- Wolf W. DU-127090 Solvay/H Lundbeck. Curr Opin Investig Drugs. 2003;4:72–76. [PubMed] [Google Scholar]
- Yoshida K, Sugita T, Higuchi H, Hishikawa Y. Effects of tandospirone on tardive dyskinesia and parkinsonian symptoms. Eur Psychiatry. 1998;13:421–422. doi: 10.1016/S0924-9338(99)80690-7. [DOI] [PubMed] [Google Scholar]